Abstract
Genes related to trp (transient receptor potential) are proposed to encode store-operated channels. We examined the ionic permeation of recombinant channels formed by stable and transient expression of the TRP homologue bCCE1 in Chinese hamster ovary (CHO) cells (CHO(CCE1)) and rat basophilic leukaemia (RBL) cells, respectively.
Store-operated currents were activated in CHO(CCE1) cells by internal dialysis of IP3 under strong buffering of intracellular Ca2+. The action of IP3 was mimicked by thapsigargin but not by IP4.
With extracellular Ca2+, Na+ and Mg2+, the store-operated currents of CHO(CCE1) rectified inwardly in the presence of internal Cs+. Outward currents were not detected below +80 mV. Identical currents were recorded with external Ba2+ and also with no external Na+ and Mg2+. In the absence of external Mg2+, the inward currents showed an anomalous mole fraction behaviour between Ca2+ and Na+. Half-maximal inhibition of Na+ currents was observed with ≈100 nM and full block with 2-5 μM external Ca2+.
In the parental CHO(-) cells, IP3 dialysis evoked inward currents that also displayed anomalous mole fraction behaviour between Ca2+ and Na+. However, half-maximal block of Na+ currents required 5 times higher Ca2+ concentrations in CHO(-) cells. Additionally, the density of Ca2+ and Na+ currents at-80 mV was 5 and 2 times larger in CHO(CCE1) cells, respectively.
In RBL cells, dialysis of IP3 evoked store-operated currents that showed 1.4-fold larger densities at-80 mV in cells expressing bCCE1.
The enhanced density of store-operated currents in CHO(CCE1) cells and in bCCE1-transfected RBL cells probably reflects the phenotype of CCE1. These results suggest a highly selective permeation of Ca2+ through recombinant channels formed by CCE1 either alone or in combination with endogenous channel proteins.
The mobilization of calcium generated by hormones and growth factors underlies vital functions in virtually all cells. In non-excitable cells, Ca2+ ions are released from intracellular stores and also enter the cell through capacitative calcium entry (CCE) channels, which open upon depletion of Ca2+ stores (Berridge, 1995; Putney, 1997). Due to the link between Ca2+ entry and Ca2+-store depletion, CCE channels are also called store-operated channels. Although the ionic currents through CCE channels have been extensively characterized (see Putney, 1997), the molecular basis for capacitative calcium entry remains elusive. A hint was provided by the finding that the transient receptor potential (trp) gene of Drosophila (Montell & Rubin, 1989) encodes proteins that form recombinant store-operated channels (Vaca et al. 1994; Gillo et al. 1996; Xu et al. 1997). Expression studies with the TRP homologues bCCE1 (originally bCCE; Philipp et al. 1996), rCCE2 (also called TRP5; Philipp et al. 1998) and TRPC1A (Zitt et al. 1996) revealed in mammals a family of ionic channels, which have in common a store-operated activation. Surprisingly, the activation of recombinant channels formed by TRPC3 and mTrp6 may not require depletion of intracellular Ca2+ stores (Zitt et al. 1997; Boulay et al. 1997). It is therefore likely that not all genes related to trp encode CCE channels. Furthermore, TRPC1A appears to form non-selective recombinant cation channels (Zitt et al. 1996) and could be therefore subunits of non-selective native CCE channels (e.g. Lückhoff & Clapham, 1994; Vaca & Kunze, 1994; Krause et al. 1996). By contrast, bCCE1 apparently forms Ca2+-selective channels (Philipp et al. 1996). Additionally, antisense directed to the CCE1 orthologue mTrp4 suppresses the capacitative calcium entry in L cells (Birnbaumer et al. 1996). Thus, CCE1 or another closely related TRP homologue could be implicated in the formation of Ca2+-selective CCE channels (e.g. Hoth & Penner, 1993; Parekh et al. 1993; Zweifach & Lewis, 1993; Yao & Tsien, 1997). It is not known, however, whether the mechanism of ion permeation through recombinant CCE1 channels accounts for a selective Ca2+ inflow, as is the case with native Ca2+-selective CCE channels (Hoth, 1995; Lepple-Wienhues & Cahalan, 1996).
The construction of a cell line stably expressing bCCE1 allowed us to study the permeation of Ca2+ and Na+ through recombinant CCE1 channels. We found that the currents of CHO cells expressing CCE1 display an anomalous mole fraction behaviour between Ca2+ and Na+. As expected for a Ca2+-selective entry pathway, micromolar external Ca2+ was sufficient to block CCE1 channel currents carried by Na+. Additionally, we expressed bCCE1 in the RBL cell line, which is used as a model in studies of store-operated currents.
METHODS
The bovine CCE1 cDNA (originally bCCE, Philipp et al. 1996) was subcloned into the expression vector pMT3 (Genetics Institute, Cambridge, MA, USA) containing the dihydrofolate reductase (DHFR) gene as a selection marker. Stable expression was carried out in a CHO(-) cell line deficient in DHFR using media and conditions previously described (Bosse et al. 1992). For detection of bCCE1 and DHFR transcripts by RT-PCR, 1 μg total RNA was reverse transcribed using oligodeoxythymidine as primer. bCCE1 cDNA fragments (293 bp) were amplified with primers 5′GGGTCAAGTATCACAACC3′ and 5′GCTGGAGTCCCGAACTC3′ and DHFR cDNA fragments (334 bp) with primers 5′CAAGAACGGAGACCTACC3′ and 5′TGGTTGATTCATGGCTTCC3′. For Northern blot analysis, 10 μg poly(A)+ RNA isolated from CHO(-) and CHO(CCE1) cells was electrophoresed and blotted as described previously (Philipp et al. 1996). The complete coding bCCE1 cDNA was used as a probe for hybridization. To control the integrity of the transferred poly(A)+ RNA, the same filter was stripped and rehybridized with a 239 bp cDNA fragment of the human glyceraldyde-3-phosphate dehydrogenase (GAPDH). For recombinant expression of bCCE1 in RBL-1 cells, we constructed the dicistronic expression plasmid pdiCCE1. This dicistronic plasmid is based on the eukaryotic vector pCAGGS (Niwa et al. 1991) and contains the bCCE1 cDNA (Philipp et al. 1996) downstream of the β-actin promoter followed by the internal ribosome entry site (IRES) of the encephalomyocarditis virus and the cDNA of the green fluorescent protein (GFP). Approximately 1 × 105 RBL cells were seeded in 35 mm dishes 1 day before transfection. The transfection mixture consisted of 5 μl SuperFect (Gibco) and 3 μg pdiCCE1. In mock transfections, we used the pCAGGS vector containing only the GFP cDNA. The IRES sequence of the pdiCCE1 vector allows the simultaneous translation of bCCE1 and GFP from one transcript and, accordingly, cells expressing GFP must also express bCCE1. Current recordings were performed only in GFP expressing cells 48-72 h after transfection.
Membrane currents were recorded in the whole-cell mode of the patch-clamp technique (Hamill et al. 1981). Since CHO cells express various ionic currents, we established conditions that favour the recording of nearly pure CCE currents (Fig. 1C). Basically, K+ channel currents were inhibited by internal Cs+ and Ca2+-activated Cl− currents by internal EGTA. Volume-regulated currents were not observed. The standard pipette solution used for internal dialysis contained (mM): 115 CsCl, 10 EGTA, 4 MgCl2 and 10 Hepes; pH 7.2 (CsOH). When indicated, BAPTA (10 mM) was used instead of EGTA in the dialysate (pH 7.2). The concentrations of 400 nM and 1 μM internal Ca2+ ([Ca2+]i) were obtained by adding 6.9 and 8.5 mM CaCl2 to the standard pipette solution (10 mM EGTA) before adjusting the pH to 7.2, respectively. To achieve rapid cell dialysis, patch pipettes had resistances of 2-3 MΩ. The external solution contained (mM): 115 NaCl, 5 KCl and 10 Hepes; pH 7.2 (NaOH). The final extracellular Na+ concentration ([Na+]o) was 120 mM. The concentrations of external free Ca2+ ([Ca2+]o) were adjusted to 2 and 5 μM with 1 mM HEDTA mixed with 0.39 and 0.61 mM CaCl2 (pH 7.2), respectively. The external Ca2+ concentration of 100 nM was obtained with 1 mM EGTA and 0.4 mM CaCl2 (pH 7.2). A Ca2+ concentration of 1 nM was assumed for the solution containing only 1 mM EGTA (pH 7.2). [Ca2+]o is given throughout as pCao. In the external solutions containing EGTA or HEDTA, the concentration of NaCl was reduced to maintain a final Na+ concentration of 120 mM after adjustment of the pH to 7.2 (NaOH). The extracellular concentration of Mg2+ ([Mg2+]o) represents the total amount of added MgCl2. The Ba2+ solution was prepared by adding 10 mM BaCl2 and 2 mM MgCl2to the external solution. In some experiments, extracellular Na+ was replaced with NMDG+ (mM): 115 NMDG, 10 Hepes; pH 7.2 (HCl).
Figure 1. Stable expression of CCE1 in CHO cells.
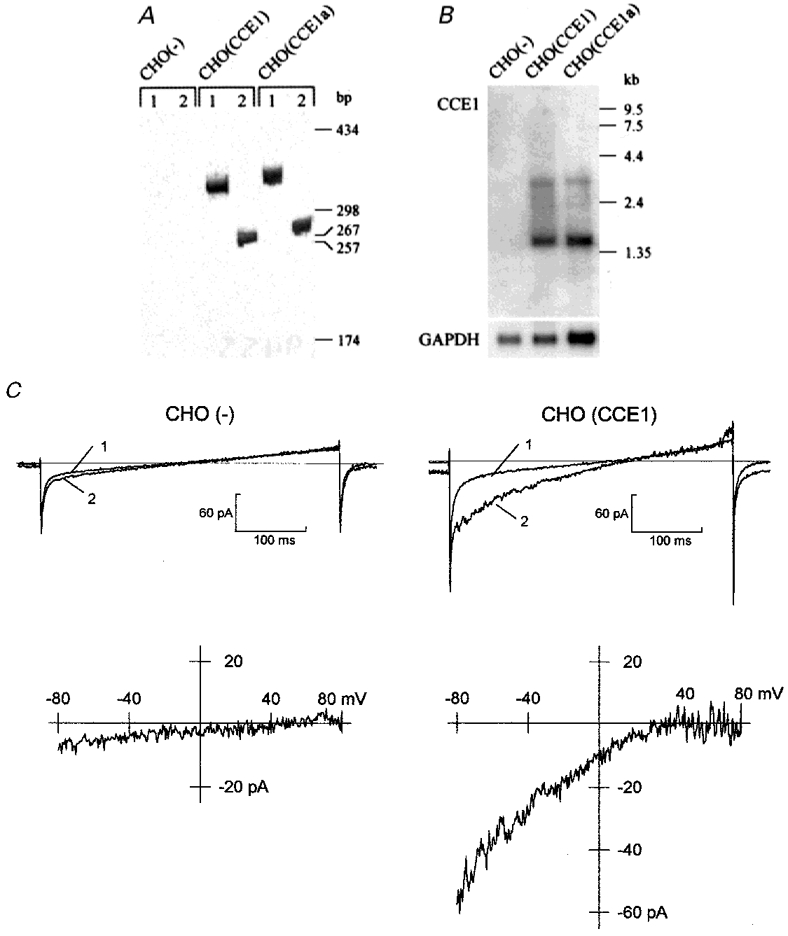
A, RT-PCR analysis of parental (CHO(-)) and bCCE1-transfected (CHO(CCE1), CHO(CCE1a)) cells. PCR primers were specific for the selection marker DHFR (1) and for bCCE1 (2). B, Northern blot analysis of CCE1 and GAPDH (glyceraldehyde-3-phosphate-dehydrogenase) expression. C, activation of ionic currents in CHO(-) and CHO(CCE1) cells by internal dialysis of 10 μM IP3. Ionic currents were elicited every 5 s by voltage-clamp ramps (400 ms) from -100 to +100 mV delivered from a holding potential of 0 mV. Leak currents (1) were collected at the start of dialysis and enhanced currents (2) after IP3 dialysis. Current-voltage relationships (lower panels) were obtained by subtracting leak currents from activated currents. Cm: 15 pF (left) and 20 pF (right). The internal dialysate contained 115 mM Cs+ and 10 mM EGTA. The external solution contained 120 mM Na+, 10 mM Ca2+ and 2 mM Mg2+.
Whole-cell currents were elicited every 5 s by voltage-clamp ramps (400 ms) from −100 to +100 mV delivered from a holding potential of 0 mV. Series resistances (Rs) and whole-cell membrane capacitance (Cm) were read from the settings provided by the patch-clamp amplifier (EPC-7, List Electronic). Data were analysed from experiments with Rs below 7 MΩ. Cm was 17.93 ± 4.58 pF (n = 131). Electronic compensation of 30-50 % was used to reduce charging time. Currents were sampled at 10 kHz and filtered at 3 kHz. Liquid junction potentials between -10 and +5 mV measured as described by Neher (1992) were not corrected. Current traces were leak subtracted using an average of three to five traces collected within the first 60 s and 6 s of recordings in CHO and RBL cells, respectively. For analysis, the maximal current observed during CCE activation was normalized to cell size using Cm. Our limit of resolution was 0.1 pA pF−1, which corresponds to inward currents of 1.5-2.5 pA at -80 mV in leak-subtracted traces. Accordingly, cells showing current densities below this limit of resolution were not included in further calculations. Experiments with different concentrations of external Ca2+ were carried out in separated cells. Data were analysed with Student's t test and are given as the mean ±s.d.
RESULTS
Stable expression of CCE1 in CHO cells
The bovine CCE1 (originally bCCE, Philipp et al. 1996) was stably expressed in CHO cells. As illustrated in Fig. 1A, RT-PCR analysis confirmed the expression of bCCE1 in two selected clones, CHO(CCE1) and CHO(CCE1a). Accordingly, Northern analysis (Fig. 1B) revealed the expression of ∼3.9 kb transcripts corresponding to the expected size of bCCE1 transcripts expressed from the pMT vector. Additional 1.5 kb transcripts may arise from rearrangements or deletion events during selection of stable cell lines (Kaufman et al. 1991). No expression of CCE1-related transcripts was detected in parental CHO(-) cells (Fig. 1A and B).
In the presence of extracellular Ca2+, Na+ and Mg2+, internal dialysis with 10 μM IP3 and 10 mM EGTA evoked in CHO(CCE1) cells inward currents that differed from native currents in CHO(-) cells (Fig. 1C). The leak-subtracted currents of CHO(CCE1) cells showed prominent inward rectification and reversed at potentials > +40 mV. In fact, no outward current was observed below +80 mV with Cs+ in the dialysate. For comparison, we calculated the densities of leak-subtracted currents at -80 mV. On average, the density of inward currents was 1.63 ± 0.85 pA pF−1 in 17 out of 18 CHO(CCE1) cells and statistically higher (P < 0.001) than in CHO(-) cells (0.32 ± 0.23 pA pF−1, n = 18). Similar currents were recorded in 5 out of 6 cells of the CHO(CCE1a) line (1.25 ± 0.68 pA pF−1) dialysed with IP3, suggesting that the enhanced densities of inwardly rectifying currents are due to the expression of bCCE1 and not to selection of a CHO clone with high density of endogenous currents. With 10 mM BAPTA instead of EGTA (Fig. 2A), the IP3-induced currents of CHO(CCE1) cells showed similar densities (1.36 ± 0.48 pA pF−1 in 8 out of 9 cells). When the dialysate contained 10 μM IP3 and 400 nM free Ca2+ (Fig. 2B), however, the inward currents attained densities of 0.62 ± 0.24 pA pF−1 in 5 out of 18 cells. No inward current was detected upon dialysis of 10 μM IP3 in the presence of 1 μM free Ca2+ (n = 5), suggesting that the action of IP3 required strong buffering of intracellular Ca2+.
Figure 2. Activation of ionic currents by IP3, IP4 and thapsigargin in CHO(CCE1) cells.
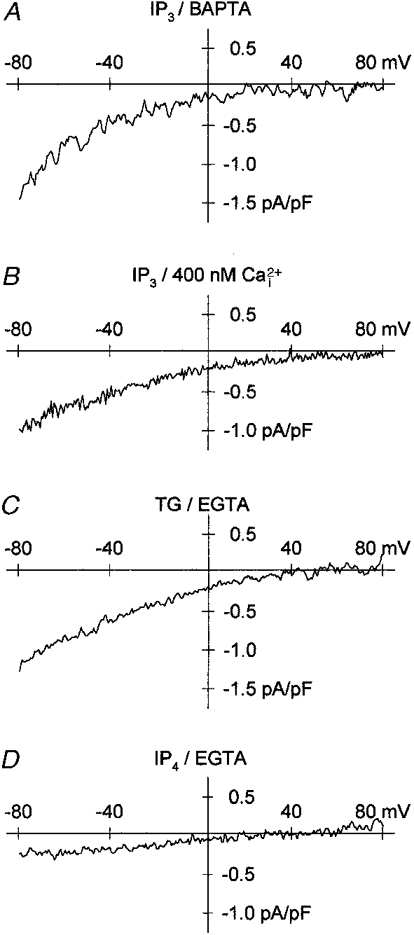
The internal solution contained 115 mM Cs+. IP3 (10 μM) was dialysed in the presence of 10 mM BAPTA (A, IP3/BAPTA) and 400 nM free Ca2+ (B, IP3/400 nM ). Thapsigargin (1 μM) was applied to cells dialysed with 10 mM EGTA (C, TG/EGTA). IP4 (20 μM) was dialysed with 10 mM EGTA (D, IP4/EGTA). Same external solution as in Fig. 1.
As previously observed in transient expression experiments (Philipp et al. 1996), the activation of inward currents in CHO(CCE1) cells was detected 140-330 s after the onset of IP3 dialysis and the inward currents remained enhanced for ≤ 40 s. In CHO(-) cells, the endogenous inward currents (Fig. 1C) activated also with a delay of 49-189 s. In order to ensure that the inwardly rectifying currents were activated by store depletion, intracellular Ca2+ stores were emptied in an IP3-independent fashion by the Ca2+-ATPase inhibitor thapsigargin (Thastrup et al. 1990). After application of 1-2 μM thapsigargin to CHO(CCE1) cells dialysed with 10 mM EGTA (Fig. 2C), we observed inward currents with densities of 1.19 ± 0.40 pA pF−1 (n = 7) similar to those induced by IP3 (see Fig. 1C). Dialysis of 20 μM IP4 and 10 mM EGTA induced currents with densities of 0.30 ± 0.12 pA pF−1 in 4 out of 6 CHO(CCE1) cells (Fig. 2D). Thus, the action of IP3 in the CHO(CCE1) cell line required strong buffering of intracellular Ca2+ and was mimicked by thapsigargin but not by IP4. These data support the idea that recombinant channels are activated via a store-operated mechanism in cells expressing bCCE1 (Philipp et al. 1996).
Ion permeation in cells expressing CCE1
Native CCE channels expressed in a number of cell types are characterized by a high selectivity for Ca2+ over Na+ (Hoth & Penner, 1993; Parekh et al. 1993; Zweifach & Lewis, 1993; Yao & Tsien, 1997). In some other instances, store-operated channels seem to be less Ca2+ selective (Lückhoff & Clapham, 1994; Vaca & Kunze, 1994; Krause et al. 1996). If CCE1 encodes channel proteins that mediate a capacitative entry pathway selective for Ca2+, it can be expected that the ion permeation through recombinant CCE1 channels shares basic properties with the permeation through Ca2+-selective channels (see Almers & McCleskey, 1984; Hess & Tsien, 1984; Hoth, 1995; Lepple-Wienhues & Cahalan, 1996). We examined whole-cell currents of CHO cells expressing bCCE1 under various ion conditions to outline the permeation of monovalent and divalent cations through recombinant CCE1 channels.
The ability of external Ca2+, Ba2+ and Na+ to act as charge carriers in the presence of internal Cs+ is illustrated in Fig. 3. Like currents recorded in external Ca2+ (Fig. 1C), the store-operated currents of CHO(CCE1) cells displayed inward rectification and densities of 1.88 ± 0.51 pA pF−1 (n = 3) in external Ba2+ (Fig. 3A). Thus, as reported for native CCE channels (Hoth, 1995), Ca2+ and Ba2+ function as charge carriers in CHO(CCE1) cells. Removal of external Na+ had no effect on store-operated currents recorded in CHO(CCE1) cells but, conversely, no current flow was detected when extracellular Ca2+ was reduced (Fig. 3B). Since external Mg2+ was present in the experiments of Fig. 3B, the permeation of Na+ was probably blocked by Mg2+. In fact, we succeeded in recording Na+ currents in CHO(CCE1) cells after removal of external Mg2+ (Fig. 3C). In addition, the complete lack of current flow in experiments with CHO(CCE1) cells (Fig. 3B) revealed also a blockade of native CCE channel currents. Accordingly, Na+ currents were also detected in CHO(-) cells after removal of Mg2+ (Fig. 3D). Thus, as in other cell systems (Lepple-Wienhues & Cahalan, 1996), Na+ acts as a charge carrier in CHO(CCE1) and CHO(-) cells only in the absence of external divalent cations. The blockade of Na+ currents by Ca2+ was assayed in the absence of external Mg2+. Under these conditions, an increase of extracellular Ca2+ strongly reduced Na+ currents in CHO(CCE1) and CHO(-) cells (Fig. 3C and D).
Figure 3. Permeation of monovalent and divalent cations through channels activated by dialysis of IP3 in CHO(CCE1) and CHO(-) cells.
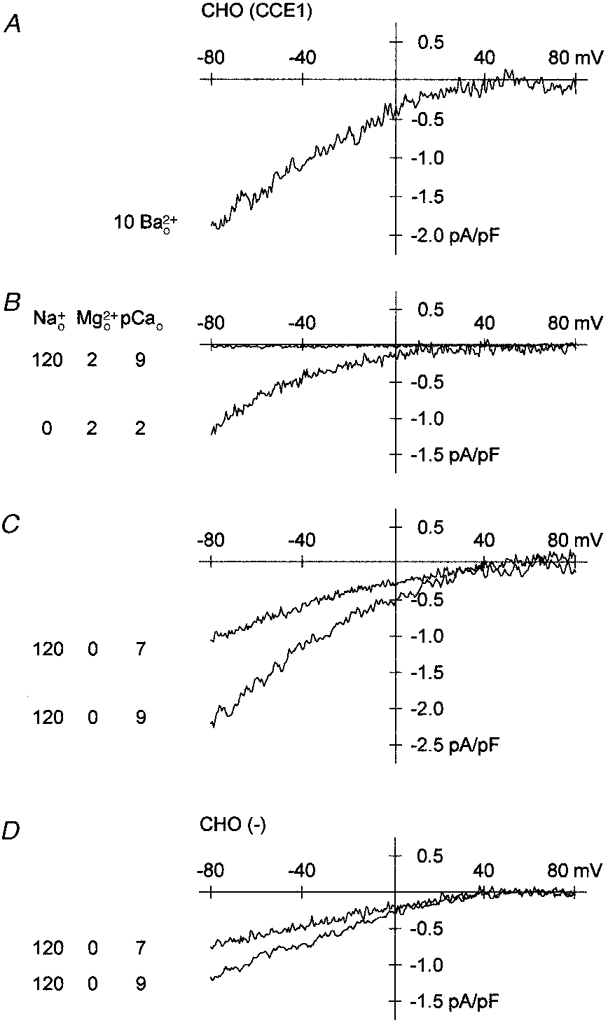
Ionic currents were recorded in CHO(CCE1) cells in the presence of extracellular Ba2+ (A) and with either external Na+ or Ca2+ in the presence of Mg2+ (B). Currents recorded with external Na+ in CHO(CCE1) (C) and CHO(-) (D) were blocked by extracellular Ca2+ in the absence of Mg2+. External concentrations of Ba2+ (), Na+ () and Mg2+ () are millimolar. The external concentration of Ca2+ is given as pCao. The intracellular concentration of Cs+ was 115 mM.
The ability of external Ca2+ to block the movements of other permeant cations is a distinct feature of the ionic permeation through voltage-dependent and store-operated Ca2+-selective channels (Almers & McCleskey, 1984; Hess & Tsien, 1984; Hoth, 1995; Lepple-Wienhues & Cahalan, 1996). Since models that include Ca2+ binding sites within the pore of Ca2+-selective channels predict an anomalous mole fraction dependence of ionic currents (Dang & McCleskey, 1998), we examined the currents of CHO(CCE1) and CHO(-) cells in various mixtures of monovalent and divalent cations. When the external concentration of Ca2+ (pCao) was varied almost 7 orders of magnitude and the extracellular Na+ concentration maintained constant, the store-operated currents measured at -80 mV in the absence of Mg2+ approached a minimum at Ca2+ concentrations in the micromolar range (Fig. 4A). On the other hand, removal of external Na+ and Mg2+ had no effect on currents measured with millimolar Ca2+ (Fig. 4A), indicating that Ca2+ rather than Na+ supports CCE currents at high Ca2+ concentrations. Mg2+ was unlikely to support current flow because no current was measured in solutions with reduced Ca2+ (Fig. 3B). Thus, like Ca2+-selective channels (Almers & McCleskey, 1984; Lepple-Wienhues & Cahalan, 1996), the CCE channels of CHO(CCE1) and CHO(-) cells display anomalous mole fraction behaviour between Ca2+ and Na+. Moreover, we found different current-pCao curves in CHO(CCE1) and CHO(-) cells (Fig. 4A). The difference that arose from the expression of bCCE1 in CHO cells is illustrated in Fig. 4B. Ca2+ currents (pCao = 2) in CHO(CCE1) cells were 5 times larger than in CHO(-) cells and Na+ currents (pCao = 9) were 2 times larger. Additionally, the ratio of Na+ currents to Ca2+ currents was 1.5 in CHO(CCE1) cells and 3.5 in CHO(-) cells. These different current-pCao relationships indicate that the expression of bCCE1 in CHO cells enhanced preferentially store-operated currents carried by Ca2+. Additionally, the Ca2+ sensitivity of currents carried by Na+ was also enhanced in CHO cells expressing bCCE1 (Fig. 3C and D). The descending limbs of the current-pCao relationships shown in Fig. 4A indicate that ∼100 nM and ∼500 nM external Ca2+ inhibited Na+ currents half-maximally in CHO(CCE1) and CHO(-) cells, respectively. Full block of Na+ currents was obtained with Ca2+ concentrations higher than 1 μM, as with Na+ currents through Ca2+-selective channels (see Almers & McCleskey, 1984; Lepple-Wienhues & Cahalan, 1996). In conclusion, CCE currents of CHO(-) and CHO(CCE1) cells showed an anomalous mole fraction behaviour between Ca2+ and Na+ in the absence of Mg2+.
Figure 4. Anomalous mole fraction dependence of ionic currents activated by depletion of Ca2+ stores in CHO(CCE1) and CHO(-) cells.
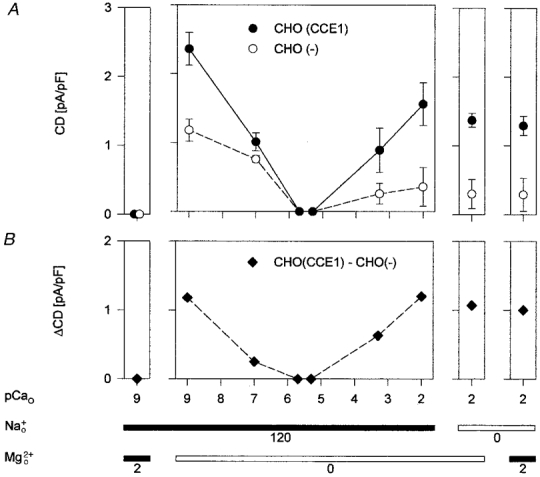
A, total current density (CD) measured at -80 mV. n = 3-8. Current densities (A) were statistically different (P < 0.001) at pCao 9 and 2. B, density of difference currents (ΔCD) calculated by subtracting mean current densities of CHO(-) cells from those of CHO(CCE1) cells. The internal dialysate contained 115 mM Cs+. The ionic composition of the external solution is shown under the panels, where and are millimolar. The external Ca2+ concentration is given as pCao.
Expression of CCE1 in RBL cells
Since the store-operated currents of CHO(CCE1) cells were larger and slightly more sensitive to external Ca2+ than native currents of CHO cells, we expressed bCCE1 in RBL cells. Upon dialysis of 10-20 μM IP3 and 10 mM EGTA in mock-transfected RBL cells (Fig. 5A), the inwardly rectifying currents increased to attain densities of 3.7 ± 0.68 pA pF−1 within 32-45 s. The expression of bCCE1 had no effect on the reversal potential (> +40 mV) and rectification properties of the store-operated currents in RBL cells (Fig. 5A). However, the density of the inward currents at -80 mV was 1.4 times larger in bCCE1-transfected RBL cells. These results support the previous finding in CHO cells, that the expression of bCCE1 enhances the density of Ca2+-selective store-operated currents.
Figure 5. Expression of bCCE1 in RBL cells.
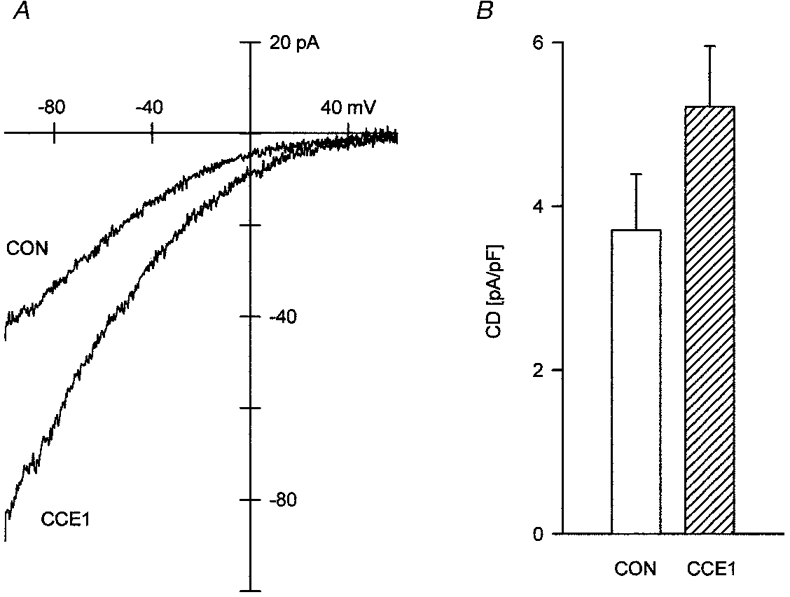
A, representative current traces evoked by dialysis of 10 μM IP3 and 10 mM EGTA in mock-transfected (CON) and bCCE1-transfected (CCE1) RBL cells. Cm: 11 pF, CON; 12 pF, CCE1. Same internal and external solutions as in Fig. 1 B., density of inward currents at -80 mV in mock- and bCCE1-transfected RBL cells. The difference is statistically significant (P < 0.001). n = 10 for CON and 11 for CCE1.
DISCUSSION
Previous expression experiments showed that HEK cells transiently transfected with bCCE1 develop a store-operated conductance that appears to be Ca2+ selective (Philipp et al. 1996). Using CHO cells that stably express bCCE1, we confirmed the functional expression of store-operated currents and studied the ionic permeation of underlying recombinant channels. Basically, we found that the store-operated currents of CHO(CCE1) cells display an anomalous mole fraction dependence on extracellular Ca2+ and Na+. Na+ currents were detected in the absence of external divalent cations and were blocked by micromolar Ca2+. Removal of external Na+ had no effect on currents recorded in millimolar Ca2+. Similarly, we found an enhancement of inward currents evoked by IP3 in RBL cells expressing bCCE1.
Considering that recombinant TRPC1 and TRPC3 co-immunoprecipate (Xu et al. 1997), it is likely that members of the TRP family assemble into heteromultimeric channels and, in principle, native TRP proteins and CCE1 may form chimeric channels in CHO(CCE1) and RBL cells. Furthermore, since internal dialysis with IP3 evoked inward currents that displayed an anomalous mole fraction behaviour even in non-transfected cells, native and recombinant currents may also aggregate to build up whole-cell currents in RBL and CHO(CCE1) cells. The idea that the properties of store-operated currents in cells expressing recombinant bCCE1 reflect the phenotype of CCE1 is underlined by previous expression studies with various TRP homologues that revealed functionally distinct currents. For instance, the mammalian TRPC1A (Zitt et al. 1996) and the dipterian TRPL (Zimmer et al. 1997) appear to form non-selective cation channels, which support linear and outwardly rectifying currents, respectively. Since TRPL and TRPC1A were expressed in CHO cells as was CCE1 in the present study, the functionally diverse currents are likely to reflect the properties not of native but of recombinant TRP channels. Similarly, the properties of store-operated currents in CHO(CCE1) cells probably represent the phenotype of bCCE1.
The recombinant channels formed in CHO cells after expression of bCCE1 parallel Ca2+-selective channels at least in two basic features. (i) Na+ permeates when Ca2+ is absent but Na+ currents are blocked by micromolar Ca2+. (ii) With millimolar concentrations of Ca2+, CCE1 currents are carried by Ca2+ and no Na+ flux occurs at these concentrations. Hence, monovalent and divalent cations apparently interact within the pore of recombinant CCE1 channels. We predict that recombinant CCE1 channels form multi-ion pores with high-affinity binding site(s) for Ca2+. Due to the high-affinity binding of extracellular Ca2+ and, probably, due to a poor permeation of intracellular Cs+, the ionic currents through CCE1 channels display inward rectification. As expected for a capacitative entry pathway selective for Ca2+, the high-affinity binding of Ca2+ prevents the permeation of monovalent cations under physiological Ca2+ concentrations and confers to recombinant CCE1 channels a Ca2+ selectivity much higher than previously expected (Philipp et al. 1996).
In conclusion, both native (Hoth & Penner, 1993; Parekh et al. 1993; Zweifach & Lewis, 1993; Lückhoff & Clapham, 1994; Vaca & Kunze, 1994; Krause et al. 1996; Yao & Tsien, 1997) and recombinant (Zitt et al. 1996; Philipp et al. 1996) store-operated channels appear to be functionally diverse. If members of the TRP family co-assemble to form store-operated channels, CCE1 is an appropriate candidate to play the role of a dominant subunit in terms of Ca2+ selectivity.
Acknowledgments
We thank M. Hoth, T. Plant, A. B. Parekh, W. Stühmer, W. Almers and B. Lindemann for the helpful discussions and criticisms of the manuscript. We thank also J. Miyazaki and M. Okave for the backbone of the pCAGGS vector. This work has been supported by the Deutsche Forschungsgemeinschaft and Fonds der Chemischen Industrie.
References
- Almers W, McCleskey W. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single file pore. The Journal of Physiology. 1984;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Capacitative calcium entry. Biochemical Journal. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. On the molecular basis and regulation of cellular capacitative calcium entry: roles for Trp proteins. Proceedings of the National Academy of Sciences of the USA. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse E, Bottlender R, Kleppisch T, Hescheler J, Welling A, Hofmann F, Flockerzi V. Stable and functional expression of the calcium channel α1 subunit from smooth muscle in somatic cell lines. EMBO Journal. 1992;11:2033–2038. doi: 10.1002/j.1460-2075.1992.tb05260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay G, Zhu X, Peyton M, Jiang M, Hurst R, Stefani E, Birnbaumer L. Cloning and expression of a novel mammalian homologue of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. Journal of Biological Chemistry. 1997;272:29672–29680. doi: 10.1074/jbc.272.47.29672. [DOI] [PubMed] [Google Scholar]
- Dang TX, McCleskey EW. Ion channel selectivity through stepwise changes in binding affinity. Journal of General Physiology. 1998;111:185–193. doi: 10.1085/jgp.111.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillo B, Chorna I, Cohen H, Cook B, Manisterky I, Chorevs M, Arnon A, Polock JA, Selinger Z, Minke B. Coexpression of Drosophila TRP and TRP-like proteins in Xenopus oocytes reconstitutes capacitative Ca2+ entry. Proceedings of the National Academy of Sciences of the USA. 1996;93:14146–14151. doi: 10.1073/pnas.93.24.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:58–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P, Tsien RW. Mechanism of ion permeation through calcium channels. Nature. 1984;309:453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hoth M. Calcium and barium permeation through calcium release-activated calcium (CRAC) channels. Pflügers Archiv. 1995;430:315–322. doi: 10.1007/BF00373905. [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1993;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ, Davies MV, Wasley LC, Michnick D. Improved vectors for stable expression of foreign genes in mammalian cells by use of the untranslated leader sequence from EMC virus. Nucleic Acids Research. 1991;19:4485–4490. doi: 10.1093/nar/19.16.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause E, Pfeiffer F, Schmid A, Schulz I. Depletion of intracellular calcium stores activates a calcium conducting non-selective cation current in mouse pancreatic acinar cells. Journal of Biological Chemistry. 1996;271:32523–32528. doi: 10.1074/jbc.271.51.32523. [DOI] [PubMed] [Google Scholar]
- Lepple-Wienhues A, Cahalan MD. Conductance and permeation of monovalent cations through depletion-activated Ca2+ channels (ICRAC) in Jurkat T cells. Biophysical Journal. 1996;71:784–794. doi: 10.1016/S0006-3495(96)79278-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückhoff A, Clapham DE. Ca2+ channels activated by depletion of internal calcium stores in A431 cells. Biophysical Journal. 1994;67:177–182. doi: 10.1016/S0006-3495(94)80467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C, Rubin G. Molecular characterisation of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods in Enzymology. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high expression transfectants with a novel eucaryotic vector. Genes. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Terlau H, Stühmer W. Depletion of InsP3 stores activates a Ca2+ and K+ current by means of a phosphatase and a diffusible messenger. Nature. 1993;364:814–818. doi: 10.1038/364814a0. [DOI] [PubMed] [Google Scholar]
- Philipp S, Cavalié A, Freichel M, Wissenbach U, Zimmer S, Trost C, Marquart A, Murakami M, Flockerzi V. Structural basis of capacitative calcium entry in mammals. EMBO Journal. 1996;15:6166–6171. [PMC free article] [PubMed] [Google Scholar]
- Philipp S, Hambrecht J, Braslavski L, Schroth G, Freichel M, Murakami M, Cavalié A, Flockerzi V. A novel capacitative calcium entry channel expressed in excitable cells. EMBO Journal. 1998;17:4274–428. doi: 10.1093/emboj/17.15.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr . Capacitative Calcium Entry. New York: Springer; 1997. [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promotor, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proceedings of the National Academy of Sciences of the USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaca L, Kunze DL. Depletion of intracellular Ca2+ stores activates a Ca2+-selective channel in vascular endothelium. American Journal of Physiology. 1994;267:C920–925. doi: 10.1152/ajpcell.1994.267.4.C920. [DOI] [PubMed] [Google Scholar]
- Vaca L, Sinkins WG, Hu Y, Kunze DL, Shilling WP. Activation of recombinant trp by thapsigargin in Sf9 insect cells. American Journal of Physiology. 1994;267:C1501–1505. doi: 10.1152/ajpcell.1994.267.5.C1501. [DOI] [PubMed] [Google Scholar]
- Xu X-ZS, Li H-S, Guggino WB, Montell C. Co-assembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 1997;89:1155–1164. doi: 10.1016/s0092-8674(00)80302-5. [DOI] [PubMed] [Google Scholar]
- Yao Y, Tsien RY. Calcium current activated by depletion of calcium stores in Xenopus oocytes. Journal of General Physiology. 1997;109:703–715. doi: 10.1085/jgp.109.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer S, Trost C, Cavalié A, Philipp S, Flockerzi V. The TRPL protein is a Ca2+-calmodulin activated non-selective cation (CAN) channel. Naunyn-Schmiedeberg's Archives of Pharmacology. 1997;355:R67. [Google Scholar]
- Zitt C, Obukhov AG, Srübing C, Zobel A, Kalkbrenner F, Lückhoff A, Schultz G. Expression of TRPC3 in chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. Journal of Cell Biology. 1997;138:1333–1341. doi: 10.1083/jcb.138.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitt C, Zobel A, Obukhov AG, Harteneck C, Kalkbrenner F, Lückhoff A, Schultz G. Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron. 1996;16:1189–1196. doi: 10.1016/s0896-6273(00)80145-2. [DOI] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proceedings of the National Academy of Sciences of the USA. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]


