Abstract
The purpose of this study was to compare the pathological effects of Shiga toxin-producing Escherichia coli (STEC) that vary in their association with bovine and human disease. Shiga toxin-producing E. coli of serotypes associated with both dysentery in calves and hemolytic uremic syndrome (HUS) in humans (O5:H-, O26:H11, O111:H-, O113:H21) were compared with O157:H7 STEC, which are associated with HUS in humans but not with disease in calves. The STEC were administered orally to 80 day-old chicks and into ligated loops in the ileum and colon of four 2- to 6-day-old calves. Examination of the ceca of the chickens 10 d postchallenge showed no adherence or tissue abnormality for any isolate. The calves were euthanized 8 to 10 h postinoculation, and sections of the intestinal loops were examined by light microscopy, transmission and scanning electron microscopy, and immunohistochemistry. All strains showed consistent focal adherence associated with mild lesions in the colon. Attaching and effacing lesions were observed with the eae-positive strains. Ileal lesions were similar to the colonic ones but were sometimes severe, with marked polymorphonuclear leukocyte proliferation in the lamina propria. It is concluded that chickens were unsuitable for studying interaction of STEC with the intestine and that there was no difference in the interaction of the ligated calf intestine with STEC of serotypes associated with disease in calves compared with O157:H7 STEC.
Introduction
Shiga toxin-producing Escherichia coli (STEC) are a heterogeneous group of E. coli that are associated with diarrhea, hemorrhagic colitis (HC), and hemolytic uremic syndrome (HUS) in humans (1,2); edema disease in pigs (3); and diarrhea and dysentery in calves (4,5,6). Those STEC that have been associated with HC and HUS in humans are called enterohemorrhagic E. coli (EHEC) (7). Shiga toxin-producing E. coli are found in the intestine of healthy cattle, which are a major source of infection for humans (8,9). Serotype O157:H7, which is the serotype most frequently implicated in human disease, is not associated with disease in calves. A few serotypes of STEC (O5:H-, O26:H11, O26:H-, O111:H-, and O113:H21) cause disease in humans and calves (1,4,5,6,10).
Known STEC virulence attributes are associated with toxin production or intestinal colonization. Since the Shiga toxins (Stx) produced by STEC isolated from cattle are the same as those produced by STEC isolated from humans, it is likely that differences in colonization-related properties may account for differences in the virulence of STEC for human and bovine hosts. Adherence of STEC to epithelial cells in vitro has been studied with tissue culture cells (11,12,13,14) or isolated intestinal epithelial cells (15). The attaching and effacing (AE) lesion induced by eae-positive STEC has been demonstrated in gnotobiotic pigs, rabbits, mice, chickens, and calves (16); however, animal infections have not been used to attempt to distinguish between virulence of STEC for human and animal hosts. At present, there is no system for determining whether an STEC isolate is a potential pathogen for humans.
Attaching and effacing lesions were induced in the ceca of chickens by O157:H7 and a few other serotypes of STEC (17,18,19), and chickens have been suggested to be a useful model for studying the interaction of STEC with the intestine. The calf is also a potentially valuable animal for studies of interaction of the intestine with STEC of bovine origin, as it permits evaluation of pathogens in their natural host and a direct comparison of the behavior of STEC that differ in virulence in calves and humans. The ligated-loop method reduces the number of animals that are needed for such studies.
The purpose of this study was to compare the pathological effects of STEC that vary in their association with bovine and human disease. Two animal models were used, namely the orally inoculated chicken and ligated intestinal loops in calves. The selection of STEC strains included a toxin-positive and toxin-negative isogenic pair of O157:H7 E. coli and an eae-negative isolate. This is the first report of its kind in which such studies have been conducted in ligated ileal and colonic loops in calves.
Materials and methods
Bacterial strains
Eight STEC strains belonging to 5 serotypes were used (Table I). The bacteria were grown overnight in Eagle's minimum essential medium (EMEM) with 5% fetal bovine serum (FBS) at 37°C in 5% CO2, centrifuged at 12 000 × g for 10 min, then resuspended in fresh medium to approximately 3 × 1010 colony-forming units per milliliter (cfu/mL) of EMEM. A strain of E. coli K-12 grown under similar conditions and uninoculated EMEM were used as negative controls in each calf.
Table I.
Animals
One 2-day-old and three 6-day-old colostrum-fed calves were used. For 24 h prior to surgery the calves were fed only electrolytes in warm water (Calf-Lyte II; Austin Canada, Joliette, Quebec). Under halothane-induced anesthesia, a paramedian incision was made, starting from the umbilicus and extending 15 cm posteriorly. Ligated loops were created in the ileum, the colon, and the cecum. A total of 10 to 12 ileal loops, 1 cecal loop, and 5 or 6 colon loops were made in each of the three 6-day-old calves. No colon loops were made in the 2-day-old calf because of technical difficulties in handling the colon in that calf. Each loop was inoculated with 1 mL of bacteria or 1 mL of EMEM. The calves were inoculated intramuscularly (IM) with 50 mg of flunixin (Banamine; Schering-Plough, Pointe Claire, Quebec) immediately after surgery and with 25 mg at 4-hour intervals thereafter. The calves were euthanized 8 to 10 h after inoculation of the loops, and samples of intestine were immediately removed from each loop for histopathologic examination and electron microscopy (EM). The infection studies followed the guidelines of the Guide to the Care and Use of Experimental Animals of the Canadian Council on Animal Care and were approved by the Animal Care Committee of the University of Guelph.
Histologic studies
Tissues were formalinized, embedded in paraffin, and sectioned at 5 μm; triplicate consecutive sections were stained with Giemsa, hematoxylin and eosin, and immunoperoxidase stains.
Immunoperoxidase staining
The identity of the isolates in ligated loops was determined by labeling the bacteria with anti-O antibody, using an indirect immunoperoxidase technique (Histostain-SP kit; Zymed Laboratories, San Francisco, California, USA). The paraffin-embedded tissue sections were collected on superfrost slides without adhesives and dried overnight at 37°C. The paraffin was melted at 60°C, and then the sections were treated with methanol. In order to inhibit endogenous peroxidase, the sections were exposed to freshly prepared 3% hydrogen peroxide in methanol for 10 min. The sections were washed with tap water and then with phosphate-buffered saline (PBS; pH 7.4). Non-specific background was eliminated by treating the samples with non-immune serum supplied with the kit. After the samples were washed twice with PBS, a 1:200 dilution of specific rabbit anti-E. coli O serum was added and allowed to remain in contact with the sections for 15 min. The sections were then washed twice for 10 min with PBS. A 50-μL volume of biotinylated goat anti-rabbit IgG antiserum was applied for 10 min, and then the sections were washed 3 times with PBS. The chromogenic substrate (supplied with the kit) was applied for 5 min, then the tissues were washed with water; counterstained with hematoxylin; washed sequentially with water, PBS, then water; dried and mounted with Glycergel (Dako Corporation, Mississauga, Ontario); and examined under the microscope.
Electron microscopy
As soon as possible after euthanasia, an approximately 4-mm-long section from each loop of calf intestine was removed and washed gently in cold PBS, then transferred to sodium cacodylate-HCl-buffered glutaraldehyde. The samples were then transferred to 2 mL of 0.1 M sodium cacodylate and held at 4°C until time for further processing. The buffer was removed from the samples, which were then stained with 10 volumes of 2% osmium tetroxide (J.B. EM Services, Dorval, Quebec) in 0.1 M cacodylate buffer (pH 7.4) for 1 h at room temperature. The tissues were washed with MQ (Milli-Q; Millipore Corp., Bedford, Massachusetts, USA) water for 15 min and then dehydrated with increasing concentrations of ethanol (50, 60, 70, 80, 90, and 100%), for 30 min at each concentration. After the last ethanol step, 100% ethanol was mixed 1:1 with an intermediate solvent, propylene oxide (VWR Canlab, Mississauga, Ontario), and added to tissue samples at room temperature for 30 min, followed by 30 min with 100% propylene oxide. A 30% concentration of complete epon in propylene oxide was added for 30 min, followed by 60% epon for 30 min. Then 100% epon was added to the samples and they were held at 4°C for 4 h. The polymerization catalyst [100% EPON + DMP30 (J.B. EM services) (0.14 g/10 mL)] was then added to the samples, which were held overnight at 4°C. The next day both the epon and the catalyst were removed, and the samples were placed in embedding molds with fresh epon and catalyst and then in an oven, at 40°C overnight and then at 60°C for a further 24 h. The hardened blocks containing the samples were sectioned with a microtome (Leica Canada, Montreal, Quebec) and stained with 5% uranyl acetate and 0.4% lead citrate (J.B. EM Services). The samples were examined in a 100S transmission electron microscope (JEOL, Peabody, Massachusetts, USA).
For the colon loop in calf D that had been injected with strain 113, it was necessary to use tissue previously embedded in paraffin to identify zones of adherent bacteria by EM. Areas of tissue with adherent bacteria were transferred to xylol overnight to remove the paraffin. The sample was then rehydrated in buffer and processed for EM as described above.
Assessment of mucosal damage and bacterial adhesion
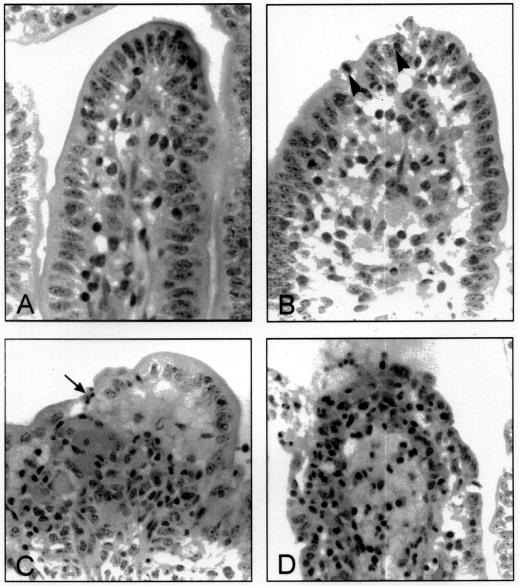
If the appearance of the intestine was similar to that in areas in which EMEM alone had been injected the histopathologic reaction was given a score of 0. Typically, the villi were slender, had an intact epithelium with regularly shaped cells, and had a low concentration of cells with few or no polymorphonuclear leukocytes in the lamina propria. Sections with irregularity in villus shape, degeneration of the epithelial cells at the tips of a low percentage of villi, and a moderate increase in neutrophils and other cells in the lamina propria were scored as mild, or grade 1. Sections demonstrating short, blunt villi with irregularly shaped epithelial cells exfoliating singly or in clumps from a moderate number of villi, as well as large numbers of neutrophils in the lamina propria that sometimes extruded through the villus tips, were assigned a rating of moderate, or grade 2. Sections demonstrating areas of villus atrophy and pseudomembrane formation, areas with loss of villus-tip epithelium and an inflammatory exudate in the lamina propria continuous with the material in the lumen, and/or some areas in which severely damaged villi showed highly vacuolated epithelial cells at their tips, with large numbers of attached bacteria, were assigned a score of severe, or grade 3. If the changes identified for a grade-3 reaction were found in most areas of the intestine, the response was scored as grade 4.
For each ligated segment, at least 2 sections stained with hematoxylin and eosin, 2 stained with Giemsa, and 2 stained by immunohistochemistry were examined. A long, continuous stretch of 50 bacteria was considered equivalent to 1 cluster. If no areas of bacterial adherence were detected, the adherence score was 0. If fewer than 5 areas of adherence per section were identified on average, the score was 1. If the average number of bacterial clusters per section was between 6 and 60, the score was 2. If the average number of clusters per section was greater than 60, the score was 3.
Results
The types of histologic reactions observed are illustrated in Figures 1 and 2. Typically, enterocytes with adherent bacteria showed pyknotic nuclei, and there was much exudate, consisting of bacteria, neutrophils, fibrin, and sloughed epithelial cells in the ileal lumen. In the colon, lesions were generally mild, and the affected epithelium was characterized by an absence of goblet cells (Figure 2).
Figure 1. Changes in villi in the ileum of calves in response to Shiga toxin-producing Escherichia coli (STEC). A. Normal villus. The villus is long and slender with intact epithelium, regularly shaped epithelial cells with prominent brush border, and a low concentration of mononuclear leukocytes in the lamina propria. B. Mild reaction (grade 1). Villi are slightly blunted, there are degenerating epithelial cells at the tip, and there is increased cellularity in the lamina propria, with neutrophils migrating into the epithelium (arrowheads). C. Moderate reaction (grade 2). Villi are short and blunt. There is shallow ulceration (arrow), and large numbers of neutrophils are present in the lamina propria and appear to be streaming into the lumen. D. Severe reaction (grade 3). The upper third of the villus is covered with degenerate epithelial cells or is ulcerated, there is massive infiltration of the lamina propria with polymorphonuclear leukocytes, and edema distends the lamina propria.
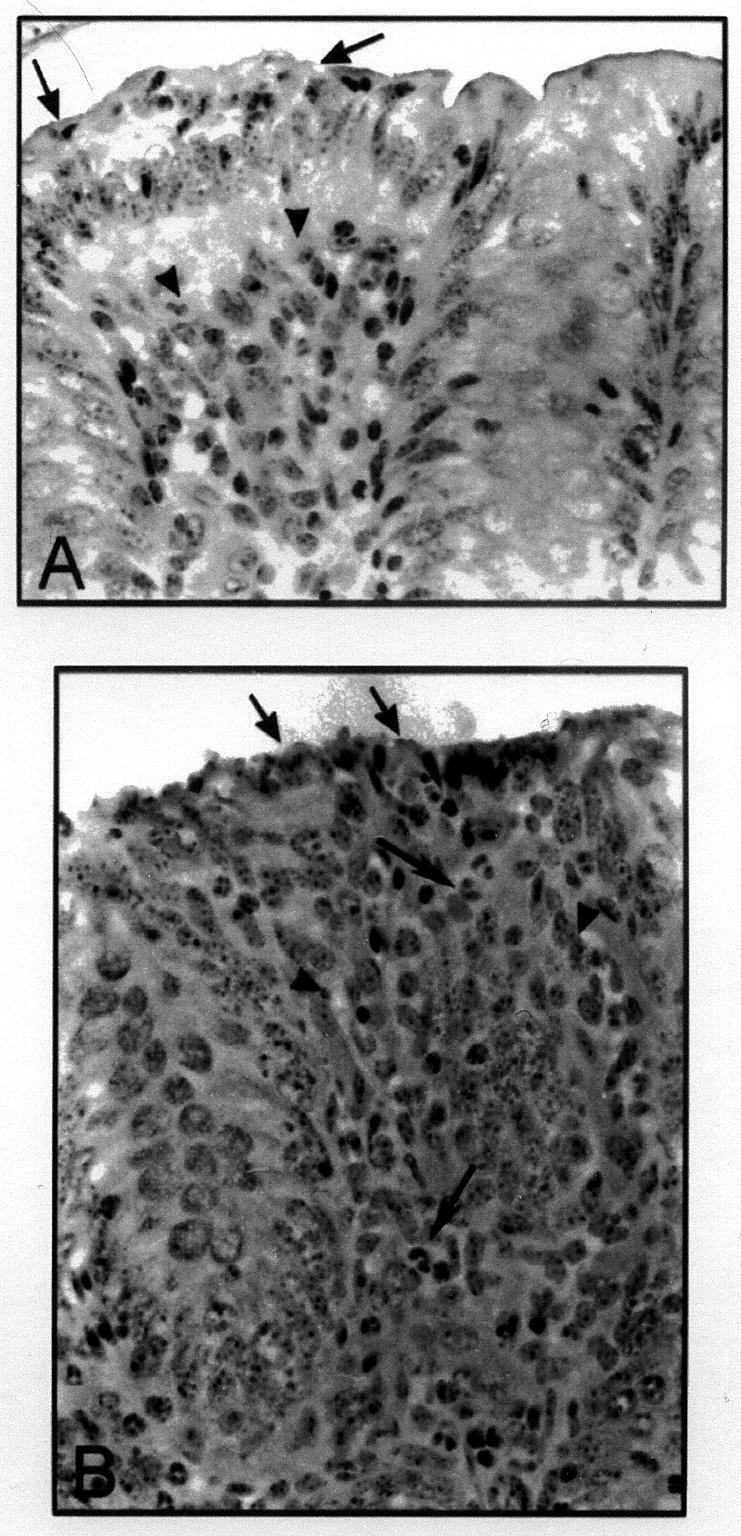
Figure 2. Changes in the appearance of the colon of calves in response to STEC. A. Mild reaction (grade 1). A mild reaction is shown at the left (arrows), adjacent to a normal area of the colon. The area to the left shows marked irregularity of the epithelium, with intraepithelial neutrophils (arrows) and increased cellularity of the lamina propria (arrowheads). B. Moderate reaction (grade 2). There is necrosis of the epithelium (black arrow), increased cellularity of the lamina propria (arrowheads) and a strong neutrophilic reaction (white arrows).
There was considerable variation from calf to calf with respect to bacterial adherence and tissue reaction, even within the same bacterial strain (Table II). Lesions were highly focal in all areas and in all animals, except for an ileal loop from calf B that was inoculated with strain 26a, in which lesions were widespread. In each field examined under 100X magnification, distinctions could be made between unaffected areas of intestine and those in which there was a tissue response (Figure 2A). Examination under 400X magnification often identified adherent bacteria in association with the lesions. The identity of adherent organisms was confirmed by the immunoperoxidase technique (Figure 3) in all cases, except for strain 113, with which the O antiserum did not react in either the immunohistochemical studies or the in vitro slide agglutination tests. The reactions to the O157:H7 isolate in the ileum were always mild (Table II).
Table II.
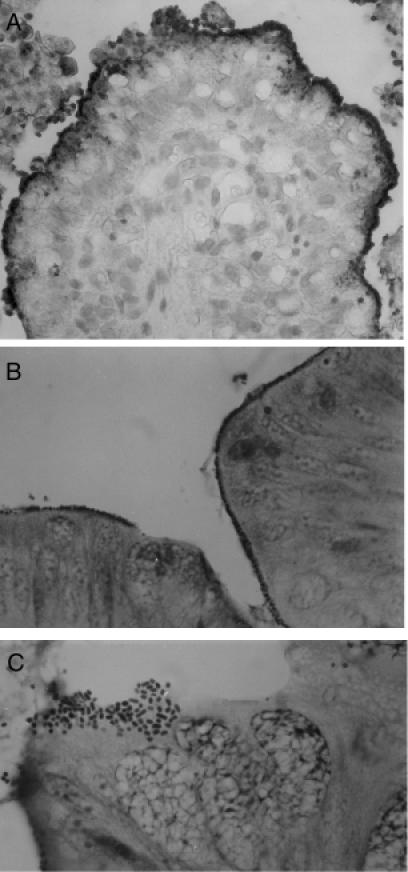
Figure 3. Adherence of STEC to ileal and colonic epithelium. A. Immunoperoxidase staining of STEC serotype O26:H11 adherent to villi and in the lumen in the ileum of a calf (40X). B. Bacteria evident as rows of dark-staining organisms at the interphase between epithelium and lumen (Giemsa, 63X). C. A cluster of bacteria adhering in a pattern similar to that seen in localized adherence to HEp-2 cells in vitro (Giemsa, 100X).
In one of the 6-day-old calves (C) there was 15 mL to 20 mL of thick, bloody fluid and grossly evident pseudomembrane formation in ileal loops inoculated with strains 5b, 26a, 26b, 111a, and 111b (Table II). In all calves, there was a mucopurulent exudate in the ileum, and in the colon the lumen content was primarily mucus.
Only one strain (5b) was inoculated in a loop at the tip of the cecum in each calf. This isolate caused focal erosion and pseudovillus formation. There was increased cellularity in the lamina propria, microerosion with increased loss of epithelial cells, and pyknotic eosinophilia.
The control ileal segments inoculated with the E. coli K-12 strain or EMEM showed long villi, with an intact epithelium consisting of regularly shaped cells. There was no polymorphonuclear leukocyte reaction in the lamina propria, except in the case of calf C; in this calf, congestion of the blood vessels in the lamina propria was evident in the control loops.
Focal bacterial adherence was observed for all STEC isolates that were tested. Adherent bacteria were much more readily observed with Giemsa stain (Figure 3B, 3C) than with hematoxylin and eosin stain, and areas of adherence were clearly identified in the immunohistochemical reactions (Figure 3A). Adherence to colonic epithelium was the most extensive with strain 157c. No bacterial adherence was detected in the control ileal loops inoculated with the E. coli K-12 strain or EMEM.
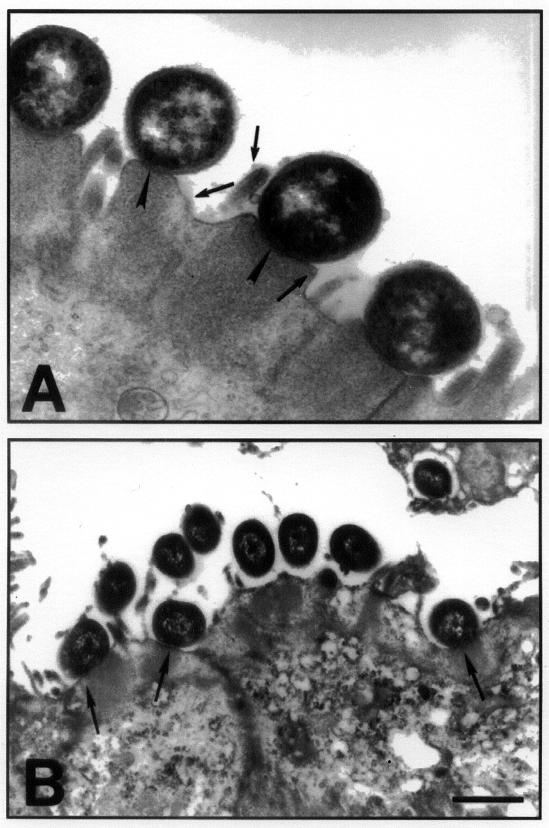
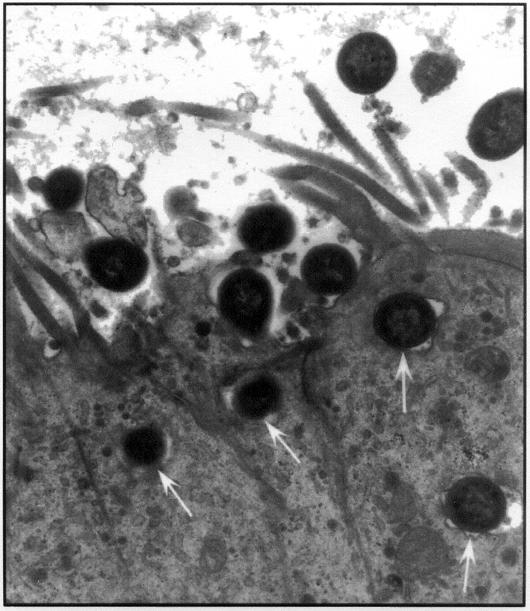
Under transmission EM, eae-positive strains were closely apposed to the epithelial cell surface in the ileum and in the colon, with effacement of microvilli and condensation of fibrillar material just below the organisms (Figure 4A). With the eaeA-negative O113:H21 strain, the bacteria were typically surrounded by an electron-lucent zone and were seen at some distance from the microvilli with no close attachment. This organism appears to be heavily capsulated both in vitro and in vivo; however, in one calf, bacteria that showed close attachment with typical AE lesions were evident in a loop inoculated with the O113:H21 organism (Figure 4B). Occasionally the bacteria were seen on cytoplasmic pedestals and there was superficial invasion (Figure 5) with each of the isolates tested.
Figure 4. Transmission electron micrograph of an eae-positive and eae-negative STEC associating with the colonic epithelium. A. The figure shows the classical AE lesion in an area of the colon infected with STEC O5:NM. Note the pedestal formation and the close association of bacteria with the colonic epithelial cells (arrowheads) and the effacement of microvilli (arrows in the lumen). B. Transmission electron micrograph of colonic mucosa infected with an eae-negative STEC of serotype O113:H21 shows that some bacteria are surrounded by an electronlucent zone and are at some distance from the epithelium. However, other bacteria (arrows) appear to be associated with typical AE lesions.
Figure 5. Invasion of the ileal epithelium by an O5:NM verotoxin-producing E. coli. Arrows point to an invading organism.
Discussion
Prior to the study in calves, we used 40-day-old broiler chickens and 40-day-old layer chickens to investigate adherence of these STEC strains to the ceca (17,18). However, we found that the orally inoculated day-old chicken was unsuitable for studying the interaction of STEC with the intestine, as no AE lesions were observed in the ceca of any of the chickens (data not shown). Earlier reports indicated that day-old chickens experimentally infected with one strain of O157:H7 STEC showed prolonged colonization of the ceca with typical AE lesions and penetration of epithelial cells (17,18). In 10-day-old chickens infected with STEC of serogroups O5 and O26 (calf origin), O103 (chicken origin), O111 and O157:H7 (human origin), and O15 (pig origin), AE lesions and/or adherence in the ceca were consistently associated only with those isolates of chicken origin (19). The findings in the present study are consistent with these findings.
One of the objectives of the present study was to compare adherence of an STEC serotype that is a human pathogen (O157:H7) with that of serotypes that are pathogens of both calves and humans (O5, O26, O111, O113); however, there was no discernible difference in the ability of the various strains to adhere to the calf intestine (Table II). The ligated calf intestine was useful for detection of those isolates that adhered; however, it may be unsuitable for determining degrees of colonization, since the bacteria are not subject to being washed away, as they are in the unligated intestine. Furthermore, the highly focal nature of the lesions might result in sampling errors.
The accumulation of fluid in the ileal loops inoculated with strains 5b, 26a , 26b, 111a, and 111b in calf C suggests that certain undefined conditions (possibly viral, bacterial, or parasitic infection) could predispose the calf to diarrhea in response to STEC serotypes O5:NM, O26:H11, and O111:H-. Since bloody diarrhea has been reproduced in gnotobiotic calves in response to STEC serotype O5:H- (20,21,22), it is clear that these organisms can induce disease without the need for other factors that damage the intestine. However, there are cases in which a combination of agents might induce disease in animals that are resistant to either agent alone, such as with enterotoxigenic E. coli and rotavirus (23,24). The absence of fluid accumulation in ligated ileal loops inoculated with strains O157:H7 and O113:H21 might mean that although these isolates adhered to the calf intestine they lacked the capacity to induce fluid production in the calf intestine. The O5 isolate from a diseased calf induced fluid production, whereas the isolate from a healthy calf did not, but this observation was limited to 2 isolates in a single calf.
There was a clear demarcation between the areas with adherent bacteria, which showed histopathologic changes, and areas that were free of bacteria, which did not show the changes. In colon samples stained with hematoxylin and eosin, the absence of goblet cells facilitated the recognition of affected areas. Other studies have inoculated calves with different serogroups of STEC, including O5:H- (4,21,22), O26:H11 (5), and O111:H- (25); these strains caused AE lesions, diarrhea, and dysentery, the lesions being seen mainly in the colon and less commonly in the ileum. Colonization may be related to factors such as distribution of receptors in the intestine, the age of the calf, and other unidentified predisposing factors that promote adherence and/or growth in the intestine.
The observed histologic lesions were similar to those described by researchers who infected gnotobiotic calves (20,21,22) or conventional calves (5,25) or examined naturally infected calves (6,21). The only difference was that invasion of epithelial cells was evident for all serotypes used in the present study but was not reported for bovine STEC in any of the other studies in calves. The marked polymorphonuclear leukocyte response seen in the ileum may be significant, as these cells have been implicated in toxin transport from the intestine, transfer to endothelial cells, and damage to tissue (26,27,28). However, unlike humans and pigs, cattle appear to lack the vascular receptors for Stx and, therefore, do not show a systemic response (29).
The immune status of the calf could influence both fluid accumulation and adherence of bacteria in the intestine. However, differences in fluid response and in adherence with strains of the same serotype that produced the same Stx suggest that antibodies against the major surface polysaccharides or antitoxic antibodies were not responsible. Host receptors for Stx could play a role in the intestinal response to toxin, as has been shown for rabbit enterocytes (30). Nothing is known of the presence or absence of receptors for Stx on the enterocytes of the ileum or colon of calves, but there would need to be marked variation in the distribution of these receptors in adjacent loops to account for the differences.
The mucus barrier can prevent bacteria from coming into contact with epithelial cells. Any change in the intestine that caused a breach in this barrier would likely promote bacterial contact with intestinal epithelial cells and hence colonization. It is noteworthy that mucus discharge was a common reaction to the presence of STEC in the colon. This reaction could be effective in both trapping bacteria in the lumen and protecting the epithelium. It would be interesting to assess whether a mucinolytic agent would promote greater adherence.
Serotype O157:H7 STEC adhered to both the colon and ileum. Dean-Nystrom and colleagues (31,32) demonstrated that O157:H7 STEC induced AE lesions and diarrhea in calves less than 36 hours old and AE lesions in weaned calves. Thus, it is likely that O157:H7 organisms attach to the intestine of calves following natural exposure. Their failure to cause disease in cattle may be due to a limited capacity for adherence and/or a lack of other properties that may be necessary to elicit a dysenteric response. This organism does not appear to cause disease in cattle, although it is consistently positive for all the potential virulence attributes of enterohemorrhagic E. coli (15,33). This suggests that some unidentified virulence factors required for disease in animals may be missing in this serotype or that regulation of virulence genes may be different in human and bovine hosts.
There was no consistent difference in adherence or tissue reaction between isolates of the same serotype from diseased and healthy cattle (Table II). One exception was the difference in fluid response to the 2 isolates of O5:H- in calf C. These observations suggest that it may be necessary to test several isolates from healthy and diseased animals, as they represent overlapping populations.
The adherence and lesions observed could not be related to the type of Stx produced by the isolates. In fact, the results seen with the O157:H7 isolate in calf D indicate that adhesion in the ligated calf colon model is not dependent on toxin production. Sjogren and colleagues (34) investigated the role of Stx by infecting rabbits with the enteropathogenic E. coli strain RDEC-I and its isogenic variant, which produced high levels of Stx1, and found that there were more severe histopathologic reactions, vascular changes, and edema in rabbits infected with the variant producing Stx1. It was concluded that Stx1 was an important virulence factor that contributed significantly towards the severity of the infection. The tests in the present system were of short duration, and it is possible that toxin production at later stages of infection might influence the kinds of lesions that are produced in the intestine.
The calf colonic loops injected with eae-negative serotype O113:H21 organisms showed non-intimate adherence, but AE lesions were also seen in some areas. The non-intimate adherence was similar to that described by Dytoc and colleagues (35), who reported that serotype O113:H21 in a rabbit model of infection caused effacement of microvilli without intimate attachment of the bacteria to the intestinal wall. It is possible that those bacteria observed to form AE lesions were eae-positive bacteria from the normal flora.
Shiga toxin-producing E. coli are found as normal intestinal flora in healthy cattle and cause dysentery only rarely (4,6,25). It is therefore likely that differences among the various serotypes may not be identifiable in the intestine of normal calves. It may be necessary to study the development of dysentery under natural conditions.
In summary, this study demonstrated that day-old chickens were not suitable for studying adherence and tissue response to verotoxin-producing E. coli. This finding is important because of the early success in chickens with one strain of O157:H7 (17) and the confirmation with the same strain (18). The two O157:H7 isolates that were investigated both adhered to the intestinal epithelium to a degree indistinguishable from that of STEC implicated in calf disease; however, the tissue response to the strain that was tested extensively was milder than that to isolates of other serotypes. Shiga toxin- producing E. coli adherence in the colon was characterized by an absence of goblet cells and a mild histologic reaction. All 5 STEC serotypes that were tested adhered to the intestinal epithelium in the ileum and colon. In one calf, in which control ileal loops showed congestion, 5 of the 8 STEC isolates tested induced a fluid reaction in the ileum. Non-intimate adherence was observed in a colon loop infected with an eae-negative O113:H21 isolate.
Footnotes
Acknowledgments
The authors acknowledge with gratitude the financial support of the Natural Sciences and Engineering Research Council of Canada and the Ontario Ministry of Agriculture, Foods, and Rural Affairs. K. Sandhu was the recipient of a Commonwealth Scholarship.
Address correspondence and reprint requests to Dr. C. L. Gyles, tel: 519-824-4120 ext. 4715, fax: 519-767-0809, e-mail: cgyles@ovc.uoguelph.ca
Received June 1, 2001. Accepted December 7, 2001.
References
- 1.Karmali MA. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev 1989;2:15–38. [DOI] [PMC free article] [PubMed]
- 2.Nataro J, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev 1998;11:142–201. [DOI] [PMC free article] [PubMed]
- 3.Gannon VP, Gyles CL. Characteristics of the Shiga-like toxin produced by Escherichia coli associated with porcine edema disease. Vet Microbiol 1990;24:89–100. [DOI] [PubMed]
- 4.Chanter N, Hall GA, Bland AP, Hayle AJ, Parsons KR. Dysentery in calves caused by an atypical strain of Escherichia coli (S102-9). Vet Microbiol 1986;12:241–253. [DOI] [PMC free article] [PubMed]
- 5.Wray C, McLaren I, Pearson GR. Occurrence of ‘attaching and effacing’ lesions in the small intestine of calves experimentally infected with bovine isolates of verocytotoxic E. coli. Vet Rec 1989;125:365–368. [DOI] [PubMed]
- 6.Janke BH, Francis DH, Collins JE, et al. Attaching and effacing Escherichia coli infection as a cause of diarrhea in young calves. J Am Vet Med Assoc 1990;196:897–901. [PubMed]
- 7.Levine MM. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis 1987;155:377–389. [DOI] [PubMed]
- 8.Griffin PM, Tauxe RV. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev 1991;13: 60–98. [DOI] [PubMed]
- 9.Chapman PA. Sources of Escherichia coli O157 and experiences over the past 15 years in Sheffield, UK. J Appl Microbiol 2000;88 Suppl:51S–60S. [DOI] [PubMed]
- 10.Karmali M, Steele BT, Petric M, Lim C. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet 1983; i:619–620. [DOI] [PubMed]
- 11.Hall GA, Dorn CR, Chanter N, Scotland SM, Smith HR, Rowe B. Attaching and effacing lesions in vivo and adhesion to tissue culture cells of vero-cytotoxin-producing Escherichia coli belonging to serogroups O5 and O103. J Gen Microbiol 1990;136:779–786. [DOI] [PubMed]
- 12.Scotland SM, Willshaw GA, Smith HR, Rowe B. Properties of strains of Escherichia coli O26:H11 in relation to their enteropathogenic or enterohemorrhagic classification. J Infect Dis 1990;162: 1069–1074. [DOI] [PubMed]
- 13.Beutin L, Geier D, Zimmermann S, Karch H. Virulence markers of Shiga-like toxin-producing Escherichia coli strains originating from healthy domestic animals of different species. J Clin Microbiol 1995;33:631–635. [DOI] [PMC free article] [PubMed]
- 14.Ismaili A, Philpott DJ, Dytoc MT, Sherman PM. Signal transduction responses following adhesion of verocytotoxin- producing Escherichia coli. Infect Immun 1995;63:3316–3326. [DOI] [PMC free article] [PubMed]
- 15.Sandhu KS, Clarke RC, Gyles CL. Virulence markers in Shiga toxin-producing Escherichia coli isolated from cattle. Can J Vet Res 1999;63:177–184. [PMC free article] [PubMed]
- 16.Moxley RA, Francis DH. Overview of animal models. In: Kaper JB, O'Brien AD, eds. Escherichia coli O157:H7 and Other Shiga Toxin-Producing E. coli Strains. Washington, DC: Am Soc Microbiol, 1998:249–260.
- 17.Beery JT, Doyle MP, Schoeni JL. Colonization of chicken ceca by Escherichia coli associated with hemorrhagic colitis. Appl Environ Microbiol 1985;49:310–315. [DOI] [PMC free article] [PubMed]
- 18.Stavric S, Buchanan B, Gleeson TM. Intestinal colonization of young chicks with Escherichia coli O157:H7 and other verotoxin-producing serotypes. J Appl Bacteriol 1993;74:557–563. [PubMed]
- 19.Sueyoshi M, Nakazawa M. Experimental infection of young chicks with attaching and effacing Escherichia coli. Infect Immun 1994;62:4066–4071. [DOI] [PMC free article] [PubMed]
- 20.Chanter N, Morgan JH, Bridger JC, Hall GA, Reynolds DJ. Dysentery in gnotobiotic calves caused by atypical Escherichia coli. Vet Rec 1984;114:71. [DOI] [PubMed]
- 21.Hall GA, Reynolds DJ, Chanter N, et al. Dysentery caused by Escherichia coli (S102-9) in calves: natural and experimental disease. Vet Pathol 1985;22:156–163. [DOI] [PubMed]
- 22.Moxley RA, Francis DH. Natural and experimental infection with an attaching and effacing strain of Escherichia coli in calves. Infect Immun 1986;53:339–346. [DOI] [PMC free article] [PubMed]
- 23.Snodgrass DR, Smith ML, Krautol FL. Interactions of rotavirus and enterotoxigenic Escherichia coli in conventionally-reared dairy calves. Vet Microbiol 1982;7:51–60. [DOI] [PMC free article] [PubMed]
- 24.Tzipori S, Smith M, Halpin C, Makin T, Krautol F. Intestinal changes associated with rotavirus and enterotoxigenic Escherichia coli. Vet Microbiol 1983;8:35–43. [DOI] [PMC free article] [PubMed]
- 25.Schoonderwoerd M, Clarke RC, van Dreumel AA, Rawluk SA. Colitis in calves: natural and experimental infection with a verotoxin-producing strain of Escherichia coli O111:NM. Can J Vet Res 1988;52:484–487. [PMC free article] [PubMed]
- 26.Liu J, Akahoshi T, Sasahana T, et al. Inhibition of neutrophil apoptosis by verotoxin 2 derived from Escherichia coli O157:H7. Infect Immun 1999;67:6203–6205. [DOI] [PMC free article] [PubMed]
- 27.Te Loo DM, Monnens LA, van Der Velden TJ, et al. Binding and transfer of verocytotoxin by polymorphonuclear leukocytes in hemolytic uremic syndrome. Blood 2000;95:3396–3402. [PubMed]
- 28.Wagner PL, Acheson DW, Waldor MK. Human neutrophils and their products induce Shiga toxin production by enterohemorrhagic Escherichia coli. Infect Immun 2001;69:1934–1937. [DOI] [PMC free article] [PubMed]
- 29.Pruimboom-Brees IM, Morgan TW, Ackermann MR, et al. Cattle lack vascular receptors for Escherichia coli O157:H7 Shiga toxins. Proc Natl Acad Sci USA 2000;97:10325–10329. [DOI] [PMC free article] [PubMed]
- 30.Mobassaleh M, Donohue-Rolfe A, Jacewicz M, Grand RJ, Keusch GT. Pathogenesis of Shigella diarrhea: evidence for a developmentally regulated glycolipid receptor for Shigella toxin involved in the fluid secretory response of rabbit small intestine. J Infect Dis 1988;157:1023–1031. [DOI] [PubMed]
- 31.Dean-Nystrom EA, Bosworth BT, Cray WC Jr, Moon HW. Pathogenicity of Escherichia coli O157:H7 in the intestines of neonatal calves. Infect Immun 1997;65:1842–1848. [DOI] [PMC free article] [PubMed]
- 32.Dean-Nystrom EA, Bosworth BT, Moon HW. Pathogenesis of Escherichia coli O157:H7 in weaned calves. Adv Exp Med Biol 1999;473:173–177. [DOI] [PubMed]
- 33.Gyles C, Johnson R, Gao A, Ziebell K, Pierard D, Aleksic S, Boerlin P. Association of enterohemorrhagic Escherichia coli hemolysin with serotypes of Shiga-like-toxin-producing Escherichia coli of human and bovine origins. Appl Environ Microbiol 1998;64:4134–4141. [DOI] [PMC free article] [PubMed]
- 34.Sjogren RW, Neill R, Rachmilewitz D, et al. Role of Shiga-like toxin 1 in bacterial enteritis: comparison between isogenic Escherichia coli strains induced in rabbits. Gastroenterology 1994;106:306–317. [DOI] [PubMed]
- 35.Dytoc MT, Ismaili A, Philpott DJ, Soni R, Brunton JL, Sherman PM. Distinct binding properties of eaeA-negative verocytotoxin-producing Escherichia coli of serotype O113:H21. Infect Immun 1994;62:3494–3505. [DOI] [PMC free article] [PubMed]