Abstract
The response to bitter-tasting substances was recorded in outside-out membrane patches excised from the taste receptor cell of the bullfrog fungiform papilla.
Application of a bitter-tasting substance, quinine or denatonium, induced channel openings under conditions in which none of the second messenger candidates or their precursors (e.g. cyclic nucleotide, inositol 1,4,5-trisphosphate, Ca2+, ATP and GTP) were present on either side of the membrane. The response could be recorded > 10 min after excision of the patch membrane. These data suggest that the channel was directly gated by the bitter-tasting substances.
No change in response was detected upon addition to the cytoplasmic side of either GDPβS (1 mM) or GTPγS (1 mM), suggesting that the G protein cascade has no direct relation to response generation.
The quinine-induced current was dose dependent. The lowest effective concentration was approximately 0.1 mM, and the saturating concentration was near 1 mM. The dose-response curve was fitted by the Hill equation with a K½ of 0.52 mM and a Hill coefficient of 3.8.
The single channel conductance measured in 120 mM NaCl solution was 10 pS. The channel was cation selective, and the ratio of the permeabilities for Na+, K+ and Cs+ (PNa:PK:PCs) was 1:0.48:0.39. The unitary conductance was dependent on the extracellular Ca2+ concentration ([Ca2+]o); 9.2 pS in a nominally Ca2+-free solution, and 4.5 pS in 1.8 mM [Ca2+]o.
The dose dependence, the ion selectivity and the dependence of the unitary conductance on [Ca2+]o were almost identical to those of the quinine-induced whole-cell current reported previously, indicating that the channel activity observed in the excised membrane is the basis of the whole-cell current.
The present observations suggest the new possibility that the cationic channel directly gated by bitter substances is involved in the bitter taste transduction mechanism.
Bitter taste is an important warning sign for animals that food is poisonous. Despite its importance, the transduction mechanism of bitter taste is still a matter of controversy. The two major current hypotheses have been proposed mainly on the basis of biochemical data; both involve metabotropic cascades. The first hypothesis involves cytoplasmic cyclic nucleotide monophosphate (cNMP). It has been shown that a taste receptor cell expresses a specific G protein (McLaughlin et al. 1992) that activates phosphodiesterase leading to the decomposition of cNMP (Price, 1973; Ruiz-Avila et al. 1995). The cationic channel, found in the frog taste receptor cell and maintained in a closed state by internal cNMP, is shown to be opened by bitter stimuli (Kolesnikov & Margolskee, 1995). The second hypothesis proposes that a bitter substance increases the cytoplasmic inositol 1,4,5-trisphosphate (IP3) concentration by activating a G protein and phospholipase C. IP3 triggers Ca2+ release from the endoplasmic reticulum, which in turn directly or indirectly induces transmitter release from the taste receptor cells (Akabas et al. 1988; Hwang et al. 1990; Spielman et al. 1994, 1996). In either system, the transduction process requires soluble cytoplasmic elements such as cNMP, IP3, Ca2+, ATP or GTP. We report here an entirely different mechanism for the bitter taste transduction. We found that bitter substances directly activate a cationic channel in the absence of known cytoplasmic factors. Channel opening was observed in outside-out membrane patches excised from frog taste receptor cells recorded when these factors were absent on either side of the membrane. A part of this work has been reported in an abstract form (Tsunenari et al. 1999).
METHODS
Preparations
Taste receptor cells of bullfrogs (Rana catesbeiana) were enzymatically isolated from fungiform papillae of the tongue as previously described (Tsunenari et al. 1996). The care of the animals was in accordance with Guideline for the Care and Use of Laboratory Animals of Keio University School of Medicine, and our experiments were approved by the University Animal Welfare Committee. In short, the animals were decapitated and double pithed. The tongue was excised and fungiform papillae were removed using scissors under a dissecting microscope. Harvested papillae (approximately 100) were incubated for 25 min in Ca2+-free saline (containing 115 mM NaCl, 2.5 mM KCl, 2 mM glucose, 2 mM Na-Hepes and 2 mM EGTA), followed by a second incubation for 25 min in normal saline (containing 115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 2 mM glucose, 2 mM Na-Hepes) supplemented by 0.2 % collagenase/dispase (Boehringer) at 27°C. After rinsing them twice with Ca2+-free saline (no EGTA added), the tissue was triturated in normal saline with a glass pipette (tip diameter, 0.2 mm). Dissociated cells were stored at 4°C for up to 10 h in normal saline, and were plated in a culture dish before recording. The bottom of the dish was made of a concanavalin A-coated coverslip. Dissociation yielded a mixture of different shaped cells found in the fungiform papillae, but taste receptor cells were easily identified by their characteristic morphology (Avenet & Lindemann, 1987; Miyamoto et al. 1988). Voltage-dependent Na+ current was also used to identify the cell type (Avenet & Lindemann, 1987; Miyamoto et al. 1988; Tsunenari et al. 1996).
Solutions
The solutions used in current recordings from excised patches were as follows. The patch pipette solution used in most experiments contained 120 mM CsCl, 2 mM Na2-EGTA and 10 mM Na-Hepes. The 120 mM NaCl solution contained 120 mM NaCl and 2 mM Na-Hepes. KCl patch pipette solution (90 mM KCl, 30 mM TEA-Cl, 2 mM K2-EGTA and 10 mM K-Hepes) was used when the K+ permeability was measured.
The Ca2+-free bath solution contained 115 mM NaCl, 2.5 mM KCl and 2 mM Na-Hepes. The 1.8 mM Ca2+ solution contained 115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2 and 2 mM Na-Hepes. The Cl−-free solution contained 120 mM sodium gluconate and 2 mM Na-Hepes. TEA-NaCl solution (90 mM NaCl, 30 mM TEA-Cl and 2 mM Na-Hepes) was used to measure the K+ permeability. All solutions were adjusted to pH 7.2, and contained 0.0005 % (w/v) Phenol Red.
Electrophysiological recordings
A culture dish was mounted on the stage of an inverted microscope with phase-contrast optics (Nikon TMD, Japan). Whole-cell and outside-out patch-clamp recordings (Hamill et al. 1981) were performed at room temperature (20-26°C). The patch pipette tip was placed in contact with the apical end or soma of the cell. After the whole-cell configuration was established, a patch membrane was excised in the outside-out configuration. The extracellular side of the patch membrane was superfused by one of the solutions listed above as indicated in each figure legend. All voltage values reported here were corrected for liquid junction potentials, measured with a microelectrode containing 3 M KCl (Neher, 1992). Membrane currents were recorded using an amplifier (Axopatch-1D, Axon Instruments), filtered at 0.5 kHz and sampled at 1 kHz by a 12-bit A/D converter (ADX-98H, Canopus, Kobe, Japan) attached to a personal computer (PC-9821Ap, NEC, Tokyo, Japan) and driven by our own software (written by T. Kurahashi). Any drift of the current baseline was corrected by Microcal Origin 5.0J (Microcal Software, Northampton, MA, USA).
Application of bitter-tasting substance
A bitter-tasting substance was dissolved in the extracellular solution and applied to the outside of the patch membrane by the following two methods. The first method of application used in most experiments was the puffer system. The test solution was drawn into a puffer pipette (tip diameter, ∼1 μm), and was ejected by pressure applied to the back opening of the pipette from a laboratory-made pressure-regulation system (Ito et al. 1995). The vehicle solution in the puffer pipette was always identical to the bath solution.
A U-shaped tube system (for detailed explanation see Krishtal & Pidoplichko, 1980; Fenwick et al. 1982) was used to measure the dose-response relation. In brief, a U-shaped polyethylene capillary tube (i.d., 600 μm) was placed near a patch pipette so that an elliptical hole (diameters, 700 and 300 μm) cut in the wall of the outer curvature of the U faced the membrane patch at a distance of ∼40 μm. The upper leg of the U-tube was connected to a peristaltic pump which continuously supplied test solution. The lower leg was an outlet connected to suction. The amount of outflow was adjusted so that it slightly exceeded the inflow. Thus, the patch membrane was continuously washed by the bath solution flowing into the elliptical hole of the U-tube. When a solenoid valve, interposed in the lower leg, was closed, a flood of a test solution quickly covered the patch membrane. Measurement of the solution conductivity revealed that the solution near the patch pipette tip was almost completely replaced by the test solution within less than 50 ms.
We used quinine, denatonium, quinidine and strychnine as test substances. Since the properties of denatonium-induced responses were almost identical to those of quinine-induced responses, we mainly used quinine in the present study.
Drugs
Quinine hydrochloride, denatonium benzoate, guanosine 5′-O- (2-thiodiphosphate) trilithium salt (GDPβS), and guanosine 5′-O- (3-thiotriphosphate) tetralithium salt (GTPγS), were purchased from Sigma. (E)-N- [(4-Hydroxy-3-methoxyphenyl)methyl]-8-methyl-6-nonenamide (capsaicin) was purchased from Research Biochemicals International.
RESULTS
Bitter substance-induced response in excised patch membrane
A patch membrane excised from an acutely dissociated taste receptor cell of the bullfrog (Fig. 1A) was held at -79 mV close to its resting potential (Tsunenari et al. 1996). Application of 1 mM quinine to the external surface of the membrane patch induced flickering inward currents (Fig. 1B). The patch pipette solution did not contain soluble cytoplasmic elements such as cNMP, IP3, Ca2+, ATP or GTP. The current trace was digitized and an amplitude histogram was constructed (Fig. 1B, right). The histogram could be fitted by the sum of two Gaussian distributions. This observation is a clear indication that the record represents single channel currents. The mean amplitude of the unitary current was approximately -1.0 pA. Current records on an expanded time scale clearly show step-like fluctuations (Fig. 1C). The channel activity was seen in patches excised from both the apical end of the cell (n = 22 patches) and the cell body (n = 58). No difference was found in the properties of active channels in patch membranes excised from these two locations. No channel opening was seen in response to puffing the bathing solution alone (not shown). To eliminate the possibility that quinine acted on the cytoplasmic side of the patch membrane after crossing the plasma membrane, we made an outside-out patch preparation using patch pipette solution containing 1 or 5 mM quinine. Channel opening was not detected with either of these preparations. To further examine whether the lack of response in these preparations was due to desensitization, we applied 1 mM quinine to the outside of the membranes and saw channel openings similar to those seen in the preparation of Fig. 1 (data not shown).
Figure 1. Currents induced by bitter substances in the outside-out membrane patches.
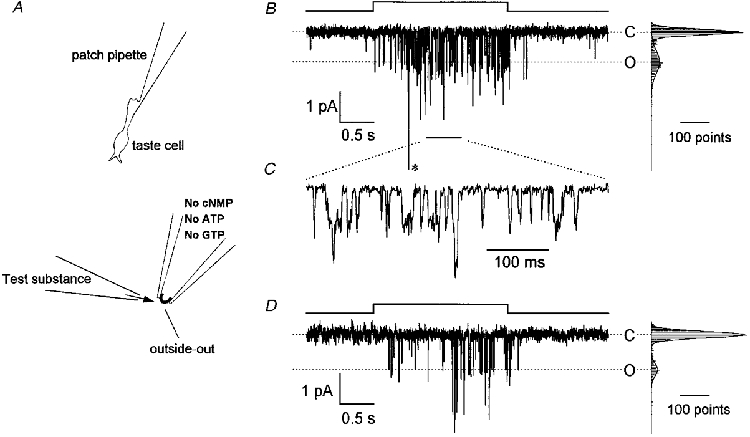
A, schematic diagram showing the experimental procedure. After the whole-cell configuration (upper panel) was established, a patch membrane was excised from an isolated taste receptor cell using a patch pipette filled with a solution containing no cNMP, Ca2+, ATP or GTP. A bitter substance was dissolved in the external solution and drawn into the puffer pipette, and applied to the outer surface of the patch membrane by pressure (lower panel). B, quinine-induced current response recorded from an outside-out membrane patch held at -79 mV. The upwards step in the pressure protocol (top) indicates the timing of 1 mM quinine application from the puffer pipette with a pressure of 20 kPa. The outside of the membrane was bathed in 120 mM NaCl (no Ca2+ added) solution and the patch pipette contained 120 mM CsCl. The amplitude histogram on the right side of the current trace was made from 2000 points during the 2 s quinine application. The histogram was fitted by the sum of two Gaussian distributions represented by continuous curves. The higher peak represents the closed state (C) and the lower peak represents the open state of one channel (O). The event labelled with an asterisk is quadruple the amplitude of the unitary current. C, part of the trace in B (indicated by a short bar below the current trace) reproduced on an expanded time scale. D, denatonium-induced current response in another outside-out patch. Denatonium (5 mM) was puff applied with a pressure of 50 kPa. The bath solution contained 115 mM NaCl and 2.5 mM KCl (no Ca2+ added). Other conditions were identical to those in B.
Most membrane patches included more than one channel. This fact hindered us from analysing the kinetics of the quinine-activated channel by constructing the open and closed time histograms. For this reason we limited our present study to the qualitative analysis of the channel.
To determine whether the response was specific to quinine or common to other bitter substances, we tested different bitter substances either in the excised patch preparation (denatonium) or in the whole-cell preparation (quinidine, denatonium and strychnine). All these substances generated the same response. Denatonium-induced channel openings are shown as an example (Fig. 1D). In the whole-cell preparation, an inward current was induced by 5 mM denatonium (150-720 pA at -54 mV, 3 cells), 1 mM quinidine (130-220 pA at -54 mV, 2 cells), or 10 mM strychnine (30 pA at -79 mV, 1 cell), and these responses showed identical reversal potentials (T. Tsunenari, T. Kurahashi & A. Kaneko, unpublished observations).
It is highly probable that the channel is directly gated by a bitter substance. Soluble cytoplasmic elements such as cNMP, IP3, Ca2+, ATP or GTP were not supplied in the patch pipette solution. If they remained on the cytoplasmic side of the patch membrane at the time of excision, they should have been washed away by the rapid dialysis of the patch pipette solution. The quinine-induced channel activity was seen in 76 % of the excised membrane patches tested more than 10 min after excision (Fig. 2A and B), although a slight rundown was unavoidable in some preparations.
Figure 2. Evaluation of whether cytoplasmic soluble elements and G proteins contribute to the response generation.
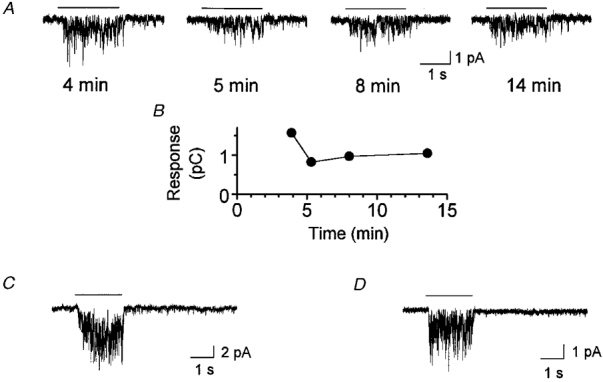
A, response of a membrane patch to quinine recorded 4, 5, 8 and 14 min after membrane excision. Quinine (5 mM) was ejected from a puffer pipette with a pressure of 10 kPa at the times indicated by the bars over the current traces. The holding voltage was -54 mV. B, magnitude of the integrated response of A as expressed by the amount of charge generated during quinine application. C, current response to quinine (1 mM, ejected from a puffer pipette with a pressure of 20 kPa) recorded 3.6 min after membrane excision with a patch pipette containing 1 mM GDPβS. The holding voltage was -79 mV. D, current response (1 mM, ejected from a puffer pipette with a pressure of 30 kPa) recorded 4.0 min after membrane excision with a patch pipette containing 1 mM GTPγS. The holding voltage was -54 mV. For all panels the bath solution contained 115 mM NaCl, 2.5 mM KCl (no Ca2+ added) and the patch pipette solution contained 120 mM CsCl.
The notion of direct gating is further supported by the observation that modulation of G protein activity was ineffective. No change was detected in quinine-induced channel opening by 1 mM GDPβS, a G protein inhibitor, in the patch pipette (representative example shown in Fig. 2C). This may indicate that the response was not induced by a G protein cascade if some soluble cytoplasmic elements remained, although this is unlikely. Experiments with GTPγS (1 mM), a G protein activator, ruled out another possibility of direct gating by G proteins of ion channels such as those known for the muscarinic K+ channels (for review see Yamada et al. 1998). Addition of GTPγS did not induce any detectable response (3 patches). It is evident that these patch membranes contained quinine-responding channels as they responded to quinine applied to the outside of the membrane (Fig. 2D).
Reversal potential and single-channel conductance of the bitter substance-induced current
The current-voltage (I-V) relation of the unitary event was almost linear (Fig. 3; 120 mM NaCl outside and 120 mM CsCl inside). Under bi-ionic conditions the reversal potential was +20.5 ± 2.3 mV (mean ±s.d., n = 3), and the single channel conductance was 10.2 ± 1.4 pS (n = 3). Under bi-ionic conditions (patch pipette solution contained 90 mM KCl and bath solution contained 90 mM NaCl; 30 mM TEA-Cl was added to both sides to block the voltage-gated K+ channels), the reversal potential was +16.9 ± 1.5 mV (n = 3), and the single channel conductance was 6.6 ± 0.1 pS (n = 3). The reversal potential was not shifted by substituting gluconate for extracellular Cl− (+18.8 ± 3.2 mV, n = 3; Fig. 3B). In a patch membrane recorded under symmetrical ionic conditions (120 mM NaCl) the reversal potential was -1.4 mV. All these observations suggest that the bitter substance-activated channel is cation selective. The ratio of the permeabilities of Cs+, K+ and Na+ (PCs:PK:PNa) estimated under bi-ionic conditions was 0.39:0.48:1. The permeability ratio and the single channel conductance were almost identical to those obtained in our previous study on the whole-cell current response (PCs:PK:PNa= 0.42:0.5:1; Tsunenari et al. 1996) and by noise analysis (12 pS; Tsunenari & Kaneko, 1997, 1998).
Figure 3. Voltage dependence of the quinine-evoked unitary events.
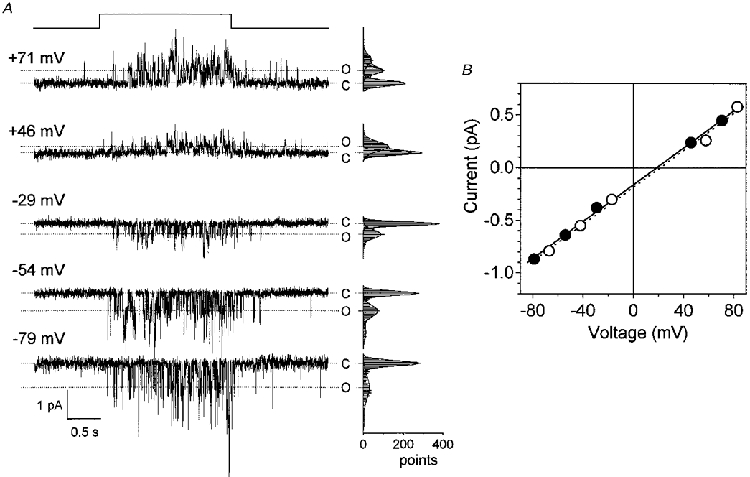
A, quinine-induced currents recorded from an outside-out membrane patch held at various holding voltages. Upward shift of the horizontal line on the top indicates the timing of quinine application. Quinine (1 mM) was ejected from a puffer pipette with a pressure of 20 kPa. The outside of the membrane was bathed in a solution containing 120 mM NaCl and 2 mM Na-Hepes. The patch pipette solution contained 120 mM CsCl, 2 mM Na2-EGTA and 10 mM Na-Hepes. Amplitude histograms to the right of each current trace were made from 2000 points during the 2 s quinine application and were fitted by the sum of Gaussian distributions represented by continuous curves. C indicates the closed state and O the open state of a single channel. B, I-V relation of the unitary current amplitude. The unitary amplitude was measured from the peaks of the Gaussian curves as shown in A.•, data from A recorded in the 120 mM NaCl solution connected by the continuous line. ○, data from the same patch membrane recorded in the Cl−-free solution connected by the dotted line (NaCl was replaced by equimolar sodium gluconate). Lines were fitted by the least-squares method.
Dose dependence of the bitter substance-induced current
The response magnitude was dose dependent (Fig. 4). In this experiment, quinine of known concentration was applied using the U-tube system, so that the concentration of quinine in the vicinity of the patch membrane was identical to that of the applied solution. The lowest effective concentration of quinine applied to the patch membrane shown in Fig. 4A was approximately 0.1 mM, and the saturating concentration was near 1 mM. At higher concentrations, channel events merged with each other, suggesting that the patch contained numerous channels. However, no sign of desensitization was seen during the 4 s application of 1-2 mM quinine (bottom traces of Fig. 4A). Similar dose dependence was obtained in two more samples. Figure 4B shows the relation between the amount of charge carried by the quinine-induced channel current and the concentration of quinine. The dose- response curve was sigmoidal and could be fitted by the Hill equation with a K½ of 0.52 mM and a Hill coefficient of 3.8. The dose-response relation shows that the excised patch membrane is as sensitive as the taste receptor cells recorded in the whole-cell configuration, in which K½ was 0.5 mM and the saturating concentration was 2 mM (Tsunenari & Kaneko, 1997, 1998; T. Tsunenari & A. Kaneko, unpublished observations).
Figure 4. Dose dependence of the quinine-evoked current response.
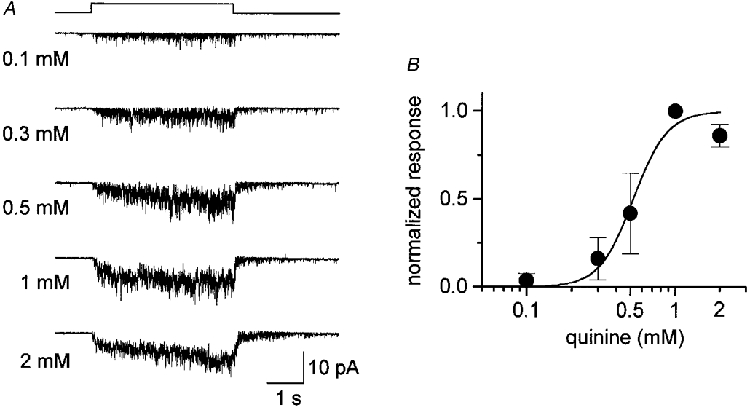
A, current responses induced by quinine at various concentrations. Test solution was applied using a U-shaped tube (see Methods) during the period indicated by the square step above the current traces. The patch membrane was held at -54 mV. The outside of the membrane was bathed in a solution containing 115 mM NaCl and 2.5 mM KCl (no Ca2+ added). B, relation between the response magnitude (total charge carried during 4 s quinine application) and the concentration of quinine. The response was normalized to that generated by 1 mM quinine. Filled circles represent the mean and bars the s.d. of three cells. The continuous curve was fitted by the Hill equation, I/Imax=cnH/(cnH+K½), with a Hill coefficient nH= 3.8 and K½= 0.52 mM, where c is the concentration of quinine.
Effects of external Ca2+ on the bitter substance-activated channel
It has been shown in whole-cell recordings of isolated taste receptor cells that the quinine-induced response is enhanced by as much as 6-fold in a nominally Ca2+-free extracellular solution (Tsunenari et al. 1996). Similar augmentation was seen in the outside-out patch preparation (Fig. 5A). The amplitude of the single channel current was -0.38 ± 0.10 pA (holding potential, -64 mV; n = 4) in 1.8 mM Ca2+ and -0.78 ± 0.09 pA (n = 3) in a nominally Ca2+-free solution (Fig. 5B). The single channel conductance was 4.5 ± 1.2 pS in 1.8 mM Ca2+ solution and 9.2 ± 1.1 pS in the Ca2+-free solution. These conductance values agree with those measured indirectly by the noise analysis of quinine-activated whole-cell current (5 pS in 1.8 mM Ca2+ and 12 pS in 0 mM Ca2+; Tsunenari & Kaneko, 1997, 1998). The most likely interpretation for the conductance decrease by Ca2+ of the quinine-induced single channel is channel flickering. The flickering frequency may have been much higher than the resolution of our recording system. We increased the sampling rate of the A/D converter by one order of magnitude (to 10 kHz), but still could not clearly resolve single channel events; this only increased the background noise. High frequency flickering by Ca2+ has been reported in cGMP-gated channels of photoreceptors (Haynes et al. 1986; Zimmerman & Baylor, 1986; Sesti et al. 1994) and in acetylcholine receptor channels (Decker & Dani, 1990; Mulle et al. 1992).
Figure 5. Effect of external Ca2+ on the quinine-induced current response.
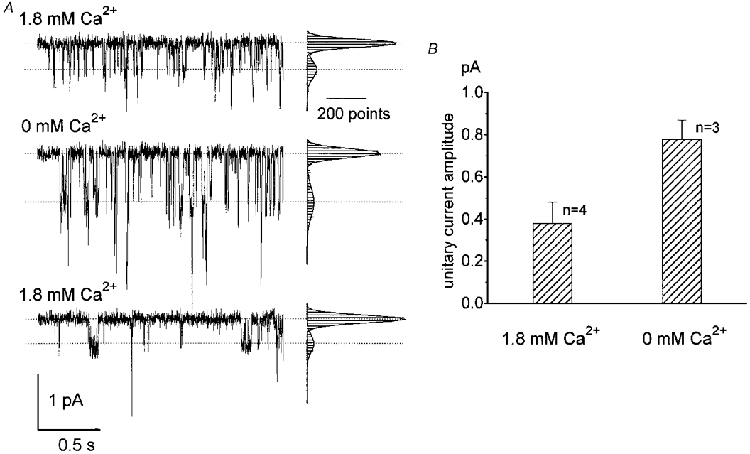
A, responses of an outside-out patch membrane held at -64 mV to a 2 s puff application of 3 mM quinine. The three records were obtained in sequence; first in solution (115 mM NaCl and 2.5 mM KCl) containing 1.8 mM Ca2+, second in Ca2+-free solution, and third upon return to the first solution containing 1.8 mM Ca2+. Histograms to the right of each current trace were made from 2000 points of the 2 s data. B, histogram showing the effect of external Ca2+ on the quinine-activated unitary current amplitude, calculated from Gaussian distributions fitted to the current amplitude histograms shown in A. Error bars indicate s.d.
DISCUSSION
In the present study we showed that bitter-tasting substances induce channel-opening events in a patch membrane excised from a taste receptor cell. The response was evoked by a variety of bitter-tasting substances, such as quinine, quinidine, denatonium and strychnine. Using the whole-cell recording method we have already demonstrated that epithelial or supporting cells of the fungiform papillae do not show detectable responses to quinine (Tsunenari et al. 1996). Therefore, it seems safe to conclude that the channel activity observed in the present study is related to the bitter taste and is unique to the taste receptor cell.
It is probable that the channel shown in the present study was directly gated by bitter substances for the following reasons. The bitter substance-induced channel opening was seen in the ‘excised’ patch. The patch membrane was exposed on both sides to very simple salt solutions which did not contain any known second messengers or their precursors. Under our recording conditions, the small molecules essential for the metabotropic cascades should have been washed away within a few seconds from the surface of the patch membrane. In fact, in the photoreceptor outer segments, which use cGMP as the second messenger for the G protein-mediated transduction system, it has been shown that the cytoplasmic factors (GTP, ATP and cGMP) are washed out immediately on truncation of the outer segment (Nakatani & Yau, 1988) or excision of the membrane patch (Watanabe & Murakami, 1992), and the dark current (and the light response) disappeared in seconds. Moreover, we obtained evidence that the quinine-induced response was not inhibited by GDPβS. This observation rules out the remaining doubt that the response we observed in the excised patch membrane was evoked by activation of the remaining second messengers, or their precursors, by G proteins. The lack of any detectable effect by GTPγS also rules out the possibility of direct control by G proteins of ionic channels. We reported previously that 8-bromo cyclic AMP partially suppresses the quinine-induced conductance (Tsunenari et al. 1996). These observations do not necessarily conflict with the present conclusion that quinine directly gated ion channels. It is possible that cyclic nucleotides or other second messengers modulate the quinine-activated channels and control their sensitivity. We tried to analyse how cyclic nucleotides may modulate the quinine-activated channel, but due to the inclusion of more than one channel in the patch membrane and to the flickering nature of the unitary current, we could not obtain quantitative data in the present study. The modulatory effects of cytoplasmic factors require further study.
The bitter substance-activated channels recorded in the present study have a selective permeability to cations. Their ionic properties are identical to those obtained in the taste receptor cells in the whole-cell current response (Tsunenari et al. 1996). The permeability ratio PNa:PK:PCs, the single-channel conductance, the dose-response relation and the effect of extracellular Ca2+ on the channel conductance are almost identical to those obtained previously. These results strongly indicate that the whole-cell current observed in the frog taste receptor cells is the sum of the channel events recorded in the present study. We wondered whether the channels could be capsaicin receptor channels, because the recently identified capsaicin receptor is also a non-selective cation channel (Caterina et al. 1997). We therefore tested three cells that responded to 5 mM quinine with 100 μm capsaicin (10 times the saturation level found in the experiment of Caterina et al. 1997), but found no response (in whole-cell recording, data not shown).
In earlier studies, blocking of K+ channels by quinine has been attributed to the underlying mechanism of bitter taste reception (Avenet & Lindemann, 1987; Kinnamon & Roper, 1988; Sugimoto & Teeter, 1991; Cummings & Kinnamon, 1992). Blockade of the voltage-dependent K+ channel, which presumably coexists with ligand-gated channels, increases the input resistance of a cell, making the inward current through the ligand-gated channel more effective. Thus, the two mechanisms can work synergistically. The present findings give new evidence to suggest that bitter taste transduction involves direct channel opening by the bitter-tasting substance. It will now be of interest to investigate whether the ionotropic and metabotropic processes coexist in bitter taste transduction, and if so how they interact.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from HFSPO, PRESTO (to T. K.) and the Ministry of Education, Science, Sports and Culture (to T. T., T. K. and A. K.), the funds from Keio Health Consulting Center and Keio Gijuku Academic Development Fund (to T. T.).
References
- Akabas MH, Dodd J, Al-Awqati Q. A bitter substance induces a rise in intracellular calcium in a subpopulation of rat taste cells. Science. 1988;242:1047–1050. doi: 10.1126/science.3194756. [DOI] [PubMed] [Google Scholar]
- Avenet P, Lindemann B. Patch-clamp study of isolated taste receptor cells of the frog. Journal of Membrane Biology. 1987;97:223–240. doi: 10.1007/BF01869225. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cummings TA, Kinnamon SC. Apical K+ channels in Necturus taste cells. Modulation by intracellular factors and taste stimuli. Journal of General Physiology. 1992;99:591–613. doi: 10.1085/jgp.99.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker ER, Dani JA. Calcium permeability of the nicotinic acetylcholine receptor: the single-channel calcium influx is significant. Journal of Neuroscience. 1990;10:3413–3420. doi: 10.1523/JNEUROSCI.10-10-03413.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick EM, Marty A, Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. The Journal of Physiology. 1982;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Haynes LW, Kay AR, Yau K-W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986;321:66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Hwang PM, Verma A, Bredt DS, Snyder SH. Localization of phosphatidylinositol signaling components in rat taste cells: role in bitter taste transduction. Proceedings of the National Academy of Sciences of the USA. 1990;87:7395–7399. doi: 10.1073/pnas.87.19.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Kurahashi T, Kaneko A. Pressure control instrumentation for drug stimulation. Nippon Seirigaku Zasshi. 1995;57:127–134. [PubMed] [Google Scholar]
- Kinnamon SC, Roper SD. Membrane properties of isolated mudpuppy taste cells. Journal of General Physiology. 1988;91:351–371. doi: 10.1085/jgp.91.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov SS, Margolskee RF. A cyclic-nucleotide-suppressible conductance activated by transducin in taste cells. Nature. 1995;376:85–88. doi: 10.1038/376085a0. [DOI] [PubMed] [Google Scholar]
- Krishtal OA, Pidoplichko VI. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5:2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Okada Y, Sato T. Membrane properties of isolated frog taste cells: three types of responsivity to electrical stimulation. Brain Research. 1988;449:369–372. doi: 10.1016/0006-8993(88)91056-6. [DOI] [PubMed] [Google Scholar]
- Mulle C, Léna C, Changeux J-P. Potentiation of nicotinic receptor response by external calcium in rat central neurons. Neuron. 1992;8:937–945. doi: 10.1016/0896-6273(92)90208-u. [DOI] [PubMed] [Google Scholar]
- Nakatani K, Yau K-W. Guanosine 3′,5′-cyclic monophosphate-activated conductance studied in a truncated rod outer segment of the toad. The Journal of Physiology. 1988;395:731–753. doi: 10.1113/jphysiol.1988.sp016943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods in Enzymology. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Price S. Phosphodiesterase in tongue epithelium: activation by bitter taste stimuli. Nature. 1973;241:54–55. doi: 10.1038/241054a0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Avila L, McLaughlin SK, Wildman D, McKinnon PJ, Robichon A, Spickofsky N, Margolskee RF. Coupling of bitter receptor to phosphodiesterase through transducin in taste receptor cells. Nature. 1995;376:80–85. doi: 10.1038/376080a0. [DOI] [PubMed] [Google Scholar]
- Sesti F, Straforini M, Lamb TD, Torre V. Gating, selectivity and blockage of single channels activated by cyclic GMP in retinal rods of the tiger salamander. The Journal of Physiology. 1994;474:203–222. doi: 10.1113/jphysiol.1994.sp020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman AI, Huque T, Nagai H, Whitney G, Brand JG. Generation of inositol phosphates in bitter taste transduction. Physiology and Behavior. 1994;56:1149–1155. doi: 10.1016/0031-9384(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Spielman AI, Nagai H, Sunavala G, Dasso M, Breer H, Boekhoff I, Huque T, Whitney G, Brand JG. Rapid kinetics of second messenger production in bitter taste. American Journal of Physiology. 1996;270:C926–931. doi: 10.1152/ajpcell.1996.270.3.C926. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Teeter JH. Stimulus-induced currents in isolated taste receptor cells of the larval tiger salamander. Chemical Senses. 1991;16:109–122. [Google Scholar]
- Tsunenari T, Hayashi Y, Orita M, Kurahashi T, Kaneko A, Mori T. A quinine-activated cationic conductance in vertebrate taste receptor cells. Journal of General Physiology. 1996;108:515–523. doi: 10.1085/jgp.108.6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunenari T, Kaneko A. Fluctuation properties of the quinine-induced current in isolated bullfrog taste cells. Japanese The Journal of Physiology. 1997;47(suppl. 2):S151. [Google Scholar]
- Tsunenari T, Kaneko A. Noise analysis of the quinine-induced current in frog taste receptor cells. Annals of the New York Academy of Sciences. 1998;855:148–149. doi: 10.1111/j.1749-6632.1998.tb10557.x. [DOI] [PubMed] [Google Scholar]
- Tsunenari T, Kurahashi T, Kaneko A. A quinine-activated cationic channel in the excised patch membrane of the bullfrog taste receptor cell. Chemical Senses. 1999 doi: 10.1111/j.1469-7793.1999.0397m.x. in the Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S-I, Murakami M. Phototransduction in cones as examined in excised membrane patch. Japanese The Journal of Physiology. 1992;42:309–320. doi: 10.2170/jjphysiol.42.309. [DOI] [PubMed] [Google Scholar]
- Yamada M, Inanobe A, Kurachi Y. G protein regulation of potassium ion channels. Pharmacological Reviews. 1998;50:723–760. [PubMed] [Google Scholar]
- Zimmerman AL, Baylor DA. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986;321:70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]


