Abstract
The effect has been examined of the accessory α2-δ and β subunits on the properties of α1G currents expressed in monkey COS-7 cells and Xenopus oocytes.
In immunocytochemical experiments, the co-expression of α2-δ increased plasma membrane localization of expressed α1G and conversely, the heterologous expression of α1G increased immunostaining for endogenous α2-δ, suggesting an interaction between the two subunits.
Heterologous expression of α2-δ together with α1G in COS-7 cells increased the amplitude of expressed α1G currents by about 2-fold. This finding was confirmed in the Xenopus oocyte expression system. The truncated δ construct did not increase α1G current amplitude, or increase its plasma membrane expression. This indicates that it is the exofacial α2 domain that is involved in the enhancement by α2-δ.
β1b also produced an increase of functional expression of α1G, either in the absence or the presence of heterologously expressed α2-δ, whereas the other β subunits had much smaller effects.
None of the accessory subunits had any marked influence on the voltage dependence or kinetics of the expressed α1G currents. These results therefore suggest that α2-δ and β1b interact with α1G to increase trafficking of, or stabilize, functional α1G channels expressed at the plasma membrane.
Voltage-dependent calcium channels and currents in native neurons and other cells have been divided into high voltage activated (HVA) and low voltage activated (LVA) (Carbone & Lux, 1984; Nowycky et al. 1985). LVA currents can be distinguished by their activation at smaller depolarizations, near to the resting potential, and by their rapid inactivation (Huguenard, 1996). At the single channel level, channels with a small unitary conductance activate over the same voltage range (Carbone & Lux, 1984). Native T-type channels are heterogeneous (Kobrinsky et al. 1994; Huguenard, 1996), suggesting that they comprise more than one subtype of channel. A new subfamily of voltage-dependent calcium channel α1 subunit genes (comprising α1G, α1H and α1I) has recently been cloned, whose structure is superficially similar to the previously cloned HVA α1 subunits A, B, C, D, E and S (Perez-Reyes et al. 1998; Cribbs et al. 1998; Lee et al. 1999), having four domains, each with a voltage sensor and a pore-forming P loop. However, there are a number of regions where the homology is very low, particularly in the intracellular linkers and the N and C termini. These novel channels, when expressed, form rapidly inactivating LVA currents that also have a small single channel conductance and slowly deactivating tail currents like native T-type currents (Carbone & Lux, 1984; Armstrong & Matteson, 1985). Recently, using an antisense approach, evidence has been obtained that T-type currents in primary sensory neurons are generated by the α1G, H and I family (Lambert et al. 1998).
The HVA channels are all thought to form heteromeric channels with the accessory subunits α2-δ, β and possibly γ. The accessory subunits, particularly β subunits, have marked effects on the assembly of functional channels at the plasma membrane. In expression systems, the β subunits increase the number of plasma membrane channels (Chien et al. 1995; Shistik et al. 1995; Brice et al. 1997) and also affect the voltage dependence and kinetics of activation and inactivation (Jones et al. 1998). Inactivation kinetics are particularly affected by different β subunits in a β-dependent manner, with β2a producing a marked slowing of inactivation (De Waard & Campbell, 1995).
It has not yet been examined whether the expression of LVA channel α1 subunits requires any known accessory subunits for trafficking to the plasma membrane, or for functional expression, or whether these accessory subunits influence the biophysical properties of the expressed channels. There are notable structural differences when comparing the α1G, H and I sequence with those of HVA channels, one contrast being that the I-II loop of the LVA channels is very large. Furthermore, they do not contain the full consensus sequence identified to be the binding site for β subunits on the I-II loop, the α interaction domain (AID). This consensus sequence, QQ-E-D/EL-GY–WI—E, is present in all the HVA channels (Walker & De Waard, 1998). The homologous sequence in α1G is GSCYEELLKYLVYILRKA, with identical residues underlined in bold, and a conserved charge in italics. However, in the C terminal part of the consensus sequence, the W is Y in α1G, a conservative substitution, compared with the W→A mutation identifying this residue as essential for β subunit binding (De Waard et al. 1996). Thus the degree of conservation of the consensus sequence may be sufficient for β interaction.
The consensus site(s) on the exofacial loops of HVA α1 subunits to which the α2-δ subunit binds have not been identified, although repeat III of α1S has been found to associate with α2 (Gurnett et al. 1997). Furthermore, the extracellular region of δ is also involved in the production of certain functional effects, although the region of α1 with which it interacts is not known (Felix et al. 1997). Thus it is not possible to determine a priori whether α1G has the capacity to bind to α2-δ. This study was therefore designed to examine whether α1G is influenced by any accessory subunits.
METHODS
Materials
The rat α2-δb cDNA (accession number M86621) was obtained from either Dr H. Chin (NIH, Bethesda, MD, USA) or Dr T. Snutch (UBC, Vancouver, Canada), rat β1b (X11394) from Dr T. Snutch and mut-3 GFP (green fluorescent protein) from Dr T. Hughes (Yale, New Haven, CT, USA). Rat β2a (M80545), β3 (M88751) and β4 (M80545) cDNAs were also used.
Molecular biology
The α1G cDNA (Perez-Reyes et al. 1998) was subcloned into the vertebrate expression vector pMT2 (Genetics Institute, Cambridge, MA, USA) (Swick et al. 1992), using standard molecular biological techniques, and the correct orientation of the insert was verified by multiple restriction digests. All other cDNAs were expressed from the same vector for expression in monkey COS-7 cells. The δ subunit (Gurnett et al. 1996) was constructed by deletion of the α2 cDNA, using the plasmid pMT2-α2-δ as a template. The forward primer was ATGGAAGAGGATGACTTCACAGCT, which binds at the beginning of the δ sequence, position 2995. The reverse primer was AGGGAAGGGCTCCTCGCTCGA, which binds at position 238, including only the signal sequence of α2. The PCR product of 6.1 kb was amplified using Pfu polymerase for 14 cycles, with the annealing temperature of 50°C for 30 s, and an extension temperature of 75°C for 13 min. This product was then treated with polynucleotide kinase and ligated to form the pMT2-δ construct, attached to the signal sequence of α2-δ, as described previously (Felix et al. 1997). The construct was sequenced for confirmation of its identity.
COS-7 cell expression
Cells were cultured and transfected by electroporation, essentially as described previously (Campbell et al. 1995a). The α1G, α2-δ or δ, β and GFP cDNAs in the vector pMT2 were used at 15, 5, 5 and 1 μg per transfection, respectively. Blank pMT2 vector was included where necessary to maintain the total cDNA at 31 μg per transfection. In some experiments in which α1G was expressed alone, the additional pMT2 vector was omitted, with no effect on the current properties. Following transfection, cells were maintained at 37°C for about 60 h, and then replated using non-enzymatic cell dissociation medium (Sigma), and maintained at 25°C for between 2 and 8 h prior to electrophysiological recording, or for 1–2 h prior to fixation for immunocytochemistry.
Ba2+ currents were recorded using the whole cell patch-clamp technique. The internal and external solutions were similar to those described previously (Campbell et al. 1995b). The patch pipette solution contained (mM): caesium aspartate, 140; EGTA, 5; MgCl2, 2; CaCl2, 0.1; K2ATP, 2; Hepes, 10; pH 7.2, 310 mosmol kg−1 with sucrose. The external solution contained (mM): tetraethylammonium (TEA) bromide, 160; KCl, 3; NaHCO3, 1.0; MgCl2, 1.0; Hepes, 10; glucose, 4; BaCl2, 1; pH 7.4, 320 mosmol kg−1 with sucrose. Whole cell currents were elicited from a holding potential (Vh) of −100 mV and recorded using an Axopatch-1D amplifier. Data were filtered at 2–5 kHz and digitized at 10–20 kHz. The junction potential between external and internal solutions was 6 mV; the values given in the figures and text have not been corrected for this. Current records are shown following leak and residual capacitance current subtraction (P/8 protocol) and series resistance compensation up to 80 %. All experiments were performed at room temperature (20–24°C). Analysis was performed using pCLAMP 6 (Axon Instruments) and Origin 5 software (Microcal Software Inc., Northampton, MA, USA).
Xenopus oocyte expression
Oocytes were obtained from X. laevis (Xenopus One) using standard techniques (Leonard & Snutch, 1991), which have been approved by the Loyola University Animal Care Committee. Frogs were anaesthetized using 1 g l−1 of tricaine, then oocytes were collected by making a 1 cm incision in the ventral abdomen and removing a portion of the ovary. The frog was sutured both on the rectus abdominus muscle sheath, and then on the skin. It was allowed to recover for 4 h before returning it to the home tank. Oocytes were prepared for microinjection as previously described (Leonard & Snutch, 1991).
Capped cRNA was synthesized using T7 RNA polymerase (Ambion). The rat α1G (Perez-Reyes et al. 1998) was contained in the vector pGEM-HEA. The rat brain α2-δb was contained in the vector pAGA. Each oocyte was injected with 5 ng of either α1G alone or plus 5 ng α2-δ cRNA in a volume of 50 nl. Oocytes were voltage clamped using a two-microelectrode voltage clamp amplifier (OC-725B, Warner Instrument Corp.). Voltage and current electrodes (1.5–1.8 MΩ tip resistance) were filled with a cushion of 1 % agarose and 3 M KCl (Schreibmayer et al. 1994). The bath solution contained the following (mM): 10 BaCl2, 36 tetraethyl ammonium, 2 CsCl2 and 5 Hepes, adjusted to pH 7.4 with methanesulfonic acid. Data were filtered at 1 kHz and acquired at 4 kHz using the pCLAMP system (Digidata 1200 and pCLAMP 6.0). All data are expressed as the means ± s.e.m. and statistical analysis was performed using Student's t test.
Immunocytochemistry
COS-7 cells were washed twice in Tris-buffered saline (TBS; 154 mM NaCl, 20 mM Tris, pH 7.4), then fixed in 4 % paraformaldehyde in TBS as described (Brice et al. 1997). The cells were permeabilized in 0.02 % Triton X-100 in TBS, and incubated with blocking solution (20 % (v/v) goat serum, 4 % (w/v) bovine serum albumin (BSA), 0.1 % (w/v) dl-lysine in TBS). The cells were incubated for 14 h at 4°C with the appropriate primary antibody diluted 1: 500 in 10 % goat serum, 2 % BSA, 0.05 % dl-lysine. The VDCC α2 antibody used in this study was raised in rabbits against a specific peptide derived from the sequence of α2. Its specificity has been described previously (Brickley et al. 1995; Brice et al. 1997). The α1G antibody was raised in rabbits against a glutathione S transferase (GST) fusion protein derived from the cytoplasmic loop between IS6 and IIS1of α1G, as described in Craig et al. (1999), in which the antibody specificity is also described. The anti-GST component of the serum was removed by adsorption on GST Sepharose 4B, and it was then affinity purified on α1G-GST Sepharose 4B. The stock concentration of affinity-purified α1G antibody was 98 μg ml−1. The primary antibodies were detected using biotin-conjugated goat anti-rabbit IgG (1: 200) (Sigma), then streptavidin FITC (1:100) (Molecular Probes, Eugene, OR, USA). Cells were examined on an MRC 1024 laser scanning confocal microscope (Bio-Rad, Hemel Hempstead, UK), with all parameters (gain, aperture) identical between experiments. Images represent 2 μm optical sections midway through the cell. Quantification was performed using Imagequant software (Molecular Dynamics, Sunnyvale, CA, USA), and results are given, following background subtraction, as arbitrary units (a.u.) of pixel density.
RESULTS
The effect of co-expression of α2-δ on the immunolocalization of α1G
COS-7 cells represent an expression system with a very low background in terms of expressed currents. No Ba2+ currents were detected in the absence of expressed α1 subunits (Berrow et al. 1997; Brice et al. 1997; Meir & Dolphin, 1998). However, by RT-PCR using primers conserved across all species whose sequences have been reported, we found evidence for a low level of mRNA for α1G/H/I, α2-δ and β3 (J. Richards and A. C. Dolphin, unpublished results), but immunocytochemistry and Western blotting showed no β subunit protein in untransfected cells (Campbell et al. 1995a; Berrow et al. 1997; Brice et al. 1997; Stephens et al. 1997). A very low level of α1G immunostaining was observed in untransfected control cells, but this was not localized to the plasma membrane (Fig. 1A; pixel density throughout cell, 5.5 ± 0.8 a.u., of which none was membrane associated, n = 10). Expression of α1G resulted in much greater immunostaining for α1G, largely at the plasma membrane (Fig. 1B; pixel density at plasma membrane, 30.8 ± 4.4 a.u., n = 10). Co-expression of α2-δ with α1G resulted in a further increase in staining intensity at the plasma membrane (Fig. 1C; pixel density at plasma membrane, 42.0 ± 5.1 a.u., n = 10, a 36.4 % increase). No staining was observed in the absence of the primary antibody (Fig. 1D). These results suggest that heterologously expressed α2-δ is either increasing translation of α1G, increasing its trafficking to the plasma membrane, or increasing its stability. This raises the possibility that since endogenous α2-δ is present in cells, it may be involved in trafficking heterologously expressed α1G when α1G is expressed alone.
Figure 1. Effect of co-expression of α2-δ on immunolocalization of expressed α1G in COS-7 cells.
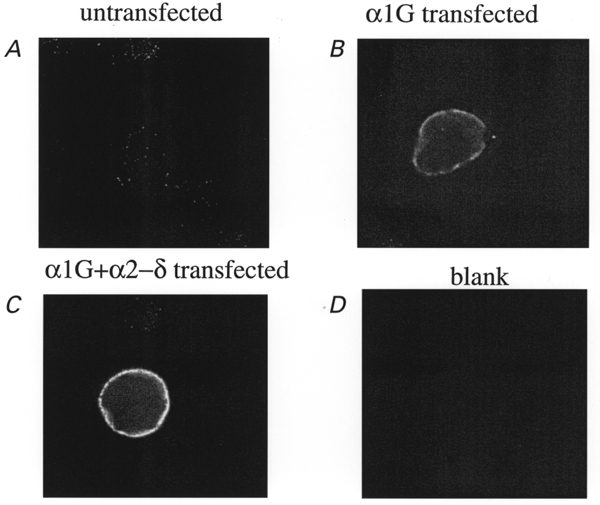
A, α1G immunolocalization in untransfected COS-7 cells; B, α1G immunolocalization in cells transfected with α1G; C, α1G immunolocalization in cells transfected with α1G + α2-δ. D, lack of immunofluorescence in the absence of the primary antibody. Experiments were performed under identical conditions in parallel cultures, and repeated 3 times with similar results. Scale bar, 20 μm.
Properties of α1G expressed in COS-7 cells
The heterologous expression of α1G alone in COS-7 cells resulted in the observation of T-type currents, using 1 mM Ba2+ as the charge carrier, in a large proportion (usually > 70 %) of GFP-positive cells (Fig. 2A and Table 1). The peak current was observed between −30 and −20 mV. The inactivation kinetics were voltage dependent at small depolarizations, reaching a voltage-independent minimum at potentials above −30 mV (Fig. 3A and Table 1). Recovery from inactivation occurred with a time constant of 107 ms (Fig. 3B). The voltage for 50 % activation (V50) was −27 mV, and the V50 for inactivation was −73 mV (Fig. 4 and Table 1). Superimposition of the activation and steady-state inactivation relationships showed no clear window current (inset in Fig. 4B).
Figure 2. I-V relationships for α1G and the effect of accessory subunits in COS-7 cells.
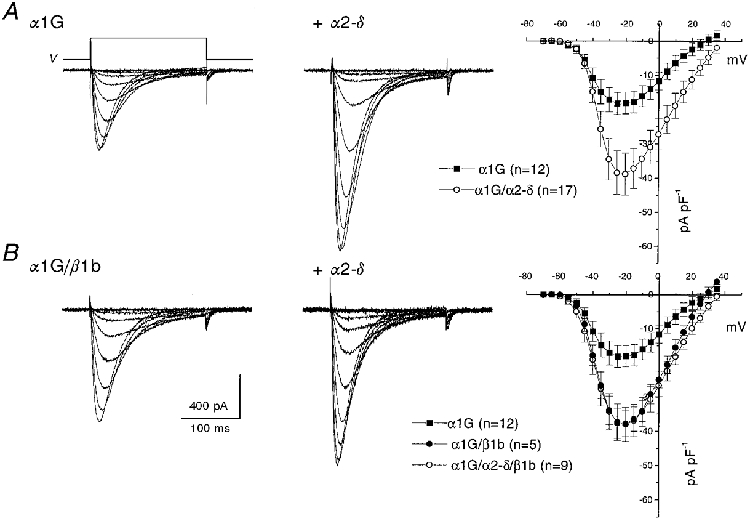
A shows α1G ± α2-δ and B shows α1G/β1b ± α2-δ. The left and centre panels show representative traces of α1G currents without (left) or with (centre) co-expression of α2-δ. Currents are shown in response to voltage steps (V) from −100 mV to between −70 and −20 mV, in 5 mV steps. The right panels show the corresponding I-V relationships (means ± s.e.m.) for the numbers of experiments given in parentheses.
Table 1.
Biophysical properties of α1G, expressed with different accessory subunit combinations in COS-7 cells
| Transfected subunits | IBa at −0 mV (pA pF−1) | Gmax (nS pF−1) | τinact at −20 mV (ms) | V50 for activation (mV) | V50 for steady-state inactivation (mV) |
|---|---|---|---|---|---|
| α1G | −19.2 ± 3.1 (15) | 0.55 ± 0.07 (15) | 13.8 ± 1.3 (7) | −27.1 ± 2.7 (10) | −72.9 ± 1.3 (8) |
| α1G/α2-δ | −40.3 ± 6.2** (17) | 0.75 ± 0.08* (17) | 13.1 ± 0.3 (6) | −27.3 ± 1.8 (8) | −69.7 ± 1.1 (6) |
| α1G/β1b | −39.8 ± 3.8** (5) | 0.87 ± 0.07** (5) | 14.2 ± 1.0 (5) | −22.3 ± 4.5 (5) | −68.7 ± 5.6 (4) |
| α1G/α2-δ/β1b | −39.5 ± 4.9** (9) | 0.79 ± 0.09** (9) | 13.6 ± 0.4 (4) | −27.4 ± 3.8 (3) | −72.0 ± 2.6 (5) |
| α1G/β2a | −27.3 ± 5.6 (9) | 0.67 ± 0.09* (9) | 11.7 ± 0.6 (3) | −22.3 ± 1.0 (3) | −69.4 ± 2.0 (5) |
| α1G/β3 | −30.7 ± 3.2* (11) | 0.65 ± 0.06 (11) | 20.3 ± 2.1 (11) | −17.0 ± 2.2* (8) | −65.5 ± 3.0* (8) |
| α1G/β4 | −23.8 ± 2.2 (6) | 0.62 ± 0.04 (6) | 12.9 ± 1.8 (5) | −20.7 ± 1.2* (5) | −67.3 ± 2.5** (4) |
The parameters determined for the different α1G and accessory subunit combinations were measured as described in Methods. The data for each subunit combination were determined from at least 3 separate transfections. Individual current density–voltage relationships were fitted with a Boltzmann equation, I = Gmax(V – Vrev)/(1 + exp(−(V – V1/2)/k)), where Gmax is the maximum conductance, Vrev is the reversal potential, k is the slope factor and V1/2 is the voltage for 50% current activation. No significant differences were observed in the Vrev or k values (results not shown). The activation V50 values given were determined from tail current analysis (Fig. 4), and steady-state inactivation V50 data were obtained using the protocol described in the legend to Fig. 4, and fitted with a single Boltzmann equation of the form I/Imax = 1/(1 + exp((V – V50)/k)). The statistical significance of the differences compared with α1G alone are indicated by
P < 0.05
P < 0.01 (Student's two-tailed t test).
Figure 3. Inactivation kinetics of α1G currents: effect of accessory α2-δ subunits in COS-7 cells.
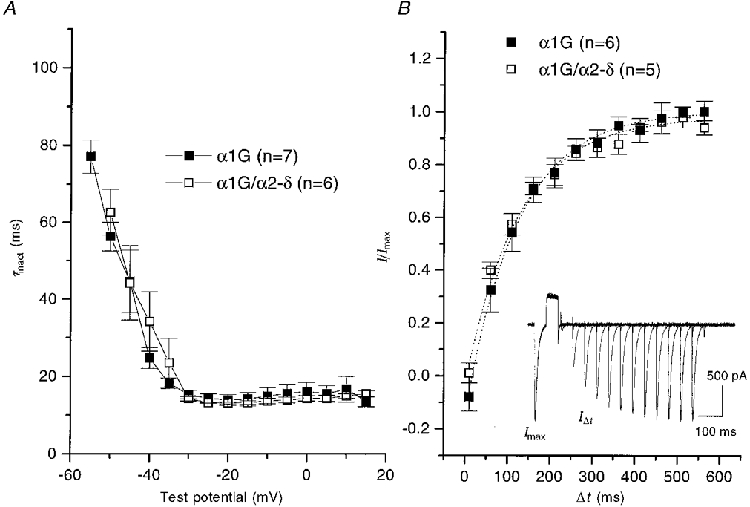
A, the inactivation phase of individual currents was fitted to a single exponential, and the mean values were plotted for α1G (▪) and α1G/α2-δ (□), for the numbers of experiments given in parentheses. B, recovery from inactivation was determined using the protocol shown in the inset, which depicts 13 overlaid traces obtained at 30 s intervals. The Vh was −100 mV. A 50 ms test step was given to −20 mV (Imax), followed by a 50 ms step to +100 mV to produce complete inactivation, followed by a variable interpulse interval (Δt), and a subsequent identical test step (IΔt). The recovery from inactivation was plotted for α1G (▪) and α1G/α2-δ (□), for the numbers of experiments given in parentheses. The data points were fitted to single exponentials (dotted lines), with τ values of 122.6 ms for α1G and 125.1 ms for α1G/α2-δ.
Figure 4. Voltage dependence of activation and inactivation of α1G currents in COS-7 cells: effect of accessory subunits.
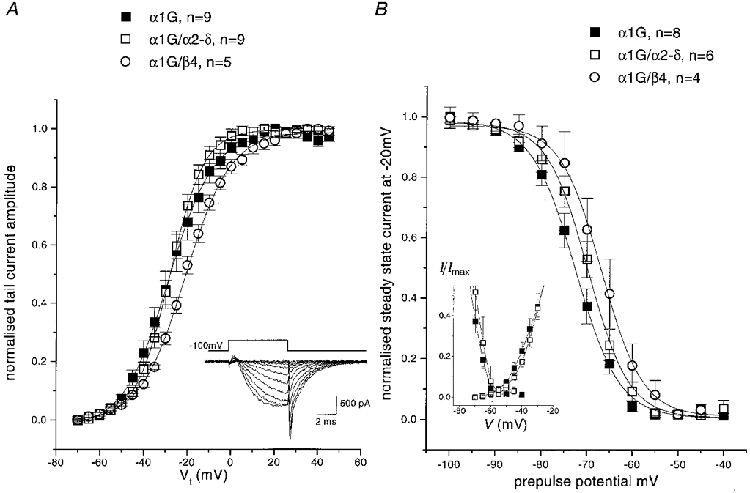
A, the current activation plots were determined from tail current amplitudes using the protocol shown in the inset, and normalized to the maximum current. Although this protocol, with a step length of 7.5 ms, gives a small error at low depolarizations because the current is not completely activated, this did not affect the V50 values. Mean ± s.e.m. values are shown for α1G (▪), α1G/α2-δ (□), α1G/β4 (^), with the numbers of experiments shown in the key. For both A and B, the curves are fits to the Boltzmann equation described in the legend to Table 1. B, steady-state inactivation curves were determined by measurement of peak current amplitude at −20 mV, following a 10 s conditioning prepulse to the potentials shown. Data were normalized before averaging the number of experiments given in the key. The symbols are the same as for A. The inset graph shows the region of overlap between the α1G and α1G/α2-δ activation and steady-state inactivation curves.
The effect of co-expression of α2-δ on the properties of α1G currents in COS-7 cells
Co-expression with α2-δ significantly increased the α1G current density at −20 mV by 2.1-fold, and increased the maximum conductance, compared with that for α1G alone (Fig. 2A and Table 1), while not significantly affecting any of the kinetic parameters measured (Figs 2A and 3A and Table 1). The kinetics of inactivation were very similar for α1G and α1G/α2-δ, reaching identical voltage-independent minima above −30 mV (Fig. 3A and Table 1). The recovery from inactivation also followed an identical time course (Fig. 3B). Furthermore, the reversal potential (Fig. 2A) was not significantly increased by α2-δ (+29.0 ± 2.3 mV, n = 15, for α1G compared with +34.3 ± 2.7 mV, n = 19, for α1G/α2-δ). The voltage dependence of activation (Fig. 4A) and the steady-state inactivation (Fig. 4B) of α1G were also not significantly affected by co-expression of α2-δ (Table 1). However, there was a small change in the value of the slope factor, k, of the Boltzmann fits to the activation data (evident from Fig. 4A), which was 9.1 ± 0.5 mV (n = 10) for α1G and 7.5 ± 0.5 mV (n = 8) for α1G/α2-δ.
In control experiments no calcium channel currents were observed in cells transfected with α2-δ alone, or with α2-δ plus a β subunit (results not shown), indicating that these accessory subunits are not recruiting an endogenous α1 subunit, as we also concluded in a previous work (Meir & Dolphin, 1998).
The effect of co-expression of α2-δ on the properties of α1G currents expressed in Xenopus oocytes
Co-injection of α2-δ with α1G cRNA in Xenopus oocytes increased the peak currents at −20 mV from −493 ± 60 nA for α1G alone to −784 ± 79 nA for α1G/α2-δ (P < 0.05, n = 40 from 4 separate experiments; Fig. 5). The average enhancement of the α1G current by α2-δ from these four experiments was 1.7 ± 0.2-fold. The shape of the current- voltage (I-V) relationship was not affected by co-injection with α2-δ, as determined following normalization of the peak currents. There was also no effect on the voltage dependence of current activation, determined from I-V relationships (for α1G the V50 for activation was −31.6 ± 0.6 mV, n = 40, and for α1G/α2-δ it was −32.2 ± 0.3 mV, n = 40). Activation kinetics were not affected (τ at −20 mV was 1.9 ms for both), and neither were inactivation kinetics (τinact at −20 mV was 11.1 ± 0.3 ms, n = 8, for α1G and 10.0 ± 0.4 ms, n = 9, for α1G/α2-δ). Furthermore, injection of α2-δ had no significant effect on the voltage dependence of steady-state inactivation as measured with 10 s conditioning prepulses (the V50 for steady-state inactivation was −63.8 ± 0.3 mV, n = 10, for α1G and −62.6 ± 0.5 mV, n = 14, for α1G/α2-δ). All the data in oocytes were therefore in full agreement with the results from COS-7 cells.
Figure 5. Effect of α2-δ on α1G expression in Xenopus oocytes.
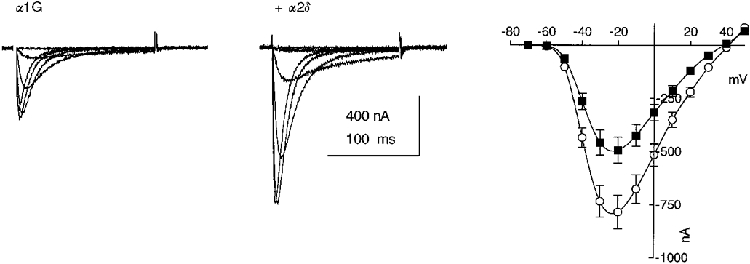
The left and centre panels show representative families of α1G currents recorded at between −60 and −10 mV in Xenopus oocytes in the absence (left) and presence (centre) of heterologously expressed α2-δ. The I-V relationships on the right represent the mean ± s.e.m. values from 40 experiments (10 oocytes from 4 different experiments) for α1G (▪), and α1G + α2δ (^).
Does heterologously expressed α1G associate with endogenous α2-δ?
Since α2-δ co-expression with α1G results in an increase in α1G immunostaining at the plasma membrane and an increase in functional expression, it is possible that α1G, when expressed alone, associates with endogenous α2-δ. We have observed a low level of endogenous α2 immunostaining in untransfected control cells (Fig. 6A; total pixel density of 38.2 ± 6.3 a.u., n = 14, from 3 separate transfection experiments), and this was not associated with the plasma membrane in most cells. The total staining for endogenous α2 was significantly increased when α1G cDNA was transfected into the cells (Fig. 6B; pixel density 82.7 ± 11.9 a.u., n = 19, P < 0.01 compared with untransfected cells), and the staining always showed some membrane association, indicating an association between the two proteins. These results suggest that expression of α1G is able to increase expression or stabilize endogenous α2-δ, and also increase membrane association of endogenous α2-δ.
Figure 6. Effect of α1G on immunolocalization of endogenous α2-δ in COS-7 cells.
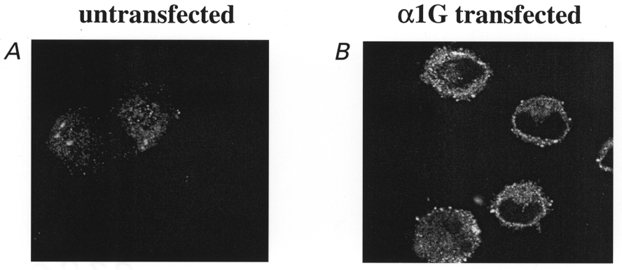
A, immunolocalization of endogenous α2 in untransfected COS-7 cells; B, immunolocalization of endogenous α2 in cells transfected with α1G. Experiments were performed under identical conditions in parallel cultures, and transfections were repeated 3 times with similar results.
Since these immunocytochemical results suggested that transfected α1G was associating with endogenous α2-δ, co-expression experiments were also performed with the δ construct, which has been found to influence some properties of α1C and α1A, but unlike α2-δ does not increase the current amplitude (Gurnett et al. 1996; Felix et al. 1997). When the δ-construct was expressed in COS-7 cells, clear immunostaining was observed at the plasma membrane (results not shown), using an antibody directed against an extracellular epitope in δ (Brickley et al. 1995). Co-expression of α1G with δ reduced the plasma membrane expression of α1G by 25 %, from immunocytochemical experiments (pixel density 22.8 ± 1.8 a.u., n = 10, for α1G/δ compared with 30.8 ± 4.4 a.u. (n = 10) for α1G expressed alone in parallel experiments). The expression level for α1G when co-expressed with δ represents a 45.5 % reduction compared with co-expression of α1G with full-length α2-δ. In agreement with this, in electrophysiological experiments, co-expression of δ with α1G did not produce any increase in the expression of α1G currents, unlike α2-δ. The current density for α1G/δ was 22.1 ± 3.2 pA pF−1 at −20 mV, and the conductance was 0.49 ± 0.04 nS pF−1 (n = 8). This result indicates that it is the α2 moiety of α2-δ that is responsible for the α1G current enhancement, in agreement with the results for α1C (Gurnett et al. 1996).
Effect of co-expression of β subunits on the properties of α1G currents in COS-7 cells
Co-expression of α1G with the β subunit β1b (Fig. 2B and Table 1) increased the α1G current density without significant effect on the reversal potential, which was +31.1 ± 1.9 mV (n = 5). In contrast, for co-expression of β2a, β3 or β4 with α1G, only a small current enhancement was observed (Table 1). Additional co-expression of α2-δ produced no further increment of current density for β1b (Table 1). There was no systematic effect of β subunits on the inactivation kinetics of α1G currents, the limiting τinact at −20 mV being similar for all subunit combinations (Table 1). There was also no effect of any β subunit on recovery from inactivation (results not shown). Co-expression with any one of the β subunits produced a small depolarization of the voltage dependence of activation of α1G, determined from tail current amplitudes (see Fig. 4A for β4). The voltage for 50 % activation (V50) was only depolarized significantly by β3 and β4 (Table 1). Similarly, the steady-state inactivation of α1G, measured after a 10 s conditioning potential step, was shifted to more depolarized potentials by co-expression of the β subunits β3 and β4 (Table 1 and, for β4, Fig. 4B). In control experiments, β1b did not induce calcium channel currents when expressed alone in COS-7 cells (results not shown).
DISCUSSION
Effect of co-expression of accessory α2-δ subunits on α1G expression
From the literature, diverse effects of α2-δ have been reported on the properties of cloned HVA channels. Many studies of the effects of accessory subunits have been performed using oocytes, before it was realized that they contain endogenous calcium channel subunits, including a Xenopus β3 subunit (Tareilus et al. 1997). Therefore, although the effect of α2-δ has been reported to increase the membrane expression of HVA α1 subunits, and to increase their open probability (Shistik et al. 1995; Gurnett et al. 1996) as well as shifting the voltage dependence of activation to more positive potentials (Qin et al. 1998), these effects may have been dependent on the presence of endogenous β subunits (Tareilus et al. 1997), and for α1A the effect of α2-δ is small in the absence of co-expressed β subunits. In HEK293 cells α2-δ produced a small increase in the density of α1E currents, but had no effect on the biophysical properties (Jones et al. 1998), whereas in COS-7 cells we observed α2-δ, in the absence of β, to shift activation of α1E to more positive potentials, while having very little effect on current density (Stephens et al. 1997). In both cell types, α2-δ was ineffective alone in targeting α1C or α1A to the plasma membrane (Brice et al. 1997; Gao et al. 1999). A recent report has shown that a novel α2-δ (α2δ-3) produces a similar enhancement of α1C and α1E current amplitudes to α2δ-1 (the α2-δ used in the current study), but these effects are only evident in the additional presence of a β subunit (Klugbauer et al. 1999).
We have previously reported that we could not detect mRNA for α2-δ in COS-7 cells (Berrow et al. 1997), but since these experiments were performed, additional α2-δ sequences have been reported, and using PCR primers conserved across species, we have now observed a faint PCR product in COS-7 cells of the size expected for α2-δ (results not shown). Consistent with this, we have also observed a low level of immunostaining for endogenous α2-δ, which, however, was not specifically plasma membrane associated (Fig. 6A). We have examined the effect of co-expression of α2-δ on immunostaining for heterologously expressed α1G, and observed a clear increase in α1G immunostaining at the plasma membrane (Fig. 1). Furthermore, the effect of heterologous expression of α1G on immunostaining for endogenous α2-δ was also studied, since we found previously that α2-δ was trafficked to the plasma membrane by HVA calcium channel α1/β combinations (Brice et al. 1997). We consistently found that immunostaining for endogenous α2-δ was greater in cells transfected with α1G than in untransfected cells, and that the staining was more plasma membrane associated, suggesting that α1G may be associating with, and stabilizing, endogenous α2-δ and also trafficking it to the plasma membrane. We still observed α1G currents when α1G was co-expressed with a truncated δ construct, lacking the α2 domain, but the current amplitude was not enhanced as it was with α2-δ, indicating that the extracellular α2 domain is required for current enhancement, in accord with previous results for α1C (Gurnett et al. 1996). In further agreement with this, α1G immunostaining was slightly reduced by δ, in contrast to the enhancement produced by α2-δ, again indicating the importance of the α2 moiety in this effect. The δ subunit acted as a ‘dominant negative’ in previous studies, reducing the effect of α2-δ on α1A expression (Gurnett et al. 1996). However, in the present study co-expression of δ with α1G did not prevent the expression of α1G currents, indicating either that it was not able to disrupt α1G interaction with endogenous α2-δ, or that such an interaction was not essential for functional expression of α1G currents. It therefore remains unclear whether the presence of endogenous α2-δ is essential for the expression of functional α1G channels, and in the future an antisense approach might be taken to deplete endogenous α2-δ, in order to answer this point.
In the present study, overexpression of exogenous α2-δ produced an approximately 2-fold increase in the amplitude of α1G currents in COS-7 cells, and a 1.7-fold increase in Xenopus oocytes, but had no effect on their kinetics or voltage dependence of activation or inactivation, or on recovery from inactivation in either system. This argues either for an effect of α2-δ on trafficking of the nascent α1G channels from the endoplasmic reticulum to the plasma membrane, or an effect to stabilize the plasma membrane channels in a functional conformation. Further studies at the single channel level will be necessary to determine whether there are effects on the open probability of the channels.
In a previous study, we have shown that overexpression of α2-δ, in undifferentiated NG108-15 cells, induced the appearance of an HVA sustained current. It is likely that this current component represented L-type current that had a very low open probability in the absence of α2-δ, as a similar component could also be induced by the L-type channel agonist Bay K8644 (Wyatt et al. 1998), and the induced HVA current was blocked by the L-type channel antagonist, nicardipine (C. N. Wyatt and A. C. Dolphin, unpublished results). There were no marked effects of α2-δ on the biophysical properties of the T-type current component itself, in terms of kinetics or voltage dependence (Wyatt et al. 1998), consistent with the observation made here. However, it should be noted that NG108-15 cells express substantial endogenous α2-δ at the plasma membrane.
Effect of co-expression of accessory β subunits on α1G expression
In the present study we have shown that co-expression of the β subunit, β1b, also has clear effects on expression of functional α1G current (Brice et al. 1997). In contrast, a recent antisense study in nodose ganglia concluded that native T-type channels were not associated with β subunits (Lambert et al. 1997), although in these cells the main β subunits observed were β2 and β3, with no β1 apparently present. However an antisense study in cardiac atrial cells has suggested that loss of β subunits may affect expression of T-type currents (Chen & Best, 1998). The consensus binding site for β subunits, which has been identified on the I-II loop of HVA α1 subunits, is not completely conserved in α1G (Perez-Reyes et al. 1998). While it has been suggested that there is another region on the C terminus, at least of α1E and α1A (Tareilus et al. 1997; Walker et al. 1998), to which certain β subunits may bind, it has been disputed whether this has any functional consequences for the biophysical properties (Jones et al. 1998). It is therefore possible that α1G may interact transiently with the β1b subunit, which may serve a chaperone function to traffic the α1G channel protein to the plasma membrane, or to stabilize the α1G once in the membrane, thus increasing current density, while having only minor effects on the biophysical characteristics of the channels. The finding that the effects of β1b and α2-δ were not additive in COS-7 cells suggests that they may be acting by a similar chaperone or stabilizing mechanism. We have observed that all β subunits increase the membrane expression of α1A (Brice et al. 1997), whereas it has been found that β1b was the only β subunit that increased the heterologous expression of α1S in Xenopus oocytes (Ren & Hall, 1997). We were unable to perform experiments on α1G/β interactions in Xenopus oocytes because β subunits stimulate the expression of endogenous oocyte calcium channels (Lacerda et al. 1994), making the results uninterpretable. This problem does not arise in COS-7 cells, as endogenous currents were not detected in these cells. It is of interest that a jellyfish α1 subunit has recently been cloned which also has only a rudimentary β binding motif (HMLDDAVKGYLDWINQAS, again with conserved residues in bold, and conserved charges in italics). This channel is also able to express in the absence of β subunits, and co-expression of a β subunit has also been reported to increase expression (Jeziorski et al. 1998).
For the HVA channels, all β subunits shift the voltage dependence of activation of the currents to more hyperpolarized potentials, with β4 producing the greatest effect for α1A, and all β subunits producing a similar effect for α1E (De Waard & Campbell, 1995; Jones et al. 1998). In contrast, the trend in the present study was for β subunits to shift the voltage dependence of activation of α1G to more depolarized potentials, although the depolarization of the V50 for activation was only statistically significant for β3 and β4. It remains to be determined whether these subtle effects on the biophysical properties of α1G are due to direct interaction with the α1G protein.
The effect of all β subunits, except β2a, is to cause an increase in the inactivation rate of HVA channels, and to shift the steady-state inactivation to more hyperpolarized potentials (Stephens et al. 1997; Walker & De Waard, 1998), the effect being particularly marked for β3 (Jones et al. 1998). In contrast, rat β2a, which is palmitoylated, produces an attenuation of inactivation of HVA currents and a shift of steady-state inactivation to more depolarized potentials (De Waard & Campbell, 1995; Costantin et al. 1998; Jones et al. 1998). No such effects were observed for β2a on α1G currents. This is in agreement with our previous study in which we investigated the effect of overexpression of β subunits on the properties of native T-type currents, expressed in isolation in undifferentiated NG108-15 cells (Wyatt et al. 1998). β2a, but not β1b, induced the appearance of a slowly inactivating HVA component of current, but neither had any effect on the biophysical properties of the T-type component. Similarly, it has recently been shown that antisense depletion of β subunits in nodose ganglia had no effect on the biophysical properties of the native T-type currents in these cells (Lambert et al. 1997). However, overexpression in Xenopus oocytes of either β2 or β4 slowed inactivation of an endogenous T-type current which had a single channel conductance of 9 pS (Lacerda et al. 1994). Part of the effect of β2 was attributed in this paper to an effect on channel assembly, stability or trafficking, in agreement with the role suggested here. It is also possible that the single channels observed by Lacerda et al. (1994) represent a small conductance mode of HVA channels, with properties very similar to T-type channels (Meir & Dolphin, 1998).
Conclusion
We have observed a clear effect of α2-δ on the amount of α1G channel protein expressed at the plasma membrane, and on the amplitude of the α1G current expressed in COS-7 cells and Xenopus oocytes. In reciprocal experiments we have also observed that α1G increased endogenous α2-δ plasma membrane expression in COS-7 cells. This provides several lines of evidence that α1G can interact with α2-δ. Nevertheless, from our results and those of others, it appears that α1G is able to form functional channels in the absence of co-expressed accessory subunits. However, as Xenopus oocytes (Singer-Lahat et al. 1992) and most cell lines used for expression studies, including COS-7 cells (present work) and HEK-293 cells (J. Richards and A. C. Dolphin, unpublished results), contain endogenous α2-δ subunits, this requires further study before it can be considered definitive.
Acknowledgments
We thank Professor C. Hopkins of the Laboratory of Molecular Cell Biology, University College London for access to the confocal microscope. This work was supported by a Wellcome Trust grant to A. C. D. and an NIH grant (HL57828) to E. P. R. We thank N. Balaguero for technical support.
References
- Armstrong CM, Matteson DR. Two distinct populations of calcium channels in a clonal line of pituitary cells. Science. 1985;227:65–67. doi: 10.1126/science.2578071. [DOI] [PubMed] [Google Scholar]
- Berrow NS, Brice NL, Tedder I, Page K, Dolphin AC. Properties of cloned rat α1A calcium channels transiently expressed in the COS-7 cell line. European Journal of Neuroscience. 1997;9:739–748. doi: 10.1111/j.1460-9568.1997.tb01422.x. [DOI] [PubMed] [Google Scholar]
- Brice NL, Berrow NS, Campbell V, Page KM, Brickley K, Tedder I, Dolphin AC. Importance of the different β subunits in the membrane expression of the α1A and α2 calcium channel subunits: studies using a depolarisation-sensitive α1A antibody. European Journal of Neuroscience. 1997;9:749–759. doi: 10.1111/j.1460-9568.1997.tb01423.x. [DOI] [PubMed] [Google Scholar]
- Brickley K, Campbell V, Berrow N, Leach R, Norman RI, Wray D, Dolphin AC, Baldwin S. Use of site-directed antibodies to probe the topography of the α2 subunit of voltage-gated Ca2+ channels. FEBS Letters. 1995;364:129–133. doi: 10.1016/0014-5793(95)00371-f. [DOI] [PubMed] [Google Scholar]
- Campbell V, Berrow N, Brickley K, Page K, Wade R, Dolphin AC. Voltage-dependent calcium channel β-subunits in combination with α1 subunits have a GTPase activating effect to promote hydrolysis of GTP by Gαo in rat frontal cortex. FEBS Letters. 1995a;370:135–140. doi: 10.1016/0014-5793(95)00813-o. [DOI] [PubMed] [Google Scholar]
- Campbell V, Berrow NS, Fitzgerald EM, Brickley K, Dolphin AC. Inhibition of the interaction of G protein Go with calcium channels by the calcium channel β-subunit in rat neurones. The Journal of Physiology. 1995b;485:365–372. doi: 10.1113/jphysiol.1995.sp020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E, Lux HD. A low voltage-activated fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984;310:501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Chen CC, Best PM. An antisense oligonucleotide targeting four β subunit genes inhibits the expression of both T and L type calcium currents in cultured atrial myocytes. Biophysical Journal. 1998;74 M-Pos350 (abstract) [Google Scholar]
- Chien AJ, Zhao XL, Shirokov RE, Puri TS, Chang CF, Sun D, Rios E, Hosey MM. Roles of a membrane-localized β subunit in the formation and targeting of functional L-type Ca2+ channels. Journal of Biological Chemistry. 1995;270:30036–30044. doi: 10.1074/jbc.270.50.30036. [DOI] [PubMed] [Google Scholar]
- Costantin J, Noceti F, Qin N, Wei XY, Birnbaumer L, Stefani E. Facilitation by the β2a subunit of pore openings in cardiac Ca2+ channels. The Journal of Physiology. 1998;507:93–103. doi: 10.1111/j.1469-7793.1998.093bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig PJ, Beattie RE, Folly EA, Banerjee MD, Reeves MB, Priestley JV, Carney SL, Sher E, Perez-Reyes E, Volsen SG. Distribution of the voltage-dependent calcium channel α1G subunit mRNA and protein throughout the mature rat brain. European Journal of Neuroscience. 1999. in the Press. [DOI] [PubMed]
- Cribbs LL, Lee J-H, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson MP, Fox M, Rees M, Perez-Reyes E. Cloning and characterization of α1H from human heart, a member of the T type Ca2+ channel gene family. Circulation Research. 1998;83:103–109. doi: 10.1161/01.res.83.1.103. [DOI] [PubMed] [Google Scholar]
- de Waard M, Campbell KP. Subunit regulation of the neuronal α1A Ca2+ channel expressed in. Xenopusoocytes Journal of Physiology. 1995;485:619–634. doi: 10.1113/jphysiol.1995.sp020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waard M, Scott VES, Pragnell M, Campbell KP. Identification of critical amino acids involved in α1-β interaction in voltage-dependent Ca2+ channels. FEBS Letters. 1996;380:272–276. doi: 10.1016/0014-5793(96)00007-5. [DOI] [PubMed] [Google Scholar]
- Felix R, Gurnett CA, De Waard M, Campbell KP. Dissection of functional domains of the voltage-dependent Ca2+ channel α2δ subunit. Journal of Neuroscience. 1997;17:6884–6891. doi: 10.1523/JNEUROSCI.17-18-06884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao TY, Chien AJ, Hosey MM. Complexes of the α1C and β subunits generate the necessary signal for membrane targeting of class C L-type calcium channels. Journal of Biological Chemistry. 1999;274:2137–2144. doi: 10.1074/jbc.274.4.2137. [DOI] [PubMed] [Google Scholar]
- Gurnett CA, de Waard M, Campbell KP. Dual function of the voltage-dependent Ca2+ channel α2δ subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. doi: 10.1016/s0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- Gurnett CA, Felix R, Campbell KP. Extracellular interaction of the voltage-dependent Ca2+ channel α2δ and α1 subunits. Journal of Biological Chemistry. 1997;272:18508–18512. doi: 10.1074/jbc.272.29.18508. [DOI] [PubMed] [Google Scholar]
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annual Review of Physiology. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- Jeziorski MC, Greenberg RM, Clark KS, Anderson PAV. Cloning and functional expression of a voltage-gated calcium channel α1 subunit from jellyfish. Journal of Biological Chemistry. 1998;273:22792–22799. doi: 10.1074/jbc.273.35.22792. [DOI] [PubMed] [Google Scholar]
- Jones LP, Wei SK, Yue DT. Mechanism of auxiliary subunit modulation of neuronal α1E calcium channels. Journal of General Physiology. 1998;112:125–143. doi: 10.1085/jgp.112.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugbauer N, Lacinova L, Marais E, Hobom M, Hofmann F. Molecular diversity of the calcium channel α2-δ subunit. Journal of Neuroscience. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrinsky EM, Pearson HA, Dolphin AC. Low- and high-voltage-activated calcium channel currents and their modulation in the dorsal root ganglion cell line ND7–23. Neuroscience. 1994;58:539–552. doi: 10.1016/0306-4522(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Lacerda AE, Perez-Reyes E, Wei X, Castellano A, Brown AM. T-type and N-type calcium channels of Xenopus oocytes: Evidence for specific interactions with β subunits. Biophysical Journal. 1994;66:1833–1843. doi: 10.1016/S0006-3495(94)80977-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert RC, McKenna F, Maulet Y, Talley EM, Bayliss DA, Cribbs LL, Lee J-H, Perez-Reyes E, Feltz A. Low-voltage-activated Ca2+ currents are generated by members of the CaVT subunit family (α1G/H) in rat primary sensory neurons. Journal of Neuroscience. 1998;18:8605–8613. doi: 10.1523/JNEUROSCI.18-21-08605.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert RC, Maulet Y, Mouton J, Beattie R, Volsen S, De Waard M, Feltz A. T-type Ca2+ current properties are not modified by Ca2+ channel β subunit depletion in nodosus ganglion neurons. Journal of Neuroscience. 1997;17:6621–6628. doi: 10.1523/JNEUROSCI.17-17-06621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-H, Daud AN, Cribbs LL, Lacerda AE, Pereverzev A, Klockner U, Schneider T, Perez-Reyes E. Cloning and expression of a novel member of the low voltage activated T type calcium channel family. Journal of Neuroscience. 1999;19:1912–1921. doi: 10.1523/JNEUROSCI.19-06-01912.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JP, Snutch TP. Oxford: IRL Press; 1991. The expression of neurotransmitter receptors and ion channels in Xenopus oocytes. [Google Scholar]
- Meir A, Dolphin AC. Known calcium channel α1 subunits can form low threshold, small conductance channels, with similarities to native T type channels. Neuron. 1998;20:341–351. doi: 10.1016/s0896-6273(00)80461-4. [DOI] [PubMed] [Google Scholar]
- Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316:440–446. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee J-H. Molecular characterization of a neuronal low-voltage-activated T type calcium channel. Nature. 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- Qin N, Olcese R, Stefani E, Birnbaumer L. Modulation of human neuronal α1E-type calcium channel by α2δ-subunit. American Journal of Physiology. 1998;274:C1324–1331. doi: 10.1152/ajpcell.1998.274.5.C1324. [DOI] [PubMed] [Google Scholar]
- Ren D, Hall LM. Functional expression and characterization of skeletal muscle dihydropyridine receptors in. Xenopus oocytes Journal of Biological Chemistry. 1997;272:22393–22396. doi: 10.1074/jbc.272.36.22393. [DOI] [PubMed] [Google Scholar]
- Schreibmayer W, Lester HA, Dascal N. Voltage clamping of Xenopus laevis oocytes utilizing agarose cushion electrodes. Pflügers Archiv. 1994;426:453–458. doi: 10.1007/BF00388310. [DOI] [PubMed] [Google Scholar]
- Shistik E, Ivanina T, Puri T, Hosey M, Dascal N. Ca2+ current enhancement by α2/δ and β subunits in Xenopus oocytes: Contribution of changes in channel gating and α1 protein level. The Journal of Physiology. 1995;489:55–62. doi: 10.1113/jphysiol.1995.sp021029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Lahat D, Lotan I, Itagaki K, Schwartz A, Dascal N. Evidence for the existence of RNA of Ca2+-channel α2/δ subunit in. Xenopus oocytes Biochimica et Biophysica Acta. 1992;1137:39–44. doi: 10.1016/0167-4889(92)90097-u. [DOI] [PubMed] [Google Scholar]
- Stephens GJ, Page KM, Burley JR, Berrow NS, Dolphin AC. Functional expression of rat brain cloned α1E calcium channels in COS-7 cells. Pflügers Archiv. 1997;433:523–532. doi: 10.1007/s004240050308. [DOI] [PubMed] [Google Scholar]
- Swick AG, Janicot M, Cheneval-Kastelic T, McLenithan JC, Lane DM. Promoter-cDNA-directed heterologous protein expression in. Xenopus laevis oocytes Proceedings of the National Academy of Sciences of the USA. 1992;89:1812–1816. doi: 10.1073/pnas.89.5.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tareilus E, Roux M, Qin N, Olcese R, Zhou JM, Stefani E, Birnbaumer L. A Xenopus oocyte β subunit: Evidence for a role in the assembly/expression of voltage-gated calcium channels that is separate from its role as a regulatory subunit. Proceedings of the National Academy of Sciences of the USA. 1997;94:1703–1708. doi: 10.1073/pnas.94.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D, Bichet D, Campbell KP, de Waard M. A β4 isoform-specific interaction site in the carboxyl-terminal region of the voltage-dependent Ca2+ channel α1A subunit. Journal of Biological Chemistry. 1998;273:2361–2367. doi: 10.1074/jbc.273.4.2361. [DOI] [PubMed] [Google Scholar]
- Walker D, De Waard M. Subunit interaction sites in voltage-dependent Ca2+ channels. Trends in Neurosciences. 1998;21:148–154. doi: 10.1016/s0166-2236(97)01200-9. [DOI] [PubMed] [Google Scholar]
- Wyatt CN, Page KM, Berrow NS, Brice NL, Dolphin AC. The effect of overexpression of auxiliary calcium channel subunits on native Ca2+ channel currents in undifferentiated NG108–15 cells. The Journal of Physiology. 1998;510:347–360. doi: 10.1111/j.1469-7793.1998.347bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


