Abstract
The synaptic localization of ion channel receptors is essential for efficient synaptic trans-mission and the precise regulation of diverse neuronal functions, such as signal integration and synaptic plasticity. Emerging evidence points to an important role of cytoskeleton-associated proteins that assemble receptors and components of the subsynaptic machinery at postsynaptic membrane specializations. This article reviews interactions of inhibitory postsynaptic neurotransmitter receptors with the receptor anchoring protein gephyrin and intracellular components involved in downstream signalling and/or control of signal transduction processes. The presently available data suggest a central synaptic organizer function for gephyrin in inhibitory postsynaptic membrane assembly and stabilization.
The surface membranes of neurons contain a wide variety of ion channel and receptor proteins that mediate intercellular communication in the nervous system. These proteins do not diffuse freely in the plasma membrane, but are localized at specific sites specialized for chemotransmission between neurons, the synapses. Notably, synapses are not only sites of interneuronal communication but also concentrate components of different intracellular signalling cascades, thereby forming modular units uniquely equipped for regulating complex cellular functions such as learning and memory processing.
A high concentration of neurotransmitter-gated ion channels underneath appropriate presynaptic nerve terminals is a prerequisite for efficient synaptic transmission. Different lines of evidence indicate that receptor-associated proteins are crucial for concentrating and anchoring such receptors in the postsynaptic membrane of central synapses. Various subtypes of excitatory glutamate receptors have been found to associate with cytosolic and cytoskeletal components via PDZ domain (named after the proteins PSD 95, Dlg and ZO-1) mediated protein-protein interactions (Sheng, 1996; Craven & Bredt, 1998). In contrast, inhibitory receptors and their interaction partners appear to associate via different mechanisms (for review, see Kirsch, 1999). The tubulin binding protein gephyrin has been shown to play a crucial role in the postsynaptic clustering of both glycine receptors (GlyRs) and GABAA receptors (GABAARs). Recently, gephyrin was also found to have a crucial role in molybdenum cofactor (MoCo) biosynthesis and to bind proteins that have been implicated in cytoskeletal dynamics and regulation of translational efficacy. Here, we discuss the putative roles of gephyrin and its newly discovered binding partners in postsynaptic membrane formation and synaptic signalling processes.
Gephyrin is essential for the postsynaptic clustering of glycine receptors
Gephyrin (Prior et al. 1992) was originally identified as a peripheral membrane protein (Schmitt et al. 1987) that copurifies with the mammalian GlyR (Pfeiffer et al. 1982; Graham et al. 1985). Experiments with cultured spinal neurons indicate that gephyrin anchors and immobilizes GlyRs on the subsynaptic cytoskeleton (Kirsch & Betz, 1995). Gephyrin binds to the large cytoplasmic loop of the GlyR β subunit via an amphipathic sequence (Meyer et al. 1995; Kneussel et al. 1999b), displays high-affinity binding to polymerized tubulin (Kirsch et al. 1991) and is required for the localization of GlyRs at postsynaptic sites (for review, see Kirsch et al. 1996). Gephyrin accumulates at developing spinal postsynaptic sites prior to the GlyR (Kirsch et al. 1993a; Bechade et al. 1996), and the loss of gephyrin expression, either via antisense depletion of primary neurons (Kirsch et al. 1993a) or by gene knockout in mice (Feng et al. 1998), prevents the synaptic clustering of GlyRs. Gephyrin is therefore thought to orchestrate the development of glycinergic postsynaptic membrane specializations (Kirsch et al. 1996).
In cultured spinal neurons, blockade of GlyR activation by the specific antagonist strychnine inhibits GlyR localization at postsynaptic sites but causes an accumulation of GlyR immunoreactivity in intracellular vesicles (Kirsch & Betz, 1998; Levi et al. 1998). A similar effect is seen when neurons are treated with L-type Ca2+ channel blockers or tetrodotoxin (TTX), which eliminates all action potentials. These data are consistent with GlyR cluster formation depending on GlyR-mediated membrane depolarization and Ca2+ influx (Kirsch & Betz, 1998). In support of this interpretation, glycine has been shown to induce Ca2+ signals sufficient to trigger transmitter release in embryonic cultured neurons (Reichling et al. 1994; Wang et al. 1994; Boehm et al. 1998). A model integrating these observations (Fig. 1) has been proposed previously (Kirsch & Betz, 1998). Accordingly, the temporal sequence of GlyR clustering in developing neurons that express both GlyR and gephyrin (panel A) is thought to start with GlyR-triggered Ca2+ influx causing an accumulation of gephyrin at sites of nerve terminal-postsynaptic cell contact (panel B). The resulting gephyrin clusters trap GlyRs diffusing laterally in the plane of the membrane, thus creating a postsynaptic GlyR matrix. Subsequently, gephyrin clusters grow at these innervated contact sites, possibly via a local reorganization of the cytoskeleton (panel C). The simultaneous endocytotic removal of extrasynaptic receptors from non-innervated plasma membrane domains then leads to a selective concentration of GlyRs at developing postsynaptic membrane specializations.
Figure 1. A model for activity-dependent GlyR clustering at developing postsynaptic membrane specializations.
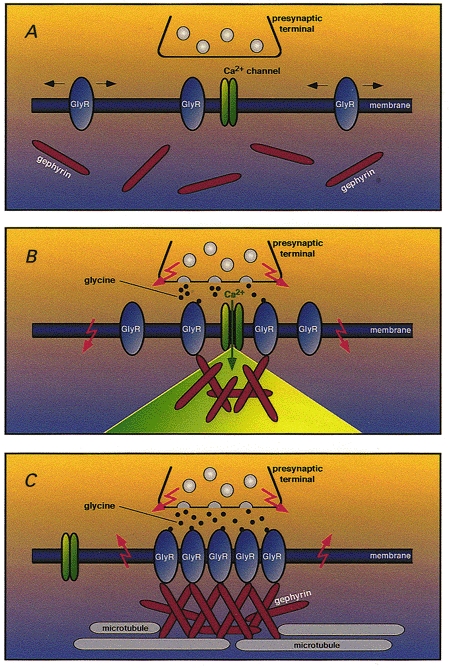
A glycinergic presynaptic terminal and a segment of the plasma membrane of a GlyR-expressing postsynaptic neuron are shown at different stages of synaptogenesis. A, immature stage: GlyRs are randomly distributed in the plasma membrane of the postsynaptic cell, whereas gephyrin is cytoplasmically localized. B, initiation of gephyrin cluster formation: release of glycine from the presynaptic terminal gates neighbouring GlyRs. This results in a depolarizing Cl− efflux (downward arrows) that activates nearby voltage-dependent Ca2+ channels. The resultant local microdomain of elevated Ca2+ concentration triggers the membrane apposition and aggregation of gephyrin at sites of GlyR activation. C, maturation stage: the growing submembraneous gephyrin aggregate traps additional GlyRs underneath the presynaptic terminal and immobilizes the receptors in the developing postsynaptic membrane by anchoring them to the subsynaptic cytoskeleton. Due to a change in Cl− equilibrium potential, GlyR activation triggers Cl− influx causing hyperpolarization (upward arrows), and thus inhibition of neuronal firing. This model is based on data by Kirsch & Betz (1998) and does not include G protein-mediated signalling pathways that may also contribute to the clustering process (Kins et al. 2000).
Recent data suggest that the model shown in Fig. 1 may require modification. First, a block of gephyrin targeting to developing postsynaptic sites has been found in one (Kirsch & Betz, 1998) but not in a second (Levi et al. 1998) of the aforementioned strychnine inhibition studies. This may reflect differences in culture conditions and/or the presence of additional presynaptic clustering factors. Second, in young cultured neurons, spontaneous formation of gephyrin-GlyR clusters at non-innervated plasma domains can be consistently observed (Kirsch & Betz, 1995; Levi et al. 1999). This has been interpreted as being indicative of a lack of activity and/or trophic factor dependence of postsynaptic receptor clustering (Levi et al. 1999), but may solely reflect the presence of a default pathway that generates extrasynaptically clustered GlyRs in the absence of nerve terminals, as is seen with nicotinic acetylcholine receptors in isolated myotubes (Godfrey et al. 1984). Third, the remarkable in vivo specificity of gephyrin targeting to inhibitory nerve terminals calls for presynaptic clustering signals other than an elevated intracellular Ca2+ concentration (Kirsch & Betz, 1998). Elevations in Ca2+ concentration are generated upon activation of various excitatory neurotransmitter receptors, none of which is colocalized with gephyrin in vivo. Additional specific clustering factors must therefore exist that regulate the signalling cascades and cytoskeletal rearrangements required for postsynaptic gephyrin apposition.
Consistent with the biochemically demonstrated binding of gephyrin to tubulin and affinity-purified GlyR, treatment with alkaloids which affect the organization of microtubules and actin microfilaments has been found to alter the number and size of postsynaptic gephyrin and GlyR clusters (Kirsch & Betz, 1995). Upon expression in heterologous cell systems, gephyrin forms intracellular aggregates which bind to heteromeric GlyR with high affinity (Kirsch et al. 1995). Singly expressed GlyR β, but not α, subunits are targeted to intracellular gephyrin aggregates in human kidney HEK 293 cells, showing that gephyrin binding is mediated by the β subunit. Notably, insertion of the high-affinity gephyrin binding motif localized in the cytoplasmic loop of the GlyR β subunit (Meyer et al. 1995) routes engineered GABAAR and NMDA receptor, as well as green fluorescent protein (GFP), to intracellular gephyrin aggregates upon cotransfection (Meyer et al. 1995; Kins et al. 1999; Kneussel et al. 1999b). These experiments prove that gephyrin acts as a dominant localization signal for GlyR β subunit-containing receptor assemblies. Thus, the interactions of gephyrin with both GlyR β subunit binding domains and subsynaptic cytoskeletal elements critically participate in the postsynaptic localization of GlyR proteins. These findings imply that homo-oligomeric embryonic GlyRs (Becker et al. 1988; Hoch et al. 1989) may be exclusively extrasynaptic.
Role of gephyrin in the synaptic localization of GABA receptors
Gephyrin is widely expressed throughout the mammalian CNS (Prior et al. 1992; Kirsch & Betz, 1993) and is found even in brain regions known to be largely devoid of glycinergic synapses (Malosio et al. 1991; Kirsch et al. 1993b; Araki et al. 1998). Immunocytochemistry revealed intense gephyrin immunoreactivity at GABAergic synapses in spinal cord (Triller et al. 1987; Bohlhalter et al. 1994; Cabot et al. 1995; Todd et al. 1996), retina (Sassoe-Pognetto et al. 1995) and olfactory bulb (Giusetto et al. 1998), as well as in cultured hippocampal (Craig et al. 1996) and cortical (Essrich et al. 1998) neurons. At the ultrastructural level, an excellent matching of postsynaptic GABAARs and gephyrin immunoreactivity has been reported for retinal synapses (Sassoe-Pognetto et al. 1995). However, at present, biochemical evidence for an association of GABAAR subunits with gephyrin is lacking. Different attempts to demonstrate binding of gephyrin to native GABAARs have proved unsuccessful (B. Schmitt & A. Stephenson, unpublished data; Meyer et al. 1995). Cotransfection of gephyrin and the GABAAR β3 subunit into mammalian cells showed only a weak interaction of these proteins in a cellular context (Kirsch et al. 1995).
More convincing evidence for a role of gephyrin in GABAAR clustering comes from studies on knockout mice (Essrich et al. 1998; Kneussel et al. 1999a). First, GABAAR γ2 subunit-deficient mice display a strong reduction in postsynaptic gephyrin and GABAAR clusters (Essrich et al. 1998); notably, both immunoreactivities were restored at postsynaptic sites upon transgenic expression of the homologous γ3 subunit (Baer et al. 1999). This suggests that different postsynaptic GABAAR subtypes colocalize with gephyrin. Second, depletion of gephyrin by antisense oligonucleotide treatment of cultured hippocampal neurons produces a decrease in punctate synaptic staining for the GABAAR γ2 subunit (Essrich et al. 1998). More importantly, brain sections and neurons from gephyrin knockout mice show a total loss of postsynaptic clustering of the GABAAR subunits γ2 and α2 (Kneussel et al. 1999a; see Fig. 2). Thus, gephyrin is essential for the postsynaptic localization of GABAARs. However, in cultured hippocampal neurons isolated from gephyrin-deficient mice, GABA and glycine currents were only marginally reduced (Kneussel et al. 1999a), suggesting that gephyrin is not required for plasma membrane insertion of inhibitory neurotransmitter receptors, but an increased accumulation of intracellular GABAAR immunoreactive microclusters suggests enhanced receptor internalization in the absence of gephyrin. These observations indicate that in addition to its role in inhibitory receptor clustering (Betz, 1998) gephyrin may also be required to stabilize inhibitory postsynaptic membrane specializations (Kneussel et al. 1999a).
Figure 2. GABAARs require gephyrin for synaptic cluster formation.
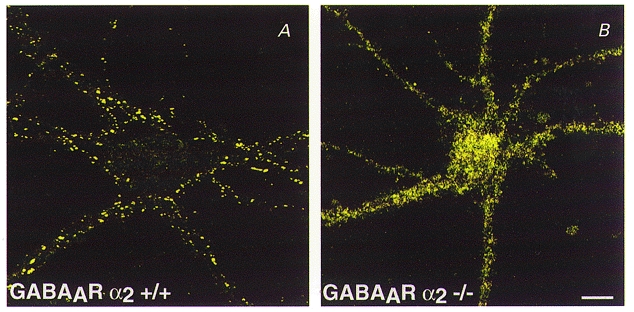
Immunochemistry of cultured hippocampal neurons from wildtype +/+ (A) and geph -/- (gephyrin-deficient) mice (B). After 21 days in vitro, the cultures were stained with antibodies specific for the GABAAR subunit α2. Note the loss of GABAA receptor clusters in neurons from geph -/- mice. These mutant neurons consistently show a significant increase in intracellular GABAA receptor subunit immunoreactivity. Scale bar, 20 μm; applies to both A and B. (Modified from Kneussel et al. 1999a.)
The highly abundant GABAAR subunit γ2 has been reported to bind GABAA receptor-associated protein (GABARAP), a protein with homology to microtubule-associated proteins (MAPs) (Wang et al. 1999). It has been suggested that GABARAP harbours a putative microtubule binding motif in its N-terminal domain, thus qualifying as a potential receptor-cytoskeleton linker. Similarly, the microtubule-associated protein MAP1-B has been shown to bind the retinal GABAC receptor ρ1 subunit, a property consistent with microtubule-mediated anchoring of this inhibitory receptor subclass (Hanley et al. 1999). Notably, the tubulin binding properties of gephyrin closely resemble those of MAPs (Kirsch et al. 1996), and a cDNA encoding MAP1-B (also known as MAP5) has been isolated in earlier attempts to clone gephyrin (Rienitz et al. 1989; Kirsch et al. 1990). At present, it is unclear whether the tubulin binding functions of gephyrin, GABARAP, MAP1-B and possibly other MAPs are crucial for postsynaptic anchoring of GABA receptors. Moreover, it remains to be established whether these proteins interact in a highly regulated postsynaptic protein complex, or whether these different tubulin binding proteins provide independent receptor-cytoskeleton linkages that are specific for distinct subunit combinations (Fig. 3). In any case, all presently available data strongly indicate that gephyrin is associated with and essential for the clustering of glycine and GABAA, but not GABAC, receptors.
Figure 3. Schematic representation of postsynaptic gephyrin and its binding partners.
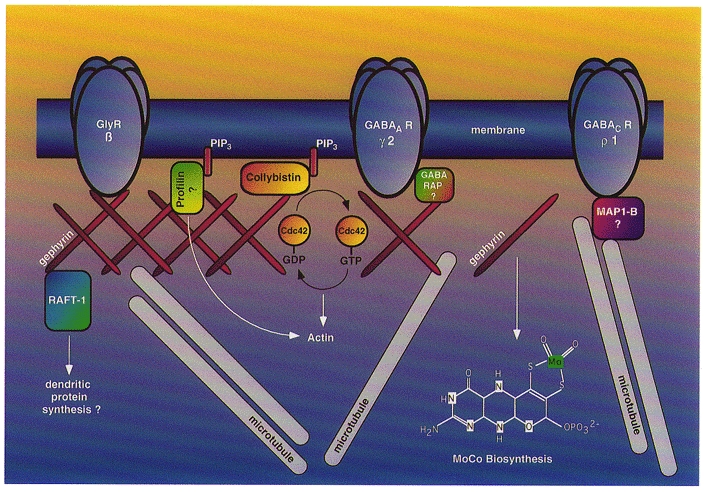
Gephyrin is depicted as a submembraneous protein scaffold that concentrates structural and membrane components at the postsynaptic membrane. Gephyrin directly binds and clusters GlyRs and is essential for the postsynaptic localization of a major population of GABAARs; these GABAARs may use the tubulin binding protein GABARAP for interaction with gephyrin. Gephyrin also binds to phosphatidylinositol 3,4,5-trisphosphate (PIP3) binding proteins involved in actin dynamics and downstream signalling, such as collybistin and profilin, and interacts with RAFT1 (rapamycin and FKBP12 target protein), a candidate regulator of dendritic protein synthesis. Additionally, gephyrin catalyses a crucial step in the biosynthesis of the molybdenum cofactor (MoCo); whether this catalysis also occurs at the synapse is presently unclear. The tubulin binding properties of gephyrin closely resemble those of MAP1-B, a protein thought to link GABAC receptors to microtubules and ensure tight anchoring of both GlyRs and GABAA receptors to subsynaptic microtubules.
A recent report by Levi et al. (1999) elegantly supports and extends this conclusion. Using motoneurons cocultured with either GABAergic dorsal root ganglia or inhibitory spinal interneurons, these authors found a selective postsynaptic accumulation of GlyRs at glycinergic, and of GABAARs at GABAergic, sites of gephyrin deposition. This result indicates that the postsynaptic gephyrin matrices induced by different types of interneurons are not equivalent, thus corroborating the postulated existence of additional receptor-specific clustering factors in inhibitory nerve terminals (Kirsch & Betz, 1998). Consistent with this view, neither GlyRs nor GABAARs were seen in the ectopic gephyrin clusters formed under the terminals of autaptic cholinergic motoneurons, although these receptor proteins are present in the surface membranes of these cells (Levi et al. 1999). In contrast, but in agreement with previous immunocytochemical (Triller et al. 1987; Bohlhalter et al. 1994; Todd et al. 1996) and electrophysiological data (Jonas et al. 1998), glycine and GABA co-releasing spinal interneurons induced mixed GlyR and GABAAR-containing postsynaptic differentiations, showing that gephyrin can provide a common anchoring scaffold for both types of inhibitory neurotransmitter receptors. The mechanisms that impose receptor selectivity on postsynaptic gephyrin are presently unknown but may relate to the high number of potential splice variants that can be generated from the highly mosaic gephyrin gene (Prior et al. 1992; M. Ramming, S. Kins, N. Werner, A. Hermann, H. Betz & J. Kirsch, unpublished results).
Other functions of gephyrin: gephyrin is required for molybdenum cofactor (MoCo) biosynthesis
Both the N- and the C-terminal regions of gephyrin display comparatively high homology to proteins from other distant species, i.e. E. coli (MogA, MoaB and MoeA), Drosophila (cinnamon) and Arabidopsis (Cnx1). Using genetic methods, all these bacterial, invertebrate and plant homologues of gephyrin have been shown to be essential for the synthesis of a coenzyme, the molybdenum cofactor (MoCo). In liver and intestinal extracts from mouse embryos deficient in gephyrin, the activities of the MoCo-dependent enzymes xanthine dehydrogenase and sulfite oxidase (for review, see Johnson & Wadman, 1995) are reduced to background levels (Feng et al. 1998). Moreover, expression of rat gephyrin was recently shown to rescue the phenotype of MoCo biosynthesis mutants in E. coli and plants (Stallmeyer et al. 1999). Gephyrin might catalyse step 3 in the MoCo biosynthesis pathway, i.e. insertion of molybdenum ions into molybdopterin, since mutations in Cnx1, which catalyses this biosynthetic step (Mendel, 1997), can be rescued by gephyrin expression in Arabidopsis (Stallmeyer et al. 1999). Collectively, these data establish a novel function of gephyrin in intermediary metabolism that is not connected to synaptogenesis, a finding which explains the ubiquitous expression of the gephyrin gene in all mammalian tissues analysed to date (Prior et al. 1992). Notably, patients suffering from hereditary MoCo deficiency display symptoms that resemble those seen upon loss of inhibitory neurotransmission, including microcephaly, severe neurological abnormalities, myoclonus and mental retardation (Johnson & Wadman, 1995). Thus, the MoCo biosynthetic functions of gephyrin may be important for brain development, but whether this is somehow connected to inhibitory postsynaptic membrane compartments remains unclear. In any case, the similar phenotypes of gephyrin-deficient mice and humans suffering from severe forms of hereditary MoCo deficiency (Feng et al. 1998) qualify the gephyrin gene as a candidate disease locus. Interestingly, the neurological symptoms and the early lethality of gephyrin knockout mice indicate a more severe phenotype as seen in naturally occurring GlyR deficiencies (Kuhse et al. 1995; Koch et al. 1996). Whether this reflects the simultaneous loss of both GlyR and GABAAR clustering or additional consequences of sulfite toxicity resulting from impaired MoCo enzyme function awaits further clarification.
Novel gephyrin binding proteins
GDP-GTP exchange factors (GEFs)
Studies at the neuromuscular junction have revealed a multitude of interacting proteins that are required for postsynaptic membrane organization (Colledge & Froehner, 1998). Therefore, attempts have been made to identify novel gephyrin binding partners. Using yeast two-hybrid screening, a novel interacting protein, termed collybistin, has been identified recently (Kins et al. 2000). Collybistin shows striking homology to dbl-like GDP-GTP exchange factors (GEFs), which regulate small G proteins of the Rho/Rac family, and share typical tandem dbl homology (DH)-pleckstrin homology (PH) domains with other GEFs. The DH domain is thought to catalyse guanylnucleotide exchange, whereas PH domains bind phosphoinositides and thereby restrict GEF activation to the membrane (for review, see Lemmon et al. 1996; Kavran et al. 1998). Collybistin exists in two splice variants, one of which (collybistin I) contains an N-terminal SH3 domain and a C-terminal coiled coil region. Expression of the variant lacking these domains (collybistin II) was found to alter the intracellular distribution of gephyrin upon heterologous coexpression. In about one-third of the transfected cells, gephyrin and collybistin were colocalized in submembraneous microaggregates. Furthermore, heteromeric GlyRs were recruited to these gephyrin-collybistin co-aggregates (Kins et al. 2000). Collybistin is therefore postulated to constitute an essential component of the signalling machinery that directs apposition of gephyrin to the developing postsynaptic membrane. Both its brain-specific expression (Kins et al. 2000) and its recently demonstrated specificity for activating the Rho-like GTPase cdc42 (Reid et al. 1999) are consistent with this protein acting as a transducer element that signals from the membrane to the subsynaptic cytoskeleton. Whether the activation of cdc42 by collybistin at presumptive postsynaptic sites is crucial for triggering formation of the subsynaptic gephyrin matrix (Kirsch & Betz, 1998; Kins et al. 2000) or is only required for cytoskeletal reorganization (Reid et al. 1999) is unknown at present. Gene targeting experiments should show whether collybistin I and II are indeed essential for GlyR and GABAAR cluster formation in vivo.
Profilin
Another protein recently found to interact with gephyrin in a glutathione-S-transferase pulldown assay (Mammoto et al. 1998) is the well-characterized actin monomer binding protein profilin (for review, see Schlüter et al. 1997). Profilin stimulates the ADP-ATP exchange reaction of the actin monomer, thereby promoting monomer incorporation into filamentous actin (Finkel et al. 1994). It also binds to different actin assembly proteins as well as to phosphatidylinositol 3,4,5-trisphosphate (PIP3; Schlüter et al. 1997). Moreover, profilins form complexes with regulators of endocytosis and synaptic vesicle recycling (Witke et al. 1998). In mammals, two profilin isoforms are encoded by different genes: profilin I is ubiquitously expressed in mouse, whereas profilin II expression is restricted to brain and skeletal muscle (Witke et al. 1998). Treatment of cultured spinal cord neurons with cytochalasin D, a drug that severs the actin cytoskeleton, has been found to reduce the size and to increase the density of postsynaptic gephyrin clusters (Kirsch & Betz, 1995). This is consistent with actin microfilaments being crucially implicated in the control of inhibitory receptor density at postsynaptic sites. Consequently, profilin-gephyrin interactions could contribute to the regulation of receptor cluster size and the modulation of signalling processes.
RAFT1
In another yeast two-hybrid screen, gephyrin was found to interact with RAFT1 (rapamycin and FKBP12 target protein; Sabatini et al. 1999). RAFT1 is an important target of the immunosuppressant rapamycin and has been suggested to play a crucial role in the regulation of the cell cycle. Binding of RAFT1 to gephyrin involves a region that displays 45 % similarity to the gephyrin binding site of the GlyR β subunit (Sabatini et al. 1999). Furthermore, RAFT1 and its yeast homologue TOR participate in signalling pathways controlling mRNA translation (for review, see Sabatini et al. 1997). RAFT1 mutants deficient in gephyrin binding failed to mediate signalling to 4EBP1, a regulator of protein synthesis that inhibits the initiation factor and cap binding protein eIF-4E (Brunn et al. 1997). The identification of RAFT1 as a binding partner of the submembraneous component gephyrin suggests that RAFT1 may act as a regulator of dendritic mRNA translation at identified synapses. Notably, high levels of GlyR α subunit mRNAs have been demonstrated subsynaptically in spinal cord dendrites (Racca et al. 1997). Therefore, a high concentration of regulators of the translational machinery, such as RAFT1, close to synaptic sites would be in line with the hypothesis that certain proteins involved in signal transduction and memory processing are locally translated in dendritic compartments (Steward et al. 1998; Steward & Halpain, 1999). Interestingly, the yeast homologues (TOR) of RAFT1, which also participate in both cell cycle regulation and translational control, affect the organization of the actin cytoskeleton via the GDP-GTP exchange factor ROM2 (Schmidt et al. 1997). Gephyrin's dual interactions with both RAFT1 and collybistin may thus allow for crosstalk between dendritic translational control mechanisms and both the subsynaptic cytoskeleton and small G protein activated pathways.
Concluding remarks
Recent experiments addressing the role of gephyrin in inhibitory postsynaptic membrane formation have revealed novel functions of this receptor-associated protein, extending its role from receptor clustering to synaptic organization and intermediary metabolism (Fig. 3). Gene targeting in mice indicates that gephyrin is not only essential for the synaptic localization of GlyRs but also required for the clustering of GABAA receptors. The signalling mechanisms that provide for the specificity of gephyrin and GlyR, or GABAAR, accumulation at glycinergic and GABAergic synapses have not been elucidated but are likely to include both activity-driven and – as yet hypothetical – clustering factor-dependent pathways. As intracellular receptor levels are increased in neurons from gephyrin-deficient mice, we speculate that gephyrin protects receptors against internalization, and thus stabilizes postsynaptic receptor clusters.
Analysis of organ extracts from gephyrin knockout mice and gene complementation experiments in bacteria and plants have established a non-synaptic role of gephyrin in MoCo biosynthesis. The large extent of sequence conservation between gephyrin and MoCo synthesizing proteins from diverse species suggests that this enzymatic role may constitute the primordial function of this ubiquitously expressed gene product. Whether gephyrin's localization at postsynaptic sites reflects a role for MoCo-dependent enzymes at the synapse will be a topic of future investigation.
The list of potential roles of gephyrin is extended further by the recent identification of novel binding partners other than neurotransmitter receptors and cytoskeletal elements. The GEF collybistin is thought to mediate membrane apposition of gephyrin and constitutes an excellent candidate for G protein-mediated signal transduction from the postsynaptic plasma membrane to the actin cytoskeleton. Similarly, the gephyrin binding protein profilin promotes actin polymerization and, interestingly, binds to targets which are downstream of Rho-type small G proteins (Imamura et al. 1997; Mammoto et al. 1998; Alberts et al. 1998). By interacting with RAFT1, gephyrin may in addition regulate the subsynaptic translational machinery thought to initiate protein synthesis in dendritic subcompartments (Sabatini et al. 1999).
In conclusion, gephyrin seems to crosslink postsynaptic receptors to intracellular proteins that regulate signalling processes. Gephyrin may therefore act as a switch that transduces changes in synaptic activity into morphological alterations of synapse structure, as postulated for synaptic plasticity underlying learning and memory processes. The rapid dynamics of synaptic gephyrin seen upon deafferentation of inhibitory inputs (Triller et al. 1993) are consistent with this hypothesis.
Acknowledgments
Work in the authors’ laboratory is supported by Deutsche Forschungsgemeineschaft, Bundesministerium für Bilding und Forschung and Fonds der Chemischen Industrie.
References
- Alberts AS, Bouquin N, Johnston LH, Treisman R. Analysis of RhoA-binding proteins reveals an interaction domain conserved in heterotrimeric G protein beta subunits and the yeast response regulator protein Skn7. Journal of Biological Chemistry. 1998;273:8616–8622. doi: 10.1074/jbc.273.15.8616. [DOI] [PubMed] [Google Scholar]
- Araki T, Yamano M, Murakami T, Wanaka A, Betz H, Tohyama M. Localization of glycine receptors in the rat central nervous system: an immunocytochemical analysis using monoclonal antibody. Neuroscience. 1988;25:613–624. doi: 10.1016/0306-4522(88)90263-1. [DOI] [PubMed] [Google Scholar]
- Baer K, Essrich C, Benson JA, Benke D, Bluethmann H, Fritschy J-M, Lüscher B. Postsynaptic clustering of γ-aminobutyric acid type A receptors by the γ3 subunit in vivo. Proceedings of the National Academy of Sciences of the USA. 1999;96:12860–12865. doi: 10.1073/pnas.96.22.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechade C, Colin I, Kirsch J, Betz H, Triller A. Expression of glycine receptor α subunits and gephyrin in cultured spinal neurons. European Journal of Neuroscience. 1996;8:429–435. doi: 10.1111/j.1460-9568.1996.tb01226.x. [DOI] [PubMed] [Google Scholar]
- Becker C-M, Hoch W, Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO Journal. 1988;7:3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H. Gephyrin, a major player in GABAergic postsynaptic membrane assembly? Nature Neuroscience. 1998;7:541–543. doi: 10.1038/2777. [DOI] [PubMed] [Google Scholar]
- Boehm S, Harvey RJ, Holst von A, Rohrer H, Betz H. Glycine receptors in cultured chick sympathetic neurons are excitatory and trigger neurotransmitter release. The Journal of Physiology. 1998;504:683–694. doi: 10.1111/j.1469-7793.1997.683bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlhalter S, Möhler H, Fritschy JM. Inhibitory neurotransmission in rat spinal cord: co-localization of glycine- and GABAA-receptors at GABAergic synaptic contacts demonstrated by triple immunofluorescence staining. Brain Research. 1994;642:59–69. doi: 10.1016/0006-8993(94)90905-9. [DOI] [PubMed] [Google Scholar]
- Brunn GJ, Hudson CC, Sekulic A, Williams JM, Hosoi H, Houghton PJ, Lawrence JC, Jr, Abraham R T. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277:99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- Cabot JB, Bushnell A, Alessi V, Mendell NR. Postsynaptic gephyrin immunoreactivity exhibits a nearly one-to-one correspondence with γ-aminobutyric acid-like immunogold-labeled synaptic inputs to sympathetic preganglionic neurons. Journal of Comparative Neurology. 1995;356:418–432. doi: 10.1002/cne.903560309. [DOI] [PubMed] [Google Scholar]
- Colledge M, Froehner SC. Signals mediating ion channel clustering at the neuromuscular junction. Current Opinion in Neurobiology. 1998;8:357–363. doi: 10.1016/s0959-4388(98)80061-5. [DOI] [PubMed] [Google Scholar]
- Craig AM, Banker G, Chang W, McGrath ME, Serpinskaya AS. Clustering of gephyrin at GABAergic but not glutamatergic synapses in cultured rat hippocampal neurons. Journal of Neuroscience. 1996;16:3166–3177. doi: 10.1523/JNEUROSCI.16-10-03166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven SE, Bredt DS. PDZ proteins organize synaptic signaling pathways. Cell. 1998;93:495–498. doi: 10.1016/s0092-8674(00)81179-4. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Lüscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nature Neuroscience. 1998;7:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Feng G, Tintrup H, Kirsch J, Nichol MC, Kuhse J, Betz H, Sanes JR. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998;282:1321–1324. doi: 10.1126/science.282.5392.1321. [DOI] [PubMed] [Google Scholar]
- Finkel T, Theriot JA, Dise KR, Tomaselli GF, Goldschmidt CP. Dynamic actin structures stabilized by profilin. Proceedings of the National Academy of Sciences of the USA. 1994;91:1510–1514. doi: 10.1073/pnas.91.4.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusetto M, Kirsch J, Fritschy JM, Cantino D, Sassoe-Pognetto M. Localization of the clustering protein gephyrin at GABAergic synapses in the main olfactory bulb of the rat. Journal of Comparative Neurology. 1998;395:231–244. doi: 10.1002/(sici)1096-9861(19980601)395:2<231::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Godfrey EW, Nitkin RM, Wallace BG, Rubin LL, McMahan UJ. Components of Torpedo electric organ and muscle that cause aggregation of acetylcholine receptors on cultured muscle cells. Journal of Cell Biology. 1984;99:615–627. doi: 10.1083/jcb.99.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D, Pfeiffer F, Simler R, Betz H. Purification and characterization of the glycine receptor of pig spinal cord. Biochemistry. 1985;12:990–994. doi: 10.1021/bi00325a027. [DOI] [PubMed] [Google Scholar]
- Hanley JG, Koulen P, Bedford F, Gordon-Weeks PR, Moss S J. The protein MAP-1B links GABAC receptors to the cytoskeleton at retinal synapses. Nature. 1999;397:66–69. doi: 10.1038/16258. [DOI] [PubMed] [Google Scholar]
- Hoch W, Betz H, Becker C-M. Primary cultures of mouse spinal cord express the neonatal isoform of the inhibitory glycine receptor. Neuron. 1989;3:339–348. doi: 10.1016/0896-6273(89)90258-4. [DOI] [PubMed] [Google Scholar]
- Imamura H, Tanaka K, Hihara T, Umikawa M, Kamei T, Takahashi K, Sasaki T, Takai Y. Bni1p and Bnr1p: downstream targets of the Rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO Journal. 1997;16:2745–2755. doi: 10.1093/emboj/16.10.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Wadman SK. Molybdenum cofactor deficiency and isolated sulfite oxidase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 1995. pp. 2271–2283. [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Kavran JM, Klein DE, Lee A, Falasca M, Isakoff SJ, Skolnik EY, Lemmon MA. Specificity and promiscuity in phosphoinositide binding by pleckstrin homology (PH) domains. Journal of Biological Chemistry. 1998;273:30497–30508. doi: 10.1074/jbc.273.46.30497. [DOI] [PubMed] [Google Scholar]
- Kins S, Betz H, Kirsch J. Collybistin, a novel brain specific GEF, induces submembrane clustering of gephyrin. Nature Neuroscience. 2000;3:22–29. doi: 10.1038/71096. [DOI] [PubMed] [Google Scholar]
- Kins S, Kuhse J, Laube B, Betz H, Kirsch J. Incorporation of a gephyrin-binding motif targets NMDA receptors to gephyrin-rich domains in HEK293 cells. European Journal of Neuroscience. 1999;11:740–744. doi: 10.1046/j.1460-9568.1999.00527.x. [DOI] [PubMed] [Google Scholar]
- Kirsch J. Assembly of signaling machinery at the postsynaptic membrane. Current Opinion in Neurobiology. 1999;9:329–335. doi: 10.1016/s0959-4388(99)80048-8. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Betz H. Widespread expression of gephyrin, a putative receptor-tubulin linker protein, in rat brain. Brain Research. 1993;621:301–310. doi: 10.1016/0006-8993(93)90120-c. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Betz H. The postsynaptic localization of the glycine receptor-associated protein gephyrin is regulated by the cytoskeleton. Journal of Neuroscience. 1995;15:4148–4156. doi: 10.1523/JNEUROSCI.15-06-04148.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch J, Betz H. Glycine-receptor activation is required for receptor clustering in spinal neurons. Nature. 1998;392:717–720. doi: 10.1038/33694. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Kuhse J, Betz H. Targeting of glycine receptor subunits to gephyrin-rich domains in transfected human embryonic kidney cells. Molecular and Cellular Neuroscience. 1995;6:450–461. doi: 10.1006/mcne.1995.1033. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Langosch D, Prior P, Littauer UZ, Schmitt B, Betz H. The 93-kDa glycine receptor associated protein binds to tubulin. Journal of Biological Chemistry. 1991;266:22242–22245. [PubMed] [Google Scholar]
- Kirsch J, Littauer UZ, Schmitt B, Prior P, Thomas L, Betz H. Neuraxin corresponds to a C-terminal fragment of microtubule-associated protein 5 (MAP5) FEBS Letters. 1990;262:259–262. doi: 10.1016/0014-5793(90)80205-w. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Malosio M, Wolters I, Betz H. Distribution of gephyrin transcripts in the adult and developing rat brain. European Journal of Neuroscience. 1993a;5:1109–1117. doi: 10.1111/j.1460-9568.1993.tb00965.x. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Meyer G, Betz H. Synaptic targeting of ionotropic neurotransmitter receptors. Molecular and Cellular Neuroscience. 1996;8:93–98. doi: 10.1006/mcne.1996.0048. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Wolters I, Triller A, Betz H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature. 1993b;266:745–748. doi: 10.1038/366745a0. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstätter JH, Laube B, Stahl S, Müller U, Betz H. Loss of postsynaptic GABAA receptor clustering in gephyrin-deficient mice. Journal of Neuroscience. 1999a;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Hermann A, Kirsch J, Betz H. Hydrophobic interactions mediate binding of the glycine receptor β-subunit to gephyrin. Journal of Neurochemistry. 1999b;72:1323–1326. doi: 10.1046/j.1471-4159.1999.0721323.x. [DOI] [PubMed] [Google Scholar]
- Koch M, Kling C, Becker C-M. Increased startle responses in mice carrying mutations of glycine receptor. NeuroReport. 1996;7:806–808. doi: 10.1097/00001756-199602290-00030. [DOI] [PubMed] [Google Scholar]
- Kuhse J, Betz H, Kirsch J. The inhibitory glycine receptor: architecture, synaptic localization and molecular pathology of a postsynaptic ion channel complex. Current Opinion in Neurobiology. 1995;5:318–323. doi: 10.1016/0959-4388(95)80044-1. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Ferguson KM, Schlessinger J. PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]
- Levi S, Chesnoy-Marchais D, Sieghart W, Triller A. Synaptic control of glycine and GABAA receptors and gephyrin expression in cultured motoneurons. Journal of Neuroscience. 1999;19:7434–7449. doi: 10.1523/JNEUROSCI.19-17-07434.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Vannier C, Triller A. Strychnine-sensitive stabilization of postsynaptic glycine receptor clusters. Journal of Cell Science. 1998;111:335–345. doi: 10.1242/jcs.111.3.335. [DOI] [PubMed] [Google Scholar]
- Malosio M, Marqueze-Pouey B, Kuhse J, Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO Journal. 1991;10:2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A, Sasaki T, Asakura T, Hotta I, Imamura H, Takahashi K, Matsuura Y, Shirao T, Takai Y. Interactions of drebrin and gephyrin with profilin. Biochemical and Biophysical Research Communications. 1998;243:86–89. doi: 10.1006/bbrc.1997.8068. [DOI] [PubMed] [Google Scholar]
- Mendel RR. Molybdenum cofactor of higher plants: biosynthesis and molecular biology. Planta. 1997;203:399–405. doi: 10.1007/s004250050206. [DOI] [PubMed] [Google Scholar]
- Meyer G, Kirsch J, Betz H, Langosch D. Identification of a gephyrin binding motif on the glycine receptor β subunit. Neuron. 1995;15:563–572. doi: 10.1016/0896-6273(95)90145-0. [DOI] [PubMed] [Google Scholar]
- Pfeiffer F, Graham D, Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. Journal of Biological Chemistry. 1982;257:9389–9393. [PubMed] [Google Scholar]
- Prior P, Schmitt B, Grenningloh G, Pribilla I, Multhaup G, Beyreuther K, Maulet Y, Werner P, Langosch D, Kirsch J, Betz H. Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein. Neuron. 1992;8:1161–1170. doi: 10.1016/0896-6273(92)90136-2. [DOI] [PubMed] [Google Scholar]
- Racca C, Gardiol A, Triller A. Dendritic and postsynaptic localizations of glycine receptor α subunit mRNAs. Journal of Neuroscience. 1997;17:1691–1700. doi: 10.1523/JNEUROSCI.17-05-01691.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling DB, Kyrozis A, Wang J, MacDermott AB. Mechanisms of GABA and glycine depolarization-induced calcium transients in rat dorsal horn neurons. The Journal of Physiology. 1994;476:411–421. doi: 10.1113/jphysiol.1994.sp020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid T, Bathoorn A, Ahmadian MR, Collard JG. Identification and characterization of hPEM-2, a guanine nucleotide exchange factor specific for Cdc42. Journal of Biological Chemistry. 1999;274:33587–33593. doi: 10.1074/jbc.274.47.33587. [DOI] [PubMed] [Google Scholar]
- Rienitz A, Grenningloh G, Hermans-Borgmeyer I, Kirsch J, Littauer UZ, Prior P, Gundelfinger ED, Schmitt B, Betz H. Neuraxin, a novel putative structural protein of the rat central nervous system that is immunologically related to microtubule-associated protein 5. EMBO Journal. 1989;8:2879–2888. doi: 10.1002/j.1460-2075.1989.tb08436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DM, Barrow RK, Blackshaw S, Burnett PE, Lai MM, Field ME, Bahr BA, Kirsch J, Betz H, Snyder SH. Interaction of RAFT1 with gephyrin required for rapamycin-sensitive signaling. Science. 1999;284:1161–1164. doi: 10.1126/science.284.5417.1161. [DOI] [PubMed] [Google Scholar]
- Sabatini DM, Lai MM, Snyder SH. Neural roles of immunophilins and their ligands. Molecular Neurobiology. 1997;15:223–235. doi: 10.1007/BF02740635. [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Kirsch J, Grünert U, Greferath U, Fritschy JM, Möhler H, Betz H, Wässle H. Colocalization of gephyrin and GABAA receptor subunits in the rat retina. Journal of Comparative Neurology. 1995;357:1–14. doi: 10.1002/cne.903570102. [DOI] [PubMed] [Google Scholar]
- Schlüter K, Jokusch BM, Rothkegel M. Profilins as regulators of actin dynamics. Biochimica et Biophysica Acta. 1997;1359:97–109. doi: 10.1016/s0167-4889(97)00100-6. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Bickle M, Beck T, Hall MN. The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2. Cell. 1997;88:531–542. doi: 10.1016/s0092-8674(00)81893-0. [DOI] [PubMed] [Google Scholar]
- Schmitt B, Knaus P, Becker CM, Betz H. The Mr 93000 polypeptide of the postsynaptic glycine receptor complex is a peripheral membrane protein. Biochemistry. 1987;26:805–811. doi: 10.1021/bi00377a022. [DOI] [PubMed] [Google Scholar]
- Sheng M. PDZs and receptor/channel clustering: rounding up the latest suspects. Neuron. 1996;17:575–578. doi: 10.1016/s0896-6273(00)80190-7. [DOI] [PubMed] [Google Scholar]
- Stallmeyer B, Schwarz G, Schulze J, Nerlich A, Reiss J, Kirsch J, Mendel R R. The neurotransmitter receptor-anchoring protein gephyrin reconstitutes molybdenum cofactor biosynthesis in bacteria, plants, and mammalian cells. Proceedings of the National Academy of Sciences of the USA. 1999;96:1333–1338. doi: 10.1073/pnas.96.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Halpain S. Lamina-specific synaptic activation causes domain-specific alterations in dendritic immunostaining for MAP2 and CAM kinase II. Journal of Neuroscience. 1999;19:7834–7845. doi: 10.1523/JNEUROSCI.19-18-07834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Watt C, Spike R C, Sieghart W. Colocalization of GABA, glycine and their receptors at synapses in the rat spinal cord. Journal of Neuroscience. 1996;16:974–982. doi: 10.1523/JNEUROSCI.16-03-00974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triller A, Cluzeaud F, Korn H. Gamma-aminobutyric acid-containing terminals can be apposed to glycine receptors at central synapses. Journal of Cell Biology. 1987;104:947–956. doi: 10.1083/jcb.104.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triller A, Sur C, Korn H. Heterogeneous distribution of glycinergic and GABAergic afferents on an identified central neuron. Journal of Comparative Neurology. 1993;338:83–96. doi: 10.1002/cne.903380107. [DOI] [PubMed] [Google Scholar]
- Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW. GABAA-receptor-associated protein links GABAA receptors and the cytoskeleton. Nature. 1999;397:69–72. doi: 10.1038/16264. [DOI] [PubMed] [Google Scholar]
- Wang J, Reichling DB, Kyrozis A, MacDermott AB. Developmental loss of GABA- and glycine-induced depolarization and Ca2+ transients in embryonic rat dorsal horn neurons in culture. European Journal of Neuroscience. 1994;6:1275–1280. doi: 10.1111/j.1460-9568.1994.tb00317.x. [DOI] [PubMed] [Google Scholar]
- Witke W, Podtelejnikov AV, Nardo Di A, Sutherland J D, Gurniak CB, Dotti C, Mann M. In mouse brain profilin I and profilin II associate with regulators of the endocytic pathway and actin assembly. EMBO Journal. 1998;17:967–976. doi: 10.1093/emboj/17.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]


