Abstract
Functional magnetic resonance imaging (fMRI) provides a means of studying neuronal circuits that control respiratory muscles in humans with better spatial and temporal resolution than in previous positron emission tomography (PET) studies.
Whole brain blood oxygenation level-dependent (BOLD) changes determined by fMRI were used to identify areas of neuronal activation associated with volitional inspiration in five healthy men. Four series of scans of each subject were acquired during voluntary breathing (active task) and mechanical ventilation (passive task). Ventilation and end-tidal PCO2 were similar between tasks. Scan data were re-aligned to correct for movement artefacts and cross-referenced breath by breath to respiratory data for selective averaging of inspiratory and expiratory images.
Group analysis identified significant increases in the fMRI signal with volitional inspiration in the superior motor cortex, premotor cortex and supplementary motor area at loci similar to those detected in earlier studies that used PET. Additional regions activated by volitional inspiration included inferolateral sensorimotor cortex, prefrontal cortex and striatum (these foci were only revealed by PET under significant inspiratory load).
This study represents the first synchronised breath-by-breath analysis of respiratory-related neuronal activity with whole brain imaging in humans. Temporal resolution is sufficient to distinguish individual breaths at a normal breathing frequency.
Control of breathing can emanate from many motor command neurones within the central nervous system, including neurones within the cortex, limbic system and brainstem (reviewed by Dick et al. 1997; Guz, 1997). This complex control system subserves both metabolic and behavioural requirements, including the voluntary control of breathing. Humans have a vast array of voluntary breathing strategies used during various behaviours including speech and while playing wind instruments. The identification of the neural pathways associated with voluntary control of breathing has been little studied in animals (with isolated exceptions in which animals have been trained to adjust their breathing pattern, e.g. Orem & Netick, 1986). The control pathways have been investigated in humans with clinical lesion studies and with electrical stimulation studies, with obvious limitations (reviewed by Plum & Leigh, 1981). More recently, positron emission tomography (PET) imaging has been used with promising results. For example, studies using PET have detected foci of enhanced regional cerebral blood flow during volitional breathing in the primary motor cortex, premotor area, supplementary motor area and cerebellum (Colebatch et al. 1991; Ramsay et al. 1993). Nonetheless, there are still many unanswered questions relating to the identification of the precise neural elements involved in the planning, co-ordination, motor activation and sensations of breathing as well as the relative time course of activation of these elements across the respiratory cycle.
The initial PET studies of breathing had a temporal resolution of approximately 90 s and a pre-processed spatial resolution of 433.5 mm3 (8.5 mm × 8.5 mm × 6 mm slice thickness). Functional magnetic resonance imaging (fMRI) permits indirect assessment of focal neuronal activity with greater spatial and temporal resolution than PET and without exposure to ionising radiation. This technique was first used in human respiratory studies by Gozal et al. (1994, 1995, 1996) studying a single sagittal ‘slice’ of brain with a temporal resolution of 7.5-9.5 s and a spatial resolution of 10.3 mm3 (1.01 mm × 2.03 mm × 5 mm slice thickness). However, single slice imaging has the major limitation of the inability to characterise other regions involved in the neural network subserving breathing control. The present study employed echo-planar fMRI using a blood oxygenation level-dependent (BOLD) pulse sequence that enabled whole brain imaging every 2 s and a spatial resolution of 84.4 mm3 (3.75 mm × 3.75 mm × 6 mm slice thickness). With this first study of respiratory control using whole brain echo-planar fMRI, we confirm the loci of motor command neurones responsible for volitional inspiration previously identified by PET (Colebatch et al. 1991; Ramsay et al. 1993) and demonstrate that fMRI can detect within-breath changes in cortical activation across the different phases of the respiratory cycle. Preliminary results of the present study have been reported previously (Evans et al. 1998).
METHODS
Subjects
Five healthy, right-handed men, aged 23-27 years, with no known respiratory or neurological problems were studied. The study was approved by the Committee for the Protection of Human Subjects at Dartmouth Medical School. Written informed consent was obtained from all subjects.
Protocol and measurements
While lying supine in the MRI scanner subjects breathed at all times through a scuba-type mouthpiece. There were two experimental conditions. During the passive task, subjects were ventilated with fixed mechanical ventilation (intermittent positive pressure ventilation, IPPV; Puritan-Bennett model 7200, CA, USA). During the active task, subjects breathed volitionally. A higher than normal tidal volume (approximately 1.6 l) and a slower than normal breathing frequency (10 breaths min−1) were selected so that breathing was entirely different from the spontaneous pattern, ensuring that breathing was under voluntary control during the active task. The ratio of inspiratory duration to expiratory duration was 1:2.
To prevent potential scanner interference, the ventilator was situated just outside the scanner room and ventilation was supplied via 9 m of standard ventilator tubing. The additional compliance of this extended tubing length was taken into account during ventilator calibration.
Training
Subjects were trained to perform the active and passive respiratory tasks on several different days prior to fMRI scanning. The adequacy of passivity during the mechanical ventilation task was assessed by the level and consistency of the airway pressure trace during fixed mechanical ventilation, as used by Colebatch et al. (1991). For the active task intermittent feedback on respiratory timing and volume were provided until this could be performed accurately without coaching from the investigators.
Mechanical ventilation (passive task)
Subjects relaxed respiratory muscles during passive normocapnic positive pressure ventilation. Ventilation was set at a relatively high level of 0.16 l min−1 kg−1, approximately twice the normal level, in order to diminish spontaneous phasic respiratory muscle activity at normocapnia (Prechter et al. 1990; Simon et al. 1991). Respiratory rate was set at 10 breaths min−1. To avoid alterations in global brain blood flow or fMRI signal associated with changes in blood gases, each subject's baseline end-tidal PCO2 was measured before the recordings, and normocapnia was maintained by the administration of adequate amounts of CO2 to the inspirate.
Volitional inspiration (active task)
Subjects performed voluntary hyperpnoea with passive expirations. During the active task subjects breathed from the ventilator circuit with the ventilator set in ‘flow-by’ (flow-triggered) mode (10 l min−1 bias flow; 1 l min−1 sensitivity). The airflow provided by the ventilator negated the resistance of the ventilator circuit and enabled subjects to breathe at minimal inspiratory pressures (Kacmarek & Hess, 1994). With the circuit used in this study, airway pressure was < -2 cmH2O during inspiration and expiration. This breathing pattern during volitional inspiration was matched to that delivered by the ventilator during the passive task. Again, normocapnia was maintained by administering inspired CO2.
Control for targeted breathing during the active task
Three subjects targeted their tidal volume and inspiratory duration to audible cues (a pre-recorded tape of the cycling ventilator) delivered through earphones (Magnacoustics, NJ, USA). In an attempt to prevent auditory bias during the passive task, a live broadcast of the remote cycling ventilator was transmitted to these subjects providing an equivalent auditory input. After having mastered the breathing tasks, the other two subjects were scanned without audible cues during both active and passive tasks.
Respiratory measurements
Airflow and pressure were measured from the ventilator pneumotachometer and pressure transducers, respectively. End-tidal PCO2 was monitored with a MRI-compatible vital signs monitor (Omnitrak 3100, Invivo, FL, USA), digitized and recorded onto magnetic disk (Superscope II, GW Instruments, MA, USA). Breath-by-breath assessment of tidal volume (VT), inspiratory duration (TI), expiratory duration (TE) and respiratory frequency (fR) was derived off-line from the airflow signal.
MRI scanning
Imaging was performed with a 1.5 T magnetic resonance scanner (General Electric Signa System, USA) using a local gradient head coil (Medical Advances, USA). High resolution T1-weighted spin echo (FOV/TR/TE/NEX/matrix, 24/450/17/0.75/256x192) contiguous sagittal anatomical images were obtained for each subject to serve as a reference for anatomical landmarks and co-ordinates (20 × 6 mm thick slices). The scanner was fine-tuned via linear shims for each subject prior to respiratory activation studies. The iterative shimming algorithm ensured optimum field homogeneity and resonance frequency; gain and flip angle were also optimised. T2*-weighted echo-planar images sensitive to BOLD signal changes were collected in the same planes and slice thickness, yielding an in-plane resolution of 3.75 mm (TR/TE/flip angle/NEX/matrix 2000/40/90/1/64x64). A functional image acquisition of 2 s enabled three whole brain images per breath to ensure that the fMRI sampling frequency was sufficient to detect any signal changes occurring at the breathing frequency (the minimum sampling rate or ‘Nyquist’ frequency is twice the fundamental signal frequency; Oppenheim & Schafer, 1986).
Scanning protocol
Four fMRI scan series (1-4) were collected for each subject. The duration of each scan series was 3 min 12 s. Each scan series consisted of a period of volitional inspiration (active task) and a period of mechanical ventilation (passive task). The breathing conditions were performed in a counterbalanced order and repeated 4 times with equal numbers of images in each condition. Scanning commenced when respiratory parameters were determined to be at steady state for the initial condition (either active or passive). The precise scan start time and breathing task transitions were recorded to magnetic disk simultaneously with the respiratory data.
Image processing and data analysis
Image data were transferred to a Silicon Graphics workstation, reconstructed and archived on optical disk. Image manipulations and statistical analyses were performed using Matlab 4.2 (Mathworks, MA, USA) and AFNI software (Medical College of Wisconsin, USA). Statistical parametric maps (SPMs) of brain activation were generated (SPM96 software, Wellcome Department of Cognitive Neurology, London, UK). Image processing began with spatial realignment of each subject's scans to the first image of each series after spin saturation. This step ensures spatial congruency and removes minor movement-related artefacts. Next, the fMRI time series was ‘brought forward’ 6 s relative to the respiratory data to compensate for the lag (including haemodynamic delay) between neural activation and peak fMRI signal, as suggested by Bandettini et al. (1993). Then, images determined to be primarily time-registered with either inspiration (I) or expiration (E) were segregated and averaged for all scans in individual subjects for both the passive and active conditions. Each subject's data were then spatially normalised to standard stereotactic space (Talairach & Tournoux, 1988; Friston et al. 1995). Prior to group analysis, the images were spatially smoothed with a three-dimensional 15 mm full-width half-maximum isotropic Gaussian kernel filter to enhance signal-to-noise ratio. Subsequent t statistics (and associated Z statistics) of the four conditions at each voxel were determined by a pixel-based analysis of covariance (ANCOVA), using a general linear model approach (Friston et al. 1995) as implemented in SPM96. Condition-related differences in fMRI signal intensity were determined by SPM{t}s (P < 0.001, Z > 3.1) with local maxima reported in x, y and z reference co-ordinates (Talairach & Tournoux, 1988).
RESULTS
Respiratory measurements
The mean respiratory variables for each subject are displayed in Fig. 1. End-tidal PCO2 and TI did not significantly differ between tasks. Although significant differences occurred in VT and in TE between active and passive tasks, these changes were small and were similar to those reported in the original PET studies of Colebatch et al. (1991) and Ramsay et al. (1993). A record of typical respiratory waveforms observed during the active and passive breathing tasks is provided in Fig. 2. The chart record demonstrates that this subject remained passive during mechanical ventilation as indicated by the uniform nature of the airflow and airway pressure traces. In addition, during the volitional (active) task the subject was able to closely match the ventilatory pattern achieved during the passive task with targeted volitional breaths of consistent VT and fR.
Figure 1.
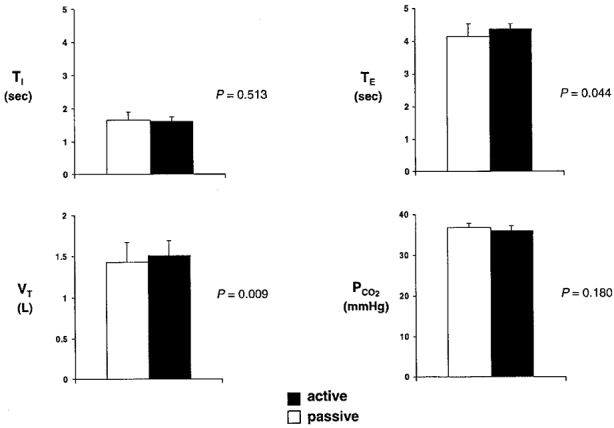
Group mean respiratory data (n= 5) during fMRI scanning: TI, inspiratory time; TE, expiratory time; VT, tidal volume; PCO2, partial pressure of end-tidal CO2. The statistical significance between the active and passive task for each variable is represented by P (Student's paired 2-tailed t test, α = 0.05).
Figure 2.
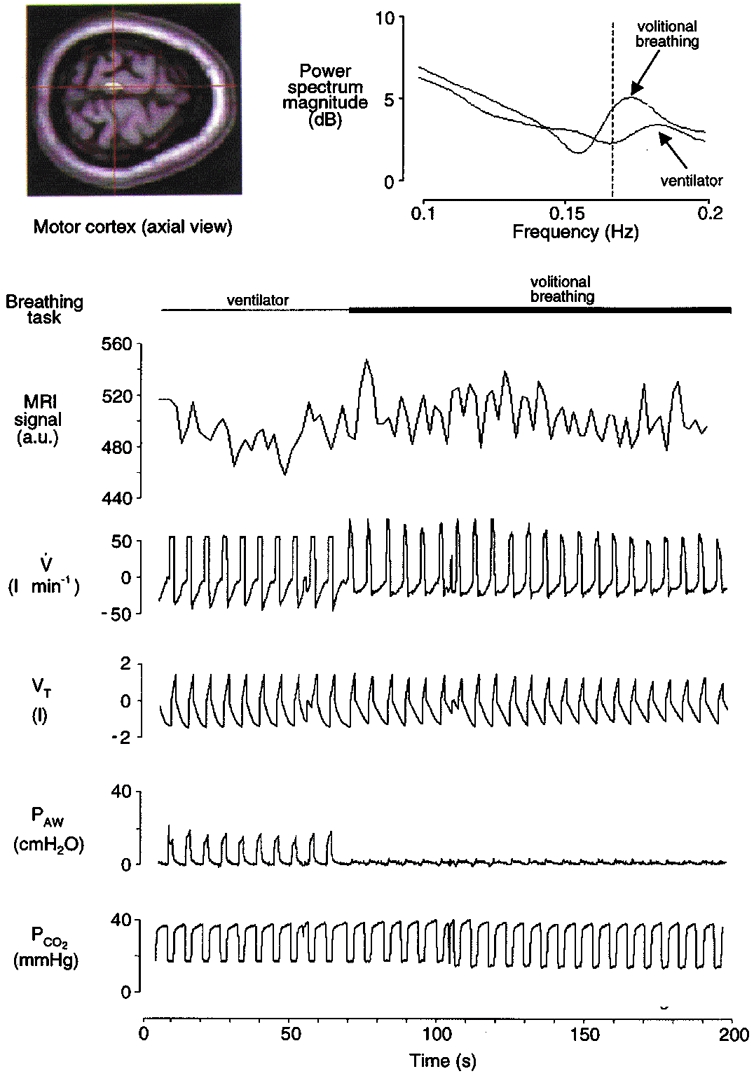
Record from a typical subject (F) during mechanical ventilation (passive task; first minute only) and volitional inspiration (active task; remainder of scan series). The left inset depicts an axial fMRI image and a region of interest in the left motor cortex (centre of cross-hairs). The fMRI signal (in arbitrary units, a.u.) at this region is time-aligned with the respiratory waveforms: V, airflow; VT, tidal volume; PAW, airway pressure; PCO2, partial pressure of end-tidal CO2. (NB, the ‘raw’ fMRI signal in the figure is unadjusted for haemodynamic lag.) There was a small average increase in fMRI signal during volitional inspiration relative to passive ventilation in this superior region of motor cortex. There were also phasic fMRI changes synchronised with inspiration and expiration that are particularly evident during the active task. This synchronisation (between neural activation and volitional inspiration) is depicted in the power spectra of these fMRI signals (right inset). The fMRI spectrum during volitional inspiration has an isolated peak near the frequency of breathing (0.167 Hz; vertical dashed line), whereas the spectrum during passive mechanical ventilation shows less specificity with breathing frequency.
Phase relationship of fMRI signal with breathing cycle
A record of the fMRI signal intensity is shown in Fig. 2 (time-aligned with the respiratory waveforms). This signal intensity plot represents the activity from a superior region of the motor cortex that had previously been found to be associated with active inspiration (Colebatch et al. 1991; Ramsay et al. 1993). In this example, there was an average ∼2 % increase in fMRI signal during volitional inspiration relative to passive mechanical ventilation. The task-related increases in fMRI signal observed in the motor cortex were of similar magnitude in the other respiratory-related foci (∼2-3 %), a finding consistent with the typical BOLD signal changes reported throughout the fMRI literature during motor tasks (e.g. Bandettini et al. 1993). In addition, the signal in this region of the motor cortex noticeably oscillated at the frequency of breathing during the active task, but fMRI changes were not as synchronised with the breathing cycle during the passive task. This finding was confirmed by power spectrum analysis of the fMRI signals. The power spectra did not have peaks at the frequency of breathing in other sample ‘control’ regions of the brain, such as in the occipital cortex. Similar mean significant increases in cortical activation as well as breath-related oscillations in fMRI were found in all subjects in the sensorimotor cortex and at other loci (see below).
Regions activated during the inspiratory phase of volitional breathing and comparison of co-ordinates between the current study and previous studies
The left panel of Fig. 3 represents regions significantly activated during volitional inspiration (group comparison of active inspiration vs. passive inspiration). There was significant activation in the left superolateral motor cortex, left inferolateral motor cortex, right supplementary motor area and right premotor area, as well as bilateral activation of both the inferolateral sensorimotor cortex and the putamen (striatum). Stereotactic co-ordinates of these maxima are noted in Table 1.
Figure 3.
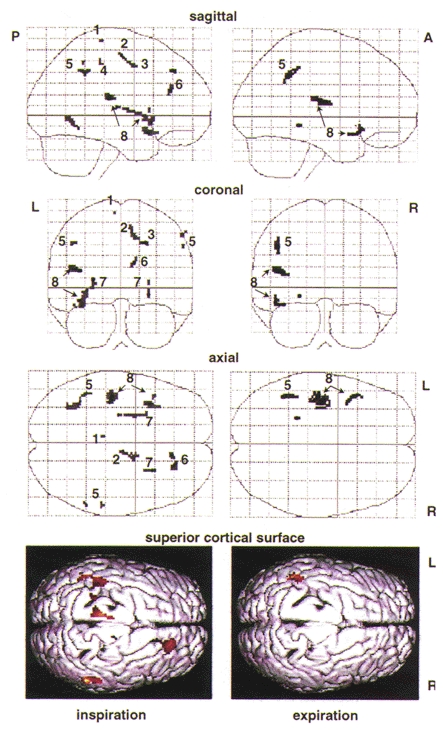
Maximum intensity projections of multisubject analyses from selective averaging of inspiratory and of expiratory images demonstrate increases in local fMRI signal intensity during volitional inspiration (left panel, active inspiration vs. passive inspiration) and expiration (right panel, ‘passive’ expiration during the volitional breathing task vs. passive expiration during mechanical ventilation). Increases in signal intensity are represented by an arbitrary grey scale (light grey indicates statistical threshold of P= 0.001, darker shades indicate increasingly higher effect size (increasing Z values)). Plots are shown in normalised stereotactic space (see Methods) in sagittal, coronal and axial planes. A, anterior; P, posterior; R, right; L, left. Relevant local maxima are labelled: 1, superior motor cortex; 2, supplementary motor area; 3, premotor area; 4, inferolateral motor cortex; 5, inferolateral sensory cortex; 6, prefrontal cortex; 7, striatum (putamen); 8, superior temporal lobe and auditory cortex. At the bottom, a cortical surface rendering in the superior axial plane is provided for three-dimensional visualisation of significant cortical surface regions that selectively activate during volitional inspiration, left, and expiration, right (red indicates statistical threshold of P= 0.01, lighter colours indicate increasingly higher effect size (increasing Z values)).
Table 1. Co-ordinates of local maxima with significant increases in fMRI signal during volitional inspiration (active inspiration vs. passive inspiration).
| Left | Right | |||||||
|---|---|---|---|---|---|---|---|---|
| Region | x | y | z | Z score | x | y | z | Z score |
| Superior motor cortex (BA 4) | −6 | −30 | 70 | 3.15 | — | — | — | — |
| Inferolateral motor cortex* | −44 | −22 | 18 | 3.62 | — | — | — | — |
| Inferolateral sensory cortex | −38 | −32 | 54 | 3.18 | 56 | −32 | 48 | 3.59 |
| −46 | −46 | 40 | 3.79 | 58 | −46 | 38 | 3.56 | |
| Supplementary motor area (BA 6) | — | — | — | — | 8 | −12 | 56 | 4.25 |
| Premotor area | — | — | — | — | 16 | −2 | 46 | 3.30 |
| Striatum | −28 | 14 | −2 | 3.26 | 26 | 14 | 6 | 3.22 |
| Prefrontal cortex (BA 8) | — | — | — | — | 22 | 36 | 42 | 3.24 |
| Medial frontal gyrus | — | — | — | — | 16 | 36 | 30 | 3.60 |
| Superior temporal gyrus* | −38 | 10 | −16 | 3.87 | — | — | — | — |
| Auditory cortex (BA 22)* | −46 | −22 | 18 | 4.62 | — | — | — | — |
Asterix denotes maxima which reach significance during expiration (volitional breathing vs. ventilator) in addition to volitional inspiration. Relevant Brodmann areas (BA) are given in parentheses. Note, two foci were located in the inferolateral sensory cortex.
There was a remarkable degree of similarity between the co-ordinates detected with volitional inspiration using fMRI in the current study and those co-ordinates detected in previous PET studies during similar breathing tasks (Colebatch et al. 1991; Ramsay et al. 1993) or loaded breathing (Fink et al. 1996). The regions detected in all studies were overlapping in virtually all instances. Furthermore, the concordance of local maxima within these regions was quite similar in virtually all regions. For example, in the right premotor area, differences in the co-ordinates of maximal activation between the current study and the PET studies ranged from 2 to 24 mm (x), 0 to 16 mm (y) and 2 to 18 mm (z) (current study versusColebatch et al. 1991; Ramsay et al. 1993; Fink et al. 1996). Differences in the left primary motor cortex ranged from 8 to 12 mm (x), 2 to 7 mm (y) and 6 to 15 mm (z) (current study versusColebatch et al. 1991; Ramsay et al. 1993; Fink et al. 1996). Differences in the supplementary motor area were 4 mm (x), 17 mm (y) and 8 mm (z) (current study versusColebatch et al. 1991). Differences in the striatum were 18 mm (x), 0 mm (y) and 10 mm (z) (current study versusFink et al. 1996).
In contrast to previous respiratory studies with PET, the present fMRI study identified significant novel activation of the right prefrontal cortex (Brodmann area 8), which is known to have neural connections with other regions activated in the current study, namely the primary motor area and the striatum (Passingham, 1993; Frackowiak et al. 1997). Another difference between the findings of the previous PET studies and the current study was the detection of regions known to be associated with auditory attention and processing in the left superior temporal lobe (including Brodmann area 22). These loci were probably activated by our subjects whilst they attended to the auditory signal used to time inspiration (cf. Binder et al. 1994; Saykin et al. 1999). Lastly, the present study identified diffuse task-related bilateral activation of the inferolateral sensorimotor cortex. The co-ordinates of these local maxima differed by as little as 2 mm (y) and 8 mm (z) but were 26-46 mm (x) inferior to loci reported by Fink et al. (1996). This relatively large difference in only one of the three planes between the current study and the previous respiratory study is most probably due to a different cortical representation of the breathing apparatus employed by the two studies (e.g. mouthpiece of present study vs. nose mask of previous PET studies). The mouthpiece used in the present study probably activated regions representing the teeth and jaw which lie inferior to those representing the face and nose along the sensorimotor homunculus described by Penfield & Boldrey (1937).
Regions activated during the expiratory phase of volitional breathing
The right panel of Fig. 3 illustrates only those regions significantly activated during the expiratory phase of the volitional breathing task (expiration during the volitional breathing task vs. expiration during mechanical ventilation). Most of the motor regions activated during volitional inspiration failed to reach significance in the analysis of expiratory scans. Significant activation was limited to the left inferolateral sensorimotor cortex and left superior temporal lobe including Brodmann area 22. Stereotactic co-ordinates of these maxima are noted in Table 1.
DISCUSSION
One objective of this study was to determine whether or not fMRI would identify the same voluntary respiratory control areas previously obtained by PET, thereby validating both techniques for use in respiratory studies. A second aim was to extend these PET observations by improvements in both temporal and spatial resolution with fMRI. The ultimate goal was to evaluate the potential of fMRI for determining the components of the distributed neural network subserving planning, co-ordination, motor activation and sensations of breathing as well as the relative time course of activation of these elements across the respiratory cycle.
Confirmation of earlier PET data, with enhanced sensitivity
The present fMRI study identified activation related to voluntary inspiration (VT= 1.43 l, fR= 10 breaths min−1) in the superior motor cortex, premotor area and supplementary motor area with stereotactic co-ordinates for local maxima remarkably similar to those reported in the earlier studies which employed entirely different scanning methodology but comparable levels of ventilation (Colebatch et al. 1991; Ramsay et al. 1993). Some of the small degree of variability between studies may be explained by improvements upon earlier editions of the normalisation software or differences in spatial resolution. Nonetheless, the differences in the co-ordinates of local maxima between fMRI and PET studies were similar in magnitude to the differences in the co-ordinates of local maxima that occurred between similar PET studies by the same research group (e.g. Colebatch et al. 1991; Ramsay et al. 1993; Fink et al. 1996). As these regions appeared in both techniques, we have great confidence in the findings.
Beyond the general concordance with previous PET studies, the fMRI technique used in the current study appears to be more sensitive than PET. For instance, the current study identified respiratory loci in the inferolateral sensorimotor cortex and striatum that were not detected in PET studies with equivalent breathing patterns, numbers of subjects and statistical thresholds (Colebatch et al. 1991; Ramsay et al. 1993), but which did emerge as significant in a PET study when subjects breathed through an inspiratory load (Fink et al. 1996). The apparent enhanced sensitivity of fMRI relative to PET could be inherent to the fMRI technique itself, improvements in its temporal or spatial resolution, the ability to selectively average the inspiratory and expiratory phases of the breathing cycle or a combination of these factors.
Temporal resolution of fMRI enables detection of within-breath changes
There is a consistent latency between onset of neural activation and change in fMRI signal of approximately 2 s that is presumed to be haemodynamic in origin (e.g. Savoy et al. 1995; Boynton et al. 1996; Dale & Buckner, 1997). It has been demonstrated that there is a further ‘rise-time’ delay of approximately 2-4 s before peak fMRI activation occurs following an isolated stimulus (Savoy et al. 1995). Furthermore, fMRI BOLD signal summation occurs during repetitive stimulation (Dale & Buckner, 1997). Debate remains concerning the absolute limit of the temporal resolution of fMRI, which has mostly been investigated using rapid sensory stimuli. Despite a haemodynamic lag, the rise-time to peak signal change and any summation during repetitive tasks, fMRI is still able to indirectly detect rapid changes in neural activation during repetitive motor tasks. For example, with finger tapping at 2 Hz, oscillations in fMRI signal at the same frequency were detected in the contralateral primary motor cortex (Rao et al. 1993). In the current study breathing frequency was restricted to 10 breaths min−1 (0.167 Hz) and whole brain images were acquired 3 times per breath, with one image per inspiration. This sampling frequency was presumably too low to detect absolute peaks of fMRI signal throughout the breathing cycle but was certainly adequate to detect significant within-breath variations in fMRI signal in the sensorimotor cortex, presumably indicating within-breath-related changes in neuronal activity in these regions.
Implications of results as related to the study of respiratory control
Studies involving electrical stimulation of brain structures in cats, dogs, monkeys, and occasionally in humans during brain surgery, testify to the widespread cortical and subcortical sites that can affect breathing (reviewed by Plum & Leigh, 1981; Hugelin, 1986; Davenport & Reep, 1995). The present study confirms the identification of several brain regions that have previously been shown to be associated with breathing and respiratory muscle activity. The region of superolateral motor cortex identified in the current study was first demonstrated to be related to diaphragmatic contraction in response to electrical stimuli by Foerster (1936). Maskill et al. (1991) provided further evidence of the superolateral cortical involvement in breathing by evoking diaphragm contraction in response to transcranial magnetic stimulation. The diaphragm contraction was maximal when the magnetic coil was placed just lateral to the vertex (3 cm to the right of the mid-line). As mentioned earlier, this same region was located more precisely using PET (Colebatch et al. 1991; Ramsay et al. 1993). The confluence of these findings gives us confidence that the superior cortical region activation during our voluntary breathing task represents pyramidal neurones associated with breathing muscles. However, the lateralised activation of this loci to the left hemisphere remains a curiosity. Lateralisation of superior motor cortical activity was also evident in the earlier PET studies of breathing. While bilateral activation was observed, the statistical parametric maps of Colebatch et al. (1991) and Ramsay et al. (1993) demonstrate a greater spatial extent of activation in the left motor cortex. We remain unsure of the implications of this lateralisation in the control of breathing.
In addition to the superior motor cortex, the voluntary breathing task was observed to activate Brodmann areas 6 and 8, including local maxima in the premotor and supplementary motor areas, with similar co-ordinates to those originally revealed by PET. These regions have been identified as crucial to the successful execution of self-initiated (voluntary) well-rehearsed movements (Passingham, 1993). Moreover, activity in the supplementary motor area has been demonstrated to be related to the differential rate/frequency and force required for various limb movement tasks (Frackowiak et al. 1997). As in the previous breathing studies which employed PET, our subjects were well trained and performed volitional breathing to target volume and frequency with remarkable accuracy. Evidence from single unit recordings during specific motor tasks in animals, lesion studies in humans and other animals, and other movement studies with PET have demonstrated connections from the supplementary motor area (Brodmann area 6) to the motor cortex, and connections of the premotor area and Brodmann area 8 to the striatum (Passingham, 1993; Frackowiak et al. 1997). The act of initiating a repetitive volitional task such as the overt hyperpnoea required by the current study and the previous PET studies would necessarily call upon the supplementary motor and premotor area in the execution of the learned breathing task (Fink et al. 1996). As in the previous PET studies the activation of the premotor and supplementary motor area was lateralised to the right. We support the hypothesis suggested by Ramsay (1993) that this laterality of premotor and supplementary motor area activity demonstrates the likelihood of ‘hemispheric specialisation’ which may be involved in tasks that require a significant degree of attention (Pardo et al. 1991). In contrast, it is not surprising that bilateral activation of the striatum was observed in the present study, as bilateral striatum activity has been associated with numerous studies of motor programming (reviewed by Passingham, 1993; Frackowiak et al. 1997). Further, the striatum has been shown to become activated in conjunction with frontal areas in PET studies of learned movements, and premotor areas are known to project to the putamen (Frackowiak et al. 1997). While completely different protocols would be required to differentiate the interaction of these cortical and subcortical regions, there is general concordance among the present study and the previous imaging studies that the superolateral motor cortex, premotor area, supplementary motor area and striatum are all activated during volitional inspiration.
Critique of methods and potential of fMRI in respiratory control studies
We studied only five subjects. Although this is similar to other studies in the imaging field, and significant results were obtained in many cortical regions (indicating some consistency among subjects), it is possible that small brain regions that are actively related to the control of breathing would be missed because of variability among subjects. In an effort to minimise such problems, our data were spatially normalised with ‘state of the art’ software (SPM 96). Future studies may have enhanced sensitivity gained from improved approaches to normalisation as the techniques of functional imaging continue to evolve.
The sampling frequency used (one whole brain image every 2 s) was adequate to detect respiratory activity at a breathing frequency of approximately 10 breaths min−1. We did not assess the limits of the fMRI technique in detection of respiratory-related activity at varying respiratory rates, nor did we adjust tidal volume to determine the threshold of sensitivity of our technique. One of our aims was to confirm and extend the PET data with improved technology. Therefore, we chose to compare overt volitional inspiration at relatively large tidal volumes versus passive inflation at the same tidal volume. We did not compare overt volitional breathing with normal spontaneous breathing, although an interesting remaining question concerns the role of the cortex in ‘automatic’ breathing. Although PET does not appear sensitive enough to detect respiratory-related neural activation during eupnoea, fMRI may be.
A feature unique to fMRI is the ability to evaluate breathing-related activity within single subjects in images on a frame-by-frame (2 Hz or less) basis as demonstrated in Fig. 2 and by Gozal et al. (1995, 1996). Limits in temporal resolution prohibit such an analysis in PET studies of breathing and typically necessitate that PET data be averaged over multiple subjects. Our experimental protocol was not designed to fully exploit single subject analysis. An fMRI experimental design which utilises more frequent and rapid on-off transients between the active and passive tasks could achieve optimal results from single subject fMRI time series analyses. It is likely that studies of this nature could demonstrate the connectivity and timing of respiratory-related activity between cortical and subcortical regions.
All current imaging modalities have specific limitations. While we have demonstrated the enhanced sensitivity and increased spatial resolution of fMRI over PET, one must still consider the current technical limitations of fMRI. The first fMRI studies of breathing by Gozal et al. (1994, 1995, 1996) were limited by the single slice fMRI pulse sequence used. Our primary goal in the present study was to improve temporal resolution, while still scanning the greatest extent of brain volume that potentially has a role in voluntary control of inspiration. While the present study demonstrates significant advancement in temporal resolution of the neuroimaging of breathing centres, we were still limited in our study design. Due to technical considerations specific to fMRI concerning the balance between temporal resolution and the field of view, we chose to concentrate on the cortex rather than the brainstem (thus allowing a comparison with previous PET studies that also had a limited field of view). An important question remains as to which brainstem respiratory neurones are active during volitional inspiration, but unfortunately this was beyond the scope of this study.
In conclusion, the present study employed fMRI to detect cortical loci related to volitional breathing in humans with greater sensitivity and better temporal resolution than previously reported in studies of respiration that used PET. The precise localisation of the cortical structures (motor cortex, supplementary motor area and premotor area) associated with the volitional control of breathing is consistent among studies of clinical lesions, electrical stimulation, magnetic stimulation, PET and the current fMRI study. Moreover, the present study identified breathing-related foci in the striatum and inferolateral sensorimotor cortex which were only revealed by PET under significant inspiratory load, and novel loci in the medial frontal gyrus (Brodmann area 8). The role of the striatum and frontal cortex has been associated with self-initiated motor acts and learned movements (Passingham, 1993; Frackowiak et al. 1997). The neurophysiological models of respiratory control that emerge from these studies are still exceedingly elementary when compared to what is known about the co-ordinated control of other muscle groups (reviewed by Lemon, 1993). Major questions remain, principally concerning the timing of neural events. This first imaging study that distinguishes within-breath activation of cortical sites documents the enormous potential of this fMRI technique for respiratory control studies. This potential could be more fully exploited by adjustments in the field of view, pulse sequences, and carefully designed respiratory protocols that either control or correct for changes in blood gases induced by the protocol. It is likely that future fMRI studies could resolve more precisely the neural elements involved in the planning, co-ordination, motor activation and sensations of breathing as well as the relative time course of activation of these elements across the respiratory cycle.
Acknowledgments
We thank our subjects for their time and co-operation. We are grateful for helpful suggestions and support contributed by Mr Robert Joyner, Drs Robert Banzett, Terrance Darcey, Andrew Daubenspeck, J. Leiter, Harold Manning, Alexander Mamourian, Henry Riordan and John Weaver, and for technical support provided by Chad Moritz, Ken Lee, Scott Slogic and DHMC Departments of Respiratory Care and Radiology. We thank Mr Robert Hamlin, Mr Steven McKoy and Mr John Battiston for their assistance. This work was supported in part by grants from the National Institutes of Health (HL46690, HL07715-03 and HL62149-01), the Ira DeCamp Foundation and the New Hampshire Hospital.
References
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magnetic Resonance in Medicine. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Binder JR, Rao SM, Hammeke TA, Yetkin FZ, Jesmanowicz A, Bandettini PA, Wong EC, Estkowski LD, Goldstein MD, Haughton VM, Hyde JS. Functional magnetic resonance imaging of the human auditory cortex. Annals of Neurology. 1994;35:662–672. doi: 10.1002/ana.410350606. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in the human V1. Journal of Neuroscience. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch JG, Adams L, Murphy K, Martin AJ, Lammertsma AA, Tochon-Danguy HJ, Clark JC, Friston KJ, Guz A. Regional cerebral blood flow during volitional breathing in man. The Journal of Physiology. 1991;443:91–103. doi: 10.1113/jphysiol.1991.sp018824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Human Brain Mapping. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Reep RL. Cerebral cortex and respiration. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. 2. New York: Marcel Dekker Inc.; 1995. pp. 365–388. [Google Scholar]
- Dick TE, Orem J, Shea SA. Behavioral control of breathing. In: Crystal RG, West JB, Weibel ER, Barnes PJ, editors. The Lung. 2. Philadelphia: Lippincott-Raven; 1997. pp. 1821–1837. [Google Scholar]
- Evans KC, Shea SA, Saykin AJ. Functional MRI localization of CNS regions associated with volitional inspiration. Neuroimage. 1998;7:S290. doi: 10.1111/j.1469-7793.1999.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Corfield DR, Murphy K, Kobayashi I, Dettmers C, Adams L, Frackowaik RSJ, Guz A. Human cerebral activity with increasing inspiratory force: a study using positron emission tomography. Journal of Applied Physiology. 1996;81:1295–1305. doi: 10.1152/jappl.1996.81.3.1295. [DOI] [PubMed] [Google Scholar]
- Foerster O. Motorische Felden und Bahen. In: Bumke O, Foerster O, editors. Handbook der Neurologie. Berlin: Springer; 1936. pp. 50–51. [Google Scholar]
- Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC. Functional organisation of the motor system. In: Frackowiak RSJ, editor. Human Brain Function. London: Academic Press; 1997. pp. 243–274. [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Friston KJ, Holmes AP, Worsley JJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:1–25. [Google Scholar]
- Gozal D, Hathout GM, Kirlew KA, Tang H, Woo MS, Zhang J, Lufkin RB, Harper RM. Localization of putative neural respiratory regions in the human by functional magnetic resonance imaging. Journal of Applied Physiology. 1994;76:2076–2083. doi: 10.1152/jappl.1994.76.5.2076. [DOI] [PubMed] [Google Scholar]
- Gozal D, Omidvar O, Kirlew KA, Gasser HM, Hamilton R, Lufkin RB, Harper RM. Identification of human brain regions underlying responses to resistive loading with functional magnetic resonance imaging. Proceedings of the National Academy of Sciences of the USA. 1995;92:6607–6611. doi: 10.1073/pnas.92.14.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Omidvar O, Kirlew KA, Hathout GM, Lufkin RB, Harper RM. Functional magnetic resonance imaging reveals brain regions mediating the response to resistive expiratory loads in humans. Journal of Clinical Investigation. 1996;97:47–53. doi: 10.1172/JCI118405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guz A. Brain, breathing and breathlessness. Respiration Physiology. 1997;109:197–204. doi: 10.1016/s0034-5687(97)00050-9. [DOI] [PubMed] [Google Scholar]
- Hugelin A. Forebrain and midbrain influence on respiration. In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology, section 3, The Respiratory System, Control of Breathing. II. Baltimore: American Physiological Society; 1986. pp. 69–91. part 1. [Google Scholar]
- Kacmarek RM, Hess D. Basic principles of ventilator machinery. In: Tobin MJ, editor. Principles and Practice of Mechanical Ventilation. New York: McGraw-Hill Inc.; 1994. pp. 91–93. [Google Scholar]
- Lemon RN. Cortical control of the primate hand. Experimental Physiology. 1993;78:263–301. doi: 10.1113/expphysiol.1993.sp003686. [DOI] [PubMed] [Google Scholar]
- Maskill D, Murphy K, Mier A, Owen M, Guz A. Motor cortical reprsentation of the diaphragm in man. The Journal of Physiology. 1991;433:105–121. doi: 10.1113/jphysiol.1991.sp018825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim AV, Schafer RW. Discrete-time Signal Processing. Englewood Cliffs: Prentice-Hall; 1986. p. 879. [Google Scholar]
- Orem J, Netick A. Behavioral control of breathing in the cat. Brain Research. 1986;366:238–253. doi: 10.1016/0006-8993(86)91301-6. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron tomography. Nature. 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Passingham RE. The Frontal Lobes and Voluntary Action. Oxford: Oxford Psychology Series 21, Oxford University Press; 1993. [Google Scholar]
- Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Plum F, Leigh R. Abnormalities of central mechanisms. In: Hornbein TF, editor. Regulation of Breathing, Lung Biology in Health and Disease. New York: Marcel Dekker; 1981. pp. 989–1067. part 2. [Google Scholar]
- Prechter GC, Nelson SB, Hubmayr RD. The ventilatory recruitment threshold for carbon dioxide. American Review of Respiratory Disease. 1990;141:758–764. doi: 10.1164/ajrccm/141.3.758. [DOI] [PubMed] [Google Scholar]
- Ramsay SC, Adams L, Murphy K, Corfield DR, Grootoonk S, Bailey DL, Frackowiak RSJ, Guz A. Regional cerebral blood flow during volitional expiration in man: a comparison with volitional inspiration. The Journal of Physiology. 1993;461:85–101. doi: 10.1113/jphysiol.1993.sp019503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz A, Lisk LM, Morris GL, Mueller WM, Estkowski LD, Wong EC, Haughton VM, Hyde JS. Functional magnetic resonance imaging of complex human movements. Neurology. 1993;43:2311–2318. doi: 10.1212/wnl.43.11.2311. [DOI] [PubMed] [Google Scholar]
- Savoy RL, Bandettini PA, O'Craven KM, Kwong KK, Davis TL, Baker JR, Weisskoff RM, Rosen BR. Pushing the temporal resolution of fMRI: studies of very brief visual stimuli, onset variability and asynchrony, and stimulus-correlated changes in noise. Proceedings of the Society for Magnetic Resonance Medicine. 1995;3:450. [Google Scholar]
- Saykin AJ, Flashman LA, McAllister TW, Johnson SJ, Guerin SJ, Brown CJ, Sparling MB, Moritz CH, Mamourian AC, Vidaver RM. Semantic, phonological and episodic memory processing in schizophrenia: functional MRI activation patterns indicate a need for new models of dysfunction. Schizophrenia Research. 1999;36(suppl.):233. [Google Scholar]
- Simon PM, Skatrud JB, Bard MS, Griffin DM, Iber C, Dempsey JA. Role of airway mechanoreceptors in the inhibition of inspiration during mechanical ventilation in humans. American Review of Respiratory Disease. 1991;144:1033–1041. doi: 10.1164/ajrccm/144.5.1033. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Coplanar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]


