Abstract
Isolated rat fetal distal lung epithelial (FDLE) cells were cultured (for 48 h) at PO2 levels between 23 and 142 mmHg. Higher PO2 levels between 23 and 142 mmHg. Higher PO2 was associated with increased short circuit current (ISC) and increased abundance of the Na+ channel protein α-ENaC. PO2 had no effect upon ISC remaining after apical application of amiloride (10 μM).
Studies of cells maintained (for 48 h) at PO2 levels of 23 mmHg or 100 mmHg, and subsequently nystatin permeabilized (50 μM), showed that high PO2 increased Na+ pump capacity. This response was apparent 24 h after PO2 was raised whilst it took 48 h for the rise in ISC seen in intact cells to become fully established. Both parameters were unaffected by raising PO2 for only 30 min.
Basolateral application of isoprenaline (10 μM) did not affect ISC in cells maintained at 23 mmHg but evoked progressively larger responses at higher PO2. The response seen at 142 mmHg was larger than at 100 mmHg, the normal physiological alveolar PO2.
Isoprenaline had no effect on Na+ pump capacity at PO2 levels of 23 mmHg or 100 mmHg, but stimulated Na+ extrusion at 142 mmHg. Increasing PO2 above normal physiological levels thus allows the Na+ pump to be controlled by isoprenaline. This may explain the enhanced sensitivity to isoprenaline seen under these slightly hyperoxic conditions.
Changes in PO2 mimicking those occurring at birth thus exert profound influence over Na+ transport in FDLE cells and the Na+ pump could be an important locus at which this control is exercised.
During fetal life, the distal lung epithelia secrete fluid into the developing airspaces (Olver & Strang, 1974) and establish a distending pressure that is crucial to lung morphogenesis (reviewed by Harding & Hooper, 1996). However, during the final stages of pregnancy, and particularly during labour, this liquid must be removed from the lungs so that the newborn infant can breathe at birth. This process is driven by the active absorption of Na+ from the lung lumen (Brown et al. 1983; Olver et al. 1986). During the perinatal period, the distal lung epithelia thus undergo a profound phenotypic transition from net secretion to net absorption. It is clear that the rise in fetal adrenaline seen during labour can trigger this switch by binding to β-adrenoceptors (Walters & Olver, 1978; Brown et al. 1983; Olver et al. 1986). However, adrenaline levels fall rapidly after birth and yet the lung retains its newly acquired, absorptive phenotype throughout adult life (for example see Matelon & O'Brodovich, 1999). It is therefore clear that factors other than acute regulation via β-adrenoceptors contribute to the control of alveolar ion transport. Recent studies have shown that pulmonary Na+ absorption is stimulated at high ambient PO2 and this raises the possibility that the increase in PO2 accompanying the newborn infant's first breaths might provide a stimulus capable of modifying the physiological properties of the alveolar epithelium (Pitkänen et al. 1996; Rafii et al. 1998). However, although these experiments established an important principal, they did not take into account the fact that alveolar PO2 does not normally rise above ∼100 mmHg (Haldane & Priestly, 1935). This earlier work thus failed to explore the effects of increasing PO2 from fetal (∼23 mmHg) to adult (∼100 mmHg) alveolar levels and so, at present, it is difficult to assess the physiological significance of this potentially important stimulus. This observation has prompted the present, detailed examination of the effects of PO2 upon ion transport in fetal distal lung epithelial (FDLE) cells.
METHODS
Isolation and culture of rat FDLE cells
Fetuses removed from anaesthetized (3 % halothane), 20 day pregnant (term = 22 days) rats were decapitated and their lungs carefully dissected free of the heart, trachea and as much of the bronchial tree as possible and collected into ice-cold, Ca2+- and Mg2+-free Hanks’ balanced salt solution (HBSS). Once all their fetuses had been removed, the anaesthetized rats were killed by exsanguination/cervical dislocation. This procedure was conducted in accordance with legislation currently in force in the UK and with the University of Dundee's animal welfare guidelines. The fetal lung tissue was then chopped into pieces (< 0.5 mm) and digested using 0.2 % trypsin-0.012 % DNAase (2 × 20 min, 37°C) followed by 0.1 % collagenase-0.012 % DNAase (15 min, 37°C); both enzyme solutions prepared in HBSS. The resultant fluid containing the digested cells was washed by centrifugation (400g, 5 min) and resuspended in Dulbecco's modified Eagle's medium containing 10 % (v/v) fetal calf serum (DMEM-FCS). The cells were incubated in a culture flask for 1 h, and the supernatant was then gently decanted in order to separate non-adherent epithelial cells from fibroblasts and smooth muscle cells which characteristically attach rapidly to plastic. After a second such fractionation, non-adherent cells were washed by repeated (4 times) centrifugation (130g, 3 min), resuspended in DMEM-FCS, and finally resuspended in serum-free PC-1 medium. Aliquots of this cell suspension were plated (1.5 × 106 cells cm−2) onto Transwell Col membranes (1 cm2, Costar, High Wycombe, Bucks, UK) and incubated for 18–24 h before any non-viable cells were removed by gently washing each culture. The remaining cells were incubated in fresh medium for a further 24 h before being used in experiments. All cells were therefore studied ∼48 h after isolation and were maintained (37°C) in a water-saturated atmosphere containing 5 % CO2. Previous work has shown that essentially all cells isolated in this way are of epithelial lineage and fibroblast contamination is negligible (Clunes et al. 1998). Moreover, in our hands, the isolation/culture protocol almost invariably (> 90 % of instances) yields cells that become integrated into coherent, epithelial layers with transepithelial resistances (Rt) > 200 Ω cm2.
Techniques used to explore the effects of changing PO2
The incubators used permitted control over PO2 by allowing the regulated introduction of N2. Experiments were therefore undertaken using cells cultured at O2 tensions between 23 mmHg and 142 mmHg. The latter value is the PO2 of water-saturated (PH2O, 47 mmHg) room air containing 5 % CO2 (PCO2, 38 mmHg). In most experiments the cells remained at a defined PO2 throughout the entire incubation period but, in some instances, cells were transferred into fresh medium that had been placed in a separate incubator set at a different PO2 and allowed to equilibrate. These shifts in PO2 were imposed 3–24 h before cultured epithelia were used in experiments. Some experiments, however, explored the effects of 30 min shifts in PO2. These were imposed once the preparations had been mounted in the Ussing chambers by changing the gas mixture used to circulate the bathing solution.
Quantification of transepithelial ion transport processes
Culture membranes bearing FDLE cells were mounted in Ussing chambers and bathed with bicarbonate-buffered saline (composition (mM): NaCl, 117; NaHCO3, 25, KCl, 4.7; MgSO4, 1.2; KH2PO4, 1.2; CaCl2, 2.5, D-glucose, 11; pH 7.3-7.4 when equilibrated with 5 % CO2) that was continuously circulated using gas lifts. Each preparation was first maintained under open circuit conditions whilst transepithelial potential difference (Vt) and resistance (Rt) were monitored. Once Vt had stabilized (10-20 min), the epithelial cells were short circuited (i.e. Vt held at 0 mV) using a DVC-1000 voltage/current clamp (World Precision Instruments, Stevenage Herts, UK). The current required to maintain Vt at 0 mV (short circuit current, ISC) was continually displayed, digitized (4 Hz) and recorded directly to computer disk using a PowerLab computer interface and associated software (ADInstruments, Hastings, East Sussex, UK). Positive currents are shown as upward deflections of the traces and were defined as those carried by cations moving from the apical to the basolateral solution. Experimentally induced changes in ISC were quantified by measuring the current flowing at the peak of a response and subtracting the current flowing measured prior to an experimental manipulation. Throughout each experiment, the preparations were frequently returned to open circuit conditions for 5–10 s so that the spontaneous Vt could be measured and Rt calculated.
Quantification of Na+ pump capacity
Short circuited epithelia were apically exposed to 10 μM amiloride in order to block their endogenous apical Na+ channels, and nystatin (50 μM) was then added to the apical solution to introduce an exogenous Na+ conductance into this membrane. As shown in Fig. 1, this caused a slowly developing rise in ISC. We have assumed this reflects the activity of the basolaterally located Na+- and K+-dependent ATPase (Na+,K+-ATPase) and is due to an increase in the internal Na+ concentration (for example see Lewis et al. 1977; Ito et al. 1997; Niisato & Marunaka, 1999). We subsequently measured the fall in ISC evoked by adding 1 mM ouabain (Fig. 1), an inhibitor of this enzyme, to the basolateral solution and have assumed that this fall in ISC provides an indicator of the overall capacity of this Na+ pump. The term capacity is taken to imply the product of the number of active Na+ pumps and the mean activity of the each pump. Changes in either of these parameters would thus have identical effects upon our estimate of pump capacity. Moreover, as ouabain never totally abolished the measured ISC (Fig. 1) we are aware that the present protocol may underestimate this parameter. However, we were forced to accept this limitation as initial experiments showed that the present concentrations of nystatin and ouabain were the highest that could be used without causing immediate loss of epithelial integrity.
Figure 1. Quantification of ouabain-sensitive ISC.
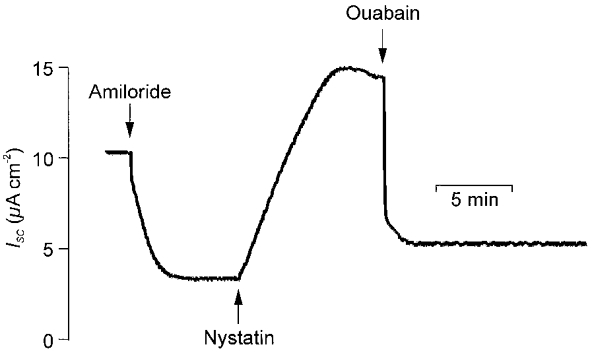
Cells maintained under adult alveolar conditions (PO2, 100 mmHg) were exposed to apical amiloride (10 μM) and nystatin (50 μM) as indicated. Nystatin evoked a slowly developing rise in ISC and ouabain (1 mM) was added to the basolateral solution once this response had reached its peak value. The fall in ISC evoked by this application of ouabain was measured in order to estimate the extrusion capacity of the basolateral Na+ pump.
Quantification of the abundance of the Na+ channel protein α-ENaC
FDLE cells grown (for 48 h) on Transwell (4.8 cm2) membranes were harvested by scraping into ice-cold 250 mM sucrose solution containing 10 mM triethanolamine-HCl (pH 7.6), DNAase I (2 μg ml−1) and protease inhibitors (26 μg ml−1 aprotinin, 10 μg ml−1 leupeptin, 10 μg ml−1 pepstatin A and 175 μg ml−1 4,2-aminoethyl-benzenesulphonyl fluoride hydrochloride) and disrupted by homogenization. The homogenate was centrifuged (15 min, 250g) to remove nuclei and large debris, and a crude membrane fraction was then prepared by high-speed centrifugation (30 min, 14000g). These membranes were resuspended in the buffered sucrose solution and stored at -70°C pending analysis. Standard techniques were then used to fractionate 40 μg aliquots of membrane protein on sodium dodecyl sulphate-polyacrylamide gels. Fractionated proteins were transferred to nitrocellulose membranes by semi-dry electrophoresis and then probed for α-ENaC and actin by Western blot analysis. The anti α-ENaC antiserum was used at a dilution of 1:500 and was kindly provided by C. Canessa (Yale University, USA). Previous studies have confirmed the specificity of this antiserum (Duc et al. 1994). Anti-actin was from Sigma (Poole, Dorset, UK). Positive bands were detected by enhanced chemiluminescence (Amersham ECL kit, Amersham, UK).
Experimental design and data analysis
Typically, we could obtain several cultured epithelial cells from each isolation procedure and so control and experimental cells were always age-matched and derived from the same litter. Pooled data are presented as means ± s.e.m. and n values refer to the number of observations made using cultures obtained from different litters. Unless otherwise stated, the statistical significance of any differences between mean values were tested using statistical tests appropriate for such a strictly paired experimental design. Where an experiment explored the effects of a single factor, the differences between mean values were assessed using Student's paired t test. Data derived from more complex protocols, however, were first inspected by analysis of variance. If this analysis showed that the data were not derived from a single population, the significance of differences between particular pairs of values was subsequently tested using Student's t test followed by Bonferroni's post hoc correction.
RESULTS
Effects of PO2 upon ISC in unstimulated cells
Cultured epithelia consistently generated ISC but larger currents were recorded from cells maintained at high PO2 (Fig. 2). Analysis of these data showed that the PO2 required for half-maximal activation of ISC was 38.2 ± 0.5 mmHg and that maximal activation occurred when PO2 was > 80 mmHg. However, only ∼80 % of the maximum observable ISC appeared to be subject to PO2-dependent control and extrapolation of the sigmoid curve fitted to these data (Fig. 2) suggested that cells maintained in an O2-free environment would generate a current of 2.2 ± 0.2 μA cm−2. Apically applied amiloride (10 μM), a drug that blocks epithelial Na+ channels, evoked a rapid fall in ISC at each O2 tension but did not abolish this current. Interestingly, the amiloride resistant ISC was always ∼2 μA cm−2 and so our data indicate that raised PO2 stimulates ISC by activating amiloride-sensitive Na+ absorption (Fig. 2).
Figure 2. The relationship between PO2 and ISC.
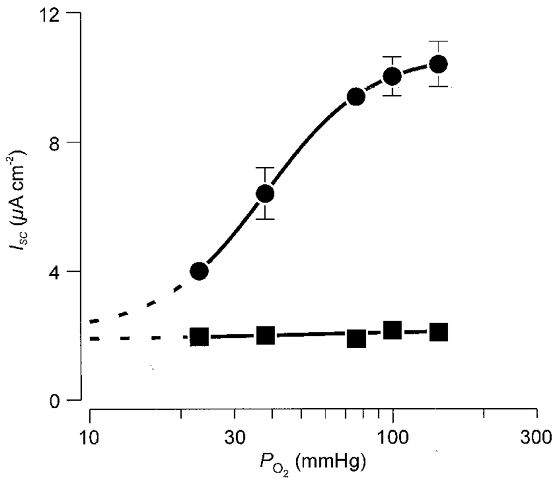
In each experiment ISC was first measured under control conditions (•) and after apical exposure to 10 μM amiloride (▪) and a second estimate of ISC was made once the current had stabilized at its new value (5-10 min). These data (n = 7) are plotted (means ±s.e.m.) against ambient PO2. The sigmoid curve was fitted to these data using a least squares regression procedure implemented in a commercially available software package (GraFit 4.1, Erithacus Software, Staines, UK). For this analysis, each point was weighted according to the reciprocal of its standard error. The figure also includes a line fitted to the data obtained from amiloride-treated cells.
Effects of PO2 upon α-ENaC protein abundance
The Na+ channel protein α-ENaC could be detected in membranes isolated from cells maintained (for 48 h) at fetal PO2 but was ∼3-fold more abundant (P < 0.05, Student's paired t test) in cells cultured at 142 mmHg (Fig. 3). Further analysis of these data revealed a statistically significant correlation (r = 0.959, P < 0.05) between the membrane abundance of α-ENaC and ambient PO2. Moreover, there was also a significant correlation between the abundance of α-ENaC and the ISC measured in unstimulated cells. The relationship between these parameters could be described almost perfectly (r = 0.996, P < 0.001) by a line whose slope was 3.37 ± 0.2 μA cm−2 (α-ENaC abundance unit)−1 and whose y intercept did not differ significantly from zero (0.34 ± 0.043 μA cm−2). The level of α-ENaC abundance is thus influenced by ambient PO2 and provides an excellent predictor of the net rate of transepithelial ion transport.
Figure 3. α-ENaC abundance in cells maintained at different PO2 levels.
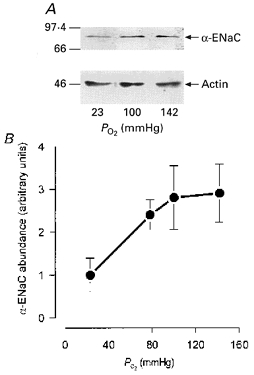
A, nitrocellulose membranes bearing fractionated proteins extracted from cultured FDLE cells that had been continuously maintained at different PO2 levels were probed using antibodies against α-ENaC and actin. Numbers to the left indicate the position of the appropriate molecular mass markers in kDa. The relative abundance of α-ENaC measured in this, and in other experiments, was measured and the pooled data from four independent analyses are plotted (means ±s.e.m.) against the ambient PO2 in B.
Responses to isoprenaline in cells maintained at different PO2 levels
Cells maintained at a range of PO2 levels (23-100 mmHg) were exposed to basolateral isoprenaline (10 μM). This evoked a barely discernible response when PO2 was 23 mmHg but elicited successively larger increases in ISC at higher PO2. Each response consisted of a slowly developing rise to a plateau that was reached after 30–40 min (Fig. 4). However, when PO2 was 142 mmHg this rise was preceded by an initial transient peak. Moreover, under these conditions, the ISC seen after 30–40 min exposure to isoprenaline was larger (P < 0.005) than at the normal adult alveolar PO2 (100 mmHg). Sensitivity to isoprenaline thus increases as PO2 rises above its normal physiological range.
Figure 4. Responses to basolaterally applied isoprenaline in cells maintained at different PO2 levels.
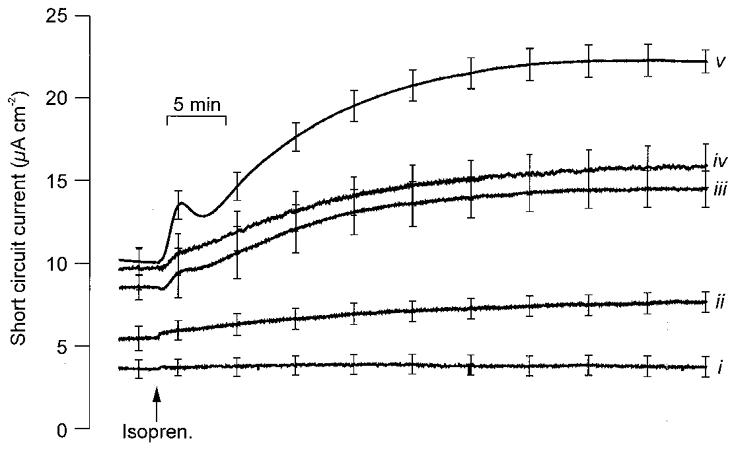
The responses evoked by basolateral application of 10 μM isoprenaline (Isopren.) in cultured epithelial cells maintained (for 48 h) at different PO2 levels (i, 23 mmHg, n = 10; ii, 38 mmHg, n = 10; iii, 76 mmHg, n = 8; iv, 100 mmHg, n = 7; v, 142 mmHg, n = 9). Traces show mean responses whilst the vertical bars indicate the s.e.m.
Responses to isoprenaline in cells transferred between fetal PO2 and adult alveolar PO2
Cells initially maintained at fetal PO2 (23 mmHg) or at adult alveolar PO2 (100 mmHg) were switched between these two atmospheres ∼24 h after isolation and basal ISC and the response to adrenaline measured after a further 24 h at the new PO2. These data were compared with the corresponding values derived from control cells maintained continuously under the original conditions. The control data confirm that cells maintained continuously at fetal PO2 (n = 6) generate smaller basal ISC (3.0 ± 0.7 μA cm−2) than cells maintained continuously at adult alveolar PO2 (8.1 ± 1.2 μA cm−2, n = 7, P < 0.05). Moreover, isoprenaline did not evoke a discernible response under fetal conditions (Fig. 5A) but consistently elicited a clear rise in ISC (5.5 ± 0.5 μA cm−2, P < 0.0001) in the adult alveolar environment (Fig. 5B). Transferring cells from the fetal to the adult alveolar environments for 24 h (n = 6) caused basal ISC to rise to a significantly elevated value (6.6 ± 0.6 μA cm−2, P < 0.01) and allowed isoprenaline to evoke a further rise in ISC (Fig. 5A, 3.1 ± 0.3 μA cm−2, P < 0.0001). However, basal ISC recorded in cells (n = 7) that had been transferred from adult alveolar to fetal PO2 (3.8 ± 0.6 μA cm−2) did not differ significantly from that seen in cells maintained continuously under fetal conditions (Fig. 5). However, isoprenaline did elicit a clear response in these cells (increase in ISC 1.2 ± 0.5 μA cm−2, P < 0.05) although the rise in ISC was smaller (P < 0.002) than in cells maintained continuously at adult alveolar PO2 (Fig. 5B).
Figure 5. Responses to isoprenaline in cells transferred between fetal and alvolar PO2.
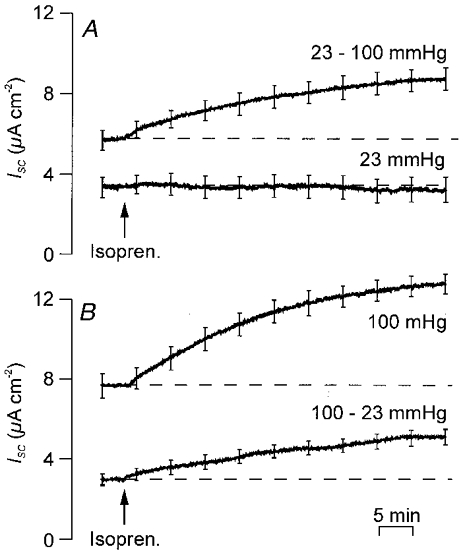
FDLE cells were maintained in gas mixtures that mimicked either the fetal (PO2 = 23 mmHg) or adult alveolar (PO2 = 100 mmHg, n = 6) environments for 24 h. Experimental cells were then transferred between the two environments and, after a further 24 h, their responses to basolateral isoprenaline (10 μM) were monitored. These data are presented (means ±s.e.m.) together with the responses of age-matched control cells that had been isolated from the same litters and continuously maintained at the original PO2. A, responses of cells initially maintained at 23 mmHg (n = 7). B, responses of cells initially maintained at neonatal PO2 (100 mmHg, n = 6).
Quantification of Na+ pump capacity
Na+ pump capacity was ∼2.5-fold greater in cells maintained at adult alveolar PO2 than in cells maintained at fetal PO2 (Fig. 6). Increased capacity was also evident in cells that had been exposed to adult alveolar PO2 for only 24 h; indeed, the currents measured under these conditions did not differ significantly from those in the cells continuously maintained at adult alveolar PO2 (Fig. 6). Increased Na+ pump capacity was also evident in cells that were initially maintained at adult alveolar PO2 but then transferred to a fetal atmosphere for 24 h, although these currents were smaller (P < 0.01) than in cells maintained continuously at 100 mmHg. Further experiments showed that the significant stimulation of the Na+ pump became apparent 6 h after PO2 was raised and that significant loss of Na+ pump capacity was apparent 6 h after PO2 was lowered (Fig. 7). Briefer shifts in PO2 had no significant effect.
Figure 6. Effects of isoprenaline upon ouabain-sensitive ISC at fetal and neonatal PO2.
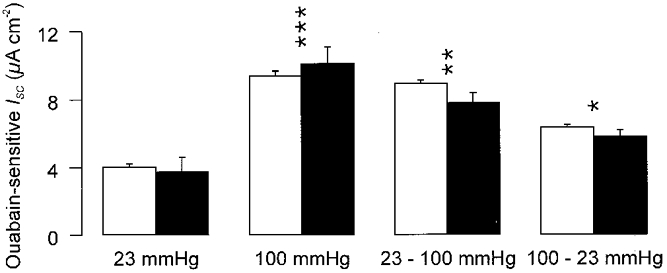
FDLE cells were initially maintained at a PO2 of 23 or 100 mmHg for 24 h and then either transferred between these two environments or allowed to remain at their original PO2. Both groups of cells were then cultured for a further 24 h before being mounted in Ussing chambers where ouabain-sensitive ISC was quantified in both unstimulated cells (□) and cells that had been exposed to isoprenaline for 30–40 min (▪). Experiments were undertaken using a strictly paired experimental protocol in which all measurements were made using cells isolated from the same litters (n = 7). Analysis of variance revealed that the data from unstimulated and isoprenaline-stimulated cells belonged to the same statistical population. We thus conclude that this drug has no effect upon the ouabain-sensitive currents recorded from cells maintained under any of the culture regimes tested. This did show, however, that the different culture regimes used provided a statistically significant source of variability between the data. Student's t test followed by Bonferroni's post hoc correction was therefore used to compare the data obtained from cells that had been exposed to increased PO2 for all or part of the 48 h culture period with the equivalent data from cells maintained continuously at 23 mmHg. The results of this analysis are indicated by asterisks (***P < 0.02, **P < 0.01, *P < 0.05).
Figure 7. PO2-evoked changes in Na+ pump capacity.
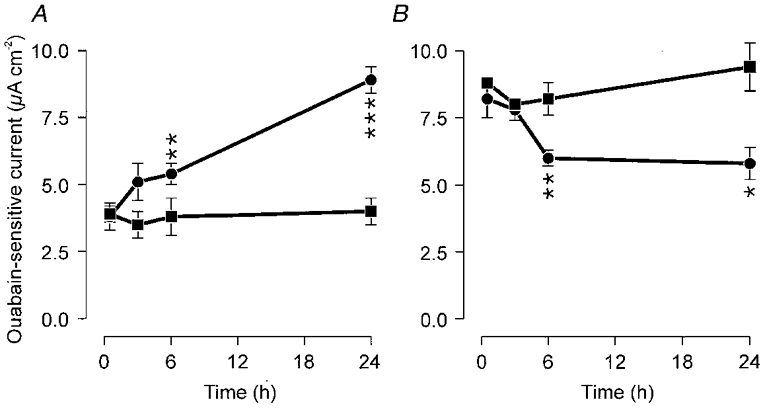
Cells isolated from pups in the same litter were maintained in vitro for a total of ≈48 h before Na+ pump capacity was quantified. A, Na+ pump capacity in cells that had first been maintained at a PO2 of 23 mmHg before being transferred to an adult alveolar (100 mmHg) atmosphere for the final 0.5-24 h of this incubation period is plotted against the length of time that the cells had been exposed to elevated PO2 (•, means ±s.e.m.). The figure also shows Na+ pump currents measured in control cells isolated from the same litters that had been maintained at 23 mmHg throughout the entire 48 h incubation period (▪). B, data from analogous experiments in which Na+ pump currents were measured in cells that had been maintained at the adult alveolar PO2 before being transferred to the fetal atmosphere for the final 0.5-24 h of the incubation period (•) or in age-matched control cells from the same litters (▪). The significance of any differences between the mean values was assessed using Student's paired t test. ***P < 0.001, **P < 0.01, *P < 0.05.
The data presented in Fig. 6 show that isoprenaline failed to have any significant effect upon Na+ pump capacity in cells maintained under any of the culture regimes shown in Fig. 5. This was surprising, as β-adrenoceptor agonist-stimulated Na+ pump current has been documented in these cells (Ito et al. 1997). However, a separate series of experiments in which cells derived from the same litters (n = 4) were maintained in water saturated room air containing 5 % CO2 (PO2 = 142 mmHg) showed that isoprenaline did evoke ∼15 % stimulation of Na+ pump capacity under these conditions (control: 10.6 ± 0.8 μA cm−2; isoprenaline-stimulated: 14.5 ± 3.2 μA cm−2; P < 0.05, Student's paired t test).
DISCUSSION
The first part of the present study shows that basal Na+ transport is enhanced in FDLE cells maintained at elevated PO2 which is consistent with previous data (Barker & Gatzy, 1993; Pitkänen et al. 1996; Rafii et al. 1998). The present study, however, extends upon this work by establishing that half-maximal stimulation of Na+ transport occurs at a PO2 of ∼40 mmHg and by showing that the effect is maximal at O2 tensions above ∼80 mmHg. We also show that α-ENaC, a protein thought to form an apical Na+ channel in FDLE cells (for example see Voilley et al. 1994; Hummler et al. 1996; Jain et al. 1999; Matelon & O'Brodovich, 1999), is more abundant in cells maintained at high PO2 for 48 h. This also accords with earlier work, which showed that raised PO2 increases the abundance of mRNA encoding α-ENaC, as well as the closely-related β- and γ-ENaC isoforms (Pitkänen et al. 1996). The expression of these proteins thus appears to be subject to PO2-dependent control in isolated FDLE cells (present study; Pitkänen et al. 1996; Rafii et al. 1998), type II pneumocytes of the adult lung (Yue et al. 1995; Planes et al. 1997; Yue & Matalon, 1997) and a number of other systems (for example see Portier et al. 1999).
Whilst it is tempting to attribute the PO2-evoked rise in Na+ transport to the increased abundance of α-ENaC, the earlier work did show that no rise in α-ENaC mRNA levels occurred unless the cells had been continuously exposed to increased PO2 for between 18 and 48 h (Pitkänen et al. 1996). Increased Na+ transport, however, was evident after only 18 h (Pitkänen et al. 1996). Moreover, Rafii et al. (1998) showed that the PO2-evoked stimulation of Na+ transport reversed within 48 h, and the present data show that the response can be completely attenuated by returning the cells to a fetal atmosphere for 24 h. The stimulation of Na+ transport is thus a reversible phenomenon. It has recently become clear, however, that the mRNA species encoding α-ENaC has a cellular half-life of over 20 h (Otulakowski et al. 1999). It is difficult to see how increased expression of such a remarkably stable mRNA species could underlie the relatively rapid and reversible changes in Na+ transport seen when FDLE are exposed to increased PO2. However, as well as evoking increased expression of the α-ENaC gene, it is possible that increased PO2 also stimulates the translation of pre-existing RNA or promotes the insertion of α-ENaC protein into the apical plasma membrane (for example see Pitkänen et al. 1996). Both of these events could allow increased apical Na+ entry to precede any genomic effects of increased PO2.
Whilst increased abundance and/or activity of α-ENaC may facilitate apical Na+ influx (Hummler et al. 1996; Jain et al. 1999; Matelon & O'Brodovich, 1999), Na+ entering the cell in this way must be extruded by basolateral Na+,K+-ATPase (Lewis et al. 1977) and the present data show that increasing PO2 from fetal to adult alveolar levels caused a ∼2.5-fold increase in the capacity of this Na+ pump. This response could be detected 6 h after PO2 was increased and was fully established by 24 h. It is unlikely to be a metabolic consequence of raising PO2 from hypoxic levels as parallel studies of these cells showed that cellular adenylate energy charge (i.e. the ratio [ATP ] + ½[ADP]/[ATP]+[ADP]+[AMP]) is maintained at value of ∼0.7 even if PO2 is lowered to 23 mmHg (Haddad & Land, 2000). Moreover, the PO2-evoked stimulation of the Na+ pump was persistent. Indeed the effect could be discerned 24 h after the cells had been returned to a fetal environment even though ISC measured in intact cells was essentially normal by this time. This discrepancy shows clearly that the rate of transepithelial Na+ transport is not simply determined by the capacity of the Na+ pump; this is consistent with the view that the rate of apical Na+ entry normally limits transepithelial Na+ transport in absorptive epithelia (for example see Lewis et al. 1977). This discrepancy also suggests strongly that the increased Na+ pump capacity is not secondary to increased Na+ entry due to increased α-ENaC abundance/activity. However, this possibility cannot be formally excluded at present but the issue may be resolved by undertaking detailed electrophysiological studies of FDLE cells (for example see Marunaka et al. 1999) maintained at different oxygen tensions.
There is, however, clear evidence from earlier studies that the activity of the Na+ pump is subject to PO2-dependent control. Hypoxia thus causes loss of Na+,K+-ATPase activity in type II pneumocytes isolated from adult rats (Planes et al. 1997) whilst rats maintained in a hypoxic environment show reduced alveolar fluid clearance and Na+,K+-ATPase activity (Suzuki et al. 1999). Furthermore, pulmonary Na+,K+-ATPase activity is increased in animals exposed to profoundly hyperoxic environments (for example see Carter et al. 1997). The present study certainly shows that the PO2-evoked stimulation of the Na+ pump is much more rapid than the increase in α-ENaC mRNA abundance and this raises the possibility that control over the Na+ pump may be one of the primary mechanisms by which increases in PO2 are transduced into increased Na+ transport. Increased Na+ pump activity may lower the intracellular Na+ concentration ([Na+]i) which could have important consequences as [Na+]i is an major determinant of apical Na+ conductance. Indeed, in salivary epithelia, G protein-dependent signalling pathways allow [Na+]i to control the activity of apical Na+ channels and regulate the number of such channels present in this membrane (Komwatana et al. 1996, 1998; Dinudom et al. 1998; Harvey et al. 1999). A PO2-evoked rise in Na+ pump capacity could thus initiate a series of events leading to enhanced Na+ transport with no need for increased α-ENaC expression. Interestingly, studies of an epithelial cell line suggest that such effects of increased PO2 upon Na+ pump capacity may reflect increased expression of genes encoding Na+,K+-ATPase subunits (Wendt et al. 1998a,b).
Many previous studies have shown that β2-adrenoceptor agonists increase Na+ absorption in FDLE cells. This response is due to (i) the activation of Na+-permeable channels in the apical membrane; (ii) the insertion of additional Na+-permeable channels into this membrane, and (iii) increased Na+ pump capacity (Tohda et al. 1994; Ito et al. 1997; Marunaka et al. 1999). However, these experiments were undertaken using cells maintained at high PO2 and, to our knowledge, the present study is the first to explore the effects of PO2 upon the FDLE cell sensitivity to a β-adrenoceptor agonist. These data show that the cells are essentially insensitive to isoprenaline at fetal PO2 but that this drug evokes progressively larger responses at successively higher O2 tensions. This was surprising as lung liquid clearance in fetal sheep is clearly subject to β-adrenoceptor-mediated control in utero (Brown et al. 1983; Olver et al. 1986). Moreover, this observation has been repeatedly confirmed in several species and the view that adrenaline stimulates lung Na+ absorption under fetal conditions has become almost axiomatic (for example see Matelon & O'Brodovich, 1999). However, the studies of fetal sheep did show that adrenaline only stimulates Na+ absorption in mature fetuses and it is known that rat lungs are relatively undifferentiated at birth (Oosterhuis et al. 1984). It is therefore possible that rat FDLE cells maintained at fetal PO2 are insensitive to isoprenaline because they normally maintain a fetal phenotype at a gestational age of 20 days. If this is accepted, then our data suggest strongly that raised PO2 rapidly causes precocious maturation of these cells (see also Pitkänen et al. 1996). This implies that the rat FDLE cells used in almost all previous studies expressed a neonatal, or even an adult, phenotype at the time they were actually used in experiments. It is therefore clear that studies of FDLE cells will only reveal the physiological changes that occur in the perinatal period if undertaken using protocols designed carefully to ensure that the fetal phenotype is maintained.
Although cells continuously maintained at fetal PO2 were insensitive to isoprenaline, this drug evoked responses in cells that had been first maintained at adult alveolar PO2 and then transferred to a fetal atmosphere for 24 h. This manoeuvre did not affect basal ISC, but it was clear that the capacity of the Na+ pump was still elevated. This raises the possibility that isoprenaline may increase apical Na+ conductance at fetal PO2, but that this does not lead to any increase in transepithelial Na+ transport unless the cells have also been exposed to increased PO2. This may increase Na+ pump capacity and provide a driving force for Na+ absorption.
Previous work suggested that β-adrenoceptor agonists could acutely regulate the activity of the Na+ pump (Ito et al. 1997) but the present study shows that this only occurs when PO2 is above the normal physiological adult alveolar value. Interestingly, the responses to isoprenaline measured in intact cells were also augmented under these conditions and this enhanced sensitivity may reflect this additional control over Na+ extrusion. Taken together with the earlier work (Tohda et al. 1994; Ito et al. 1997; Marunaka et al. 1999), our data suggest that β-adrenoceptor agonists normally control apical Na+ conductance but that control over the Na+ pump also occurs if PO2 rises above its normal physiological range. This may well be a pathological response to hyperoxia.
The present data, in common with those recently presented by Round et al. (1999), thus show that physiologically relevant increases PO2 have the potential to influence ion transport processes in the alveolar region (Bland & Boyd, 1986; Barker & Gatzy, 1993; Pitkänen et al. 1996; Rafii et al. 1998). However, whilst previous work has generally attributed these effects to PO2-dependent control of α-ENaC expression, our data raise the possibility that the Na+ pump may be an important locus at which such control is exercised.
Acknowledgments
This study was made possible by grants from the Wellcome Trust and Tenovus and a Post Graduate Studentship from the University of Dundee (S.J.R.). The authors are grateful to Helen Murphie for her skilled technical assistance and to Andrew Collett, Stephan Land, Aleksander Jovanovic and Sarah Inglis (University of Dundee) for their helpful comments and discussions. S.M.W. is particularly grateful to Yoshinori Marunaka and Naomi Niisato (University of Toronto) for kindly demonstrating the nystatin-permeabilization technique.
References
- Barker PM, Gatzy JT. Effect of gas composition on liquid secretion by explants of distal lung of fetal rat in submersion culture. American Journal of Physiology. 1993;265:L512–517. doi: 10.1152/ajplung.1993.265.5.L512. [DOI] [PubMed] [Google Scholar]
- Bland RD, Boyd CAR. Cation transport in lung epithelial cells derived from fetal, newborn and adult rabbits. Journal of Applied Physiology. 1986;61:507–515. doi: 10.1152/jappl.1986.61.2.507. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Olver RE, Ramsden CA, Strang LB, Walters DV. Effects of adrenaline and of spontaneous labour on the secretion and absorption of lung liquid in the fetal lamb. The Journal of Physiology. 1983;344:137–152. doi: 10.1113/jphysiol.1983.sp014929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter EP, Wangensteen OD, O'Grady SM, Ingbar DH. Effects of hyperoxia on type II cell Na-K-ATPase function and expression. American Journal of Physiology. 1997;272:L542–551. doi: 10.1152/ajplung.1997.272.3.L542. [DOI] [PubMed] [Google Scholar]
- Clunes MT, Collett A, Baines DL, Bovell DL, Murphie H, Inglis SK, McAlroy HL, Olver RE, Wilson SM. Culture substrate-specific expression of P2Y2 receptors in distal lung epithelial cells isolated from foetal rats. British Journal of Pharmacology. 1998;124:845–847. doi: 10.1038/sj.bjp.0701942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinudom A, Harvey KF, Komwatana P, Young JA, Kumar S, Cook DI. Nedd4 mediates control of an epithelial Na+ channel in salivary duct cells by cytosolic Na+ Proceedings of the National Academy of Sciences of the USA. 1998;95:7169–7173. doi: 10.1073/pnas.95.12.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc C, Farman N, Canessa CM, Bonvalet JP, Rossier BC. Cell-specific expression of epithelial sodium channel alpha, beta, and gamma subunits in aldosterone-responsive epithelia from the rat: localisation by in situ hybridization and immunocytochemistry. Journal of Biological Chemistry. 1994;127:1907–1921. doi: 10.1083/jcb.127.6.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad JJE, Land SC. O2-evoked regulation of HIF-α and NF-κB in perinatal lung epithelium requires glutathione biosynthesis. American Journal of Physiology. 2000 doi: 10.1152/ajplung.2000.278.3.L492. in the Press. [DOI] [PubMed] [Google Scholar]
- Haldane JS, Priestly JG. Respiration. 2. Oxford: Clarendon Press; 1935. [Google Scholar]
- Harding R, Hooper SB. Regulation of lung expansion and lung growth before birth. Journal of Applied Physiology. 1996;81:209–224. doi: 10.1152/jappl.1996.81.1.209. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Dinudom A, Komwatana P, Jolliffe CN, Day ML, Parasivam G, Cook DI, Kumar S. All three WW domains of murine Nedd4 are involved in the regulation of epithelial sodium channels by intracellular Na+ Journal of Biological Chemistry. 1999;274:12525–12530. doi: 10.1074/jbc.274.18.12525. [DOI] [PubMed] [Google Scholar]
- Hummler E, Baker P, Gatzy J, Berrmann F, Verdumo C, Schmidt A, Boucher R, Rossier RC. Early death due to defective neonatal lung liquid clearance in α-ENaC-deficient mice. Nature Genetics. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- Ito Y, Niisato N, O'Brodovich H, Marunaka Y. The effects of brefeldin A on terbutalin-induced sodium absorption in fetal rat distal lung epithelium. Pflügers Archiv. 1997;434:492–494. doi: 10.1007/s004240050425. [DOI] [PubMed] [Google Scholar]
- Jain L, Chen X-C, Malik B, Al-Khalili O, Eaton DC. Antisense oligonucleotides against the α-subunit of ENaC decrease lung epithelial cation channel activity. American Journal of Physiology. 1999;276:L1046–1051. doi: 10.1152/ajplung.1999.276.6.L1046. [DOI] [PubMed] [Google Scholar]
- Komwatana P, Dinudom A, Young JA, Cook DI. Cytosolic Na+ controls an epithelial Na+ channel via the Go guanine nucleotide-binding regulatory protein. Proceedings of the National Academy of Sciences of the USA. 1996;93:8107–8111. doi: 10.1073/pnas.93.15.8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komwatana P, Dinudom A, Young JA, Cook DI. Activators of epithelial Na+ channels inhibit cytosolic feedback control. Evidence for the existence of a G protein-coupled receptor for cytosolic Na+ Journal of Membrane Biology. 1998;162:225–232. doi: 10.1007/s002329900360. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Eaton DC, Clausen C, Diamond JM. Nyastatin as a probe for investigating the electrical properties of a tight epithelium. Journal of General Physiology. 1977;70:427–440. doi: 10.1085/jgp.70.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marunaka Y, Niisato N, O'Brodovich H, Eaton DC. Regulation of an amiloride-sensitive Na+-permeable channel by a β2-adrenergic agonist, cytosolic Ca2+ and Cl− in fetal rat alveolar epithelium. The Journal of Physiology. 1999;515:669–683. doi: 10.1111/j.1469-7793.1999.669ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matelon S, O'Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, bipohysical properties and physiological significance. Annual Review of Physiology. 1999;61:627–661. doi: 10.1146/annurev.physiol.61.1.627. [DOI] [PubMed] [Google Scholar]
- Niisato N, Marunaka Y. Activation of the Na+-K+ pump by hyposmolality through tyrosine kinase-dependent Cl− conductance in Xenopus renal epithelial A6 cells. The Journal of Physiology. 1999;518:417–432. doi: 10.1111/j.1469-7793.1999.0417p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olver RE, Ramsden CA, Strang LB, Walters DV. The role of amiloride-blockable sodium transport in adrenaline-induced lung liquid reabsorption in the fetal lamb. The Journal of Physiology. 1986;376:321–340. doi: 10.1113/jphysiol.1986.sp016156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olver RE, Strang LB. Ion fluxes across the pulmonary epithelium and the secretion of lung liquid in the fetal lamb. The Journal of Physiology. 1974;241:327–357. doi: 10.1113/jphysiol.1974.sp010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterhuis WP, Mooren PG, Charles R, Lamers WH. Perinatal development of the lung in rat and spiny mouse: its relation to altricial and precocial timing at birth. Biology of the Neonate. 1984;45:236–243. doi: 10.1159/000242011. [DOI] [PubMed] [Google Scholar]
- Otulakowski G, Raffii B, Bremner HR, O'Brodovich H. Structure and hormone responsiveness of the gene encoding the α-subunit of the rat amiloride-sensitive epithelial sodium channel. American Journal of Respiration: Cellular and Molecular Biology. 1999;20:1028–1040. doi: 10.1165/ajrcmb.20.5.3382. [DOI] [PubMed] [Google Scholar]
- Pitkänen OM, Tanswell AK, Downey G, O'Brodovich H. Increased PO2 alters the bioelectric properties of the fetal distal lung epithelium. American Journal of Physiology. 1996;270:L1060–1066. doi: 10.1152/ajplung.1996.270.6.L1060. [DOI] [PubMed] [Google Scholar]
- Planes C, Escubet B, Blot-Charbaud M, Friedlander G, Farman N, Clerici C. Hypoxia down regulates expression and activity of epithelial sodium chanels in rat alveolar epithelial cells. American Journal of Respiration: Cellular and Molecular Biology. 1997;17:508–518. doi: 10.1165/ajrcmb.17.4.2680. [DOI] [PubMed] [Google Scholar]
- Portier F, van den Abbeele T, Lecain E, Sauvaget E, Escorbet B, Tran Ba, Huy P, Herman P. Oxygen modulates Na+ absorption in middle ear epithelium. American Journal of Physiology. 1999;276:C312–317. doi: 10.1152/ajpcell.1999.276.2.C312. [DOI] [PubMed] [Google Scholar]
- Rafii B, Tanswell AK, Otulakowski G, Pitkanen O, Belcastro-Taylor R, O'Brodovich H. O2-induced ENaC expression is associated with NF-kB activation and blocked by superoxide scavenger. American Journal of Physiology. 1998;275:L764–770. doi: 10.1152/ajplung.1998.275.4.L764. [DOI] [PubMed] [Google Scholar]
- Round JEC, Junor RWJ, Gallacher ME, Walters DV. The effects of in vivo pulmonary oxygenation on lung liquid production in near-term fetal sheep. Experimental Physiology. 1999;84:725–738. [PubMed] [Google Scholar]
- Suzuki S, Noda M, Sugita M, Ono S, Koike K, Fujimura S. Impairment of transalveolar fluid transport and lung Na+-K+-ATPase function by hypoxia in rats. Journal of Applied Physiology. 1999;87:962–968. doi: 10.1152/jappl.1999.87.3.962. [DOI] [PubMed] [Google Scholar]
- Tohda H, Foskett JK, O'Brodovich H, Marunaka Y. Cl− regulation of a Ca2+-activated nonselective cation channel in β-agonist-treated fetal distal lung epithelium. American Journal of Physiology. 1994;266:C104–109. doi: 10.1152/ajpcell.1994.266.1.C104. [DOI] [PubMed] [Google Scholar]
- Voilley N, Lingueglia E, Champigny G, Mattei MG, Waldmann R, Lazdunski M, Barbry P. The lung amiloride-sensitive Na+ channel: biophysical properties, pharmacology, ontogenesis, and molecular cloning. Proceedings of the National Academy of Sciences of the USA. 1994;91:247–251. doi: 10.1073/pnas.91.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters DV, Olver RE. The role of catecholamines in lung liquid absorption at birth. Pediatric Research. 1978;12:239–242. doi: 10.1203/00006450-197803000-00017. [DOI] [PubMed] [Google Scholar]
- Wendt CH, Sharma R, Bair R, Towle H, Ingbar DH. Oxidant effects on epithelial Na, K-ATPase gene expression and promoter function. Environmental Health Perspectives. 1998a;106:1213–1217. doi: 10.1289/ehp.98106s51213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt CH, Towle H, Sharma R, Duvick S, Kawakami K, Gick G, Ingbar DH. Regulation of Na-K-ATPase gene expression by hyperoxia in MDCK cells. American Journal of Physiology. 1998b;274:C356–364. doi: 10.1152/ajpcell.1998.274.2.C356. [DOI] [PubMed] [Google Scholar]
- Yue G, Matalon S. Mechanisms and sequelae of increased alveolar fluid clearance in hyperoxic rats. American Journal of Physiology. 1997;272:L407–412. doi: 10.1152/ajplung.1997.272.3.L407. [DOI] [PubMed] [Google Scholar]
- Yue G, Russell WJ, Benos DJ, Jackson RM, Olman MA, Matelon S. Increased expression and activity of sodium channels in alveolar type II cells of hyperoxic rats. Proceedings of the National Academy of Sciences of the USA. 1995;92:8418–8422. doi: 10.1073/pnas.92.18.8418. [DOI] [PMC free article] [PubMed] [Google Scholar]


