Abstract
We present evidence that adenosine triphosphate (ATP) plays a major role in excitatory neuro-neuronal transmission in ascending and descending reflex pathways to the longitudinal (LM) and circular muscle (CM).
A partitioned bath was used for the pharmacological isolation of a segment of guinea-pig ileum (∼6 cm in length), allowing drugs to be selectively applied to an intermediate region between the region where mucosal stimulation was applied and that where mechanical recordings were made.
Brush stroking the mucosa (3 strokes) elicited a synchronous contraction of the LM and CM both above (ascending excitation) and below (descending excitation) the site of stimulation. All reflexes were abolished when tetrodotoxin (1 μm) was applied to the intermediate chamber.
Hexamethonium (300 μm) added to the intermediate chamber abolished the ascending contraction in 15 % of oral preparations (from 26 preparations, 18 animals) and the descending contraction in 13 % of anal preparations studied (from 53 preparations, 48 animals). In the remaining 85 % of oral preparations, hexamethonium usually attenuated the oral contraction of the LM and CM. However, in the remaining 87 % of anal preparations, hexamethonium had no effect on the anal contraction of the LM and CM.
Oral and anal reflexes that were hexamethonium resistant were either abolished or attenuated by the further addition of the P2 purinergic receptor antagonist pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS, 10 μm) or α,β-methylene ATP (50–100 μm) to the intermediate chamber.
1,1-Dimethyl-4-phenyl-piperazinium iodide (DMPP, 20 μm) or α,β-methylene ATP (50–100 μm) stimulated both ascending and descending excitatory pathways, when applied to the intermediate chamber.
In conclusion, ascending and descending neuro-neuronal transmission in excitatory nervous pathways to the LM and CM is complex and clearly involves neurotransmitter(s) other than acetylcholine (ACh). We suggest mucosal stimulation releases ACh and ATP in both ascending and descending excitatory reflex pathways that synapse with excitatory motoneurons to the LM and CM.
More is known about the enteric nervous system of the guinea-pig ileum than of any other species. It has developed as the model preparation for understanding how enteric neurons generate the peristaltic reflex. In this tissue, immunohistochemical and electrophysiological studies have resulted in a detailed understanding of the neural projections in the myenteric plexus (Costa et al. 1996). It is now known that there is only one major class of ascending interneuron in the guinea-pig ileum (Brookes et al. 1997) and that orally directed transmission in this tissue is markedly attenuated or abolished by hexamethonium (Smith & Furness, 1988; Holzer, 1989; Smith et al. 1990; Tonini & Costa, 1990; Johnson et al. 1996; Spencer et al. 1999b).
In contrast, anally directed transmission in descending interneurons of the guinea-pig ileum is considerably more complex. There are at least four major classes of descending interneuron in this tissue, three of which are cholinergic (Costa et al. 1996). Surprisingly, it is generally believed that these descending interneurons largely utilize neurotransmitters other than ACh, since descending neuro-neuronal transmission in the ileum has consistently been reported to be resistant to hexamethonium (Smith et al. 1990; Johnson et al. 1996; Spencer et al. 1999b). Therefore, the identity of the neurotransmitter substance(s) involved in descending transmission from enteric interneurons has largely remained unclear. However, the neurotransmitter substance released from descending pathways would, like ACh in ascending interneurons, most probably have to be a fast-acting transmitter, operating through ligand-gated ion channels, since neurons in both reflex pathways communicate essentially via fast excitatory postsynaptic potentials (fEPSPs) (Hirst et al. 1975, 1976; Bornstein et al. 1991; Smith et al. 1992, 1999; Brookes et al. 1997).
Although much evidence has been presented suggesting a role for ATP as a neurotransmitter at many neuromuscular synapses in autonomic smooth muscle (see reviews by Burnstock, 1972, 1997; Hirst & Edwards, 1989), little attention has been focused on a role for ATP as a neuro-neuronal transmitter in the enteric nervous system of mammals. There is increasing evidence that ATP may mediate fast synaptic transmission in some ganglia of the central nervous system (Pankratov et al. 1998). In fact, recent evidence has been presented that fast synaptic transmission in the enteric nervous system is likely to involve purinergic as well as nicotinic receptors (Galligan & Bertrand, 1994; Zhou & Galligan, 1996, 1998; Barajas-Lopez et al. 1998; Lepard & Galligan, 1999). However, a role for purinergic transmission during physiological reflexes has not been shown. Furthermore, it is unclear which classes of enteric neuron may involve such transmission.
In the current study, we have used the partitioned organ bath technique to investigate the identity of the neurotransmitter substances released from ascending and descending interneurons involved in the transmission of the orally and anally directed reflex pathways. We present evidence that ACh and ATP both act as excitatory neurotransmitters, involved in ascending and descending neuro-neuronal transmission to motoneurons in the LM and CM.
METHODS
Guinea-pigs weighing 200–350 g were killed by exposure to a rising concentration of CO2 gas, in accordance with the animal ethics committee, University of Nevada School of Medicine. The abdominal cavity was opened and 10 cm of terminal ileum was removed and the mesenteric attachment trimmed away. The luminal contents were flushed out with Krebs solution and the ileum was placed into a modified cold Krebs solution (composition below). To record the simultaneous responses of the LM and CM and prevent mechanical interactions between the movements of the two muscle layers, the LM and myenteric plexus were dissected free of the CM and mucosa in either the oral or anal region, to create a longitudinal muscle-myenteric plexus (LMMP) preparation (see Spencer et al. 1999b). To do this, a longitudinal incision (approximately 15 mm in length) was made along the oral or anal extremity. This section was pinned mucosal-side uppermost, so that the mucosa and submucosa could be removed from this region, to expose the underlying CM layer. Strips of CM were then removed from the exposed oral or anal regions to expose the myenteric plexus (see Smith & Robertson, 1998; Spencer et al. 1999b). Therefore, preparations consisted of a flap of LM with attached myenteric plexus that remained in continuity with the oral or anal region of the ileum, depending on which experiments were performed (Fig. 1). The free end of the dissected region (see LMMP in Fig. 1) was connected via a thread to an independent tension transducer (see below) mounted orally or anally to the mucosal stimulus. The mechanical activity of the CM was monitored using small clips (Micro-serrefines no. 18055-04; Fine Science Tools Inc., Foster City, CA, USA). Changes in LM and CM tension were monitored using isometric tension transducers (Kent Scientific Corporation, USA) (see Smith & McCarron, 1998). Initial resting tensions of the LM and CM were routinely set to 10 mN, so that we could directly compare the reflex responses of the guinea-pig ileum with those we observed in the guinea-pig distal colon (Smith & McCarron, 1998). In the latter preparation, we used identical stimulating and recording methods to those in the current study and relaxation responses of the LM and CM were clearly shown to occur anal to mucosal stimuli (see Smith & McCarron, 1998; Spencer et al. 1999a). Preparations were bathed in oxygenated Krebs solution at 37°C.
Figure 1. Partitioned organ bath.
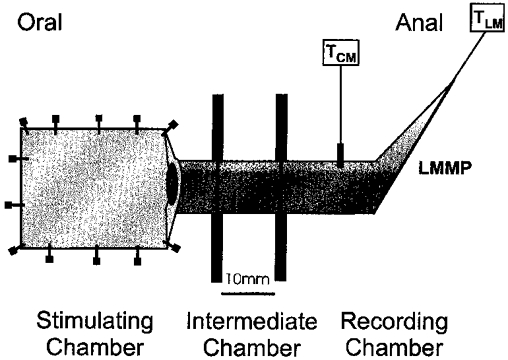
A diagrammatic representation of the preparation used for simultaneously recording CM and LM tension (TCM and TLM), oral and anal to a mucosal stimulus. A segment of ileum (∼6 cm in length) was mounted in a three-chambered organ bath, so that the tissue spanned three regions, a stimulating and recording chamber separated by an intermediate chamber. The CM over the terminal 15 mm was dissected off the myenteric plexus to create a flap of LM with attached myenteric plexus (LMMP) that remained in continuity with the remaining segment of tissue. This was performed to minimize passive mechanical interactions between the LM and CM. The mucosal surface was pinned uppermost in the stimulating chamber, so as to expose the mucosa for stimulation, and LM and CM tension were monitored simultaneously in the recording chamber.
Partitioned organ bath technique and protocol for mucosal stimulation
To pharmacologically isolate certain regions of ileum, the partitioned organ bath technique was used (see Tonini & Costa, 1990; Smith & McCarron, 1998). This technique allows the selective addition of drugs to specific neural components active during orally and anally directed reflexes.
Oral and anal reflexes were elicited by mechanical distortion of the mucosa. The mucosa was stimulated by brush stroking with an artists' brush as described previously (Smith & Furness, 1988; Smith et al. 1990, 1999b; Smith & McCarron, 1998). The mucosa was stimulated at a frequency of approximately 1–2 Hz, delivering three strokes in series.
Drugs and solutions
The following drugs were used in the current study. α,β-Methylene adenosine 5′-triphosphate (α,β-methylene ATP), 1,1-dimethyl-4-phenyl-piperazinium iodide (DMPP), hexamethonium bromide and tetrodotoxin (TTX) were all obtained from Sigma. Pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS, a P2 purinergic receptor antagonist) was obtained from RBI. All stock solutions were prepared in distilled water. For experiments where PPADS and α,β-methylene ATP were used as antagonists, a minimum exposure period of 30 min was given.
The composition of the modified Krebs solution was (mM): NaCl, 120.35; KCl, 5.9; NaHCO3, 15.5; NaH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.5; and glucose, 11.5. The solution was gassed continuously with a mixture containing 3 % CO2-97 % O2 (v/v), pH 7.3-7.4. In some experiments, the composition of the Krebs solution was further modified to block all synaptic transmission in the small intestine (see Bywater, 1994). This was performed by a 10-fold reduction in the Ca2+ concentration and by raising the Mg2+ concentration 10-fold, to stabilize the extracellular ionic composition across the cell membrane. Thus this solution contained (mM): NaCl, 120.35; KCl, 5.9; NaHCO3, 15.5; NaH2PO4, 1.2; MgSO4, 12.0; CaCl2, 0.25; and glucose, 11.5.
Measurements and statistics
Student's paired t tests, or analysis of variance (ANOVA) with multiple comparison procedure (Dunnett's method) were used where appropriate. A minimum significance level of P < 0.05 was used throughout. In the Results section, n refers to the number of animals on which experiments were performed and data are presented as means ±s.e.m. Measurements were made of the amplitude, half-width and area under contractile responses for the LM and CM. These values were obtained using Acqknowledge 3.2.6 (BIOPAC Systems, Inc., Santa Barbara, CA, USA) and tests for statistical significance were made using SigmaPlot 5.0 (Jandel Scientific, San Rafael, CA, USA). To facilitate comparison of responses between different animals, reflex responses elicited following three brush strokes to the mucosa only were analysed, as described in Spencer et al. (1999b).
RESULTS
Control mechanical reflex responses of the LM and CM during the ascending excitatory reflex
In response to brush stroking the mucosa (3 strokes) in the anal stimulating chamber, a synchronous contraction was elicited in the LM and CM in the oral recording chamber (ascending excitation; Fig. 2a). Contractions of the LM and CM had peak amplitudes of 10.9 ± 1.1 mN (half-width, 2.6 ± 0.1 s; 30 preparations, n = 20) and 21.7 ± 3.9 mN (half-width, 2.6 ± 0.2 s; 30 preparations, n = 20), respectively. The area under the contractile responses of the two muscle layers was 24.1 ± 2.3 and 46.5 ± 9.3 mN s for LM and CM, respectively.
Figure 2. Involvement of cholinergic and purinergic neuro-neuronal transmission underlying the ascending excitatory reflex.
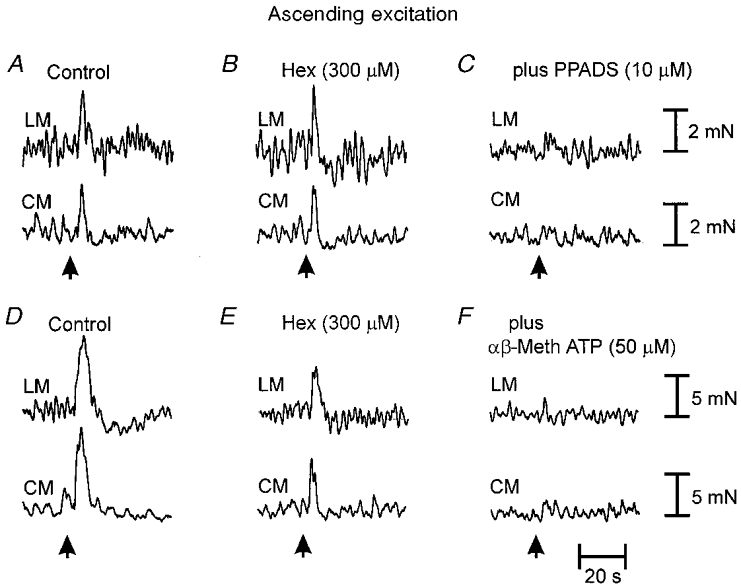
A, brush stroking the mucosa (3 strokes; arrow) in the anal stimulating chamber elicited a synchronous contraction of the LM and CM in the oral recording chamber. B, addition of hexamethonium (Hex, 300 μm) to the intermediate chamber was without effect on ascending neuro-neuronal transmission. C, further addition of PPADS (10 μm) to the intermediate chamber abolished the ascending excitatory reflex to both muscle layers. D, in a different preparation, three control brush strokes to the mucosa elicited synchronous contractions of the two muscle layers. E, the addition of hexamethonium (300 μm) to the intermediate chamber attenuated ascending neuro-neuronal transmission. F, the further addition of α,β-methylene ATP (50 μm) abolished non-nicotinic ascending transmission to both muscles.
Effects of hexamethonium and DMPP on ascending neuro-neuronal transmission to the LM and CM
The involvement of nicotinic neurotransmission in ascending pathways was tested by selective application of hexamethonium to the intermediate chamber. It was found that in four out of 26 preparations (n = 18), hexamethonium (300 μm) abolished the oral contraction in both muscles, while in 19 preparations, the contraction of both muscle layers in the oral recording chamber was significantly attenuated, by 27 % in the LM (from 22.6 ± 2.4 to 16.6 ± 2.0 mN s) and by 48 % in the CM (from 35.2 ± 5.3 to 18.4 ± 5.3 mN s; 19 preparations, n = 11, P < 0.05; cf. Fig. 2D and E). In three other preparations, however, hexamethonium was without any notable effect on ascending transmission (cf. Fig. 2a and B). To test the possibility that in ascending interneurons, nicotinic receptor activation alone is sufficient to elicit ascending excitation in the LM and CM, DMPP (20 μm) was applied to the intermediate chamber. In all preparations studied (14 preparations, n = 10) application of DMPP (20 μm) to the intermediate chamber elicited a powerful and synchronous contraction of both the LM (amplitude, 14.7 ± 3.5 mN) and CM (amplitude, 7.4 ± 0.9 mN) in the oral recording chamber (Fig. 3B). DMPP-induced motor responses were indistinguishable from reflex responses elicited by mucosal stimulation. These responses were abolished by hexamethonium (900 μm).
Figure 3. Stimulatory effects of DMPP and α,β-methylene ATP on ascending and descending neuro-neuronal transmission to the LM and CM.
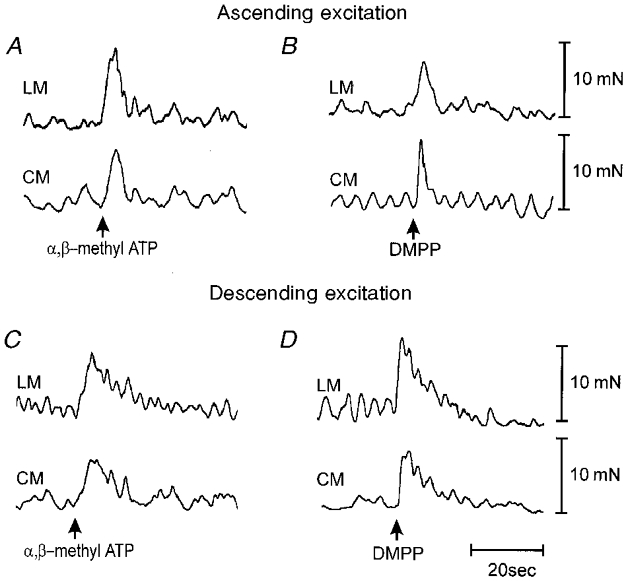
A, immediately following application to the intermediate chamber, α,β-methylene ATP (100 μm) elicited an ascending excitation in both the LM and CM in the oral recording chamber. These responses occurred in the presence of hexamethonium in the intermediate chamber. B, similarly, DMPP (20 μm) added to the intermediate chamber elicited a powerful ascending excitation in the oral recording chamber. C, in the presence of hexamethonium (300 μm), addition of α,β-methylene ATP (100 μm) to the intermediate chamber also elicited a descending excitation in the anal recording chamber. D, similarly, addition of DMPP (20 μm) to the intermediate chamber elicited a descending excitation in the anal recording chamber.
Effects of purinergic antagonists on hexamethonium-resistant ascending neuro-neuronal transmission to the LM and CM
In preparations where hexamethonium did not block the oral reflex contraction when applied to the intermediate chamber, PPADS (10 μm) was subsequently added to this chamber, to test for the involvement of purinergic transmission. PPADS (10 μm) abolished hexamethonium-resistant transmission in three out of six preparations (Fig. 2C), and in the remaining three preparations tested, hexamethonium-resistant transmission was almost blocked, leaving residual contractions almost within the recording noise (see Fig. 4a). Overall, from six preparations studied, PPADS significantly attenuated orally directed hexamethonium-resistant transmission (P < 0.05, n = 6). Hexamethonium and PPADS-resistant transmission was abolished by addition of TTX (1 μm) to the intermediate chamber (2 preparations). A summary of the data showing the effects of hexamethonium and PPADS on the ascending excitatory reflex from these six preparations is shown in Fig. 4A.
Figure 4. Graphical representation of the effects of nicotinic and purinergic antagonists on neuro-neuronal transmission underlying ascending and descending excitatory reflexes.
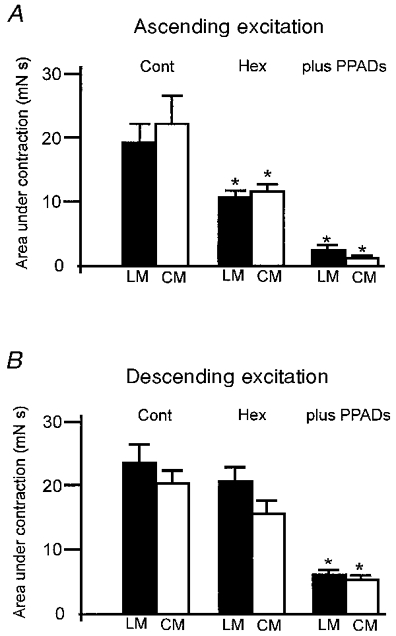
A, brush stroking the mucosa (3 strokes) in the anal stimulating chamber elicited synchronous contractions of the LM and CM in the oral recording chamber. Hexamethonium (300 μm) added to the intermediate chamber significantly attenuated ascending neuro-neuronal transmission when compared with control responses. The addition of the P2 receptor antagonist PPADS (10 μm) further attenuated hexamethonium-resistant ascending neuro-neuronal transmission. B, brush stroking the mucosa in the oral stimulating chamber elicited contractions of the LM and CM in the anal recording chamber. Hexamethonium (300 μm) added to the intermediate chamber did not significantly reduce descending neuro-neuronal transmission. However, the subsequent addition of PPADS to the intermediate chamber significantly reduced descending neuro-neuronal transmission to the LM and CM. * P < 0.05, ANOVA.
To further test whether ATP may be an excitatory neurotransmitter involved in hexamethonium-resistant transmission, α,β-methylene ATP (50–100 μm) was applied to the intermediate chamber. In 10 out 16 preparations tested (n = 13), application of α,β-methylene ATP (50–100 μm) abolished hexamethonium-resistant reflex contractions of both the LM and CM (cf. Fig. 2E and F). In five of the remaining six preparations that were studied, α,β-methylene ATP (50–100 μm) added to the intermediate chamber significantly attenuated hexamethonium-resistant transmission to both muscle layers, by 63 % in the LM (from 16.1 ± 1.8 to 6.0 ± 3.1 mN s) and 83 % in the CM (from 19.0 ± 7.9 to 3.2 ± 1.2 mN s; P < 0.05, n = 5).
It is particularly noteworthy that immediately upon application of α,β-methylene ATP (50–100 μm) to the intermediate chamber, this drug initially stimulated ascending excitatory pathways and elicited a powerful synchronous contraction of both muscle layers in the oral recording chamber (Fig. 3a). These responses occurred in the presence of hexamethonium, supporting the existence of excitatory purinergic receptors in ascending hexamethonium-resistant neuro-neuronal pathways to the LM and CM. Contractions elicited by α,β-methylene ATP (50–100 μm) had amplitudes of 4.9 ± 1.1 mN in the LM and 7.3 ± 2.2 mN in the CM (12 preparations, n = 9).
Effects of low Ca2+-high Mg2+ solution in the intermediate chamber on ascending neuro-neuronal transmission to the LM and CM
To test whether oral reflex responses involved chains of ascending interneurones making synaptic connections within the intermediate chamber, the composition of the Krebs solution in this chamber was altered to a low Ca2+, high Mg2+ solution to block all synapses in this region (see Bywater, 1994). It was found that in seven out of 20 preparations (n = 14) this solution applied to the intermediate chamber abolished reflex contractions in the oral recording chamber (cf. Fig. 5a and B). In the remaining 13 preparations, application of the low Ca2+-high Mg2+ Krebs solution to the intermediate chamber significantly attenuated the oral reflex contraction of both muscles, by 56 % in the LM (from 25.0 ± 2.9 to 11.1 ± 1.2 mN s) and by 67 % in the CM (from 39.5 ± 8.9 to 12.8 ± 2.0 mN s; P < 0.05, n = 13).
Figure 5. Effects of blockade of synaptic transmission on ascending excitatory and descending excitatory reflex pathways.
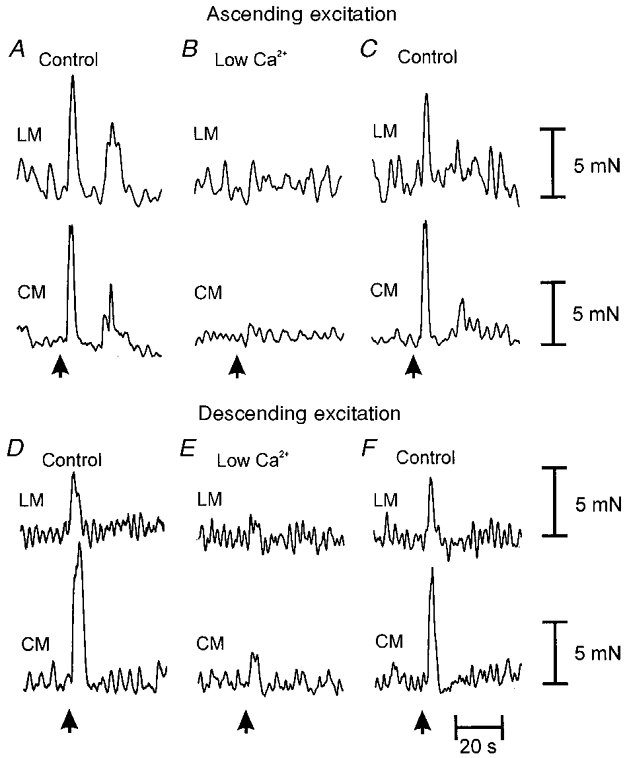
Mucosal stimulation (arrows) elicited synchronous contractions of the LM and CM oral (A) and anal (D) to the mucosal stimulus. Selective addition of a low Ca2+-high Mg2+ solution to the intermediate chamber abolished ascending (B) and almost completely abolished descending (E) neuro-neuronal transmission, confirming the presence of synaptic transmission in this region of ileum. Replacement of this solution with the normal Krebs solution in the intermediate chamber restored ascending (C) and descending (F) neuro-neuronal transmission to the LM and CM.
Anal reflex responses of the longitudinal and circular muscle to mucosal stimulation
In response to mucosal stimulation in the oral stimulating chamber, a synchronous contraction was elicited in the LM and CM in the anal recording chamber (see Fig. 6a and D). Consistent with previous studies on this tissue, no descending relaxation of either muscle layer was recorded anal to the stimulus (see Spencer et al. 1999b). Contractions of the LM and CM had peak amplitudes of 8.6 ± 1.1 mN (half-width, 3.0 ± 0.2 s; 57 preparations, n = 51) and 9.5 ± 2.1 mN (half-width, 2.7 ± 0.2 s; 57 preparations, n = 51). The area under the contractile responses of the two muscles was 21.2 ± 2.7 and 19.3 ± 4.0 mN s for LM and CM, respectively.
Figure 6. Involvement of cholinergic and purinergic neuro-neuronal transmission underlying the descending excitatory reflex.
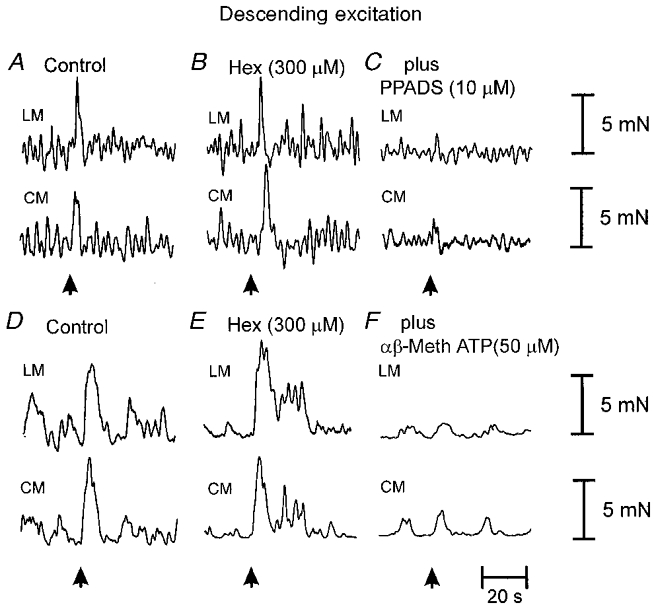
A, brush stroking the mucosa (3 strokes; arrow) in the oral stimulating chamber elicited a synchronous contraction of the LM and CM in the anal recording chamber. B, addition of hexamethonium (300 μm) to the intermediate chamber was without effect on descending neuro-neuronal transmission to the LM and CM. C, the further addition of PPADS (10 μm) to the intermediate chamber essentially abolished descending transmission. D–F, in another preparation, hexamethonium (300 μm) added to the intermediate chamber (E) also was without effect on descending excitatory transmission to both muscle layers. However, the further application of α,β-methylene ATP (50 μm) to the intermediate chamber abolished descending neuro-neuronal transmission (F).
Effects of hexamethonium and DMPP on descending neuro-neuronal transmission to the LM and CM
The identity of the possible neurotransmitter substances involved in anally directed neuro-neuronal transmission to the LM and CM was investigated. The role of nicotinic transmission in descending pathways was tested by applying hexamethonium to the intermediate chamber. It was found that in seven out of 53 preparations (n = 48), hexamethonium (300 μm) added to the intermediate chamber abolished reflex-evoked contractions of the LM and CM in the anal recording chamber. In only three preparations, hexamethonium (300 μm) reduced the reflex responses of both muscles (LM, 10.2 ± 2.6 to 8.4 ± 2.3 mN; and CM, 18.1 ± 5.8 to 9.2 ± 1.9 mN; P > 0.05, n = 3), while in the remaining 43 preparations, hexamethonium (300 μm) was completely without effect on descending transmission to the LM and CM (cf. Fig. 6a and B). To test whether stimulation of nicotinic receptors on descending interneurons elicited descending excitation in the anal recording chamber, similar to mucosal stimulation, DMPP (20 μm) was added to the intermediate chamber. Immediately upon addition of DMPP to the intermediate chamber, a powerful contraction occurred synchronously in the LM (amplitude, 9.6 ± 1.4 mN; 9 preparations, n = 7) and CM (amplitude, 6.5 ± 1.5 mN; 9 preparations, n = 7) in the anal recording chamber (Fig. 3D). Responses to DMPP in the anal recording chamber were blocked by the addition of hexamethonium (900 μm) to the intermediate chamber.
Effects of purinergic antagonists on hexamethonium-resistant descending neuro-neuronal transmission to the LM and CM
To test for the involvement of purinergic transmission in descending non-nicotinic pathways, PPADS was added to the intermediate chamber in the continuing presence of hexamethonium. In seven out of 21 preparations tested (n = 21), PPADS (10 μm) abolished descending transmission into the anal recording chamber (cf. Fig. 6B and C). In the remaining 14 preparations, PPADS (10 μm) significantly attenuated hexamethonium-resistant transmission by 57 % in the LM and by 63 % in the CM (P < 0.05, n = 14). A summary of the data showing the effects of hexamethonium and PPADS on the descending excitatory reflex from these 14 preparations is shown in Fig. 4B.
As a further test of whether hexamethonium-resistant transmission may involve purinergic transmission, we applied α,β-methylene ATP (50–100 μm) to the intermediate chamber. In seven out of 12 preparations tested (n = 10), α,β-methylene ATP abolished hexamethonium-resistant descending transmission to the LM and CM (cf. Fig. 6E and F). In the remaining five preparations, α,β-methylene ATP significantly attenuated hexamethonium-resistant transmission, by 43 % in the LM (from 17.1 ± 4.9 to 9.7 ± 5.3 mN s) and by 72 % in the CM (from 16.3 ± 5.2 to 4.6 ± 2.1 mN s; P < 0.05, n = 5). As was found with the ascending excitatory reflex, α,β-methylene ATP (100 μm) added to the intermediate chamber initially acted as an agonist, and elicited a powerful descending excitatory reflex to both the LM and CM in the anal recording chamber (Fig. 3C). These contractions occurred synchronously, and appeared identical to those reflex responses elicited by mucosal stimulation. These responses occurred in the presence of hexamethonium (300 μm), supporting the existence of hexamethonium-resistant excitatory purinergic transmission from descending interneurons to LM and CM motoneurons. The amplitudes of these contractions were 7.7 ± 2.4 mN in the LM and 4.2 ± 0.8 mN in the CM (9 preparations, n = 7).
Effects of low Ca2+-high Mg2+ solution in the intermediate chamber on descending neuro-neuronal transmission to the LM and CM
A low Ca2+-high Mg2+ solution was applied in the intermediate chamber to confirm the presence of descending neuronal synapses in this region of ileum. In four out of 16 preparations (n = 15), addition of the low Ca2+-high Mg2+ solution to the intermediate chamber reduced the descending excitatory reflex to both muscles, essentially to within the recording noise (cf. Fig. 5D and E). However, in the remaining 12 preparations, this solution attenuated, but did not block, the reflex responses, by 31 % in the LM (from 18.0 ± 2.0 to 12.4 ± 2.3 mN s) and by 51 % in the CM (from 15.5 ± 2.8 to 7.9 ± 1.6 mN s; P < 0.05, n = 12). Replacement of the low Ca2+-high Mg2+ solution with the normal Krebs solution in the intermediate chamber restored the descending excitatory reflex in the anal recording chamber (Fig. 5F). It is noteworthy that in five other preparations studied, low Ca2+-high Mg2+ solution was without any effect on descending transmission when applied to the intermediate chamber. In three of these five preparations we further applied low Ca2+-high Mg2+ solution to the oral stimulating chamber. This was found to attentuate (by approximately half), but did not block, reflex responses in the anal recording chamber. This suggests the existence of long anally projecting mucosal sensory neurons from the stimulating chamber into the recording chamber (see Brookes et al. 1995).
Effects of PPADS before hexamethonium on ascending and descending neuro-neuronal transmission to the LM and CM
We investigated whether blockade of P2 receptors with PPADS would significantly attenuate ascending and descending neuro-neuronal transmission, prior to the application of hexamethonium. We found that PPADS (10 μm) alone in the intermediate chamber was without effect on ascending (20 preparations, n = 15, P > 0.05) or descending transmission (6 preparations, n = 6, P > 0.05) to either muscle layer (Fig. 7). However, in the presence of PPADS, subsequent addition of hexamethonium (300 μm) to the intermediate chamber was found to significantly attenuate or abolish ascending (6 preparations, n = 6, P < 0.05; Fig. 7) and descending transmission (4 preparations, n = 4, P < 0.05; Fig. 7) to the LM and CM, suggesting that the presence of hexamethonium or PPADS alone does not sufficiently reduce the safety factor for neuro-neuronal transmission to subthreshold levels (see Discussion). In two preparations from one animal, addition of PPADS (10 μm) and hexamethonium (300 μm) to the intermediate and oral stimulating chambers did not affect descending transmission in the anal recording chamber. However, in these two preparations, the further addition of PPADS (10 μm) to the anal recording chamber abolished the descending excitatory reflex.
Figure 7. Effects of PPADS applied before hexamethonium on ascending and descending neuro-neuronal transmission.
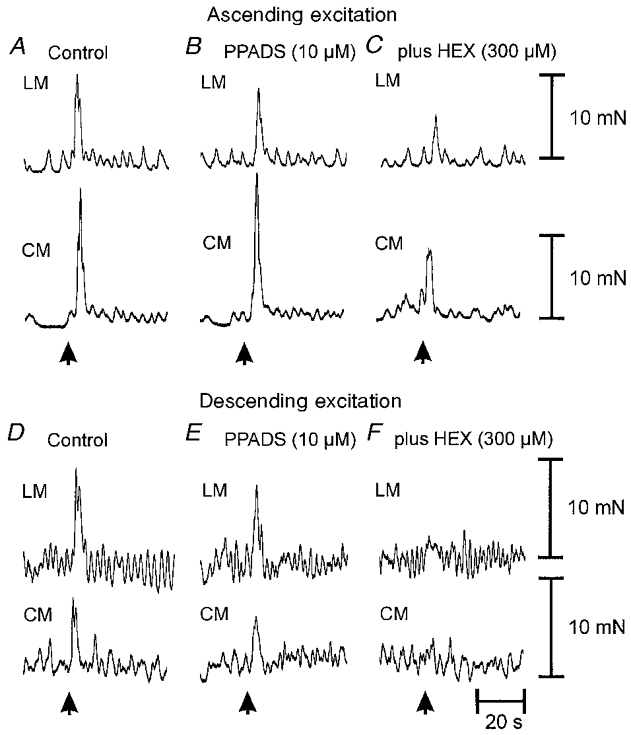
A, control ascending excitatory reflex in the oral recording chamber, following mucosal stimulation (3 brush strokes; arrow) in the anal stimulating chamber. B, application of PPADS (10 μm) to the intermediate chamber was without affect on ascending neuro-neuronal transmission. C, in the presence of PPADS, the further addition of hexamethonium (300 μm) to the intermediate chamber markedly attenuated ascending transmission to both muscles. D, control descending excitatory reflex reponses in the anal recording chamber, following mucosal stimulation (3 strokes) in the oral stimulating chamber. E, PPADS (10 μm) added to the intermediate chamber was without effect on descending neuro-neuronal transmission. F, in the presence of PPADS (10 μm), further addition of hexamethonium (300 μm) to the intermediate chamber abolished descending transmission to both muscle layers.
DISCUSSION
A primary finding of this study is the demonstration that, following mechanical stimulation of the mucosa, both endogenous ACh and ATP act as excitatory neuro-neuronal transmitters in both ascending and descending pathways that synapse with motoneurons to LM and CM of the guinea-pig small intestine.
A partitioned organ bath technique was used to localize the actions of particular drugs to specific intrinsic neural components activated following mucosal stimulation (see Tonini & Costa, 1990; Smith & McCarron, 1998; Spencer et al. 1999a). This allows an investigation into the identity of putative neurotransmitter substances involved in ascending and descending neuro-neuronal pathways.
Ascending excitatory and descending excitatory reflex pathways
Recently, we showed that in the guinea-pig distal colon, mucosal stimulation elicited a polarized mechanical reflex, whereby an oral contraction and anal relaxation response occurred in the neighbouring smooth muscles (Smith & McCarron, 1998; Spencer et al. 1999a). In contrast, in the guinea-pig ileum, we found that mucosal stimulation elicited contractions both oral (ascending excitation) and anal (descending excitation) to the stimulus, which occurred synchronously in the LM and CM. In agreement with our previous study (Spencer et al. 1999b), no anal relaxation preceded the descending contraction in the ileum (see Alvarez & Zimmerman, 1927; Alvarez, 1948). In fact, many investigators have also reported the presence of contraction and absence of relaxation anal to reflex stimuli in the small bowel of the dog (Hukuhara et al. 1936), rabbit (Alvarez & Zimmerman, 1927), human (White et al. 1934), guinea-pig (Spencer et al. 1999b), chicken (Hodgkiss, 1986) and other species (see review by Alvarez, 1948).
Neuro-neuronal transmission in ascending reflex pathways
Tonini & Costa (1990) also used the partitioned organ bath to examine the role of nicotinic transmission underlying the ascending excitatory reflex responses of the CM. They showed that hexamethonium almost blocked the ascending excitatory reflex, but a residual contraction persisted. Their intermediate chamber consisted of partitions separated by 4 mm, whereas the partitions forming our intermediate chamber were set 10 mm apart, making synaptic connections within our chamber more likely. We found that a low Ca2+-high Mg2+ solution applied to the intermediate chamber was capable of abolishing ascending transmission in seven out of 20 preparations. However, in the remaining 13 preparations, application of the low Ca2+-high Mg2+ solution to the intermediate chamber significantly attenuated, but did not block, ascending transmission. This suggests that ascending interneurons longer than ∼10 mm are also involved in orally directed transmission in these tissues. The effects of hexamethonium on the ascending excitatory reflex to the LM and CM differed in each animal, when applied to the intermediate chamber. We were surprised that hexamethonium did not always block, or in some cases even reduce, the ascending excitatory reflex. This is because there is only one major class of ascending interneuron in the guinea-pig ileum (Brookes et al. 1997) and we had assumed, like others, that ascending transmission is largely, if not solely, of cholinergic origin. Our results suggest that, in addition to ACh, there are other non-nicotinic neurotransmitters that may be released from ascending interneurons during the ascending excitatory reflex to the LM and CM. In support of this conclusion, Holzer (1989) reported that after prolonged exposure to hexamethonium the ascending excitatory reflex recovers somewhat, suggesting that there was some neuronal adaptation to non-nicotinic transmission. Following mucosal stimulation, or distension (Hirst et al. 1975; Bornstein et al. 1991; Smith et al. 1992), the predominant synaptic response in orally or anally situated myenteric neurons is a burst of fEPSPs; that is, fast excitatory potentials, caused by neurotransmitters that act through ligand-gated ion channels. In fact, ATP has been suggested to mediate a component of the fEPSPs in myenteric neurons (Galligan & Bertrand, 1994; Zhou & Galligan, 1996; Lepard & Galligan, 1999), but a role for ATP during physiological reflex stimulation had not been shown. A major finding of the current study was that hexamethonium-resistant ascending excitatory transmission was either significantly attenuated or abolished by the P2 purinergic receptor antagonist PPADS, or α,β-methylene ATP, providing strong evidence that neuro-neuronal transmission in ascending interneurons is likely to involve ATP as well as ACh. Furthermore, the observation that α,β-methylene ATP initially activated ascending excitatory pathways that are likely to mediate ascending excitatory reflexes, while in the presence of hexamethonium, suggests that P2 receptor activation is functionally important in hexamethonium-resistant ascending pathways to the LM and CM.
Neuro-neuronal transmission in descending reflex pathways
In contrast to the ascending excitatory pathway, the identity of the neurotransmitter substances involved in descending transmission in the guinea-pig ileum has largely remained elusive. The difficulty in identifying the major neurotransmitter substances has most probably been due to the fact that there are at least four major classes of descending interneuron in this tissue (Costa et al. 1996). In the current study, we found that in a small proportion of preparations tested (7 out of 53), hexamethonium abolished descending excitation in the anal recording chamber, when applied to the intermediate chamber. This suggests that ACh is involved in descending neuro-neuronal transmission to both muscle layers, but that the safety factor for transmission due to other non-cholinergic transmitters is sufficiently high (suprathreshold) for descending transmission to persist. This is similar to the effects of PPADS, since we did not observe any response to PPADS alone in the intermediate chamber. Only when hexamethonium was applied after PPADS was there a consistent depression of excitatory responses observed in ascending or descending pathways. This shows that the safety factor for neurotransmission in the enteric nervous system must be sufficiently attenuated with one of the antagonists in order to observe any effect with the other antagonist. Since in the majority of preparations, hexamethonium did not affect neuro-neuronal transmission in descending pathways, this strongly suggests that both cholinergic and non-cholinergic fast transmitters are released in the myenteric plexus. A major finding of this study was the identification that purinergic transmission plays a major role in non-cholinergic descending neuro-neuronal transmission to motoneurons of the LM and CM. We were particularly encouraged to find that in preparations where low Ca2+-high Mg2+ solution added to the intermediate chamber did not attenuate ascending or descending transmission, hexamethonium and purinergic antagonists also had no effect, suggesting that these antagonists were not acting as local anaesthetics on neurotransmission.
Perhaps the most convincing evidence that ACh and ATP act as excitatory transmitters in ascending and descending pathways was given by the effects of DMPP and α,β-methylene ATP added to the intermediate chamber. When recording LM and CM activity in the oral or anal recording chambers, application of these drugs to the intermediate chamber revealed that both these agents initially acted as powerful stimulants of ascending excitatory and descending excitatory pathways to both muscle layers.
Conclusions
Our results suggest that ascending and descending neuro-neuronal transmission to the LM and CM is far more complex than we had originally thought, and may involve a number of ‘fast’ excitatory neurotransmitters. The findings of the current study indicate that in addition to ACh, ATP is likely to be an important non-cholinergic excitatory neurotransmitter, most probably in the transmission between ascending interneurons and between descending interneurons. Since the one class of ascending interneuron and three out of the four classes of descending interneuron are cholinergic (Costa et al. 1996), ATP is likely to be co-localized with ACh.
Acknowledgments
Michelle Walsh is a visiting research scholar from the University of Ulster (Coleraine, UK). The National Institutes of Health (USA) provided financial support for the project (grant no. NIDAA 10793 to T.K.S.).
References
- Alvarez WC. An Introduction to Gastroenterology. 4. New York: Paul B. Hoeber; 1948. pp. 37–48. [Google Scholar]
- Alvarez WC, Zimmermann A. The absence of inhibition ahead of peristaltic rushes. American Journal of Physiology. 1927;83:52–59. [Google Scholar]
- Barajas-Lopez C, Espinosa-Luna R, Zhu Y. Functional interactions between nicotinic and P2X channels in short-term cultures of guinea-pig submucosal neurons. The Journal of Physiology. 1998;513:671–683. doi: 10.1111/j.1469-7793.1998.671ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Smith TK, Trussell DC. Synaptic responses evoked by mechanical stimulation of the mucosa in morphologically identified myenteric neurones of the guinea-pig ileum. Journal of Neuroscience. 1991;11:723–731. doi: 10.1523/JNEUROSCI.11-02-00505.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ, Meedinya AC, Jobling P, Costa M. Orally projecting interneurones in the guinea-pig small intestine. The Journal of Physiology. 1997;505:473–491. doi: 10.1111/j.1469-7793.1997.473bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ, Song Z-M, Ramsey GA, Costa M. Long aboral projections of Dogiel type II, AH neurons within the myenteric plexus of the guinea-pig small intestine. Journal of Neuroscience. 1995;15:4013–4022. doi: 10.1523/JNEUROSCI.15-05-04013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacological Reviews. 1972;24:509–581. [PubMed] [Google Scholar]
- Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Bywater RA. Activity following colonic distension in enteric sensory fibres projecting to the inferior mesenteric ganglion in the guinea-pig. Journal of the Autonomic Nervous System. 1994;46:19–26. doi: 10.1016/0165-1838(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Costa M, Brookes SJ, Steele PA, Gibbins I, Burcher E, Kandiah CJ. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–967. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Bertrand PP. ATP mediates fast synaptic potentials in enteric neurons. Journal of Neuroscience. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Edwards FR. Sympathetic neuroeffector transmission in arteries and arterioles. Physiological Reviews. 1989;69:546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Hirst GD, Holman ME, Mckirdy HC. Nervous pathways excited during peristalsis. In: Bulbring E, Shuba MF, editors. Physiology of Smooth Muscle. New York: Raven Press; 1976. pp. 309–311. [Google Scholar]
- Hirst GDS, Holman ME, Mckirdy HC. Two descending nerve pathways activated by distension of guinea-pig small intestine. The Journal of Physiology. 1975;244:113–127. doi: 10.1113/jphysiol.1975.sp010786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkiss JP. Intrinsic reflexes underlying peristalsis in the small intestine of the domestic fowl. The Journal of Physiology. 1986;380:311–328. doi: 10.1113/jphysiol.1986.sp016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Ascending enteric reflex: multiple neurotransmitter systems and interactions. American Journal of Physiology. 1989;256:G540–545. doi: 10.1152/ajpgi.1989.256.3.G540. [DOI] [PubMed] [Google Scholar]
- Hukuhara T, Masuda K, Kinose S. Pflügers Archives für die Gesamte Physiologie des Menschen und der Tiere. Berlin: Julius Springer, Linkstrasse 23; 1936. Ueber das ‘Gesetz des Dames’; p. W.9. [Google Scholar]
- Johnson PJ, Bornstein JC, Yuan SY, Furness JB. Analysis of the contributions of acetylcholine and tachykinins to neuro-neuronal transmission in motility reflexes in the guinea-pig ileum. British Journal of Pharmacology. 1996;118:973–983. doi: 10.1111/j.1476-5381.1996.tb15495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepard KJ, Galligan JJ. Analysis of fast synaptic pathways in myenteric plexus of guinea-pig ileum. American Journal of Physiology. 1999;113:1522–1534. doi: 10.1152/ajpgi.1999.276.2.G529. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Castro E, Miras-Portugal MT, Krishtal O. A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurons of the rat hippocampus. European Journal of Neuroscience. 1998;10:3898–3902. doi: 10.1046/j.1460-9568.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Distension-evoked ascending and descending reflexes in the circular muscle of guinea-pig ileum: an intracellular study. Journal of the Autonomic Nervous System. 1990;29:203–217. doi: 10.1016/0165-1838(90)90146-a. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Convergence of reflex pathways excited by distension and by stimulation of the mucosa of the guinea-pig ileum. Journal of Neuroscience. 1992;12:1502–1510. doi: 10.1523/JNEUROSCI.12-04-01502.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Burke EP, Shuttleworth CWR. Topographical and electrophysiological characteristics of highly excitable S-neurons in the myenteric plexus of the guinea-pig ileum. The Journal of Physiology. 1999;517:817–830. doi: 10.1111/j.1469-7793.1999.0817s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Furness JB. Reflex changes in circular muscle activity elicited by stroking the mucosa: an electrophysiological analysis in the isolated guinea-pig ileum. Journal of the Autonomic Nervous System. 1988;25:205–218. doi: 10.1016/0165-1838(88)90025-2. [DOI] [PubMed] [Google Scholar]
- Smith TK, Furness JB, Costa M, Bornstein JC. An electrophysiological study of the projections of motor neurons that mediate non-cholinergic excitation in the circular muscle layer of guinea-pig small intestine. Journal of the Autonomic Nervous System. 1988;22:115–128. doi: 10.1016/0165-1838(88)90085-9. [DOI] [PubMed] [Google Scholar]
- Smith TK, Mccarron SL. Nitric oxide modulates cholinergic reflex pathways to the longitudinal and circular muscle in the isolated guinea-pig distal colon. The Journal of Physiology. 1998;512:893–906. doi: 10.1111/j.1469-7793.1998.893bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Robertson WJ. Synchronous movements of the longitudinal and circular muscle during peristalsis in the isolated guinea-pig distal colon. The Journal of Physiology. 1998;506:563–577. doi: 10.1111/j.1469-7793.1998.563bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer N, Mccarron SL, Smith TK. Sympathetic inhibition of ascending and descending interneurons during the peristaltic reflex in the isolated guinea-pig distal colon. The Journal of Physiology. 1999a;519:539–550. doi: 10.1111/j.1469-7793.1999.0539m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer N, Walsh M, Smith TK. Does the guinea-pig ileum obey the ‘law of the intestine’? The Journal of Physiology. 1999b;517:889–898. doi: 10.1111/j.1469-7793.1999.0889s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonini M, Costa M. A pharmacological analysis of the neuronal circuitry involved in distension-evoked enteric excitatory reflex. Neuroscience. 1990;38:787–795. doi: 10.1016/0306-4522(90)90071-b. [DOI] [PubMed] [Google Scholar]
- White HL, Rainey WR, Monaghan B, Harris AS. Observations on the nervous control of the ileocaecal sphincter and on intestinal movements in an unanesthetized human subject. American Journal of Physiology. 1934;108:449–457. [Google Scholar]
- Zhou X, Galligan JJ. P2X purinoceptors in cultured myenteric neurons of guinea-pig small intestine. The Journal of Physiology. 1996;496:719–729. doi: 10.1113/jphysiol.1996.sp021722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Galligan JJ. Non-additive interaction between nicotinic cholinergic and P2X purine receptors in guinea-pig enteric neurons in culture. The Journal of Physiology. 1998;513:685–697. doi: 10.1111/j.1469-7793.1998.685ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


