Abstract
Four experiments tested the hypothesis that movement-induced discharge of somatosensory receptors attenuates cutaneous reflexes in the human lower limb. In the first experiment, cutaneous reflexes were evoked in the isometrically contracting tibialis anterior muscle (TA) by a train of stimuli to the tibial nerve at the ankle. The constancy of stimulus amplitudes was indirectly verified by monitoring M waves elicited in the abductor hallucis muscle. There was a small increase in the reflex excitation (early latency, EL) during passive cycling movement of the leg compared with when the leg was stationary, a result opposite to that hypothesized. There was no significant effect on the magnitude of the subsequent inhibitory reflex component (middle latency, ML), even with increased rate of movement, or on the latency of any of the reflex components.
In the second experiment, the two reflex components (EL and ML) elicited in TA at four positions in the movement cycle were compared with corresponding reflexes elicited with the limb stationary at those positions. Despite the markedly different degree of stretch of the leg muscles, movement phase exerted no statistically significant effect on EL or ML reflex magnitudes.
In the third experiment, taps to the quadriceps tendon, to elicit muscle spindle discharge, had no effect on the magnitude of ML in TA muscle. The conditioning attenuated EL magnitude for the first 110 ms. Tendon tap to the skin over the tibia revealed similar attenuation of EL.
The sural nerve was stimulated at the ankle in the fourth experiment. TA EMG reflex excitatory and inhibitory responses still showed no significant attenuation with passive movement. Initial somatosensory evoked potentials (SEPs), measured from scalp electrodes, were attenuated by movement.
The results indicate that there is separate control of transmission in Ia and cutaneous pathways during leg movement. This suggests that modulation of the cutaneous reflex during locomotion is not the result of inhibition arising from motion-related sensory receptor discharge.
Cutaneous reflexes are altered during human activities such as cycling (Brown & Kukulka, 1993) and walking (Van Wezel et al. 1997; Zehr et al. 1998). Whether increased somatosensory receptor discharge contributes to these alterations is not clear. The matter needs clarification, to delimit the control sources accounting for the movement-induced reflex modulation.
Reflexes arise from stimulation of cutaneous nerves of the human leg at non-nociceptive intensities. They are expressed as excitatory and inhibitory responses in many leg muscles (Duysens et al. 1996). Typically, latencies of excitatory responses occur as early as 45 ms post-stimulus, with inhibitory response latencies in some muscles ranging between 70 and 120 ms (Duysens et al. 1993). There are individual differences in reflexes between muscles (arising from stimulation of different nerves), i.e. local signs (for review, see Brooke et al. 1997a). Both types of response, EL and ML, are modulated during active movements such as walking and pedalling, compared with responses obtained when subjects sit or stand (see review, Brooke et al. 1997a). Activity of the muscle is necessary in most cases for the reflex response to be observed in the electromyogram (EMG). As the reflex response is related to the position of the limb in the cycle of movement (Yang & Stein, 1990; Duysens et al. 1990, 1996) and does not appear to relate directly to the excitability of the motoneuronal membrane, as reflected by the ongoing EMG, it has been speculated that the reflex modulation arises from control by central pattern generating circuits (Duysens et al. 1990, 1996). However, information on the role of motion-related sensory discharge has not been available, a deficit that has recently been specifically noted (Duysens et al. 1996). Remedying this deficit was the focus of the present work.
Sensory receptors discharge in muscle, tendon and joint due to movement. This discharge has been strongly associated with reflex gain attenuation and with phasic modulation for Ia monosynaptic autogenic reflexes, as evidenced by modulation of H reflex magnitudes (see the review of Brooke et al. 1997a). Passive movement of the leg (Misiaszek et al. 1995a;Brooke et al. 1995a) or leg segments (Brooke et al. 1993) is associated with very powerful attenuation of the magnitude of the reflex excitation, even to complete suppression in some cases. Phasic discharge of the muscle spindle activated by the movement appears to be a significant initiator of this attenuation (Cheng et al. 1995a,b;Misiaszek et al. 1995b). Premotoneuronal, probably presynaptic, inhibition has been strongly implicated in this proprioceptive reflex attenuation (Misiaszek et al. 1995a;Cheng et al. 1995b). Experiments on acute anaesthetized dogs (Misiaszek et al. 1995b) and on humans (Brooke et al. 1995b) have shown that the attenuation can be elicited over a spinal route.
It seemed likely that such movement-induced modulation could also be contributing to the regulation of the longer latency reflexes arising from activation of the cutaneous afferents in the leg. There is early work showing that nociceptive cutaneous reflexes are modulated by passive movement in spinally injured patients (Dimitrijevic & Nathan, 1968). The present study tested the same hypothesis but for the response to non-nociceptive stimuli presented to cutaneous fibres in the tibial or sural nerve at the ankle of the adult, healthy human.
In the first experiment, the proposition was tested that attenuation of the cutaneous reflex EMG activity is correlated with the angular velocity of movement of the leg (and therefore the rate of muscle spindle discharge). Stimuli were delivered at the point in the movement where the knee was most fully flexed. The movement was elicited passively, with a motor driving an ergometer pedal to which the subject's foot was attached. In this way the influence of the descending commands for active muscular generation of the movement pattern were removed. So that the reflex could be observed in the tibialis anterior muscle, that muscle was tonically contracted voluntarily by the subject throughout the leg movements.
The results of the first experiment clearly failed to support the hypothesis. Very little effect of movement velocity was observed in the reflex activity. This suggested that there is a fundamental difference between the movement-induced modulations of autogenic Ia and of cutaneous afferent reflexes. The issue was studied with two further techniques. The propositions were tested that (a) the cutaneous reflex activity is modulated over the cycle of slow passive pedalling movement (putatively reflecting the discharge of a set of somatosensory receptors activated at particular phases in the movement cycle), and (b) stretch of the quadriceps muscle by tendon tap elicits sustained attenuation of the cutaneous reflex activity (as in the case of soleus H reflexes; Cheng et al. 1995a).
Neither of these propositions were supported by the data. Thus, cutaneous and H reflexes appear to be conditioned quite differently by movement-induced sensory discharge. The matter was further explored through stimulation of a different nerve, the purely cutaneous sural nerve. Again, passive movement did not significantly alter the reflex in TA. In this latter study, when the biopotentials occurring at the scalp (SEPs, somatosensory evoked potentials) were observed, there was attenuation with movement, for SEPs elicited by either trains of stimuli or single pulses.
From the four experiments conducted it is clear that the reflex EMG in the tibialis anterior muscle, following trains of stimuli which activate cutaneous nerve fibres, is not conditioned by the sensory receptor discharge elicited when the leg is passively moved. In contrast, there is attenuation of the response to such stimuli at scalp electrodes over the somatosensory cortex. Part of this work has been published in abstract form (Brooke et al. 1997b).
METHODS
Subjects
Twenty-one volunteer subjects (21-36 years) of both sexes participated in the experiments. Each subject gave informed consent and none reported any history of neurological, cardiovascular, or musculoskeletal deficits. The experimental procedures were approved by the University of Guelph Ethics Committee.
Protocol
For each experiment, subjects sat with the head, neck, back and arms supported in a slightly reclined customized metal chair positioned behind a modified cycle ergometer. The right foot was strapped to the right pedal of the cycle with the ankle firmly held at 90 deg by a clinical brace. Rotation of the ergometer pedal crank was accomplished by using a variable speed motor connected by a belt to the ergometer chain wheel (accuracy ± 0·1 r.p.m.). This allowed the right leg to be moved passively, or to be held in a static posture with specific hip and knee angles. The left foot remained in a stationary supported position throughout the experiments, with hip, knee, and ankle angles of approximately 100 deg. For all experiments, subjects were instructed to maintain a mild isometric contraction of the TA muscle (approximately 10 % of maximum voluntary isometric contraction) throughout the experimental trials, while keeping all other leg muscles relaxed. The low-pass-filtered full-wave-rectified EMG of TA was displayed on an oscilloscope and monitored by subject and experimenter to ensure this consistent level of TA activity. The consistency was subsequently confirmed by analysis of the EMG records. Fatigue was prevented by allowing the subjects to relax the TA muscle between experimental conditions.
Cutaneous reflex muscle activity was evoked in TA by electrical stimulation of either the tibial or sural nerve at the ankle. For experiments 1-3, stimuli were delivered via bipolar surface electrodes placed with the anode distal over the predicted path of the right tibial nerve posterior to the medial malleolus. TA reflex activity was elicited from delivery of three 0·3 ms square wave pulses separated by 10 ms applied to the tibial nerve (De Serres et al. 1995), using a Grass S88 stimulator with SIU5 stimulus isolation unit. The voltage-controlled stability of the stimulus delivered to the tibial nerve was confirmed by maintaining a small constant M wave in the EMG of abductor hallucis which resulted from direct stimulation of motoneuronal axons serving that muscle, using a single 1 ms square wave test pulse. Trials using this single pulse to evoke M waves were interdigitated randomly among the trials featuring stimulus trains used to evoke cutaneous reflexes. In experiments 4 and 5, the electrodes were placed posterior to the lateral malleolus of the right ankle, approximating the position of the sural nerve. The sural nerve stimulation consisted of a train of five 1 ms square wave pulses with an interstimulus interval of 4 ms (Duysens et al. 1996). The intensity of the stimulus delivered to the sural nerve (a purely cutaneous nerve in humans) was based on the subject's perceptual threshold (PT, the voltage at which the stimulus was first perceived by the subject). Stimulation intensity was 2·5 × PT (Duysens et al. 1992). The stability was checked by periodic re-evaluations of the perceptual threshold as the conditions progressed over the course of the experiment.
EMG recordings of tibialis anterior and soleus muscles were obtained with surface Ag-AgCl electrodes oriented longitudinally approximately 2 cm apart over the predicted path of the muscle fibres. Electroencephalographic recordings for experiment four were made with scalp electrodes in an Electro-Cap system (ECI, Eaton, OH, USA). SEPs were recorded from Cz ‘(2 cm caudal to Cz) referenced to Fpz’ (2 cm caudal to Fpz) in accordance with the International 10-20 system. Impedance at all EMG and EEG recording and stimulation sites was less than 10 kΩ and 3 kΩ, respectively, measured at 30 Hz (Grass EZM5 impedance meter). EMG and EEG recordings were amplified (1000 × and 40 000 ×, respectively) and filtered (bandpass 3-300 Hz, 1-100 Hz, respectively) with isolated bioelectric amplifiers (Grass P511 and SA Instrumentation, respectively). The data were digitized at an analog computer interface (Keithley DAS8), at a sampling rate of 1000 Hz, and were stored on a computer for subsequent analysis.
In the first experiment, 20 triplet stimuli and 10 single-pulse stimuli (the latter interspersed between trains for M wave monitoring) were delivered to the right tibial nerve for each movement condition (30, 60 and 90 r.p.m. passive movement of the leg, or stationary) in six subjects. The stimuli were presented at the same phase of the cycle in each trial (0 deg, which was the point of fullest knee flexion). Each condition was represented twice in the order of presentation of movement conditions to subjects, i.e. a replicated Latin square design.
In the second experiment, 30 tibial nerve triplet stimuli were randomly presented in a Latin square design at one of four equally spaced positions throughout the pedal cycle (close to the fullest flexion, 340 deg, and extension, 160 deg, and intermediary between these two, at 70 deg and 250 deg), to eight subjects. Stationary, non-movement controls consisted of blocks of 20 trials of triplet stimuli applied at each of the four different phase positions. Movement trials involved 20 r.p.m. passive movement applied to the leg. This slow rate of movement was selected to allow detection of phase-related presynaptic effects, which at higher rates of movement could remain between one measurement point and the next (Cheng et al. 1995a). The order of the phase positions was randomized across subjects. Single pulse M wave stimuli at each phase position were randomly interdigitated throughout movement and stationary controls.
In the third experiment, for five subjects the right leg was immobilized with a hip angle of 80 deg, and a knee angle of 90 deg. The patellar ligament (tendon of quadriceps) was tapped briskly with a tendon hammer, with the head of the tendon hammer perpendicular to the ligament. This evoked a stretch reflex in vastus medialis. The reflex amplitudes were monitored during the experiment to ensure that they remained consistent throughout the experiment. The head of the tendon hammer contained a pressure-sensitive electrical contact switch that was activated upon contact with the knee. In this manner, the timing of the tibial nerve stimulus could be controlled relative to the tap onset. Triplet stimuli were activated from the hammer switch. The delay in closing the switch was approximately 10 ms. Including this delay, the stimuli were delivered after the start of the skin contact at 10, 20, 35, 60, 85, 110, 410 and 1010 ms. Twenty triplet stimuli were given with each delay, the presentation order of delays being random. The stimulus intensity was again controlled for by using single-pulse stimuli randomly dispersed throughout the trials. Among three of the subjects the experiment was repeated using a tap to skin over the tibia below the patellar ligament (as opposed to tapping on the ligament), so as not to cause a stretch reflex in the vastii muscle.
In the fourth experiment, randomly presented blocks of 40 sural nerve stimuli were given for each task condition (no movement and passive 60 r.p.m. movement) and were repeated (total of 80 samples for each condition). The order of presentation was random by Latin square over the four subjects. The blocks consisted of either five-pulse train stimulation evoking SEPs and TA reflex activity, or single-pulse stimulation intended only to evoke SEPs.
Data analysis
From a subject's control trials, the onset latencies and durations of EL and ML responses were established and applied to all trials, control and movement, for that subject. All filtered raw EMG data were full-wave rectified prior to averaging. A total of 20 samples were averaged for each condition. There was a period of 30 ms of pre-stimulus data that was used to establish the background level of tonic activity in the muscle. The variance over the 30 ms interval was used to establish a 95 % confidence band about the mean tonic EMG activity over this pre-stimulus period. This confidence band was used to determine the occurrence of significant reflex excitation (EL) or inhibition (ML) which occurred following the stimulation. Activity that exceeded the band was denoted as excitation and activity below the band was labelled as inhibition. EL and ML responses were considered independent, in part, because it was possible to identify the presence of ML responses without any preceding EL excitation. The ML responses were, in comparison to EL, far more robust, occurring in all subjects and task conditions and was, as a result, considered the main component of the reflex response. In contrast, EL was occasionally absent in individual subjects or specific task conditions.
Response latencies of excitation and/or inhibition were determined by identifying the point at which the activity was equal to the mean tonic activity when working backwards from the point at which the activity exceeded the 95 % band. The termination of the response was similarly determined by denoting the point at which the activity returned to the mean tonic level after re-entering the 95 % confidence interval. Response durations were calculated for discrete bursts of excitation and/or inhibition by subtracting the onset latency from the timing of response termination. The magnitude of responses was calculated by determining the area, over the response duration, of the full-wave-rectified activity subtracted from the mean level of tonic EMG activity (see Fig. 1). The tonic level of contraction was also calculated for individual trials and used as an indication of the constancy of the background activation in tibialis anterior.
Figure 1. Average EMG responses to tibial nerve stimulation.
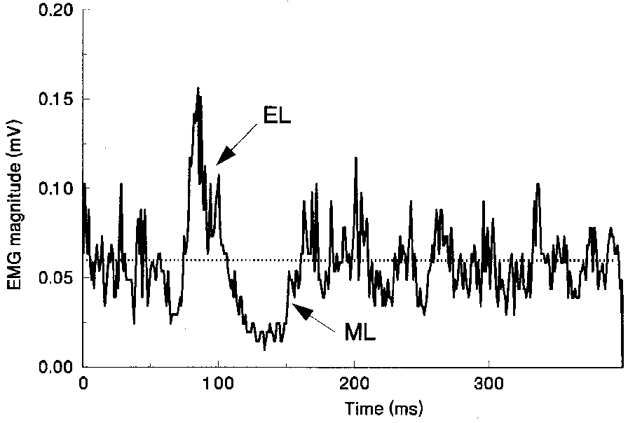
Averaged full-wave-rectified EMG responses in tibialis anterior to triplets of electrical stimuli applied to the tibial nerve to evoke the cutaneous reflex in one subject. Two components of the reflex, EL (excitation) and ML (inhibition) are denoted relative to the mean prestimulus tonic activity (dotted line) in the muscle.
Responses with more synchronous waveforms, including M waves and SEPs, were analysed by measuring the peak-to-peak amplitudes from the raw, non-rectified traces. Peak-to-peak amplitudes of M waves evoked in response to single-pulse stimulations measured in abductor hallucis were calculated for each sample. SEP traces showing large amplitude peaks representing EMG activity or eye movements, or large DC shifts, were rejected. The remaining, artifact-free, SEP traces were averaged across conditions and subjects for experiment four. From these average traces, peak-to-peak amplitudes were measured for the initial responses, a positive deflection (P1) followed by a negative deflection (N1) peaking at approximately 50 and 80 ms, respectively. When necessary the data were log transformed to conform to the assumption of homogeneity of variances and so that the residual errors were normally distributed. Such log transformation was necessary on three occasions within different experiments.
Statistical differences between means of EMG reflex magnitudes and SEPs for conditions were assessed at P = 0·05 by one- or two-way analyses of variance, blocked on subjects, followed by a priori (i.e. planned) comparisons of means with Bonferroni t corrections which utilized the appropriate analysis of variance error term.
RESULTS
Experiment 1: rate of passive movement of the leg and reflex response in tibialis anterior following tibial nerve stimulation
Raw and full-wave-rectified reflex EMG traces are shown in Fig. 2A for control and movement conditions for one subject. It can be seen that at approximately 70 ms after the start of the train of three stimuli, there is reflex excitation in the TA muscle (an EL response), followed by strong inhibition that has an onset latency of approximately 110 ms (ML). The mean latencies (with standard deviations) over the group of subjects for EL and ML were 67·9 ± 12·3 and 105·2 ± 15·6 ms, respectively. In this, and all experiments that follow, there were no statistically significant differences due to the varied task conditions in latencies for either EL or ML.
Figure 2. Rate of passive movement effects on reflex magnitudes.
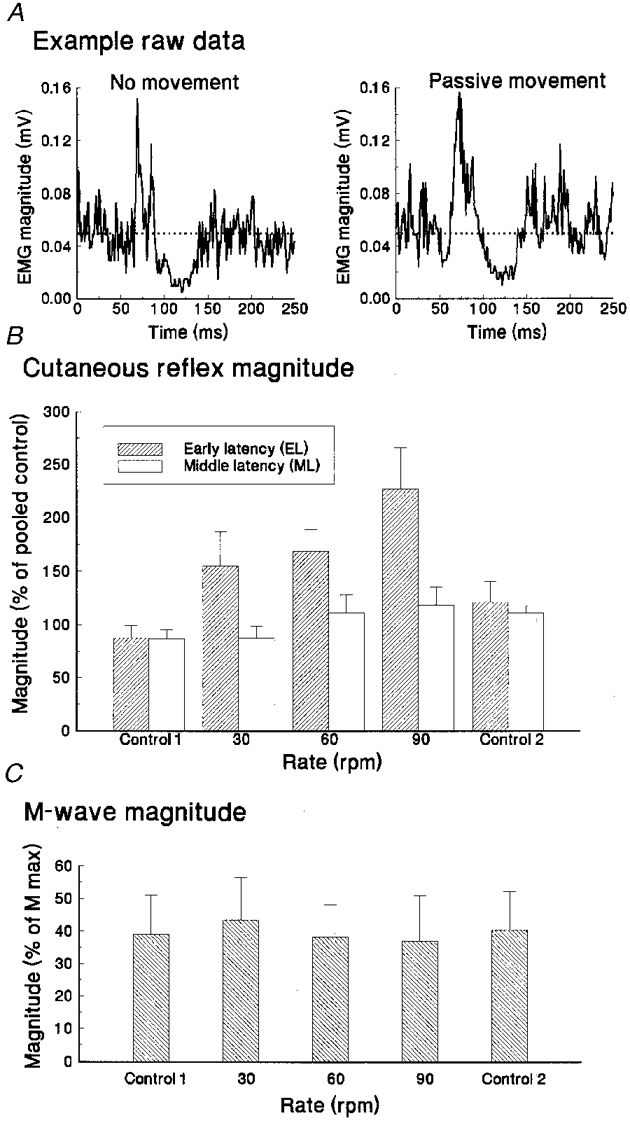
A, averaged full-wave-rectified tibialis anterior EMGs for movement and non-movement (stationary) task conditions from a single subject. B, average EL and ML reflex magnitudes (and standard deviations) for the group of five subjects, over control and movement conditions (30, 60 and 90 r.p.m.). C, group average M wave response magnitude, measured from the EMG of abductor hallucis muscle, sampled from interdigitated single-pulse trials.
Over the group of five subjects, the mean magnitudes of the two reflex components in the TA muscle are shown in Fig. 2B for the control and movement conditions. Mean EL activity was significantly increased (F (2,4) = 4·49, P= 0·02) during passive movement compared with reflexes evoked during stationary control. This direction of change was the opposite of that hypothesized. Mean ML activity showed no statistically significant change (F (2,4) = 2·10, P= 0·13).
There were no statistically significant differences between means of M wave amplitudes for the four phase positions at rest or for movement (Fig. 2C) or for the level of tonic contraction levels for TA over conditions (F (2,4) = 1·20, 0·75, P= 0·13, 0·54), respectively.
Experiment 2: phase of leg movement and reflex response in tibialis anterior following tibial nerve stimulation
As the cycle of leg movement develops, different phases are associated with the activation of different sensory receptors. To assess the putative influence of such receptor discharge on the cutaneous reflex pathway, stimuli were delivered at four equidistant phase positions in the cycle of movement and also with the leg static in the posture for each phase point. The mean magnitudes of the resulting reflex components in tibialis anterior from eight subjects are shown in Fig. 3A. There were no significant differences in the magnitudes of EL or ML responses as a function of phase position for the delivery of stimuli (F (3,7) = 0·23, 0·43, P= 0·88, 0·73, respectively).
Figure 3. Movement phase effects on reflex magnitudes.
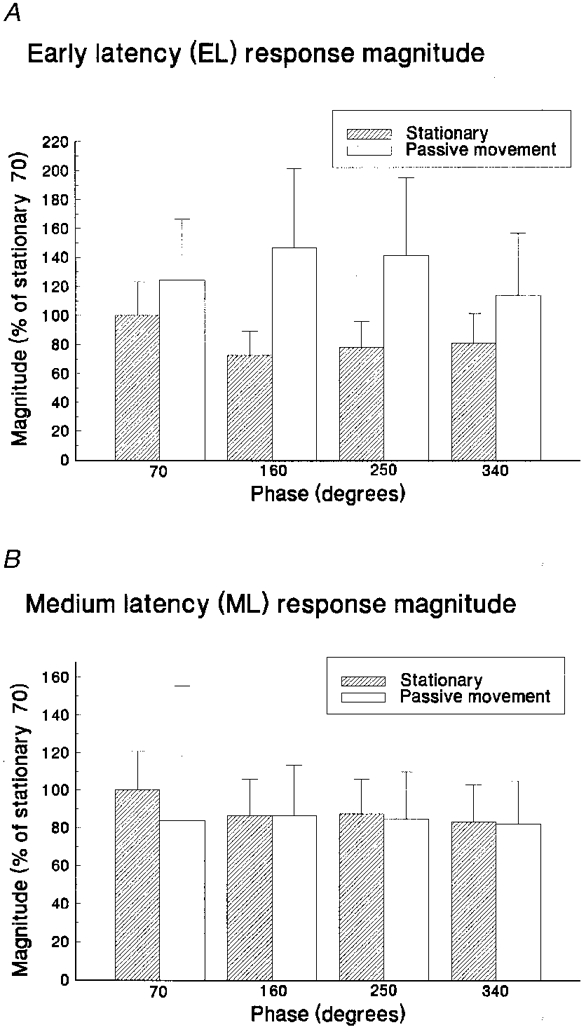
Average reflex magnitudes (and standard deviations) for stationary and passive movement task conditions measured at different phase positions (360 deg, fullest flexion; 180 deg, fullest extension). Excitatory (EL) and inhibitory (ML) reflex magnitudes are displayed in A and B, respectively. Responses are expressed as a percentage of the average magnitude measured at 70 deg during stationary control trials.
The mean magnitudes of the M waves, from interdigitated single-pulse trials, were not significantly different across the task conditions (F (3,7) = 0·3, P= 0·77). Tonic contraction levels sampled in the EMG of TA prior to stimulus delivery also were not significantly different across conditions (F (3,7) = 0·7, P= 0·51).
Experiment 3: quadriceps tendon tap and reflex response in tibialis anterior following tibial nerve stimulation
The tap conditioning was delivered at different intervals before the test (cutaneous) stimulus. The amplitude of this conditioning stimulus, as represented by the amplitude of the stretch response in the vastii muscles, was monitored during the experiment to ensure constancy of the stimulus. In the TA muscle, the EL reflex response was significantly attenuated by the conditioning (F (8,25) = 6·09, P = 0·002) for all stimulus delays less than 400 ms (see Fig. 4A, dashed line). The conditioning did not significantly affect the ML reflex magnitude at any time point (F (8,32) = 1·30, P = 0·28) (see Fig. 4B, dotted line).
Figure 4. Mechanical tap effects on reflex magnitudes.
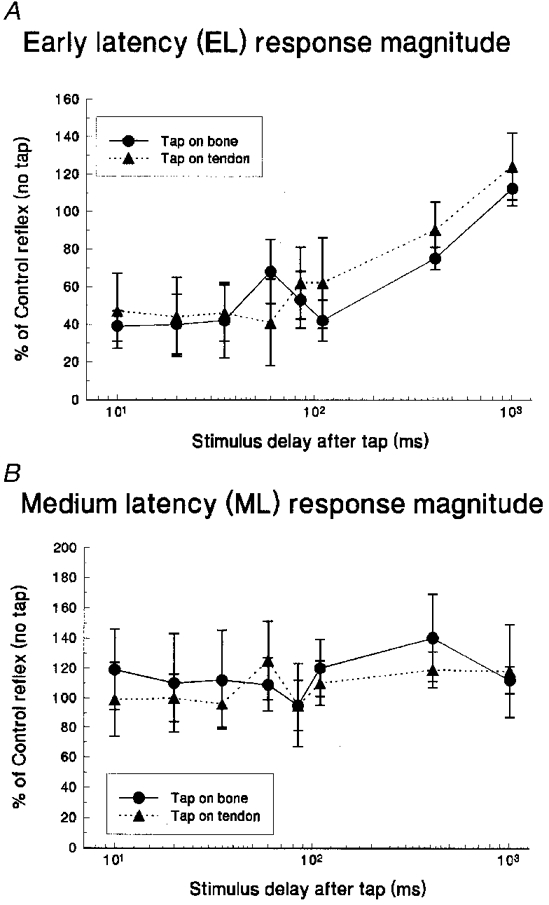
Average reflex magnitudes (and standard deviations) measured in the EMG of TA muscle at different intervals after the initiation of tapping of skin over the patellar ligament or over the tibia. Excitatory (EL) and inhibitory (ML) reflex magnitudes from tibial nerve stimulation are displayed on A and B, respectively. Responses are expressed as a percentage of the average magnitude measured without any conditioning tap.
The depression of EL with ligament tap suggested a conditioning effect from vastii muscle spindles. Yet this was contrary to the interpretation of the first two experiments. The procedure was therefore repeated on three subjects, but this time with the taps applied to the skin over the tibial bone away from the TA tendonous tissue. There was no activation of vastii muscles in response to the bone tap, indicating a lack of activation of muscle receptors in this case. There continued to be significant depression of EL (Fig. 4A). The similarity in the EL attenuation between the conditioning stimuli applied to ligament and to bone suggested that any conditioning influence of tapping appears to arise from cutaneous receptors rather than the stretch of vastii muscle spindles.
M wave magnitudes collected with interspersed single-pulse stimulation were consistent throughout experimental trials and were not significantly different across the duration of the experiment (when it was divided into four different time intervals, to provide averages of adequate size) (F (3,12) = 0·84, P = 0·50). As with the previous experiments, the variability of the M wave magnitudes was low (mean within-subject coefficient of variation was 17 %) indicating the stability of the electrical stimulus between task conditions. Similarly, tonic contraction levels sampled in the EMGs of the TA muscle prior to stimulus delivery were not significantly different across conditions (F (9,36) = 1·06, P = 0·42).
Experiment 4: passive movement of the leg, and SEPs and TA reflexes elicited from sural nerve stimulation
SEPs were elicited by single pulses or by trains of five pulses of stimulation, with the leg at rest and when passively moved at 60 r.p.m. The single pulses were randomly interdigitated with the trains. Figure 5 shows SEP data from a single subject averaged from 80 samples for both movement and the resting control state for single-pulse (Fig. 5A) and train (Fig. 5B) stimuli. The earliest record of the arrival of the stimulus at the somatosensory cortex is shown by P1-N1. On average the latency of the P1 peak was 50·7 ± 1·2 ms. There were significant differences between mean SEP amplitudes when comparing rest with passive movement conditions (F (1,3) = 9·63, P = 0·05). On average the magnitude of the P1-N1 potential was reduced by approximately 70 % when conditioned by passive movement (Fig. 5C). There were no significant differences between the single and train stimulation conditions.
Figure 5. Passive movement effects on sural nerve SEP magnitudes.
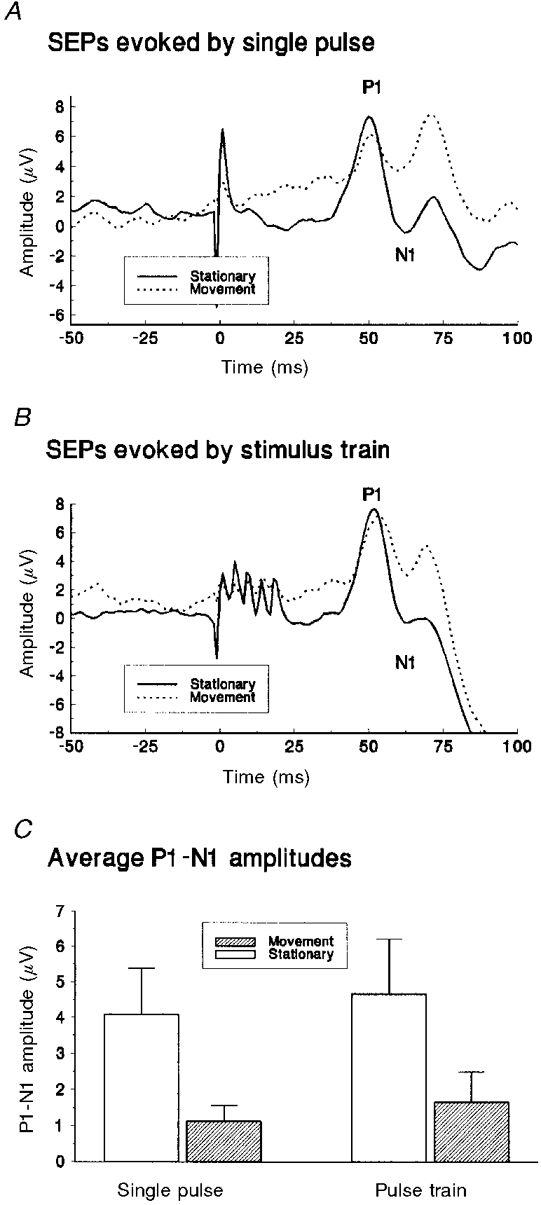
Average somatosensory evoked potentials from sural nerve stimulation, measured from a single subject for movement and non-movement (stationary) task conditions. Responses following a single pulse and those from a train of stimuli are shown in A and B, respectively, with 0 ms indicating the start of stimulation. C, averaged P1-N1 response magnitudes for a group of four subjects, following single and train pulses for movement and non-movement (stationary) task conditions.
EL and ML reflex EMG components in TA muscle were less easily evoked from the sural nerve, especially the early excitation. Two of nine subjects showed EL components, the amplitudes of which did not change significantly during passive movement (F (1,1) = 1·73, P = 0·41). All nine subjects showed ML responses and again there was no change in the mean amplitude of this component during passive movement (F (1,8) = 0·17, P = 0·69).
The tonic contraction levels sampled in the EMGs of the TA muscle prior to stimulus delivery were not significantly different across conditions (F (1,8) = 0·75, P = 0·55).
DISCUSSION
Four experimental approaches were used to test whether passive movement-induced attenuation occurred in a cutaneous reflex of the human leg. The hypothesis was that sensory receptor activation as a consequence of passive movement would attenuate these reflexes. This hypothesis was advanced as an attempt to generalize from a substantial body of work showing such attenuation in the H reflex pathway (Brooke et al. 1997a). The generalization and hypothesis were not supported by the present results. Specifically, neither the early excitatory component of the reflex (EL) nor the subsequent inhibitory component (ML), in the tonically contracted tibialis anterior muscle following stimulation of the tibial nerve at the ankle, were attenuated by (1) passive movement at different speeds, (2) different leg postures during the passive movement, representing different states of somatosensory receptor discharge in the leg, or (3) activation of quadriceps muscle spindles by tapping the patellar ligament. Attenuation arising consequent to passive movement was not seen with stimulation of either the tibial or sural nerves.
The rejection of this hypothesis is important because it clears the way for a conceptual split between the control of transmission in H reflex (putatively Ia monosynaptic; Misiaszek et al. 1995c) and cutaneous reflex pathways in the human leg, where until recently the view was that they were modulated similarly, and through similar mechanisms (Dietz, 1992). Rather, there is gain control during movement that is specific to the sensory modality. Ia-mediated reflexes are substantially attenuated by concomitant motion-related sensory discharge. Such discharge will occur whenever leg movement is of sufficient amplitude and frequency to exceed that of normal stance. Even rates of pedalling movement as low as one every 6 s (10 cycles min−1) appear to be sufficient to evoke inhibition believed to be mediated by presynaptic inhibition acting on the Ia afferent axon close to the motoneuronal synapse (Brooke et al. 1997a). Sensori-sensory conditioning in relation to specific behaviour is seen in both vertebrates and invertebrates (Prochazka, 1989; Watson, 1992). In mammals, presynaptic control of peripheral afferent transmission during locomotion can also occur through input from the central pattern generator (Dubuc et al. 1988). However, recent work on the decerebrate cat suggests that the route from sensory receptor discharge is the more important one for the attenuation of the Ia fibres in the monosynaptic path during movement (Gossard, 1996). It is likely that this ‘automatic’ attenuation establishes a baseline suppression of that Ia pathway, upon which further modulation can be overlaid from more complex centres of control when active walking or pedalling occurs.
The cutaneous reflexes studied did not show this attenuation arising from motion-related sensory discharge. Such baseline suppression appears not to occur for the cutaneous reflex and is perhaps not functionally necessary. The reflex usually requires ongoing contraction for it to be expressed in the muscle, whereas this is not a requirement for the stretch reflex, which can exert powerful muscular effects, even at phases during active locomotion featuring inactivity of soleus (Yang et al. 1991; Sinkjaer et al. 1996). Accumulating evidence has led to the proposal that the modulation of cutaneous reflex amplitudes, during active locomotor movement of the legs in humans, arises from the involvement of the central pattern generator for locomotion (Duysens et al. 1992, 1996). The latter paper came to this conclusion but cautioned that the data did not rule out a role for motion-related sensory input. The absence of attenuation of reflex gains, in response to passive movement, further implicates the spinal cord and brainstem network of oscillatory interneurons for locomotion as the likely cause of the cutaneous reflex modulation.
There is a qualifier to the above. If sensory discharge evoked by active movement led to postsynaptic effects on the motoneurons in the cutaneous reflex pathway, our experiments would not have detected them. This is because contraction of the muscle is necessary in most cases for the reflex to occur. Tonic contractions would tend to stabilize the background activation of motoneurons, compensating for fluctuating postsynaptic effects. On the other hand, there were no marked EMG fluctuations in the voluntary tonic contractions as might be expected if postsynaptic inhibition resulted from the stimulus.
In contrast to the lack of movement-related attenuation of the cutaneous reflexes noted above, the experimental procedures did evoke two changes in the early reflex excitation, EL. When the tap was delivered to the skin over the tibia or over the patellar ligament, EL was powerfully attenuated for approximately 110 ms after the conditioning stimulus. This was evidently mediated by cutaneous and not muscle receptors, which would have been differentially activated by tapping at these two sites. Inhibition of TA from stimulation of the skin adjacent to the kneecap has been reported by Hagbarth (1960). We therefore suspect that the attenuation of EL is sensori-sensory conditioning within the cutaneous modality. The effect could be oligosegmental or brainstem mediated (Gonzalez et al. 1993). As the onset latency is brief, we prefer the segmental interpretation.
The second change in EL was an increase in amplitude with a high rate of passive movement of the leg. It is not presently clear from which sensory sources this facilitation arises. The lack of significant change in EL at different phases of the movement suggests that muscle stretch receptor and joint receptor discharges do not influence EL at the levels of muscular activity used in the studies presently reported, and thus do not account for the increase in EL with high velocity of movement. This facilitation of EL may be the result of the sum of cutaneous discharge from the leg at those rates of movement. Such discharge can facilitate H reflexes through partially alleviating presynaptic inhibition of Ia afferents (Iles, 1996). This is thought to occur via spinal interneurons which are also activated during corticospinal suppression of presynaptic inhibition. An alternative to the alleviation of presynaptic inhibition is that the high velocity of movement might have activated some Golgi tendon organs whose discharge led to facilitation of the cutaneous reflex excitation.
Cortical SEPs evoked by single cutaneous stimuli and by trains of five stimuli were both attenuated by the passive movement of the leg. This replicates and extends other recent work from our laboratory on SEPs from cutaneous afferents of the lower limb (Brooke et al. 1997c). This movement-induced attenuation of cortically evoked responses to sural nerve stimulation, with absence of movement-induced changes in reflex responses measured from the TA muscle, suggests a difference in control of sensory paths during passive movement. This split may be related to a similar difference in processing of afferent input seen between SEPs and cutaneous reflexes when stance is perturbed in different ways (Dietz et al. 1989). The two control mechanisms, for cutaneous reflexes and for SEPs, could both be exerted at thalamo-cortical levels, as the reflex responses we studied in TA appear, at least in part, to follow a transcortical path (Nielsen et al. 1997) and upper limb cutaneous input to somatosensory cortex is reported to be attenuated at the thalamo-cortical level by passive movement in the monkey (Chapman et al. 1988).
There is a depression of SEPs in the early stance phase of human walking (Duysens et al. 1995), and the responsiveness of motor cortical cells to cutaneous stimulation is also depressed at this phase of the locomotor cycle in the cat (Palmer et al. 1985). Similar mechanisms may underlie our present results. The attenuation of SEPs seen in early stance is felt to arise from somatosensory receptor discharge consequent to a phase of the movement itself (Duysens et al. 1995). The effect could be occlusion from concomitant activation of receptors in the feet (though occlusion is likely not to be the case at the cortical receptive cell; Palmer et al. 1985) or through other receptor discharge.
The observation that taps applied to the skin attenuated the EL component of the reflex response and that movement attenuated cortical SEPs, suggests that technical deficiencies were not the cause of the failure to support the hypothesis that movement-related reafference attenuates cutaneous reflexes. The reflexes could be elicited clearly and conditioning effects could be demonstrated, but not the attenuation that was hypothesized. The data from the interpolated M wave procedure provided reassurance that the stimulus stayed constant through the trials. Similar reassurance came from the periodic checking of perceptual threshold when stimulating the purely cutaneous sural nerve. A tonic contraction in the target muscle was necessary for the reflex to be expressed and the level of this contraction was controlled within a narrow band. It is unlikely that the pressure of the ankle brace played a major role in the failure of the hypothesis since the use of such a brace was present in some, but not all the experiments where movement strongly attenuated H reflexes (Brooke et al. 1997a).
The work currently reported has focussed on the muscle most frequently studied in this field of cutaneous reflexes, tibialis anterior. It was easiest for subjects to maintain a stable tonic contraction in this muscle. We do note, though, that the response in tibialis anterior is one of the most clearly modulated of all the muscles studied during walking and running (Duysens et al. 1990, 1996; Yang & Stein, 1990; De Serres et al. 1995). The absence of attenuation of reflex gain in tibialis anterior, due to motion-related sensory discharge, is a serious failure of the research hypothesis. However, the present work is limited to responses evoked in a single, albeit responsive, muscle using electrical stimulation of posterior tibial or sural nerve. Further studies will need to extend the observations to determine the extent of generalization to alternative muscles and stimulation protocols.
One physiological concept that may emerge from such work is the differential modulation of muscle spindle and cutaneous pathways during ongoing movement. Cutaneous reflexes in the human leg are strongly modulated through the action of the central pattern generator during movement, even reversing in sign (Yang & Stein, 1990) or over muscles (Duysens et al. 1990), but appear from this study not to be inhibited by the sensory discharge due to the movement itself. Conversely, the H reflex is strongly attenuated by that motion-related sensory discharge (Brooke et al. 1997a) but the attenuation is probably less attributable to the central pattern generator (Gossard, 1996). This split between the two afferent groups in their susceptibility to conditioning via movement-induced sensory discharge may carry functional implications.
The Ia component of the stretch reflex contributes to static bipedal balance, especially in the soleus muscle. Studies of stretch reflex activation, with balance perturbations that are imperceptible to the subject, reveal the sensitivity of this part of the neural control of upright posture (Fitzpatrick et al. 1992, 1996) at a time when the reflex gain is high. Such high gain is functionally appropriate, as quiet, upright stance is maintained substantially by forward sway which is corrected by plantar flexion, to which soleus is a major contributor. However, soleus H reflexes are significantly depressed once the amplitude of movement exceeds that of upright posture, as for example in passive stepping (Brooke et al. 1995a). This attenuation appears only to be released transiently when central commands, such as those for active locomotion, elicit force in the autogenic, and possibly close synergistic, muscles. This default foundation of attenuation is held to have a strong presynaptic inhibitory component.
The muscular discharge arising from activation of afferents serving cutaneous receptors of the lateral border and plantar surface of the foot (the sural and tibial nerve dermatomes) is not attenuated for larger amplitude passive movements such as those of pedalling. There is no evidence of additional presynaptic inhibition. Rather, the cutaneous afferent discharge may proceed forward to sites where it is modulated by the complex interneuronal circuits of the central pattern generator. Perhaps the cutaneous receptor discharge during locomotion is an integral tonic part of the production of the locomotor pattern (Pearson, 1993). As such, afferents transmitting from the cutaneous receptors might be controlled from other modules in the pattern generating network and be less susceptible to presynaptic inhibition from movement-induced sensory conditioning.
Acknowledgments
We acknowledge grant support from The ALS Society of Canada to J. D. B., from the Natural Sciences and Engineering Research Council of Canada to J. D. B. and to W. E. M. and Graduate Scholarship support to W. R. S., and from the National Institute of Health to W. E. M.
References
- Brooke JD, Cheng J, Collins D, McIlroy WE, Misiaszek J, Staines WR. Sensori-sensory afferent conditioning with leg movement: gain control in spinal reflex and ascending paths. Progress in Neurobiology. 1997a;51:393–421. doi: 10.1016/s0301-0082(96)00061-5. [DOI] [PubMed] [Google Scholar]
- Brooke JD, Cheng J, Misiaszek JE, Lafferty K. Amplitude modulation of the soleus H reflex in the human during active and passive stepping movements. Journal of Neurophysiology. 1995a;73:102–111. doi: 10.1152/jn.1995.73.1.102. [DOI] [PubMed] [Google Scholar]
- Brooke JD, McIlroy WE, Angerilli PA, Peritore GF, Staines WR. XXXIIIth International Congress of the Physiological Sciences. Russia: St Petersburg; 1997b. Separation in control of cutaneous versus spinal paths in humans; p. P078·02. [Google Scholar]
- Brooke JD, McIlroy WE, Collins DF, Misiaszek JE. Mechanisms within the human spinal cord suppress fast reflexes to control the movement of the legs. Brain Research. 1995b;679:255–260. doi: 10.1016/0006-8993(95)00239-m. [DOI] [PubMed] [Google Scholar]
- Brooke JD, Misiaszek JE, Cheng J. Locomotor-like rotation of either hip or knee inhibits soleus H-reflexes in humans. Somatosensory and Motor Research. 1993;10:316–325. doi: 10.3109/08990229309028843. [DOI] [PubMed] [Google Scholar]
- Brooke JD, Staines WR, Cheng J, Misiaszek JE. Modulation of cerebral somatosensory evoked potentials arising from tibial and sural nerve stimulation during rhythmic active and passive movements of the human lower limb. Electromyography and Clinical Neurophysiology. 1997c;37:451–461. [PubMed] [Google Scholar]
- Brown DA, Kukulka CG. Human flexor reflex modulation during cycling. Journal of Neurophysiology. 1993;69:1212–1224. doi: 10.1152/jn.1993.69.4.1212. [DOI] [PubMed] [Google Scholar]
- Chapman CE, Jiang W, Lamarre Y. Modulation of lemniscal input during conditioned arm movements in the monkey. Experimental Brain Research. 1988;72:316–334. doi: 10.1007/BF00250254. [DOI] [PubMed] [Google Scholar]
- Cheng J, Brooke JD, Misiaszek JE, Staines WR. The relationship between the kinematics of passive movement, the stretch of extensor muscles of the leg and the change induced in the gain of the soleus H-reflex in humans. Brain Research. 1995a;672:89–96. doi: 10.1016/0006-8993(94)01321-8. [DOI] [PubMed] [Google Scholar]
- Cheng J, Brooke JD, Staines WR, Misiaszek JE, Hoare J. Long-lasting conditioning of the human soleus H-reflex following quadriceps tendon tap. Brain Research. 1995b;681:197–200. doi: 10.1016/0006-8993(95)00273-s. [DOI] [PubMed] [Google Scholar]
- De Serres SJ, Yang JF, Patrick SJ. Mechanism for reflex reversal during walking in human tibialis anterior muscle revealed by single motor unit recording. The Journal of Physiology. 1995;488:249–258. doi: 10.1113/jphysiol.1995.sp020963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V. Human neuronal control of automatic functional movements: interaction between central programs and afferent input. Physiological Reviews. 1992;72:33–69. doi: 10.1152/physrev.1992.72.1.33. [DOI] [PubMed] [Google Scholar]
- Dietz V, Horstmann GA, Berger W. Perturbations of human posture: influence of impulse modality on EMG responses and cerebral evoked potentials. Journal of Motor Behavior. 1989;21:357–372. doi: 10.1080/00222895.1989.10735489. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, Nathan PW. Studies of spasticity in man. 3. Analysis of reflex activity evoked by noxious cutaneous stimulation. Brain. 1968;71:349–368. doi: 10.1093/brain/91.2.349. [DOI] [PubMed] [Google Scholar]
- Dubuc R, Cabelguen J-M, Rossignol S. Rhythmic fluctuations of dorsal root potentials and antidromic discharges of primary afferents during fictive locomotion. Journal of Neurophysiology. 1988;60:2014–2036. doi: 10.1152/jn.1988.60.6.2014. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AAM, Murrer L, Dietz V. Backward and forward walking use different patterns of phase-dependent modulation of cutaneous reflexes in humans. Journal of Neurophysiology. 1996;76:301–310. doi: 10.1152/jn.1996.76.1.301. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AAM, Nawijn S, Berger W, Prokop T, Altenmüller E. Gating of sensation and evoked potentials following foot stimulation during human gait. Experimental Brain Research. 1995;105:423–431. doi: 10.1007/BF00233042. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AAM, Trippel M, Dietz V. Phase-dependent reversal of reflexly induced movements during human gait. Experimental Brain Research. 1992;90:404–414. doi: 10.1007/BF00227255. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AAM, Trippel M, Dietz V. Increased amplitude of cutaneous reflexes during human running as compared to standing. Brain Research. 1993;613:230–238. doi: 10.1016/0006-8993(93)90903-z. [DOI] [PubMed] [Google Scholar]
- Duysens J, Trippel M, Horstmann GA, Dietz V. Gating and reversal of reflexes in ankle muscles during human walking. Experimental Brain Research. 1990;82:351–358. doi: 10.1007/BF00231254. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Loop gain of reflexes controlling human standing measured with the use of postural and vestibular disturbances. Journal of Neurophysiology. 1996;76:3994–4008. doi: 10.1152/jn.1996.76.6.3994. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC, Taylor JL, McCloskey DI. Ankle stiffness of standing humans in response to imperceptible perturbation: reflex and task-dependent components. The Journal of Physiology. 1992;454:533–547. doi: 10.1113/jphysiol.1992.sp019278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H, Jimenez I, Rudomin P. Reticulospinal actions on primary afferent depolarization of cutaneous and muscle afferents in the isolated frog neuraxis. Experimental Brain Research. 1993;95:261–270. doi: 10.1007/BF00229784. [DOI] [PubMed] [Google Scholar]
- Gossard J-P. Control of transmission in muscle group Ia afferents during fictive locomotion in the cat. Journal of Neurophysiology. 1996;76:4104–4112. doi: 10.1152/jn.1996.76.6.4104. [DOI] [PubMed] [Google Scholar]
- Hagbarth K-E. Spinal withdrawal reflexes in the human lower limbs. Journal of Neurology, Neurosurgery and Neuropsychiatry. 1960;23:222–227. doi: 10.1136/jnnp.23.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. The Journal of Physiology. 1996;491:197–207. doi: 10.1113/jphysiol.1996.sp021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiaszek JE, Cheng J, Brooke JD. Movement-induced depression of soleus H-reflexes is consistent in humans over the range of excitatory afferents involved. Brain Research. 1995a;702:271–274. doi: 10.1016/0006-8993(95)01129-2. [DOI] [PubMed] [Google Scholar]
- Misiaszek JE, Barclay JK, Brooke JD. Inhibition of canine H-reflexes about the knee arises from muscle mechanoreceptors in quadriceps. Journal of Neurophysiology. 1995b;73:2499–2506. doi: 10.1152/jn.1995.73.6.2499. [DOI] [PubMed] [Google Scholar]
- Misiaszek JE, Cheng J, Brooke JD, Staines WR. Do contralateral influences onto soleus H reflexes require complex spinal mechanisms. Society for Neuroscience Abstracts. 1995c;21:1434. [Google Scholar]
- Nielsen J, Petersen N, Fedirchuk B. Evidence suggesting a transcortical pathway from cutaneous foot afferents to tibialis anterior motoneurones in man. The Journal of Physiology. 1997;501:473–484. doi: 10.1111/j.1469-7793.1997.473bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CI, Marks WB, Bak MJ. The responses of cat motor cortical units to electrical cutaneous stimulation during locomotion and during lifting, falling and landing. Experimental Brain Research. 1985;58:102–116. doi: 10.1007/BF00238958. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Common principles of motor control in vertebrates and invertebrates. Annual Reviews of Neuroscience. 1993;16:265–297. doi: 10.1146/annurev.ne.16.030193.001405. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Sensorimotor gain control: a basic strategy of motor systems. Progress in Neurobiology. 1989;33:281–307. doi: 10.1016/0301-0082(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Larsen B. Soleus stretch reflex modulation during gait in humans. Journal of Neurophysiology. 1996;76:1112–1120. doi: 10.1152/jn.1996.76.2.1112. [DOI] [PubMed] [Google Scholar]
- Van Wezel BMH, Ittenhoff FAM, Duysens J. Dynamic control of location-specific information in tactile cutaneous reflexes from the foot during human walking. Journal of Neuroscience. 1997;17:3804–3814. doi: 10.1523/JNEUROSCI.17-10-03804.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DHD. Presynaptic modulation of sensory afferents in the invertebrate and vertebrate nervous system. Comparative Biochemistry and Physiology. 1992;103A:227–239. doi: 10.1016/0300-9629(92)90573-9. [DOI] [PubMed] [Google Scholar]
- Yang JF, Stein RB. Phase-dependant reflex reversal in human leg muscles during walking. Journal of Neurophysiology. 1990;63:1109–1117. doi: 10.1152/jn.1990.63.5.1109. [DOI] [PubMed] [Google Scholar]
- Yang JF, Stein RB, James KB. Contribution of peripheral afferents to the activation of the soleus muscle during walking in humans. Experimental Brain Research. 1991;87:679–687. doi: 10.1007/BF00227094. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB, Komiyama T. Function of sural nerve reflexes during human walking. Journal of Physiology. 1998;507:305–314. doi: 10.1111/j.1469-7793.1998.305bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


