Abstract
Although it is generally accepted that the acid/base ratio of tissue, as represented by the pH, is strictly regulated to maintain normal function, recent studies in the nervous system have shown that neuronal activity can result in significant shifts in pH. In the vertebrate retina, many cellular phenomena, including neuronal activity, are regulated by a circadian clock. We thus investigated whether a circadian clock regulates the pH of the retina.
pH-sensitive microelectrodes were used to measure the extracellular pH of the in vitro goldfish retina superfused with a bicarbonate-based Ringer solution in the subjective day and night; that is, under conditions of constant darkness.
These measurements demonstrated that a circadian clock regulates the pH of the vertebrate retina so that the pH is lower at night compared to the day. This day-night difference in retinal pH was observed at two different values of Ringer solution pH, indicating that the circadian phenomenon is independent of the superfusion conditions.
The circadian-induced shift in pH was several times greater than light-induced pH changes and large enough to influence synaptic transmission between retinal neurons.
These findings indicate that a circadian clock regulates the pH of the vertebrate retina. Thus, an intrinsic oscillator in neural tissue may modulate metabolic activity and pH as part of normal daily function.
Although all living tissue produces acid due to metabolic activity, it has been accepted that the acid/base ratio of tissue, as represented by the pH, is strictly regulated to maintain normal function. In the nervous system, however, recent studies have shown that neuronal activity can result in shifts in pH that are large enough to influence enzyme and channel functions (Chesler & Kaila, 1992). For example, in the vertebrate retina, light stimulation produces an alkalinization of the extracellular space that surrounds photoreceptor cells (Borgula et al. 1989; Oakley & Wen, 1989; Yamamoto et al. 1992). In contrast, dark adaptation produces an acidification.
In addition to the effects of light and dark adaptation, many cellular phenomena in the vertebrate retina are now known to be regulated by a circadian clock (Reme et al. 1991; Cahill & Besharse, 1995), a type of biological oscillator that is intrinsic to neural tissue and that has persistent rhythmicity with a period of approximately 24 h in the absence of external timing cues (e.g. constant darkness) (Block et al. 1993). For example, in the vertebrate retina a circadian clock regulates dopamine content and release (Wirz-Justice et al. 1984; Kolbinger et al. 1990), melatonin production and release (Besharse & Iuvone, 1983; Tosini & Menaker, 1996), retinomotor movements (Burnside & Nagle, 1983), opsin synthesis (Korenbrot & Fernald, 1989) and neuronal light responses (Wang & Mangel, 1996). Because neuronal activity in the retina is regulated by a circadian clock, we thus investigated whether a circadian clock regulates the pH of the vertebrate retina.
METHODS
Goldfish (Carassius auratus), approximately 15 cm in length, were maintained at 19°C on a 12 h light-12 h dark cycle for at least 14 days before an experiment. The care and use of the goldfish were in accordance with federal and institutional guidelines. Anaesthetized (methanesulfonate, MS-222, 100 mg l−1, Sigma) fish were decapitated and pithed. Their eyes were then enucleated and their retinae isolated from the pigment epithelium. The intact neural retinae were then mounted photoreceptor side-up in a Teflon experimental chamber. Prior to surgery, fish were kept in darkness from 8 to 50 h. All surgical procedures were performed under red (0.2 μW cm−2) or infrared illumination with no differential effect on the results. Intact, isolated retinae were superfused at 0.5 ml min−1 with a Ringer solution that contained (mM): 130 NaCl, 2.5 KCl, 20 NaHCO3, 0.7 CaCl2, 1.0 MgCl2 and 20 glucose, as described previously (Harsanyi & Mangel, 1993; Harsanyi et al. 1996). Oxygenation of the superfusate with a mixture of 97 % O2 and 3 % CO2 maintained the superfusate at a pH of 7.65 in the retinal chamber, except where noted. Following surgery, retinae were superfused in the dark for 60 min before pH measurements were made. Dim light stimuli (scotopic intensity, 5 s in duration) were flashed occasionally to monitor the physiological condition of the tissue by recording light-induced retinal electroretinogram (ERG) responses.
Double-barrelled pH-sensitive microelectrodes, which were used to measure extracellular pH, were constructed using methods described previously for Ca2+-sensitive microelectrodes (Dmitriev et al. 1999b), except that the electrodes were filled with hydrogen ionophore I- cocktail B (Fluka) and had tip diameters of 4–6 μm. Absolute pH values in the Ringer solution and retina were determined by calibrating measurements of the pH microelectrode with those of a commercially available (Corning) glass pH electrode placed in the Ringer solution.
Standard intracellular recording procedures were employed to monitor horizontal cell membrane potential and light-evoked responses. Intracellular pipettes were filled with 2 M potassium acetate and had resistances between 100 and 200 MΩ. Cone horizontal cells were identified with spectral and intensity response curves and by response waveform (Harsanyi & Mangel, 1993; Harsanyi et al. 1996). The maximum, unattenuated intensity (Io) of full-field white light stimuli from a 100 W tungsten-halogen lamp was 2.0 × 103μW cm−2. Intensity values indicated in the text are relative to Io. Calibrated neutral density filters were used to control light intensity and narrow-band interference filters were used to control stimulus wavelength.
RESULTS
pH-sensitive microelectrodes were used to measure the extracellular pH (pHo) of the goldfish retina in the subjective day and night; that is, under conditions of constant darkness (Fig. 1). The electrodes were placed in the Ringer solution above the retina and moved towards the tissue in 100 μm steps. When the pH electrodes were within 500–700 μm of the retina, the pH of the superfusion solution began to decrease due to the influence of the tissue. In all of the experiments, a clear pH gradient was recorded in the solution above the retina (Fig. 1). When the pH electrode touched the photoreceptor surface of the retina a small shift in the potential of its indifferent barrel occurred. A much larger shift of field potential always occurred during the next step, apparently indicating the penetration of the outer limiting membrane. The pH always reached a minimum value when the electrode reached the retina. In fact, pHo in the different retinal layers was slightly different (approximately 0.01- 0.02 pH units), but it was many times smaller than the difference between retinal pHo and the pH in the superfusion solution (Fig. 1).
Figure 1. A circadian clock regulates the extracellular pH of the fish retina.
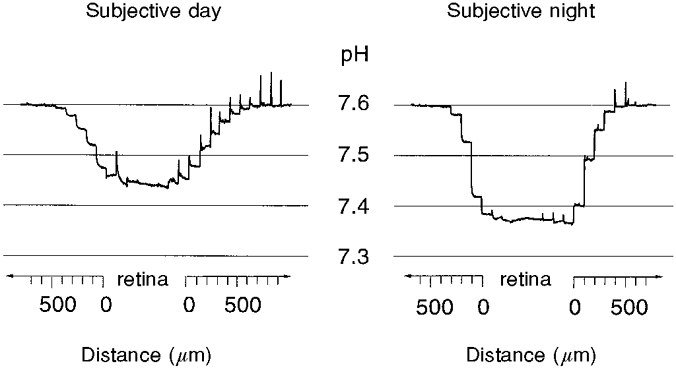
Extracellular pH (pHo) is shown as a function of distance (μm) from a superfused goldfish retina in the subjective day and night. pH-sensitive microelectrodes were advanced through the Ringer solution to the retina, then through the retina, and finally withdrawn. Retinal pHo was always lower than Ringer solution pH and the difference between retinal and Ringer solution pH was greater in the subjective night than in the subjective day. The electrodes were moved in 100 μm steps every 30 s. Fast ‘spikes’ on the records are movement artifacts.
Retinal pHo was lower than the Ringer solution pH in both the subjective day and subjective night, but was lowest during the subjective night. During the subjective day, the mean difference between the retinal pHo and the Ringer solution pH was 0.216 ± 0.006 (±s.e.m.; n = 34), whereas during the subjective night the mean difference between the retinal pHo and the Ringer solution pH was 0.294 ± 0.007 (n = 34; P < 0.01, Mann-Whitney U test). Thus the pH gradient at night was 36 % greater than the pH gradient during the day. This day-night difference clearly indicates that the retina produces more acid at night than in the day; that is, the mass metabolic activity of the retina is higher during the night. A similar circadian rhythm in retinal pH has also recently been reported in the rabbit retina (Dmitriev & Mangel, 1998).
The difference between the retinal pHo and the Ringer solution pH clearly exhibited a circadian rhythm for more than two full circadian cycles (Fig. 2). As can be seen, the difference between the retinal pHo and the Ringer solution pH was greater during the subjective night (Zeitgeber (ZT) 15 and 21, where ZT 0 is dawn and Zeitgeber means ‘time-giver’), than during the subjective day (ZT 3 and 9). In addition, the day-night difference between the retinal pHo and the Ringer solution pH could be entrained by altering the previous light-dark cycle. The data shown in Fig. 2 were obtained from animals maintained for 2 weeks in a visual environment that was phase advanced by 6 h.
Figure 2. The mean difference between retinal and Ringer solution pH exhibits a circadian rhythm.
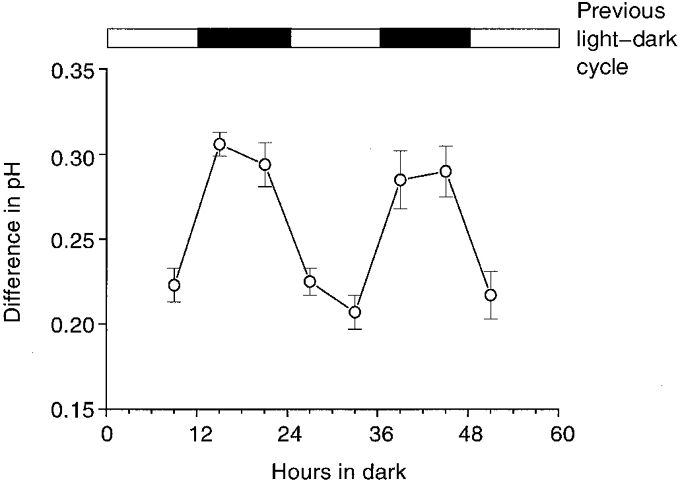
Before the fish were maintained in constant darkness, they were entrained to a 12 h light-12 h dark cycle for at least 14 days. The light-dark cycle is indicated at the top of the figure. Retinae were prepared in either the subjective day or the subjective night. The time of subjective day or night indicated corresponds to the time that pH measurements were made. Each data point represents the mean value ±s.e.m. for 7–12 measurements.
The circadian rhythm in retinal pHo was independent of the Ringer solution pH. The data described above were obtained with a Ringer solution pH of about 7.65. Thus, the mean value of the retinal pHo was 7.45 during the subjective day and 7.37 during the subjective night. When a lower (pH 7.30) superfusate pH was used by increasing the proportion of CO2 in the O2/CO2 gas mixture, retinal light-induced activity was suppressed and the viability of the preparation was shortened considerably. However, the retinal tissue still maintained its pH at a lower level than that of the superfusion solution and this difference depended on circadian time (0.106 ± 0.009, n = 9, during the subjective day and 0.140 ± 0.005, n = 10, during the subjective night; P < 0.05). Thus, even when the superfusate pH was considerably lower (7.30 vs. 7.65), the pH gradient at night was 32 % greater than the pH gradient during the day.
Although light stimulation modulates retinal pHo, as mentioned previously, circadian-induced changes in retinal pHo were several times greater than light-induced changes (Fig. 3). The amplitude of the light-induced pH changes in the isolated goldfish retina was dependent on retinal depth and was largest in the most distal portion of the retina, namely the subretinal space, the extracellular space surrounding the photoreceptors. Figure 3 illustrates the largest light-induced pH changes we were able to obtain. In no case (n = 8 different retinae) did light stimulation increase retinal pHo by more than 0.02 pH units; most often, light-induced pH changes were smaller in amplitude than this. In fact, longer duration (30 s) and brighter (0.02 μW cm−2) stimuli usually produced changes in retinal pHo of about 0.01 pH units in amplitude. These results thus indicate that circadian clock regulation of the metabolic activity of the retina, as represented by retinal pHo, is several times greater than light-dark-induced changes.
Figure 3. Light-evoked pH changes are smaller than clock-induced changes.
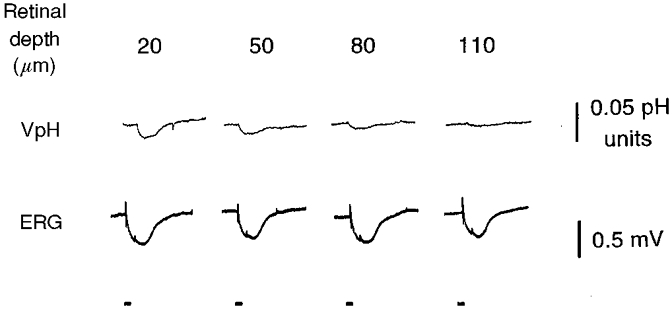
Light-evoked changes in pHo (upper row of records) are shown as a function of retinal depth. VpH is the potential recorded by the pH-sensitive microelectrode. A pH increase is indicated by a downward movement of the trace. The numbers above the records show the depth in micrometres from the receptor surface of the retina. The largest light-evoked pH changes were recorded in the most distal part of the retina (in the extracellular space that surrounds the photoreceptor cells) and had an amplitude of less than 0.02 pH units. Simultaneously recorded transretinal electroretinograms (ERG – bottom row) demonstrate the stable physiological condition of the retina. Bars under the records indicate the occurrence of 5 s light flashes (-6 logIo).
The magnitude of the circadian-induced change in retinal pHo, illustrated here, is large enough to influence synaptic transmission between retinal neurons. Cone horizontal cells in the goldfish retina are second order cells that receive synaptic contact from cone photoreceptor cells (Stell, 1967). Figure 4 shows that a decrease in Ringer solution pH from 7.6 to 7.4, which would reduce retinal pHo by approximately 0.1 pH units, reduced the size of goldfish cone horizontal cell light responses by about 50 % (see also Harsanyi & Mangel, 1993). Our measurements have indicated that changes in Ringer solution pH will produce changes in retinal pHo that are smaller in amplitude. For example, as noted above, a decrease in Ringer solution pH of 0.35 pH units (from 7.65 to 7.30) during the subjective day reduces retinal pHo by 0.24 pH units (from 7.43 to 7.19). These data thus indicate that an experimental reduction in the Ringer solution pH, which decreases retinal pHo by an amount similar to that of the clock-induced night-time decrease, significantly decreases the size of horizontal cell light responses.
Figure 4. Clock-induced pH changes may modulate synaptic transmission in the retina.
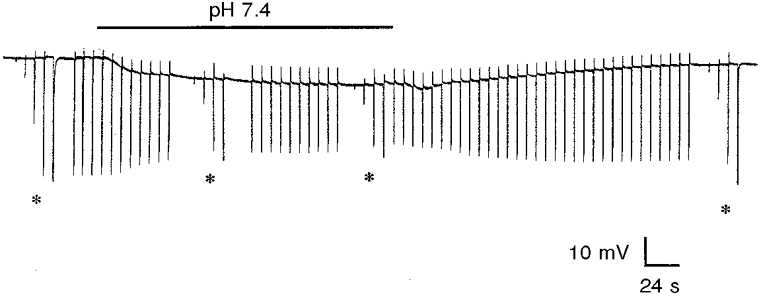
A decrease in Ringer solution pH from 7.6 to 7.4 (indicated by the bar above the record) during the day, which reduces retinal pHo by about 0.1 pH units, reduced the size of cone horizontal cell light responses by about 50 %. Full-field white light stimuli were repetitively flashed at −3 logIo, except when an intensity- response series (-5 logIo to −1 logIo) was obtained (indicated by asterisks).
DISCUSSION
The findings presented here indicate that a circadian clock regulates the pH of the fish retina so that retinal pHo is lower during the night than during the day. The clock-induced shift in pH is several times greater than light-induced pH changes and large enough to influence synaptic transmission between cones and cone horizontal cells, the first synapse of the retina. Because a similar circadian rhythm in retinal pH has also recently been reported in the rabbit retina (Dmitriev & Mangel, 1998), it is likely that most, if not all, vertebrate retinae exhibit a day-night difference in pH.
Although shifts in pHo due to neuronal activity have been reported in studies of in vitro central nervous system (CNS) tissue (Kraig et al. 1983; Chesler & Kaila, 1992), the current findings are the first demonstration that pHo in the CNS can be modulated by a circadian clock, an endogenous neural process. Moreover, the fact that an endogenous process produces acid in the retina suggests that the circadian-induced changes in pHo observed in the in vitro fish retina also occur in vivo. In fact, in support of this idea, Kraig et al. (1983) have shown that stimulus-induced shifts in the pHo of rat cortex occur to a similar extent in vivo and in vitro, suggesting that changes in blood flow in vivo do not alter or eliminate activity-dependent and circadian clock-induced pHo shifts.
The finding that the clock decreases retinal pHo at night suggests that the clock increases the production of acid in the retina at night in a sustained manner over the course of many hours. Such a sustained increase in acid production at night is most probably due to an increase in energetic metabolism at night, and in fact such an increase has been demonstrated recently (Dmitriev et al. 1999a). Moreover, a sustained increase in acid production requires an intracellular source, suggesting that there is one, or more, type of neuron in the retina that produces more acid at night. A change in acid-base transport, for example, can probably not account for a sustained decrease in pHo, because such a change would result in substantial depletion of intracellular acid, leading to neuronal dysfunction. In contrast, light-evoked changes in pHo (Fig. 3), which are smaller in size and more transient, and often of mixed polarity (Borgula et al. 1989; Oakley & Wen, 1989; Dmitriev & Mangel, 1998), might be due to changes in acid-base transport or carbonic anhydrase activity.
Although the clock-induced decrease in retinal pH at night probably reflects a clock-induced increase in retinal metabolic activity (Dmitriev et al. 1999a), the processes whereby the clock affects pH and metabolic activity, as well as the location of the clock itself, are not known. The decrease in pH and the increase in metabolic activity at night may be due to clock-induced ionic conductance changes and/or to clock-induced changes in acid-base transport that result from the action of neurotransmitters such as melatonin (Cassone et al. 1988; Cosci et al. 1997) and dopamine (Shulman & Fox, 1996). Alternatively, the clock may be directly increasing the activity of specific enzymes in retinal metabolic pathways at night.
A circadian clock-induced increase in the concentration of protons during the night may serve as a clock signal for the night. Recent work has shown that a circadian clock regulates the light responses of goldfish cone horizontal cells (Wang & Mangel, 1996). Due to the action of the clock, the responses are cone mediated during the day and are rod mediated, slower and smaller in size during the night. A decrease in Ringer solution pH from 7.6 to 7.4, which reduces retinal pHo by approximately 0.1 pH units, an amount comparable to the extent of circadian regulation, reduced the size of fish cone horizontal cell light responses by about 50 % (Fig. 4) (see also Harsanyi & Mangel, 1993). A decrease in retinal pHo may reduce cone horizontal cell light responses by decreasing transmitter release from cones (Barnes et al. 1993). Thus, the clock-induced decrease in retinal pHo during the night may contribute to the suppression of cone horizontal cell light responses observed during the night. Circadian clock regulation of the pH and metabolic activity of the retina may therefore modulate synaptic transmission in the retina. The clock-induced decrease in retinal pHo during the night may also increase rod photoreceptor sensitivity by stabilizing rhodopsin (Barlow et al. 1993). Studies in which 2-deoxyglucose has been used to measure metabolic activity have indicated that a circadian clock also regulates metabolic activity in the suprachiasmatic nucleus (Schwartz & Gainer, 1977). Thus, it is possible that circadian clock regulation of pH and metabolic activity is a general property of neural circadian clock tissue.
In summary, a circadian clock regulates the extracellular pH of the fish retina so that the pH is lower at night than in the day. The clock-induced shift in pH is several times greater than light-induced pH changes and large enough to influence synaptic transmission between retinal neurons. Thus, an intrinsic oscillator in neural tissue may modulate metabolic activity and pH as part of normal daily function.
Acknowledgments
This work was supported in part by grants to S.C.M. from the National Institutes of Health (EY05102), the National Science Foundation (IBN-9312878) and the Plum Foundation, and by a National Eye Institute CORE Grant (P30 EY03039) to the University of Alabama at Birmingham.
References
- Barlow RB, Birge RR, Kaplan E, Tallent JR. On the molecular origin of photoreceptor noise. Nature. 1993;366:64–66. doi: 10.1038/366064a0. [DOI] [PubMed] [Google Scholar]
- Barnes S, Merchant V, Mahmud F. Modulation of transmission gain by protons at the photoreceptor output synapse. Proceedings of the National Academy of Sciences of the USA. 1993;90:10081–10085. doi: 10.1073/pnas.90.21.10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Iuvone PM. Circadian clock in Xenopus eye controlling retinal serotonin N-acetyltransferase. Nature. 1983;305:133–135. doi: 10.1038/305133a0. [DOI] [PubMed] [Google Scholar]
- Block GD, Khalsa SBS, Mcmahon DG, Michel S, Guesz M. Biological clocks in the retina: cellular mechanisms of biological timekeeping. International Review of Cytology. 1993;146:83. doi: 10.1016/s0074-7696(08)60381-2. [DOI] [PubMed] [Google Scholar]
- Borgula GA, Karwoski CJ, Steinberg RH. Light-evoked changes in extracellular pH in frog retina. Vision Research. 1989;29:1069–1077. doi: 10.1016/0042-6989(89)90054-0. [DOI] [PubMed] [Google Scholar]
- Burnside B, Nagle B. Retinomotor movements of photoreceptors and retinal pigment epithelium: mechanisms and regulation. Progress in Retinal Research. 1983;2:67–109. [Google Scholar]
- Cahill GM, Besharse JC. Circadian rhythmicity in vertebrate retinas: regulation by a photoreceptor oscillator. Progress in Retinal and Eye Research. 1995;14:267. [Google Scholar]
- Cassone VM, Roberts MH, Moore RY. Effects of melatonin on 2-deoxy-14C-glucose uptake within rat suprachiasmatic nucleus. American Journal of Physiology. 1988;255:R332–337. doi: 10.1152/ajpregu.1988.255.2.R332. [DOI] [PubMed] [Google Scholar]
- Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends in Neurosciences. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Cosci B, Longoni B, Marchiafava PL. Melatonin induces membrane conductance changes in isolated retinal rod receptor cells. Life Sciences. 1997;60:1885–1889. doi: 10.1016/s0024-3205(97)00150-1. [DOI] [PubMed] [Google Scholar]
- Dmitriev AV, Mangel SC. Extracellular pH in the rabbit retina exhibits a diurnal rhythm. Investigative Ophthalmology and Visual Science. 1998;(suppl. 39):S567. [Google Scholar]
- Dmitriev AV, Nagy TR, Mangel SC. A circadian clock regulates the metabolic activity of the fish retina. Investigative Ophthalmology and Visual Science. 1999a;(suppl. 40):S611. [Google Scholar]
- Dmitriev A, Pignatelli A, Piccolino M. Resistance of retinal extracellular space to Ca2+ level decrease: implications for the synaptic effects of divalent cations. Journal of Neurophysiology. 1999b;82:283–289. doi: 10.1152/jn.1999.82.1.283. [DOI] [PubMed] [Google Scholar]
- Harsanyi K, Mangel SC. Modulation of cone to horizontal cell transmission by calcium and pH in the fish retina. Visual Neuroscience. 1993;10:81–91. doi: 10.1017/s0952523800003242. [DOI] [PubMed] [Google Scholar]
- Harsanyi K, Wang Y, Mangel SC. Activation of NMDA receptors produces dopamine-mediated changes in fish retinal horizontal cell light responses. Journal of Neurophysiology. 1996;75:629–639. doi: 10.1152/jn.1996.75.2.629. [DOI] [PubMed] [Google Scholar]
- Kolbinger W, Kohler K, Oetting H, Weiler R. Endogenous dopamine and cyclic events in the fish retina, 1: HPLC assay of total content, release, and metabolic turnover during different light/dark cycles. Visual Neuroscience. 1990;5:143–149. doi: 10.1017/s0952523800000183. [DOI] [PubMed] [Google Scholar]
- Korenbrot JI, Fernald RD. Circadian rhythm and light regulate opsin mRNA in rod photoreceptors. Nature. 1989;337:454–457. doi: 10.1038/337454a0. [DOI] [PubMed] [Google Scholar]
- Kraig RP, Ferreira-Filho CR, Nicholson C. Alkaline and acid transients in cerebellar microenvironment. Journal of Neurophysiology. 1983;49:831–850. doi: 10.1152/jn.1983.49.3.831. [DOI] [PubMed] [Google Scholar]
- Oakley B, Wen R. Extracellular pH in the isolated retina of the toad in darkness and during illumination. The Journal of Physiology. 1989;419:353–378. doi: 10.1113/jphysiol.1989.sp017876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reme C, Wirz-Justice A, Terman M. The visual input stage of the mammalian circadian pacemaking system: I. Is there a clock in the mammalian eye? Journal of Biological Rhythms. 1991;6:5–29. doi: 10.1177/074873049100600104. [DOI] [PubMed] [Google Scholar]
- Schwartz W, Gainer JH. Suprachiasmatic nucleus: use of 14C-labeled deoxyglucose uptake as a functional marker. Science. 1977;197:1089–1091. doi: 10.1126/science.887940. [DOI] [PubMed] [Google Scholar]
- Shulman LM, Fox DA. Dopamine inhibits mammalian photoreceptor Na+, K+-ATPase activity via a selective effect on the alpha3 isozyme. Proceedings of the National Academy of Sciences of the USA. 1996;93:8034–8039. doi: 10.1073/pnas.93.15.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell WK. The structure and relationship of horizontal cells and photoreceptor-bipolar synaptic complexes in goldfish retina. American Journal of Anatomy. 1967;121:401–424. doi: 10.1002/aja.1001210213. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mangel SC. A circadian clock regulates rod and cone input to fish retinal cone horizontal cells. Proceedings of the National Academy of Sciences of the USA. 1996;93:4655–4660. doi: 10.1073/pnas.93.10.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A, van Prada M, Reme CE. Circadian rhythm in rat retinal dopamine. Neuroscience Letters. 1984;45:21–25. doi: 10.1016/0304-3940(84)90323-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto F, Borgula GA, Steinberg RH. Effects of light and darkness on pH outside rod photoreceptors in the cat retina. Experimental Eye Research. 1992;54:685–697. doi: 10.1016/0014-4835(92)90023-l. [DOI] [PubMed] [Google Scholar]


