Abstract
We used large conductance Ca2+-activated K+ (BKCa) channel activity as a probe to characterize the inhibitory/stimulatory G protein (Gi/Gs) signalling pathways in intact cells from pregnant (PM) and non-pregnant (NPM) myometrium.
Isoprenaline (10 μM) enhanced the outward current (Iout) in PM cells and inhibited Iout in NPM cells. Additional application of the α2-adrenoceptor (α2-AR) agonist clonidine (10 μM) further enhanced the isoprenaline-modulated Iout in PM cells but partially antagonized Iout in NPM cells. Clonidine alone did not affect Iout. The specific cAMP kinase (PKA) inhibitor H-89 (1 μM) abolished the effects of isoprenaline and clonidine. The specific BKCa channel blocker iberiotoxin (0·1 μM) inhibited Iout by ≈80 %; the residual current was insensitive to isoprenaline.
Inhibition of Gi activity by either pertussis toxin or the GTPase activating protein RGS16 abolished inhibitory as well as stimulatory effects of clonidine on Iout.
Transducin-α, a scavenger of Gi βγ dimers, converted the stimulatory action of clonidine on Iout into an inhibitory effect. Free transducin-βγ enhanced both the stimulatory and the inhibitory effects of isoprenaline on Iout.
The results demonstrate that BKCa channel activity is a sensitive probe to follow adenylyl cyclase–cAMP–PKA signalling in myometrial smooth muscle cells. Both Giα-mediated inhibition and Giβγ-mediated stimulation can occur in the same cell, irrespective of pregnancy. It is speculated that the coupling between α2-AR and Gi proteins is more efficient during pregnancy and that Giβγ at high levels simply override the inhibitory action of Gi α.
It is well established that catecholamines influence the contractile state of the myometrium in response to the hormonal status (Marshall, 1981). Four adrenoceptors (α1-, α2-, β1- and β2-ARs) have been shown to be present in the pregnant and non-pregnant myometrium (Bottari et al. 1985). The β-ARs couple via stimulatory G proteins (Gs) to myometrial adenylyl cyclases (ACs), promote the conversion of ATP to cyclic AMP and induce, at least in the pregnant myometrium, relaxation by various mechanisms including effects on [Ca2+]i, myosin light chain kinase and large conductance Ca2+-activated potassium channels (BKCa channels) (Anwer et al. 1992; Wray, 1993). Both β1- and β2-ARs coexist in the myometrium with a higher proportion of the β2-subtype. During pregnancy, this subtype is selectively upregulated by progesterone at the level of gene transcription and converted to a high affinity state (Vivat et al. 1992). Three different ARs of the α2-type (α2A-C) have been identified in human myometrium and an alteration of the α2A/α2B-subtypes expression pattern during pregnancy has been described (Mhaouty et al. 1995; Bouet-Alard et al. 1997; Adolfsson et al. 1998). In most cell systems investigated so far, activation of α2-ARs inhibits AC via inhibitory G proteins (Gi), although α2-ARs have also been reported to mediate stimulation of AC, activation of K+ channels and of Na+-H+ exchange, and mobilization of intracellular Ca2+ (for review see Lomasney et al. 1991). The role of α2-ARs in the control of pregnancy-related changes of the AC system and uterine contractility is complex. Clonidine, a potent α2-AR agonist, potentiated the β-AR-stimulated AC activity in rat myometrium at midpregnancy by coupling to Gi proteins (Mhaouty et al. 1995). Conversely, a switch from the stimulatory to an inhibitory input to AC via the α2-AR-Gi protein signalling pathway was described at late term (Mhaouty et al. 1995). These data suggested a differential expression of AC isoforms at midpregnancy and term. Whereas AC types II and IV are co-stimulated by βγ-dimers of Gi proteins in the presence of Gsα, other cyclases are inhibited by Gi proteins (Tang & Gilman, 1991; Gao & Gilman, 1991; Federman et al. 1992). Although RNA blot analysis revealed that the expression of AC transcripts in the rat myometrium remained stable over the second half of pregnancy (Mhaouty-Kodja et al. 1997), this cannot exclude possible changes at the protein level. At the present time this issue cannot be settled as no specific antibody that allows quantification directly of each AC type is available.
AC activity in pregnant and non-pregnant myometria is usually determined by biochemical methods on plasma membranes prepared from freshly isolated tissues. In the present study, it was our purpose to investigate the AC-cAMP cascade as influenced by β- and α2-AR agonists in intact smooth muscle cells from pregnant and non-pregnant myometria. We show here by means of patch-clamp techniques, that the activity of BKCa channels reflects the status of the cellular AC-cAMP signalling pathway with a sensitivity superior to biochemical methods. Moreover, direct application of RGS proteins (regulators of G protein signalling), transducin-α (TDα) and transducin-βγ (TDβγ) to the interior of cells via the patch-pipette allowed us to address the questions of differential AC expression, and of G protein subunits responsible for co-stimulatory and inhibitory effects at midpregnancy and in the non-pregnant state. In addition, the effects of adrenaline and noradrenaline, the physiological ligands on myometrial β- and α2-ARs, were investigated.
METHODS
Materials
The catalytic subunit of cAMP kinase was prepared as described previously (Zhou et al. 1996). The cAMP-dependent protein kinase (PKA) inhibitor H-89 was obtained from Biomol (Hamburg, Germany), iberiotoxin (IbTX) from Latoxan (Rosans, France), (±)-adrenaline and (±)-isoprenaline from Sigma (Deisenhofen, Germany), (±)-noradrenaline and yohimbine from RBI (Natick, MA, USA), A23187 from Molecular Probes (Eugene, OR, USA), and pertussis toxin from List Biological Laboraories (Campbell, CA, USA). All drugs were dissolved in PSS (see below); solutions with IbTX contained 0.1 % bovine albumin fraction V (Sigma). Collagenase type H (lot 57H6832), hyaluronidase type I-S, and papain was purchased from Sigma, and 1,4-dithio-d,l-threitol (DTT) was from Gerbu Biotechnik (Gaiberg, Germany).
Purification of proteins
His6-RGS16 was expressed in the Escherichia coli strain BL21(DE3) and purified over a Ni2+-NTA column (Qiagen) as described before (Chen et al. 1996). Bleached bovine rod outer segment (ROS) membranes were prepared from bovine retinae as described previously (Papermaster & Dreyer, 1974). The bovine retinae were obtained from a local abattoir. Transducin (TD) was eluted from the membranes by hypotonic elution in the presence of 100 μM GTP, and the subunits were separated by affinity chromatography on Blue Sepharose (Bio-Rad) (Wieland et al. 1991). The buffers in which the proteins were eluted from the columns were changed to the intracellular pipette solution (see below) by repeated extensive dialysis and proteins were stored in aliquots at -80°C. The protein concentration was determined according to Bradford (1976) with IgG as standard.
Animals
All experimental procedures were carried out according to the animal welfare guidelines of the University Hospital Eppendorf. Female Wistar rats were obtained from a colony bred and maintained at the animal house of the University Hospital Eppendorf. In order to obtain myometria from pregnant animals, females were caged with males overnight, and successful mating was determined by the presence of spermatozoa in the vaginal smear (day 1 of pregnancy). Animals were killed on days 11 or 12 of gestation which corresponds to midpregnancy. Myometria were dissected from the rats following anaesthesia with halothane then cervical dislocation.
Tissue collection from pregnant and non-pregnant women
Myometrial samples were obtained from non-labouring women (37-39 weeks gestation) undergoing elective Caesarian sections. No tocolytic agents had been administered to the mother 24 h prior to Caesarian delivery. The reasons for Caesarean section included breech presentation, previous Caesarean section, and cephalo-pelvic disproportion. All biopsies were taken from the upper border of the lower isthmic uterine incision. Non-pregnant myometrium was obtained from uteri of normal cycling premenopausal patients undergoing hysterectomy for benign disease. Immediately after collection, the tissue was carried to the laboratory in ice-cold physiological saline solution (PSS (mM): 127 NaCl, 5.9 KCl, 2.4 CaCl2, 1.2 MgCl2, 11 glucose and 10 Hepes; adjusted to pH 7.4 with NaOH). Informed consent was obtained from the patients and the study had the approval of the Ethics Committee Hamburg, Germany.
Cell preparation
After the connective tissue and endometrium was removed, the uterine smooth muscle was cut into cubes of 1–2 mm side length and incubated at 37°C in Ca2+-free PSS including 0.7 mg ml−1 papain, 1 mg ml−1 DTT and 1 mg ml−1 fat-free bovine serum albumin. Thirty minutes later, the tissue pieces were transferred into PSS solution containing 50 μM Ca2+, 1 mg ml−1 collagenase, 1 mg ml−1 hyaluronidase and 1 mg ml−1 albumin, and digested for another 10–15 min at 37°C. Single cells were released by gentle trituration and stored in PSS at room temperature. After isolation, 30–40 % of the cells were relaxed, and only these cells were used for electrophysiological studies. Experiments were conducted within 6 h of cell isolation.
Recording techniques
Standard patch-clamp recording techniques were used to measure currents in the inside-out or whole-cell patch configuration (Hamill et al. 1981). Patch electrodes were fabricated from borosilicate glass capillaries (World Precision Instruments) and filled with prefiltered solutions of different composition (see below). Currents were recorded at 25°C with a List Electronics EPC-7 patch-clamp amplifier, connected via a 16 bit A/D interface to a pentium IBM clone computer. The data were filtered at 1 kHz by a 10 pole Bessel filter and sampled at 3 kHz. Data aquisition and analysis was performed with an ISO-3 multitasking patch-clamp program (MFK, Niedernhausen, Germany). The pipette resistance ranged from 2–3 MΩ in whole-cell and 8–9 MΩ for the excised-patch experiments. The amplitude of single-channel currents was derived from an amplitude distribution histogram. Average channel activity (NPo) in patches was determined from recordings using the following expression:
where Po is the open probability, T is the duration of the measurement, tj is the time spent with j = 1, 2, … N channels open, and N is the maximum number of channels seen. NPo was determined over a 3–5 min period immediately before and after the addition of a compound. The cell or patch under investigation was studied either after equilibration with the bath solution or during continuous superfusion by a gravity-flow microperfusion device consisting of multiple glass tubes assembling into a common tip with an 80 μm orifice and mounted on a hydraulic micromanipulator. Solution changes were achieved via remote-controlled solenoid valves (N. Graf, Planegg, Germany).
Solutions
For inside-out experiments, the bath solution (cytosolic surface of the patch) contained (mM): 134 KCl, 6 NaCl, 1.2 MgCl2, 5 EGTA, 11 glucose, 3 K2-ATP and 10 Hepes (pH 7.4); and the patch pipette (extracellular patch surface) was filled with (mM): 140 KCl and 10 Hepes (pH 7.4). Depending on the experiment, the free Ca2+ concentration was changed from 0 to 0.3 μM by changing the Ca2+ concentration in the corresponding solution. The appropriate amounts of CaCl2 were added, and the pH adjusted according to a computer program (Mermi et al. 1991) on the basis of the binding constants of Fabiato (Fabiato, 1988) and checked by fura-2 fluorescence.
For whole-cell patch-clamp experiments, the intracellular (pipette) solution contained (mM): 126 KCl, 6 NaCl, 1.2 MgCl2, 5 EGTA, 11 glucose, 3 K2-ATP, 0.1 Na3GTP and 10 Hepes (pH 7.4). The free Ca2+ concentration was 0.3 μM. The bath was superfused with PSS.
Statistics
Data are expressed as means ± standard error of the mean (s.e.m.). Statistical significance was determined by Student's t test for paired and unpaired data. A P value less than 0.05 was considered to be significant.
RESULTS
Modulation of BKCa channel activity by PKA
In the presence of symmetrically high potassium (140 mM) and at different membrane potentials, inside-out patches of freshly isolated smooth muscle cells from non-pregnant (NPM) and pregnant (PM) rat myometrium exhibited single channel currents with distinct amplitudes. The majority of channel openings, however, conducted currents with an amplitude 4–6 times larger than those conducted by the other channels. The current-voltage relationship of these high conductance channels was linear in the voltage range between -60 and +60 mV and showed a reversal potential close to 0 mV. The mean unitary conductances of these channels were 260 ± 10 and 267 ± 13 pS in patches excised from six NPM cells and seven PM cells, respectively. As shown in Fig. 1A and C, in the absence of Ca2+ in the bath solution no openings of BKCa channels could be detected. Addition of 0.3 μM Ca2+ resulted in the immediate appearance of channel activity with an open probability (NPo) of 0.17 and 0.32 in the NPM and PM cells, respectively. Thus, large conductance Ca2+-activated K+ channels (BKCa channels) were clearly identified in rat myometrial cells. Further superfusion of the cytosolic surface of the inside-out patch with 300 nM of the catalytic subunit of cyclic AMP kinase (PKA) had differential effects; NPo decreased to 0.08 in the NPM cell (Fig. 1A) but increased to 1.49 in the PM cell (Fig. 1C). A summary of the results with PKA is presented in Fig. 1B and D; PKA decreased NPo in NPM cells by 62 % (before, 0.18 ± 0.01; after PKA, 0.07 ± 0.005; n = 5) and increased NPo in PM cells by 426 % (before, 0.31 ± 0.03; after PKA, 1.32 ± 0.11; n = 5). The results show that (1) basal NPo is about twice as high in cells from pregnant myometrium and (2) PKA regulates NPo in opposite directions with a large increase at midpregnancy.
Figure 1. Differential regulation of BKCa channel activity by PKA.
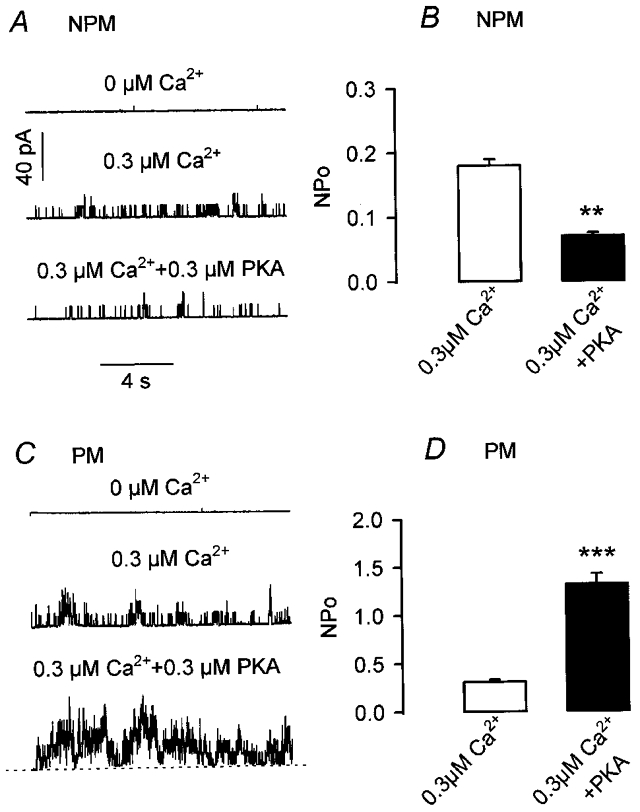
Repesentative single channel current recordings in inside-out patches from a non-pregnant (NPM; A) and a pregnant (PM; C) rat uterine myocyte. Channel openings are upward deflections. Dashed line in C indicates the closed state level. Holding potential was +40 mV and [Ca2+]i is as indicated. The catalytic subunit of PKA (300 nM) was applied to the cytosolic surface of the patch. B and D, combined results from experiments with PKA in five NPM (B) and five PM cells (D), obtained from three rats in each group; [Ca2+]i was 0.3 μM. **P < 0.01; ***P < 0.001 versus control without PKA.
Effects of isoprenaline and clonidine on Iout
Similar results to those shown in Fig. 1 were obtained when whole-cell outward currents (Iout) were recorded from relaxed myocytes treated with the β-AR agonist isoprenaline (Fig. 2). When cells were clamped from a holding potential of -60 to +80 mV in 10 mV increments for 200 ms, 70 % of all cells responded with a non-inactivating Iout, whereas 30 % showed a small inactivating early component superimposed on a much larger non-inactivating component as has been described before (Wang et al. 1998). Although Iout of both cell types was sensitive to IbTX (see later), cells with only a non-inactivating component were used in the present study. As shown by the current-voltage relations of Fig. 2, application of 10 μM isoprenaline induced a pronounced decrease of Iout in NPM cells (Fig. 2A), whereas an increase was observed in PM cells (Fig. 2B). When the effects of isoprenaline were maximal after 4 min, cells were additionally superfused with 10 μM clonidine, a selective α2-AR agonist. As expected from its inhibitory effect on AC, clonidine antagonized the isoprenaline effect by partially restoring Iout in NPM cells (Fig. 2A). In PM cells, however, clonidine did not antagonize, but further augmented the isoprenaline-enhanced current at all potentials. Clonidine alone produced no significant effect on Iout when applied in the absence of isoprenaline (not shown). Representative Iout traces obtained from a NPM and a PM cell clamped for 200 ms to +80 mV are also presented in Fig. 2. It is shown that the control current amplitude is larger in the PM cell, which corresponds with the higher NPo in inside-out patches, and currents did not inactivate during depolarizing steps. The enhanced current amplitude in PM cells was not due to myometrial smooth muscle hypertrophy because the difference between current amplitudes in NPM and PM cells persisted after normalizing currents to membrane capacitance as shown by the current-voltage relations in Fig. 2. Measurement of membrane capacitance of NPM and PM cells (n = 11 in both cases) disclosed a 2.3-fold larger cell surface of PM cells as compared with NPM cells. The stimulation and inhibition pattern of Iout in the presence of the α2- and β-agonist was observed not only in rat but also in human myometria. As shown in Fig. 3, 10 μM isoprenaline decreased Iout of human NPM cells clamped for 200 ms from -60 to +80 mV by 42 % (before, 55 ± 10 pA pF−1; after isoprenaline, 32 ± 6 pA pF−1; n = 6) and increased Iout in PM cells by 38 % (before, 78 ± 11 pA pF−1; after isoprenaline, 108 ± 15 pA pF−1; n = 5). Additional superfusion of the cells with 10 μM clonidine resulted in an increase of Iout to 89 % of the control (49 ± 8 pA pF−1) in NPM and to 200 % of the control (156 ± 19 pA pF−1) in PM cells.
Figure 2. Opposite effects of clonidine on isoprenaline-modulated Iout.
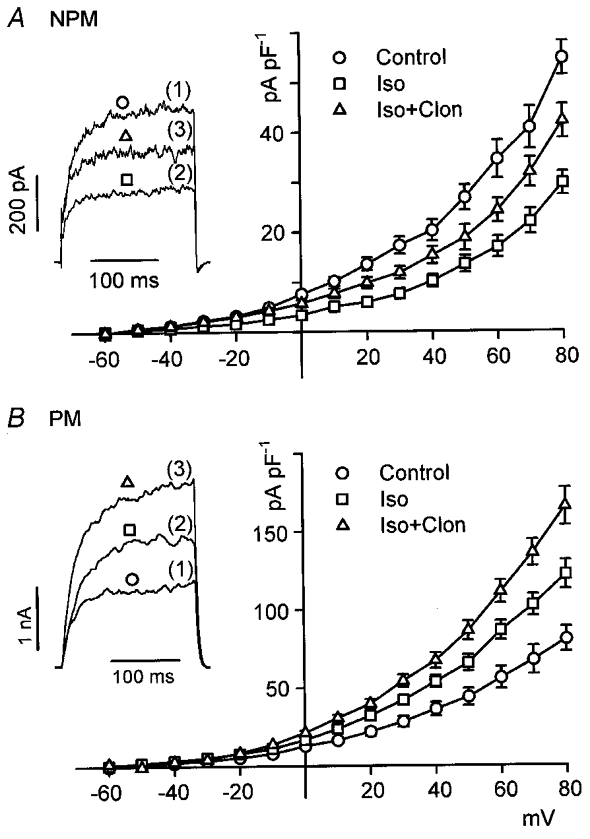
Current-voltage relations of Iout from non-pregnant (NPM; n = 6; A) and pregnant (PM; n = 6; B) rat uterine myocytes are shown. Cells were obtained from four animals in each group. Mean current densities are plotted against the respective test potential. Currents were evoked by applying a 200 ms depolarizing pulse every 10 s in 10 mV increments from a holding potential of -60 mV. Cells were superfused with 10 μM isoprenaline (Iso) first and then additionally with 10 μM clonidine (Clon). The insets show representative Iout recordings elicited by 200 ms pulses from a holding potential of -60 to +80 mV. Numbers in parentheses indicate the sequence of drug applications. The pipette solution contained 0.3 μM Ca2+.
Figure 3. Effects of isoprenaline and clonidine on human uterine myocytes.
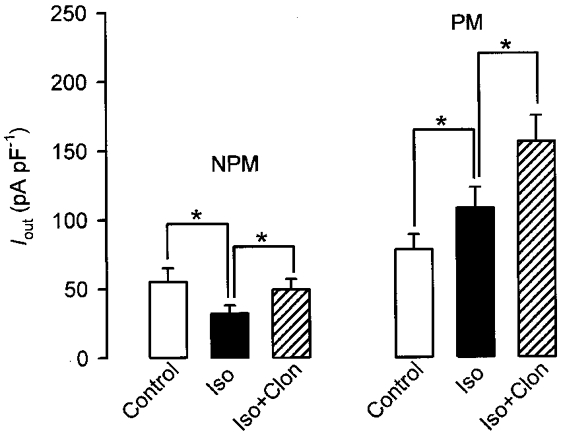
Iout was elicited by applying a 200 ms depolarizing pulse every 10 s from a holding potential of -60 to +80 mV. The uterine myocytes were obtained from non-pregnant (NPM) and pregnant (PM) women. Cells were superfused with 10 μM isoprenaline (Iso) first and then additionally with 10 μM clonidine (Clon). Bars represent the mean current densities of four NPM and five PM cells. Each cell was from a different donor. The pipette solution contained 0.3 μM Ca2+. *P < 0.05.
Selective effects of isoprenaline on the IbTX-sensitive component of Iout
In order to investigate to what extent BKCa channels were involved in the effects of isoprenaline, their contribution to whole-cell Iout was determined first. Currents were elicited in rat NPM and PM cells every 10 s by a depolarizing pulse of 200 ms from a holding potential of -60 to +80 mV. Superfusion with the Ca2+ ionophore A23187 (1 μM) resulted in a marked increase of Iout from 0.6 to 1.87 nA in the NPM cell (Fig. 4A) and from 1.5 to 3.1 nA in the PM cell (Fig. 4C). Subsequent addition of 100 nM IbTX in the presence of A23187 reduced the current to 0.17 nA in the NPM and to 0.4 nA in the PM cell. A summary of effects induced by A23187 and IbTX on Iout is shown in Fig. 4B and D. The mean current density increased in five NPM and five PM cells from 50.6 ± 8.9 (control) and 82.3 ± 14 pA pF−1 (control) to 209 ± 56.3 and 378 ± 47 pA pF−1 in the presence of 10 μM A23187 and decreased to 11.6 ± 7.4 and 17 ± 9.5 pA pF−1 in the presence of A23187 plus 100 nM IbTX, respectively. Because 300 nM IbTX did not further decrease Iout, it can be concluded that the residual current of 23 % in NPM cells and of 21 % in PM cells was due to the opening of channels other than BKCa channels. In a second step, it was tested whether the residual current was sensitive to isoprenaline. By utilizing the same stimulation protocol as that in Fig. 4, BKCa channels were blocked by 100 nM IbTX and the effect of 10 μM isoprenaline on the amplitude of the residual Iout was then determined. As shown in Fig. 5C and D, 100 nM IbTX decreased the mean Iout density in six NPM and six PM cells from 54.3 ± 4.6 to 12.2 ± 1.3 pA pF−1 (i.e. by 77.5 %) and from 85.8 ± 5.2 to 17.1 ± 2.3 pA pF−1 (i.e. by 80 %), respectively. Additional superfusion of the cells with isoprenaline produced no significant effect on the amplitude of the residual current. Figure 5A and B presents original current traces from a NPM and PM cell and it is shown that in comparison with IbTX alone, neither the current amplitude nor its activation kinetics was influenced by the catecholamine. In conclusion, Fig. 5 demonstrates that about 80 % of whole-cell Iout elicited by depolarizing steps from a holding potential of -60 to +80 mV in NPM and PM cells is conducted by BKCa channels and only this fraction of total Iout is modulated by isoprenaline.
Figure 4. Calcium and iberiotoxin sensitivity of Iout.
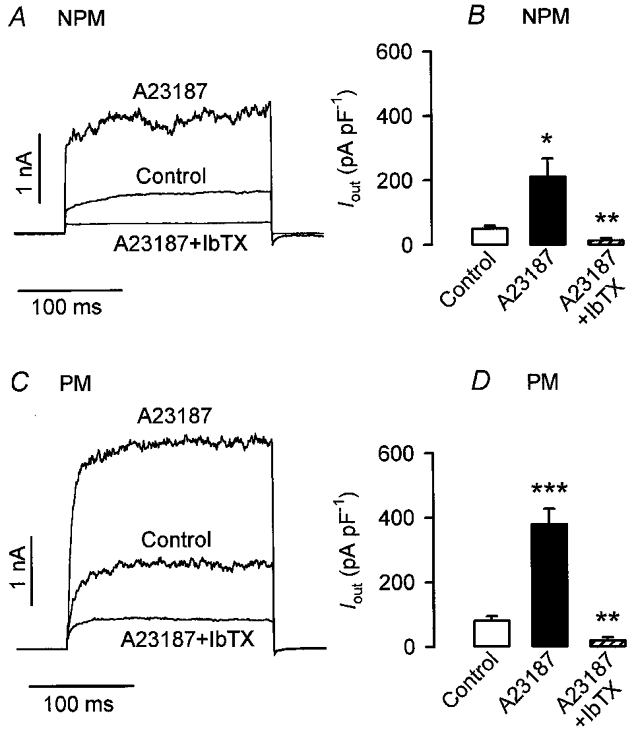
Currents were evoked in non-pregnant (NPM) and pregnant (PM) rat uterine myocytes by applying 200 ms depolarizing pulses from a holding potential of -60 to +80 mV. Original current recordings from a NPM (A) and a PM (C) cell exposed to 1 μM A23187 first and then additionally to 100 nM iberiotoxin (IbTX) are shown. B and D, summary of the effects of A23187 and of A23187 plus IbTX on Iout of five NPM and PM cells, obtained from five and four rats, respectively. Bars represent mean current densities. *P < 0.05; **P < 0.01; ***P < 0.001 versus control.
Figure 5. Isoprenaline fails to influence the iberiotoxin-insensitive Iout.
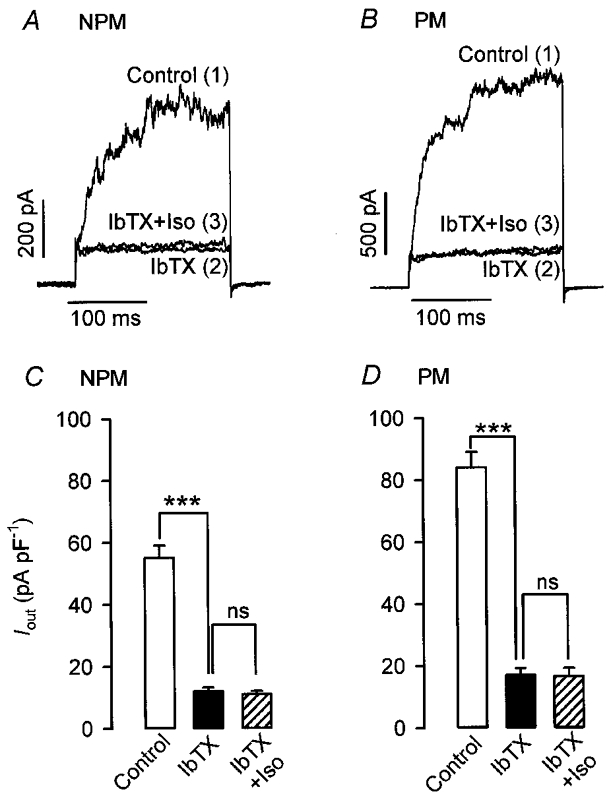
Currents were evoked in non-pregnant (NPM) and pregnant (PM) rat uterine myocytes by applying 200 ms depolarizing pulses from a holding potential of -60 to +80 mV. Original current recordings from a NPM (A) and a PM (B) cell exposed to 100 nM iberiotoxin (IbTX) first and then additionally to 10 μM isoprenaline (Iso) are shown. Numbers in parentheses denote the sequence of drug applications. C and D, average results from six NPM and PM cells, obtained from five rats in each group. Bars represent mean current densities. ***P < 0.001 versus control; ns, not significant.
Influence of H-89 on the isoprenaline- and clonidine-modulated Iout
Signal transduction initiated by activation of β- and α2-ARs in myometrial smooth muscle cells is mediated via the cAMP-PKA cascade. It was therefore important to investigate whether inhibition of PKA would reverse the effects of isoprenaline and clonidine on Iout of NPM and PM cells. The experiments shown in Fig. 6 were carried out with 1 μM H-89, a specific inhibitor of PKA at this concentration (Hidaka & Kobayashi, 1992). As in the experiments before, Iout was elicited by depolarizing the cells for 200 ms from -60 to +80 mV. It is shown that H-89 completely reversed the effect of 10 μM isoprenaline in six NPM and six PM cells (Fig. 6A). The same inhibitory effect of H-89 was observed in eight NPM and six PM cells which had been treated previously with isoprenaline plus clonidine (Fig. 6B). Thus, the findings clearly indicate that both receptor agonists produce their effects on BKCa channels exclusively via the cAMP-PKA cascade.
Figure 6. The PKA inhibitor H-89 inhibits the effects of isoprenaline and clonidine on Iout.
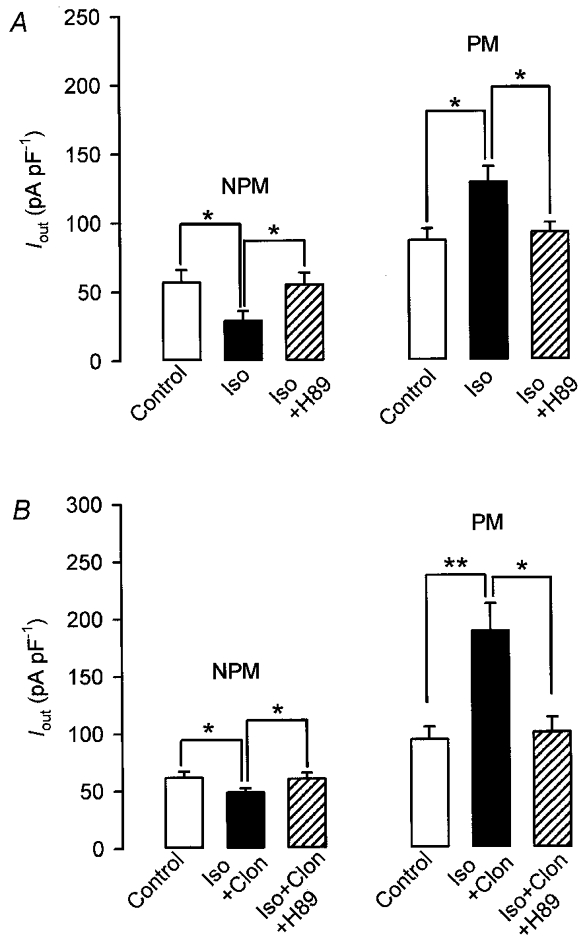
Currents were evoked by applying a 200 ms depolarizing pulse every 10 s from a holding potential of -60 to +80 mV. After recording control currents from non-pregnant (NPM) and pregnant (PM) rat uterine myocytes, cells were superfused with 10 μM isoprenaline first and then additionally with 1 μM H-89 (A) or with 10 μM isoprenaline plus 10 μM clonidine first and then additionally with 1 μM H-89 (B). Bars represent mean current densities of six NPM and six PM cells from four rats in each group in A and of eight NPM and six PM cells from five and four rats, respectively, in B. *P < 0.05; **P < 0.01.
Influence of PTX and RGS16 on the clonidine-modulated Iout
In order to test whether Gi proteins are involved in both the inhibitory and stimulatory effects on Iout when clonidine was applied in the presence of isoprenaline, experiments with RGS16, a GTPase-activating protein for members of the Giα subfamily, were conducted. Myocytes from non-pregnant and pregnant myometria were loaded via the patch-electrode with the RGS16 protein for 20 min (electrode concentration, 1 μM). Thereafter, Iout was elicited by depolarizing the cells from -60 to +80 mV in 10 mV increments for 200 ms to obtain current-voltage relations. As shown in Fig. 7, the inhibitory and stimulatory effects of 10 μM isoprenaline in nine NPM (Fig. 7A) and ten PM cells (Fig. 7B), respectively, were preserved in the presence of RGS16. Addition of 10 μM clonidine, however, produced no further change in current. Identical results to those shown in Fig. 7 with RGS16 were obtained in seven NPM and seven PM cells, incubated for 5 h in PSS containing 400 ng ml−1 pertussis toxin (PTX; data not shown). This loss of effectiveness of clonidine in the presence of RGS16 or PTX indicates that Gi proteins are involved in the inhibitory as well as in the stimulatory effect of clonidine.
Figure 7. RGS16 abolishes the effects of clonidine on isoprenaline-modulated Iout.
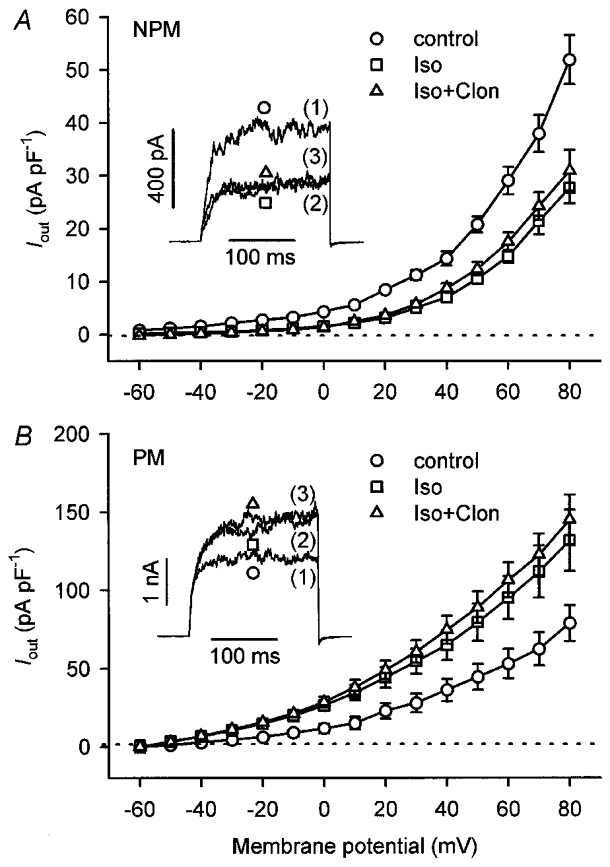
Current-voltage relations from non-pregnant (NPM; n = 9; A) and pregnant (PM; n = 10; B) rat uterine myocytes are shown. NPM and PM cells were obtained from six and seven animals, respectively. Mean current densities are plotted against the respective test potential. Currents were evoked by applying 200 ms depolarizing pulses in 10 mV steps from a holding potential of -60 mV. Cells were first dialysed via the patch-pipette for 20 min with 1 μM RGS16, thereafter superfused with 10 μM isoprenaline (Iso), followed by the additional application of 10 μM clonidine (Clon). Note that the control- and the isoprenaline-modulated Iout were not influenced by RGS16. The insets show representative Iout recordings elicited by 200 ms pulses from -60 to +80 mV. Numbers in parentheses indicate the sequence of drug applications. The pipette solution contained 0.3 μM Ca2+.
Influence of TDα on the clonidine-modulated Iout
It is well known that both α-subunits and the βγ-dimers of G proteins regulate the activity of effectors. In order to differentiate between the two types of subunits, experiments with TDα, the α-subunit of a heterotrimeric G protein that mediates signal transduction in vertebrate photoreceptor cells, were conducted on NPM and PM cells. It was reasoned that TDα, a scavenger of βγ-dimers dissociated from heterotrimeric Gi proteins upon activation of α2-ARs, should specifically antagonize βγ-mediated effects, whereas effects dependent on α-subunits should be preserved. The experimental design was similar to that of Fig. 7; 0.3 μM TDα was dialysed via the patch-pipette into the myocytes for 20 min before Iout was elicited in the absence and presence of isoprenaline and isoprenaline plus clonidine. Figure 8A and B shows the results obtained in a NPM and PM cell clamped for 200 ms from -60 to +80 mV. There were no changes in the response of both cells to isoprenaline but the clonidine effect was markedly altered in the presence of TDα. The inhibitory effect of 10 μM isoprenaline on Iout was completely antagonized by 10 μM clonidine in the NPM cell (before 580 pA, after isoprenaline 280 pA, plus clonidine 580 pA), whereas the stimulating effect of isoprenaline was partially reversed in the PM cell (before 1.7 nA, after isoprenaline 3.0 nA, plus clonidine 2.65 nA). Mean values obtained from seven NPM and nine PM cells are summarized in Fig. 8C. Note that the inhibitory effect of clonidine on Iout in nine PM cells exposed to isoprenaline was statistically significant (P < 0.05; isoprenaline 195.1 ± 23.1 % of control, plus clonidine 154.2 ± 18.3 % of control). Taken together, the results presented in Fig. 8 indicate that clonidine acts in NPM cells predominantly via Giα whereas βγ-subunits of Gi proteins seem to be involved in the stimulatory effect in PM cells.
Figure 8. Transducin-α reverses the effect of clonidine on Iout in pregnant uterine myocytes.
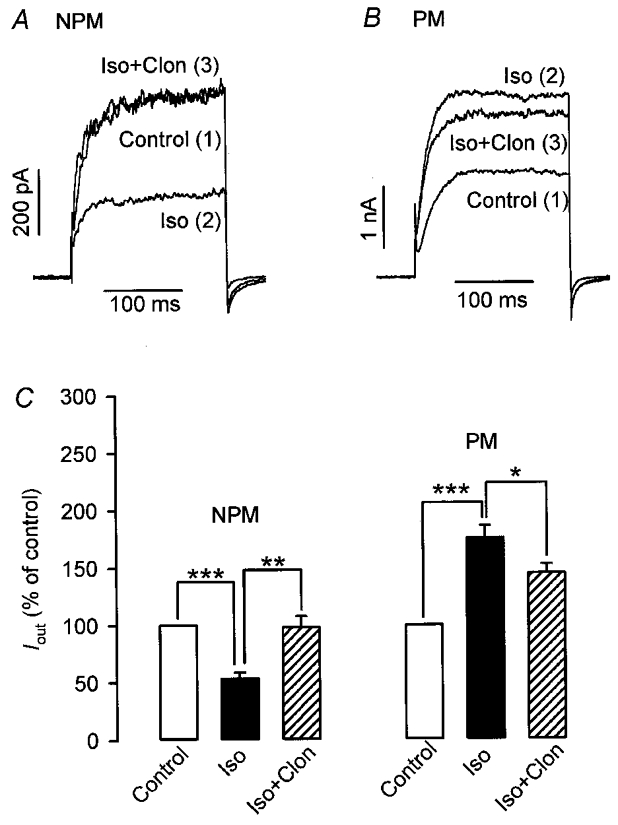
Currents were elicited in non-pregnant (NPM) and pregnant (PM) rat uterine myocytes every 10 s by 200 ms depolarizing pulses from -60 to +80 mV. Before drug application, cells were dialysed via the patch-pipette for 20 min with 0.3 μM transducin-α (TDα). Original current recordings showing that 10 μM clonidine (Clon) completely revoked the effect of 10 μM isoprenaline (Iso) in the TDα-loaded NPM cell (A) whereas the potentiating effect of clonidine was switched by TDα to an antagonistic action in the PM cell (B). Numbers in parentheses indicate the sequence of drug applications. C, average results from NPM (n = 7) and PM (n = 9) cells, obtained from five and six rats, respectively. Current densities were used to calculate the percentage values. *P < 0.05; **P < 0.01; ***P < 0.001.
Influence of TDβγ on the isoprenaline-modulated Iout
Among the effector molecules regulated by Gi proteins in myometrial smooth muscle cells are different subtypes of ACs, some of which are co-stimulated by βγ-dimers of Gi proteins in the presence of stimulatory Gsα (induced by isoprenaline). In order to test the hypothesis that cyclases co-stimulated by βγ-dimers are specifically expressed during pregnancy, NPM and PM cells were exposed to isoprenaline and simultaneously dialysed via the patch-pipette with 1 μM transducin-βγ (TDβγ). The voltage protocol was the same as before. First, NPM and PM cells were dialysed with TDβγ and the current was recorded over 20 min (Fig. 9). Although a tendency to increase Iout was observed with TDβγ, it never reached statistical significance in seven cells. A second group of cells (nine NPM and PM cells, respectively) was superfused with 10 μM isoprenaline and it can be seen that the inhibitory (Fig. 9A) and the stimulatory effect (Fig. 9B) was maximal within 3 min and remained quite stable thereafter. A third group of cells (five NPM and five PM cells) was superfused with isoprenaline while the patch-pipette contained 1 μM TDβγ. Figure 9 demonstrates that the effects of 10 μM isoprenaline on Iout were potentiated by TDβγ over a period of 20 min in both NPM and PM cells. This effect was specific, because dialysis of equimolar concentrations of TDα and TDβγ (1 μM each, data not shown) did not further increase the isoprenaline-modulated Iout. Thus, the results suggest that both NPM and PM cells express ACs which are co-stimulated by βγ-dimers.
Figure 9. Transducin-βγ enhances the effects of isoprenaline on Iout.
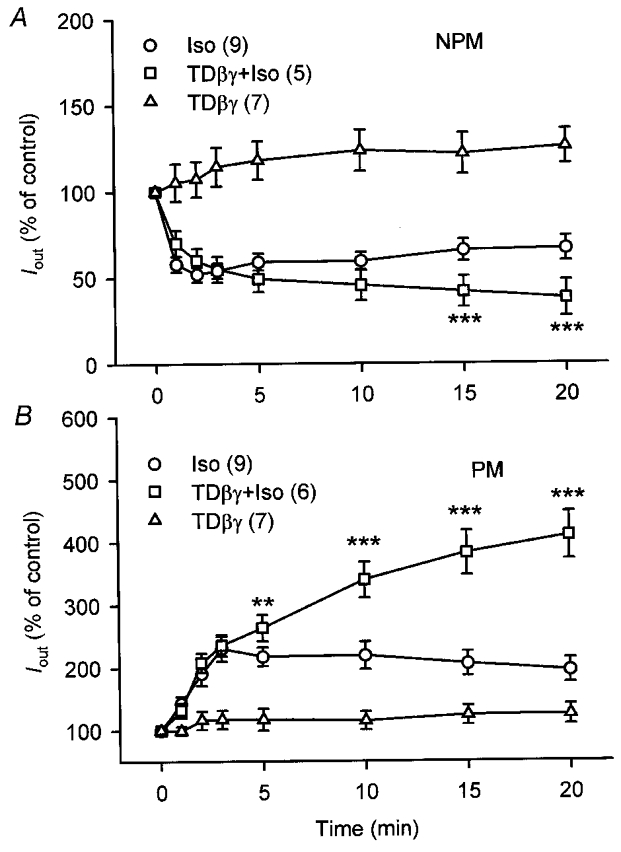
Currents were evoked in non-pregnant (NPM; A) and pregnant (PM; B) rat uterine myocytes by applying a 200 ms depolarizing pulse every 10 s from -60 to +80 mV. Recordings were started immediately after membrane disruption. A and B, time course of Iout with either 10 μM isoprenaline (Iso) in the bath, 10 μM isoprenaline in the bath and 1 μM transducin-βγ (TDβγ+ Iso) in the patch-pipette, or TDβγ in the pipette but no agonist in the bath (TDβγ). Mean values from three different groups are shown in both A and B. Numbers in parentheses denote the number of cells. NPM cells were obtained from five (▵), six (^) and seven rats (□), PM cells from five (▵), six (^) and five rats (□). Current densities were used to calculate the percentage values. **P < 0.01; ***P < 0.001 versus Iso alone.
Effects of adrenaline and noradrenaline on Iout
The physiological ligands for α- and β-ARs are noradrenaline and adrenaline. It was therefore interesting to investigate the effects of both catecholamines on Iout. As shown in Fig. 10A, when NPM cells were depolarized for 200 ms from -60 to +80 mV, Iout was elicited which was unaffected by superfusing the cells with either 10 μM noradrenaline (92.0 ± 10.2 % of control; n = 7) or adrenaline (102.5 ± 9.3 % of control, n = 6). Subsequent addition of 10 μM yohimbine, a selective antagonist of α2-ARs, however, disclosed the β-AR-mediated inhibitory effect of noradrenaline (52.6 ± 5.2 % of control; n = 7) and of adrenaline (56.1 ± 5.3 % of control; n = 6). Contrary to NPM cells, PM cells responded with a pronounced increase in Iout in the presence of noradrenaline (210.4 ± 20.1 % of control; n = 6) or adrenaline (195.0 ± 19.8 % of control; n = 6; Fig. 10B). Additional blockade of α2-ARs by 10 μM yohimbine resulted in a partial decrease of the catecholamine-stimulated current to 162.4 ± 17.1 % (noradrenaline) and 155.1 ± 16.3 % (adrenaline) of the respective control values. The results indicate that α2- and β-AR-mediated effects on Iout cancel each other in NPM cells whereas Iout was markedly augmented by the co-stimulatory signalling induced by the simultaneous stimulation of both adrenoceptors during pregnancy.
Figure 10. Adrenaline and noradrenaline enhance Iout only during pregnancy.
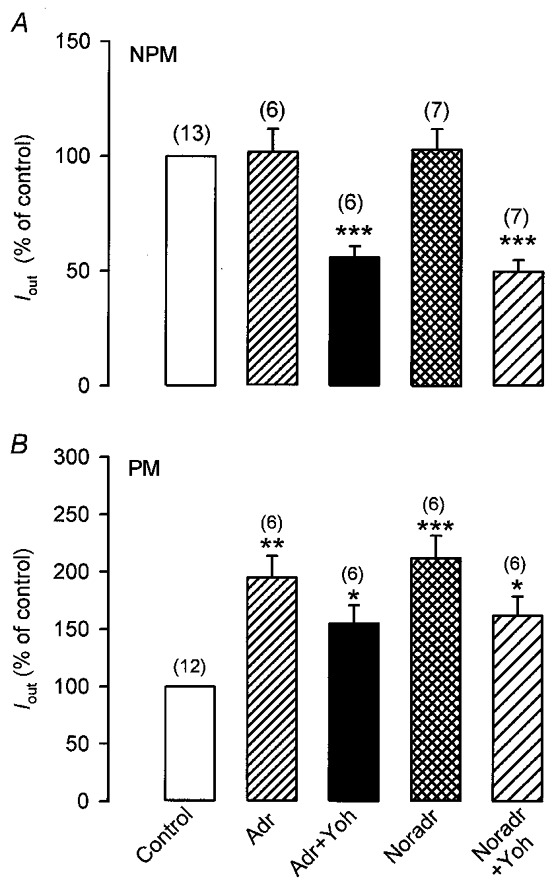
Currents were evoked in non-pregnant (NPM) and pregnant (PM) rat uterine myocytes by applying a 200 ms depolarizing pulse every 10 s from -60 to +80 mV. NPM (A) and PM (B) cells were superfused with 10 μM adrenaline (Adr) or 10 μM noradrenaline (Noradr), thereafter 10 μM yohimbine (Yoh) was added. Note that yohimbine disclosed the β-AR-mediated effects of both catecholamines on Iout. Numbers in parentheses denote the number of cells. Cells for each group (Adr and Noradr) in A and B were obtained from five rats. Current densities were used to calculate the percentage values. The pipette solution contained 0.3 μM Ca2+. *P < 0.05; **P < 0.01; ***P < 0.001 versus control.
DISCUSSION
Myometrial smooth muscle cells are richly endowed with BKCa channels (Kihira et al. 1990; Anwer et al. 1993; Pérez et al. 1993; Zhou et al. 1998). As sensors of both voltage and intracellular Ca2+, BKCa channels are responsible for membrane repolarization that follows depolarization and the accompanying increase in cytosolic free Ca2+ during an action potential (Parkington & Coleman, 1990; Kaczorowski et al. 1996). Because contraction of the myometrium is closely related to its electrical properties, which consist of characteristic spontaneous bursts of spike discharges at irregular intervals, BKCa channels are important regulators of smooth muscle contractility (Parkington & Coleman, 1990; Anwer et al. 1993). The membrane electrical and contractile properties of the myometrium vary markedly during the normal ovulatory cycle and pregnancy, presumably as a consequence of hormonal influences (Kao, 1989; Parkington & Coleman, 1990; Toro et al. 1990). For example, whereas uterine quiescence is important to maintain pregnancy, contractility is gradually activated before parturition giving rise to powerful rhythmic contractions culminating in the delivery of the fetus. Evidence has been presented that BKCa channels contribute to the myometrial tone at the different stages of pregnancy by changes in their density, voltage activation and Ca2+ sensitivity (Khan et al. 1993; Wang et al. 1998; Song et al. 1999). BKCa channels are important targets for regulation by cAMP- and cGMP-dependent protein kinases (Alioua et al. 1998; Nara et al. 1998; Zhou et al. 1998). Recently it has been reported that BKCa channels are differentially regulated by cAMP- and cGMP kinase in pregnant and non-pregnant myometrial cells (Pérez & Toro, 1994; Zhou et al. 1999). The present study confirms these observations with cAMP kinase by showing inhibition and activation of BKCa channel activity in inside-out patches from non-pregnant and pregnant rat myometrial cells, respectively. The mechanism by which BKCa channels are differentially regulated by cAMP- and cGMP kinase is presently unknown but may involve expression of isoforms of BKCa channels, different heteropolymeric assembly of channel subunits and/or expression of associated proteins with modulatory function. β-AR agonists are widely used for prevention of premature labour due to their relaxing effects on the myometrium. They are thought to act via a cascade that involves stimulatory G proteins (Gs), AC, cAMP and finally PKA activation. Myometrial relaxation is then induced by pleiotropic mechanisms including activation of BKCa channels (Anwer et al. 1992; Hamada et al. 1994). The present study shows that the β-AR agonist isoprenaline mimics the effects of PKA by augmenting Iout in pregnant and inhibiting the current in non-pregnant myometrial cells.
Besides β-ARs, transcripts of α2A- and α2B-ARs have been identified in rat myometria (Mhaouty et al. 1995; Bouet-Alard et al. 1997). It is well established that myometrial α2-ARs are functionally linked to inhibition of AC through activation of Gi proteins (Wu et al. 1988; Breuiller et al. 1990). In accordance with this signalling pathway, clonidine, a ligand with high affinity for the rat myometrial α2-AR (Mhaouty et al. 1995), partially reversed the isoprenaline effect on Iout in myocytes from non-pregnant myometrium. Conversely, clonidine potentiated the isoprenaline-stimulated Iout in cells from pregnant myometria. The latter finding corresponds with the observation that clonidine (10 μM) enhanced the effect of isoprenaline on AC activity in the rat pregnant myometrium (Mhaouty et al. 1995). Thus, the electrophysiological data closely resemble those of AC activity in membrane preparations. Contrary to single channel data, whole-cell Iout of myometrial cells is composed of various currents (Toro et al. 1990; Wang et al. 1998). Experiments with IbTX, a specific and potent blocker of BKCa channels (Galvez et al. 1990), disclosed that about 75–80 % of Iout was conducted by BKCa channels in cells from pregnant and non-pregnant myometria. This relatively high contribution of IK(Ca) to Iout differs from that reported earlier in pregnant rat myometrial cells (Wang et al. 1998). The difference may be due to the higher IbTX concentration used in the present study (100 nM instead of 1 nM) and to the fact that we analysed only cells displaying non-inactivating outward currents. Because isoprenaline did not significantly change the residual current, IbTX-sensitive IK(Ca) was the only current component sensitive to the cAMP-PKA cascade, at least under the present experimental conditions. Furthermore, the abolition of all effects on Iout produced by isoprenaline or isoprenaline plus clonidine by H-89, a specific inhibitor of PKA (Hidaka & Kobayashi, 1992), clearly demonstrates the causal relation between kinase activation and channel activity. As a result, IK(Ca) seems to be an efficient tool to monitor the intracellular cAMP-PKA cascade in myometrial cells. In contrast to data reported from rat, no stimulatory effect of isoprenaline on AC activity could be detected in membranes from human pregnant or non-pregnant myometria (Gsell et al. 2000). These data were explained by the low β-AR density in human as compared with rat myometrium (Cohen-Tannoudji et al. 1991; Engelhardt et al. 1997; Gsell et al. 2000). Contrary to AC activity, Iout recorded in cells from human myometrium responded to isoprenaline, although on a smaller scale, in exactly the same way as in rat cells, indicating that the electrophysiological approach is more sensitive in detecting subtle changes in cAMP-PKA signalling than the AC assay in isolated membranes. It is interesting to note that because of its high Ca2+ sensitivity, BKCa channel activity has been used successfully to monitor [Ca2+]i in the subsarcolemmal space of arterial smooth muscle cells (Ganitkevich & Isenberg, 1996), and in several types of non-excitable cells (for review see Marty, 1989).
Positive and negative signal integration by clonidine in the presence of isoprenaline could be due to the expression of different AC isoforms in the pregnant and non-pregnant myometrium. Nine different types of ACs that differ in their regulatory mechanism and their tissue-specific distribution have been described so far (Sunahara et al. 1996). Among the seven ACs identified in the rat myometrium (Mhaouty-Kodja et al. 1997), types II and IV share the property of being stimulated by the βγ-subunits of Gi/Go inhibitory proteins in the presence of activated Gsα (Tang & Gilman, 1991; Gao & Gilman, 1991; Federman et al. 1992). The demonstration that both the negative and the positive input of clonidine to Iout was completely abolished in cells loaded with RGS16 or treated with PTX, clearly shows that Gi/Go proteins were involved in both signal transduction pathways (see Fig. 11). RGS16 is a GTPase-activating protein that preferentially acts on members of the Giα-subfamily and converts the active GTP-bound α-subunit to the inactive GDP-bound form, which then reassembles with the βγ-dimer (for review see Wieland & Chen, 1999), whereas PTX uncouples Gi proteins from the receptor. Apparently, when the concentration of RGS16 is sufficiently high in the cell, Gi-/Go-mediated effects can be completely blocked, whereas the Gsα-mediated effect of isoprenaline on Iout remained unchanged. In order to test which G protein subunit was responsible for the clonidine effects, we took advantage of TDα which acts as a scavenger of βγ-dimers of Gi/Go proteins (Federman et al. 1992). When βγ-dimers were released by α2-AR activation in non-pregnant myometrial cells loaded with TDα and simultaneously superfused with isoprenaline, Iout was completely reversed (Fig. 8A) as compared with the partial recovery seen in the absence of TDα (Fig. 2A). This result is compatible with the view that recovery of Iout was due to unopposed Giα because the pool of free βγ-dimers available for reassembling with Giα protein was strongly reduced by TDα and was also not available for co-stimulation of AC isoforms. However, when the same experiment was repeated in myocytes from pregnant myometrium, clonidine produced a significant inhibition rather than a potentiation of the isoprenaline-stimulated Iout (Fig. 8B). Consequently, Giβγ must have been responsible for the synergistic effect of clonidine and isoprenaline on Iout when TDα was absent (Fig. 2B). The α2-AR-mediated decrease of Iout, however, reflects very probably the unopposed inhibitory effect of free Giα subunits in the presence of TDα. Thus, stimulatory and inhibitory signal transduction pathways coexist in the pregnant myometrial cell and the resulting effect depends on the prevalence of either subunit. In accordance with this view, TDβγ, when applied in high concentrations to the interior of myocytes, acted synergistically with isoprenaline irrespective of whether the cells were from pregnant or non-pregnant myometria. Again this finding supports the concept of opposing roles of Giα and Gi βγ on AC activity and is contradictory to the idea that βγ-stimulated AC isoforms are exclusively expressed in pregnant myometrium. We cannot exclude, however, that the amount of βγ-sensitive AC isoforms might be upregulated during pregnancy. Another possible explanation is a more efficient coupling between α2-ARs and Gi/Go, thus βγ-dimers at high levels may simply override the inhibitory effect of Giα. This interpretation is supported by an earlier report demonstrating an increase of the expression of Giα2 and Gi βγ in rat myometria at midpregnancy (Tanfin et al. 1991).
Figure 11. Scheme illustrating bivalent regulation of cAMP synthesis by G proteins.
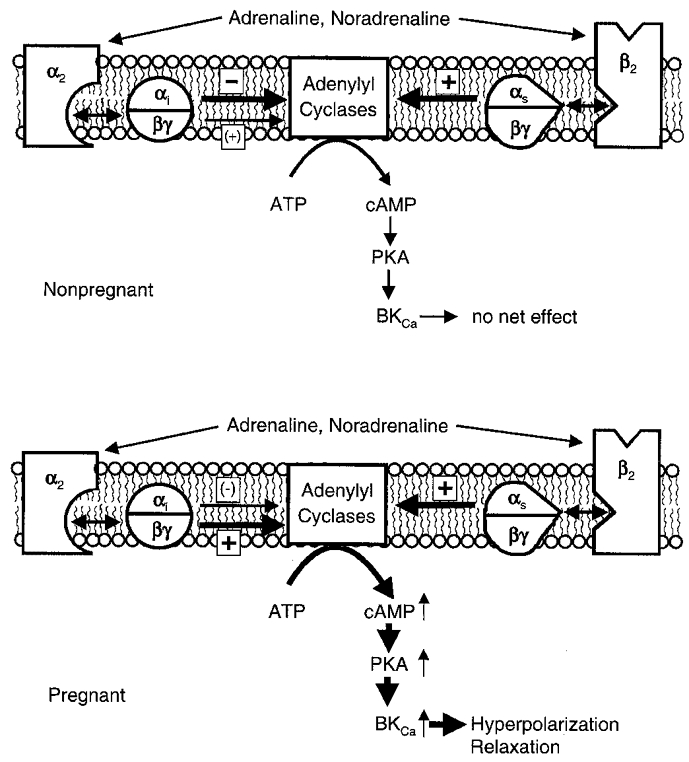
Shown is the α2- and β-AR coupling to AC in cells of the non-pregnant (upper panel) and pregnant myometrium (lower panel). Catecholamines are the physiological AR ligands which act simultaneously through receptors coupled to stimulatory (s) and inhibitory (i) G proteins. The thickness and position of arrows between G protein subunits and AC indicate the predominant effect and the subunit involved in the respective signal transduction pathway. Note that the G proteins must bind GTP and dissociate before they become active.
Adrenaline and noradrenaline are the physiological ligands of β- and α2-ARs. As expected from the inhibitory coupling of the α2-AR to AC in the non-pregnant myometrium, the β-AR-mediated reduction of Iout was completely prevented by either catecholamine. In the pregnant myometrium, however, where the signalling pathways of both ARs act synergistically, a strong increase in Iout was observed, exceeding that of β-AR activation alone. The female reproductive tract has a rich adrenergic innervation (Marshall, 1981) and it is therefore conceivable that the two types of regulation are physiologically meaningful, the latter by reinforcing uterine quiescence during pregnancy, the former by rendering the myometrium insensitive to the β/α2-AR pathway in the non-pregnant state. Figure 11 summarizes the results obtained with catecholamines and in addition indicates which subunit of the Gi protein dominates in the non-pregnant and pregnant state. There are many ways by which the cAMP-PKA signalling cascade regulates myometrial tone (Wray, 1993). It should be kept in mind, however, that BKCa channels are negative feedback regulators of the membrane potential and thus play an important role in this process. The efficacy of β-AR agonists in suppressing premature labour appears to be well established. The present data suggest that their tocolytic effectiveness may be improved by the simultaneous application of α2-AR agonists which are able to increase the level of co-stimulatory βγ-dimers of Gi/Go.
In summary, the present data establish that registration of BKCa channel activity can be used as a reliable probe to monitor cAMP-PKA signalling in myometrial smooth muscle cells. The results demonstrate that the opposing effects of β- and α2-AR agonists on BKCa channel activity in the non-pregnant myometrium switch to a synergistic action during pregnancy. This switch is probably not due to an exclusive expression of βγ-stimulated AC isoforms but might result from a more efficient coupling between α2-AR and inhibitory G proteins.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to T.W.
References
- Adolfsson PI, Dahle LO, Berg G, Svensson SP. Characterization of α2-adrenoceptor subtypes in pregnant human myometrium. Gynecologic and Obstetric Investigation. 1998;45:145–150. doi: 10.1159/000009944. [DOI] [PubMed] [Google Scholar]
- Alioua A, Tanaka Y, Wallner M, Hofmann F, Ruth P, Meera P, Toro L. The large conductance, voltage-dependent, and calcium-sensitive K+ channel, hSlo, is a target of cGMP-dependent protein kinase phosphorylation in vivo. Journal of Biological Chemistry. 1998;273:32950–32956. doi: 10.1074/jbc.273.49.32950. [DOI] [PubMed] [Google Scholar]
- Anwer K, Oberti C, Perez GJ, Perez-Reyes N, McDougall JK, Monga M, Sanborn BM, Stefani E, Toro L. Calcium-activated K+ channels as modulators of human myometrial contractile activity. American Journal of Physiology. 1993;265:C976–985. doi: 10.1152/ajpcell.1993.265.4.C976. [DOI] [PubMed] [Google Scholar]
- Anwer K, Toro L, Oberti C, Stefani E, Sanborn BM. Ca2+-activated K+ channels in pregnant rat myometrium: modulation by a beta-adrenergic agent. American Journal of Physiology. 1992;263:C1049–1056. doi: 10.1152/ajpcell.1992.263.5.C1049. [DOI] [PubMed] [Google Scholar]
- Bottari SP, Vokaer A, Kaivez E, Lescrainier JP, Vauquelin G. Regulation of alpha- and beta-adrenergic receptor sub classes by gonadal steroids in human myometrium. Acta Physiologica Academiae Scientiarum Hungaricae. 1985;65:335–346. [PubMed] [Google Scholar]
- Bouet-Alard R, Mhaouty-Kodja S, Limon-Boulez I, Coudouel N, Maltier JP, Legrand C. Heterogeneity of α2-adrenoceptors in human and rat myometrium and differential expression during pregnancy. British Journal of Pharmacology. 1997;122:1732–1738. doi: 10.1038/sj.bjp.0701555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the priciple of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breuiller M, Rouot B, Litime MH, Leroy MJ, Ferré F. Functional coupling of the α2-adrenergic receptor-adenylate cyclase complex in the pregnant human myometrium. Journal of Clinical Endocrinology and Metabolism. 1990;70:1299–1304. doi: 10.1210/jcem-70-5-1299. [DOI] [PubMed] [Google Scholar]
- Chen C-K, Wieland T, Simon MI. RGS-r, a retinal specific RGS protein, binds an intermediate conformation of transducin and enhances recycling. Proceedings of the National Academy of Sciences of the USA. 1996;93:12885–12889. doi: 10.1073/pnas.93.23.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Tannoudji J, Vivat V, Heilmann J, Legrand C, Maltier JP. Regulation by progesterone on the high-affinity state of myometrial beta-adrenergic receptor and of adenylate cyclase activity in pregnant rat. Journal of Molecular Endocrinology. 1991;6:137–145. doi: 10.1677/jme.0.0060137. [DOI] [PubMed] [Google Scholar]
- Engelhardt S, Zieger W, Kassubek J, Michel MC, Lohse M, Brodde OE. Tocolytic therapy with fenoterol induces selective downregulation of β-adrenergic receptors in human myometrium. Journal of Clinical Endocrinology and Metabolism. 1997;82:1235–1242. doi: 10.1210/jcem.82.4.3885. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods in Enzymology. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Federman AD, Conklin BR, Schrader KA, Reed RR, Bourne HR. Hormonal stimulation of adenylyl cyclase through Gi-protein βγ subunits. Nature. 1992;356:159–161. doi: 10.1038/356159a0. [DOI] [PubMed] [Google Scholar]
- Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. Purification and characterization of a unique, potent, peptidyl-probe for the high-conductance calcium-activated potassium channel from the venom of the scorpion Buthus tamulus. Journal of Biological Chemistry. 1990;265:11083–11090. [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Dissociation of subsarcolemmal from global [Ca2+] in myocytes from guinea-pig coronary artery. The Journal of Physiology. 1996;490:305–318. doi: 10.1113/jphysiol.1996.sp021145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Gilman AG. Cloning and expression of a widely distributed (type IV) adenylyl cyclase. Proceedings of the National Academy of Sciences of the USA. 1991;88:10178–10182. doi: 10.1073/pnas.88.22.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gsell S, Eschenhagen T, Kaspareit G, Nose M, Scholz H, Behrens O, Wieland T. Apparent upregulation of stimulatory G protein α subunits in the pregnant human myometrium is mimicked by elevated smoothelin expression. FASEB Journal. 2000;14:17–26. doi: 10.1096/fasebj.14.1.17. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Nakaya Y, Hamada S, Kamada M, Aono T. Activation of K+ channels by ritodrine hydrochloride in uterine smooth muscle cells from pregnant women. European Journal of Pharmacology. 1994;288:45–51. doi: 10.1016/0922-4106(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cell and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hidaka H, Kobayashi R. Pharmacology of protein kinase inhibitors. Annual Review of Pharmacology and Toxicology. 1992;32:377–397. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- Kaczorowski GJ, Knaus H-G, Leonard RJ, McManus OB, Garcia ML. High-conductance calcium-activated potassium channels; structure, pharmacology and function. Journal of Bioenergetics and Biomembranes. 1996;28:255–267. doi: 10.1007/BF02110699. [DOI] [PubMed] [Google Scholar]
- Kao CY. Electrophysiological properties of uterine muscle. In: Wynn RM, Jollie WP, editors. Biology of the Uterus. New York and London: Plenum Press; 1989. pp. 403–453. [Google Scholar]
- Khan RN, Smith SK, Morrison JJ, Ashford MLJ. Properties of large-conductance K+ channels in human myometrium during pregnancy and labour. Proceedings of the Royal Society. 1993;B 251:9–15. doi: 10.1098/rspb.1993.0002. [DOI] [PubMed] [Google Scholar]
- Kihira M, Matsuzawa K, Tokuno H, Tomita T. Effects of calmodulin antagonists on calcium-activated potassium channels in pregnant rat myometrium. British Journal of Pharmacology. 1990;100:353–359. doi: 10.1111/j.1476-5381.1990.tb15808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomasney JW, Cotecchia S, Lefkowitz RJ, Caron MG. Molecular biology of α-adrenergic receptors: implications for receptor classification and for structure-function relationships. Biochimica et Biophysica Acta. 1991;1095:127–139. doi: 10.1016/0167-4889(91)90075-9. [DOI] [PubMed] [Google Scholar]
- Marshall JM. Effects of ovarian steroids and pregnancy on adrenergic nerves of uterus and oviduct. American Journal of Physiology. 1981;240:C165–174. doi: 10.1152/ajpcell.1981.240.5.C165. [DOI] [PubMed] [Google Scholar]
- Marty A. The physiological role of calcium-dependent channels. Trends in Neurosciences. 1989;12:420–424. doi: 10.1016/0166-2236(89)90090-8. [DOI] [PubMed] [Google Scholar]
- Mermi J, Yajima M, Ebner F. The control of the contraction of myocytes from guinea-pig heart by the resting membrane potential. British Journal of Pharmacology. 1991;104:705–713. doi: 10.1111/j.1476-5381.1991.tb12492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhaouty S, Cohen-Tannoudji J, Bouet-Alard R, Limon-Boulez I, Maltier JP, Legrand C. Characteristics of the α2/β2-adrenergic receptor-coupled adenylyl cyclase system in rat myometrium during pregnancy. Journal of Biological Chemistry. 1995;270:11012–11016. doi: 10.1074/jbc.270.18.11012. [DOI] [PubMed] [Google Scholar]
- Mhaouty-Kodja S, Bouet-Alard R, Limon-Boulez I, Maltier JP, Legrand C. Molecular diversity of adenylyl cyclases in human and rat myometrium. Journal of Biological Chemistry. 1997;272:31100–31106. doi: 10.1074/jbc.272.49.31100. [DOI] [PubMed] [Google Scholar]
- Nara M, Dhulipala PDK, Wang Y-X, Kotlikoff MI. Reconstitution of β-adrenergic modulation of large conductance, calcium-activated potassium (Maxi-K) channels in Xenopus oocytes. Identification of the cAMP-dependent protein kinase phosphorylation site. Journal of Biological Chemistry. 1998;273:14920–14924. doi: 10.1074/jbc.273.24.14920. [DOI] [PubMed] [Google Scholar]
- Papermaster DS, Dreyer WJ. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974;13:2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- Parkington HC, Coleman HA. The role of membrane potential in the control of uterine motility. In: Carsten ME, Miller JD, editors. Uterine Function. Molecular and Cellular Aspects. New York and London: Plenum Press; 1990. pp. 195–248. [Google Scholar]
- Pérez GJ, Toro L. Differential modulation of large-conductance KCa channels by PKA in pregnant and nonpregnant myometrium. American Journal of Physiology. 1994;266:C1459–1463. doi: 10.1152/ajpcell.1994.266.5.C1459. [DOI] [PubMed] [Google Scholar]
- Pérez GJ, Toro L, Erulkar SD, Stefani E. Characterization of large-conductance, calcium-activated potassium channels from human myometrium. American Journal of Obstetrics and Gynecology. 1993;168:652–660. doi: 10.1016/0002-9378(93)90513-i. [DOI] [PubMed] [Google Scholar]
- Song M, Barila B, Toro L, Stefani E. Changes of expression levels of the maxi K channel during pregnancy. Biophysical Journal. 1999;76:A412. abstract. [Google Scholar]
- Sunahara RK, Dessauer CW, Gilman AG. Complexity and diversity of mammalian adenylyl cyclases. Annual Review of Pharmacology and Toxicology. 1996;36:461–480. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- Tanfin Z, Goureau O, Milligan G, Harbon S. Characterization of G proteins in rat myometrium. A differential modulation of Gi2α and Gi3α during gestation. FEBS Letters. 1991;278:4–8. doi: 10.1016/0014-5793(91)80070-j. [DOI] [PubMed] [Google Scholar]
- Tang W-J, Gilman AG. Type-specific regulation of adenylyl cyclase by G protein βγ-subunits. Science. 1991;254:1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- Toro L, Stefani E, Erulkar S. Hormonal regulation of potassium currents in single myometrial cells. Proceedings of the National Academy of Sciences of the USA. 1990;87:2892–2895. doi: 10.1073/pnas.87.8.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivat V, Cohen-Tannoudji J, Revelli JP, Muzzin P, Giacobino JP, Maltier JP, Legrand C. Progesterone transcriptionally regulates β2-adrenergic receptor gene in pregnant rat myometrium. Journal of Biological Chemistry. 1992;267:7975–7978. [PubMed] [Google Scholar]
- Wang SY, Yoshino M, Sui JL, Wakui M, Kao PN, Kao CY. Potassium currents in freshly dissociated uterine myocytes from nonpregnant and late-pregnant rats. Journal of General Physiology. 1998;112:737–756. doi: 10.1085/jgp.112.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland T, Chen C-K. Regulators of G-protein signalling: a novel protein family involved in timely deactivation and desensitization of signalling via heterotrimeric G proteins. Naunyn-Schmiedeberg's Archives of Pharmacology. 1999;360:14–26. doi: 10.1007/s002109900031. [DOI] [PubMed] [Google Scholar]
- Wieland T, Ulibarri I, Gierschik P, Jakobs KH. Activation of signal-transducing guanine-nucleotide-binding regulatory proteins by guanosine 5′-[γ-thio] triphosphate. Information transfer by intermediately thiophosphorylated βγ subunits. European Journal of Biochemistry. 1991;196:707–716. doi: 10.1111/j.1432-1033.1991.tb15869.x. [DOI] [PubMed] [Google Scholar]
- Wray S. Uterine contraction and physiological mechanisms of modulation. American Journal of Physiology. 1993;264:C1–18. doi: 10.1152/ajpcell.1993.264.1.C1. [DOI] [PubMed] [Google Scholar]
- Wu YY, Goldfien A, Roberts JM. Alpha adrenergic stimulation reduces cyclic adenosine 3′,5′-monophosphate generation in rabbit myometrium by two mechanisms. Biology of Reproduction. 1988;39:58–65. doi: 10.1095/biolreprod39.1.58. [DOI] [PubMed] [Google Scholar]
- Zhou X-B, Ruth P, Schlossmann J, Hofmann F, Korth M. Protein phosphatase 2A is essential for the activation of Ca2+-activated K+ currents by cGMP-dependent protein kinase in tracheal smooth muscle and chineses hamster ovary cells. Journal of Biological Chemistry. 1996;271:19760–19767. doi: 10.1074/jbc.271.33.19760. [DOI] [PubMed] [Google Scholar]
- Zhou X-B, Schlossmann J, Hofmann F, Ruth P, Korth M. Regulation of stably expressed and native BK channels from human myometrium by cGMP- and cAMP-dependent protein kinase. Pflügers Archiv. 1998;436:725–734. doi: 10.1007/s004240050695. [DOI] [PubMed] [Google Scholar]
- Zhou X-B, Wang G-X, Schlossmann J, Ruth P, Hüneke B, Korth M. Regulation of BKCa channels by endogenous cGMP-dependent protein kinase in excised patches from pregnant and nonpregnant human myometrium. Naunyn-Schmiedeberg's Archives of Pharmacology. 1999;359:R75. abstract. [Google Scholar]


