Abstract
The aims of this study were to investigate (a) if renal Na+ handling was normal in Cftrtm2camΔF508 cystic fibrosis mice, (b) whether adaptation to dietary salt depletion was preserved and (c) whether Cftrtm2camΔF508 mice exhibited enhanced amiloride-sensitive Na+ absorption.
In Na+-replete animals (maintained on a 0.32 % NaCl diet) given a 150 mM NaCl i.v. maintenance infusion, there was no difference in fractional Na+ excretion (FENa) between wild-type (0.42 ± 0.06 %, n = 12) and Cftrtm2camΔF508 mice (0.47 ± 0.13 %, n = 7). Amiloride infusion significantly increased FENa in both wild-type (3.14 ± 0.83 %, n = 6) and Cftrtm2camΔF508 mice (3.47 ± 0.63 %, n = 9), though with no significant difference between genotypes.
A 14 day dietary salt restriction (animals maintained on a 0.03 % NaCl diet) and maintenance infusion with a 15 mM NaCl vehicle caused a reduction in FENa to 0.14 ± 0.05 %, n = 8 in wild-type mice and 0.14 ± 0.04 %, n = 8 in Cftrtm2camΔF508 mice. No significant difference in the ability to adapt to low salt conditions was apparent comparing the two genotypes.
Treatment of salt-restricted mice with amiloride resulted in a blunted natriuresis in both wild-type mice (FENa = 1.10 ± 0.16 %, n = 7) and Cftrtm2camΔF508 mice (FENa = 1.97 ± 0.29 %, n = 9). The natriuresis induced by amiloride was significantly greater in Cftrtm2camΔF508 mice than in wild-type controls.
In conclusion, Cftrtm2camΔF508 mice exhibit normal renal salt excretion when either salt replete or salt restricted. Enhanced amiloride-sensitive FENa is consistent with increased Na+ absorption via the amiloride-sensitive sodium channel ENaC, in cystic fibrosis kidney, but this was only observed during salt restriction.
The cystic fibrosis transmembrane conductance regulator (CFTR) protein is widely expressed throughout the nephron and collecting duct system of the kidney (Morales et al. 1996). There remains some controversy about the precise localisation of CFTR protein along the nephron, with conflicting data from both immunocytochemical (Crawford et al. 1991; Devuyst et al. 1996) and electrophysiological studies (Segal et al. 1993; Rubera et al. 1998) for expression in the proximal tubule. Data for distal renal epithelia are more consistent, with functional CFTR expression demonstrated in the distal convoluted tubule (Tauc et al. 1996; Rubera et al. 1998), principal cells of the cortical collecting duct (Ling et al. 1994; Letz & Korbmacher, 1997) and in the inner medullary collecting duct (Husted et al. 1995; Vandorpe et al. 1995). There is more general agreement that CFTR is an apically located channel in the kidney, though isolated reports of basolaterally expressed CFTR-like channels also exist (Segal & Boulpaep, 1992).
In addition to being a cAMP-regulated Cl− channel (Anderson et al. 1991), it is now accepted that CFTR may be a regulator of other ion channels. Of particular interest is the interaction between CFTR and the amiloride-sensitive Na+ channel ENaC, which has been demonstrated in the airway (Mall et al. 1998), sweat ducts (Reddy et al. 1999) and gastrointestinal tract (Grubb & Boucher, 1997; Mall et al. 1999). A similar interaction may enhance Na+ absorption via ENaC in principal cells of the cortical collecting duct, where these channels are functionally co-expressed in the apical membrane (Letz & Korbmacher, 1997). Unfortunately confirmation of this interaction when CFTR expression is lost in renal principal cells is difficult to interpret because there is also a proposed interaction with the secretory K+ channel ROMK (Ho, 1998). Despite these properties of CFTR, patients with cystic fibrosis (CF) do not typically display renal dysfunction. To date, very few studies have been performed to investigate renal function in either CF patients or transgenic mouse models which have been exposed to renal stress; the physiological role of CFTR in the kidney remains unclear.
Perhaps the most relevant physiological stress to apply experimentally in CF is salt depletion. To date around 100 cases describing a ‘pseudo-Bartter's Syndrome’ of hypochloraemic metabolic alkalosis in CF children have been reported in the literature. This condition is generally ascribed to excessive salt loss in sweat, though it is not clear if primary renal salt wasting (as in true Bartter's Syndrome), or a reduced ability of the kidney to retain salt when insensible losses are high, contributes to this condition.
The aims of the present study were: (1) to determine if renal salt handling was altered by loss of wild-type CFTR expression, (2) to assess whether renal adaptation to sustained salt depletion was preserved in the absence of wild-type CFTR, (3) to determine whether there is any evidence for a functional interaction between CFTR and ENaC in the kidney. To achieve these aims, in vivo clearance experiments were performed using a ΔF508-CFTR transgenic mouse model (Cftrtm2cam) (Colledge et al. 1995). This model was selected since around 70 % of CF patients are homozygous for the ΔF508 mutation.
METHODS
Animals and genotyping
Mice were originally produced by Colledge et al. (1995). The ΔF508 mutation was introduced into the Cftr gene by targeted replacement using a construct with a three base pair deletion between nucleotides 1522 and 1524 in exon 10. Mice were bred from heterozygotes and genotyped by a PCR method modified from Rommens et al. (1990). PCR primers were redesigned to amplify the region surrounding the three base pair deletion across the GenBank sequences (mouse, M84614; rabbit, U40227; sheep, U20418 and human, M28668). Genomic PCR primer sequences were 5′-ATT AAG CAC AGT GGA AGA-3′ and 5′-CTC ATC ATA GGA AAC ACC-3′. A crude preparation of genomic murine DNA was used as the PCR template by placing ∼0.5 mm2 of dried blood spotted onto Whatman 3 mm filter paper into 23 μl of PCR reaction mix. This was left for 15 min at room temperature, overlaid with mineral oil and heated to 96°C for 10 min in a thermal cycler. The PCR was started by addition of 0.5 U Red-Hot Taq (Advanced Biotechnologies, Epsom, UK). Amplification conditions were 94°C for 30 s, 50°C for 30 s, 72°C for 60 s for 30 cycles, with a final extension of 10 min at 72°C. The PCR reaction mix was 0.2 μM of each primer, PCR buffer IV (Advanced Biotechnologies: 20 mM (NH4)2SO4, 75 mM Tris-HCl, 0.1 % Tween 20, pH 8.8), 3.5 mM MgCl2 and 0.3 mM each of dATP, dCTP, dGTP, dTTP in a final reaction volume of 25 μl. PCR products were resolved by electrophoresis on a 7 % non-denaturing polyacrylamide gel in 1× TBE running buffer (mM: 130 Tris, 45 boric acid, 2.5 EDTA) for 3 h at 220 V. PCR products were visualised by staining with ethidium bromide and the genotype obtained from the size of the PCR product (wild-type 99 bp, mutant ΔF508 96 bp).
Anaesthesia and surgery
Adult male mice were used throughout (see Tables 1 and 2 for ages and weights) and were obtained from the Field Laboratories, Western Bank, University of Sheffield, UK. Animals were specific pathogen free and were housed in a temperature- (20-22°C) and humidity- (40-60 %) controlled room with a 12 h light-dark cycle. Animals were weighed and anaesthetised with an initial intraperitoneal injection of 100 mg kg−1 sodium thiopentone, (Thiovet, C-Vet Veterinary Products, Leyland, UK). Ketamine (10 mg kg−1) and/or xylazine (1.5 mg kg−1) (Research Biochemicals International, Natick, MA, USA) was given intraperitoneally if additional maintenance anaesthesia was required. Ketamine/xylazine was used in preference to sodium thiopentone for maintenance anaesthesia, since its subsequent administration did not cause acute depression of blood pressure in preliminary experiments. Animals were then surgically prepared for renal clearance measurements. Briefly, polyethylene cannulae (Portex, outer diameter 0.63 mm, bore 0.50 mm) were placed in the right jugular vein for intravenous infusion and in the left carotid artery for continuous blood pressure monitoring and blood sampling. The bladder was also cannulated via a suprapubic incision, for urine collection. A tracheostomy was performed to maintain a clear airway and pure oxygen was blown over the neck area throughout.
Table 1.
Plasma variables and renal excretion in salt-replete mice
| Weight(g) | Age(days) | MAP(mmHg) | GFR(−l min−1) | Hct(%) | PNa(mM) | PCl(mM) | |
|---|---|---|---|---|---|---|---|
| WTvehicle (n = 12) | 29 ± 1 | 67 ± 4 | 90 ± 2 | 348 ± 45 | 46 ± 1 | 148 ± 3 | 108 ± 3 |
| * | |||||||
| CFvehicle (n = 7) | 26 ± 2 | 77 ± 3 | 94 ± 3 | 396 ± 53 | 45 ± 1 | 146 ± 2 | 114 ± 1 |
| * | * | ||||||
| WTamiloride (n = 6) | 30 ± 2 | 64 ± 8 | 98 ± 4 | 389 ± 70 | 45 ± 2 | 143 ± 3 | 109 ± 2 |
| * | |||||||
| CFamiloride (n = 9) | 25 ± 1 | 67 ± 4 | 92 ± 3 | 325 ± 26 | 46 ± 1 | 145 ± 3 | 119 ± 2 |
| * | * | * | * |
| V̇(μl min−1) | UVNa(nmol min−1) | CNa(μl min−1) | UVCl (nmol min−1) | CCl (μl min−1) | |
|---|---|---|---|---|---|
| WTvehicle (n = 12) | 4.5 ± 0.5 | 206 ± 42 | 1.4 ± 0.3 | 466 ± 41 | 4.4 ± 0.5 |
| * | |||||
| CFvehicle (n = 7) | 2.9 ± 0.3 | 238 ± 56 | 1.6 ± 0.4 | 435 ± 89 | 3.8 ± 0.8 |
| * | * | * | |||
| WTamiloride (n = 6) | 6.6 ± 0.5 | 1434 ± 174 | 9.9 ± 1.0 | 960 ± 104 | 8.8 ± 1.1 |
| * | *† | *† | † | *† | |
| CFamiloride (n = 9) | 6.2 ± 0.9 | 1560 ± 259 | 10.6 ± 1.5 | 1037 ± 96 | 8.7 ± 0.8 |
| † | † | *† | † | *† |
WT, wild-type; CF, Cftrtm2camΔF508; MAP, mean arterial blood pressure; Hct, haematocrit; Px, plasma concentration of x, V̇, urine flow rate; UVx, urine output of x; Cx, clearance of x.
Significant difference between salt-replete and salt-depleted animals (e. g. CFvehicle, Table 1versus CFvehicle, Table 2).
Significant effect of amiloride.
Table 2.
Plasma variables and renal excretion in salt-depleted mice
| Weight (g) | Age (days) | MAP(mmHg) | GFR (μl min−1) | Hct(%) | PNa(mM) | PCl(mM) | |
|---|---|---|---|---|---|---|---|
| WTvehicle (n = 8) | 33 ± 2 | 100 ± 11 | 94 ± 4 | 353 ± 44 | 46 ± 1 | 141 ± 4 | 108 ± 3 |
| * | |||||||
| CFvehicle (n = 8) | 31 ± 1 | 118 ± 10 | 88 ± 3 | 369 ± 65 | 47 ± 1 | 141 ± 2 | 105 ± 4 |
| * | * | ||||||
| WTamiloride (n = 7) | 34 ± 2 | 123 ± 13 | 90 ± 1 | 289 ± 45 | 46 ± 1 | 140 ± 2 | 106 ± 2 |
| * | |||||||
| CFamiloride (n = 9) | 32 ± 1 | 124 ± 9 | 90 ± 3 | 365 ± 22 | 47 ± 1 | 133 ± 4 | 107 ± 4 |
| * | * | * | * |
| V̇(μl min−1) | UVNa(nmol min−1) | CNa(μl min−1) | UVCl(nmol min−1) | CCl(μl min−1) | |
|---|---|---|---|---|---|
| WTvehicle (n = 8) | 4.5 ± 1.6 | 50 ± 11 | 0.3 ± 0.1 | 304 ± 81 | 2.8 ± 0.7 |
| * | * | ||||
| CFvehicle (n = 8) | 4.6 ± 0.8 | 51 ± 10 | 0.3 ± 0.1 | 206 ± 59 | 2.1 ± 0.7 |
| * | * | * | |||
| WTamiloride (n = 7) | 2.2 ± 0.4 | 429 ± 68 | 2.8 ± 0.5 | 194 ± 41 | 1.8 ± 0.4 |
| * | *† | *† | * | * | |
| CFamiloride (n = 9) | 6.0 ± 1.0 | 959 ± 138 | 6.6 ± 0.9 | 566 ± 139 | 5.3 ± 1.3 |
| ‡† | *‡† | * | * |
WT, wild-type; CF, Cftrtm2camΔF508; MAP, mean arterial blood pressure; Hct, haematocrit; Px, plasma concentration of x, V̇, urine flow rate, UVx, urine output of x, Cx = clearance of x.
Significant difference between WT and CF for equivalent treatments (e. g. WTvehicleversus CFvehicle).
Significant difference between salt-replete and salt-depleted animals (e.g. CFvehicle, Table 1 versus CFvehicle, Table 2).
Significant effect of amiloride.
Experimental protocols
Two series of experiments were performed in which mice were either salt replete or exposed to dietary salt restriction. For each level of salt intake, the effect of amiloride infusion was assessed and compared between Cftrtm2camΔF508 cystic fibrosis mice and wild-type controls. Mice in the Na+-replete series were maintained on a standard chow diet containing 0.32 % w/w NaCl. To induce a state of salt depletion mice were transferred to a chow containing 0.03 % NaCl for 14 days prior to experimentation (diets obtained from Special Diets Services, Essex, UK). All animals were given access to tap water ad libitum. In salt-replete animals the intravenous infusion solution was 155 mM NaCl, whereas in salt-depleted animals a low-Na+ solution was used containing 15 mM NaCl to avoid acutely salt reloading the animals during post surgery equilibration. Immediately following implantation of the venous cannula, until the end of surgery, all animals received intravenous infusion at a rate of 0.3 ml h−1 to replace fluid loss due to surgery. After surgery a 45 min equilibration period was observed, followed by a 60 min experimental clearance period over which urine was collected for analysis. 3H[Inulin] (Amersham Life Sciences, UK) was included in the infusion solutions to allow the estimation of the glomerular filtration rate (GFR). During the first 5 min of equilibration a 1.5 μCi priming dose of 3H[inulin] was given in 0.1 ml infusate. Thereafter the infusion rate was 0.3 ml h−1, containing a 4.5 μCi h−1 maintenance dose of 3H[inulin]. All animals further received either intravenous amiloride at a rate of 2 mg kg−1 h−1, started at the beginning of the equilibration period, or an equivalent DMSO vehicle infusion (equivalent to a total dose of 0.2 μl g−1 of DMSO over the whole experiment). A dose of 2 mg kg−1 h−1 amiloride was selected, based on previous studies in the rat, which have shown that this dose is selective for inhibition of the amiloride-sensitive Na+ channel in the collecting duct, and is insufficient to inhibit Na+-H+ exchange in the proximal nephron (Shalmi et al. 1998). Preliminary experiments established that these protocols produced stable plasma 3H[inulin] activity, [Na+], [Cl−], and haematocrit during the 60 min experimental clearance period. For this reason, a terminal plasma sample was used for calculation of renal clearances. After the terminal blood sample had been taken, animals were killed by anaesthetic overdose. 3H[Inulin] was assayed by liquid scintillation counting, Na+ was determined by single channel flame photometry (Sherwood, Model 410, Scientific Laboratory Supplies, Nottingham, UK) and Cl− was measured by electrometric titration (Jenway PCLM3, Essex, UK).
Data presentation and statistics
Renal clearance and fractional excretion values were calculated using standard formulae. Data are expressed throughout as means ±s.e.m. derived from n animals in each group. The effects of diet, amiloride and genotypes were initially assessed using a multivariate analysis, inequalities were then located using single factor analysis of variance for each dietary group and differences between genotypes determined by Student's unpaired t tests with a Bonferroni modification to accommodate multiple comparisons. P = 0.05 was taken as the level of statistical significance.
RESULTS
Animals
Adult male mice were used throughout to reduce variation due to pooling animals of different sex. It was possible to rear Cftrtm2camΔF508 mice on regular chow diets without causing mortality due to intestinal obstruction. The initial rate of growth was less in Cftrtm2camΔF508 mice (data not shown), though the final adult weights were not significantly different from wild-type animals (see Tables 1 and 2). Pre-weaning mortality was negligible in wild-type animals, but was consistently around 20 % in Cftrtm2camΔF508 mice. No special difficulties were noted in maintaining Cftrtm2camΔF508 mice under anaesthesia. It should be noted, however, that in both wild-type and Cftrtm2camΔF508 animals, the experimental success rate fell from around 80 % in salt-replete to around 50 % in salt-depleted animals. Experimental failure was characterised by cardiorespiratory depression upon induction of anaesthesia, with a progressive fall in mean arterial pressure throughout the protocol, usually resulting in anuria. The need for additional experiments in these groups accounts for the increased average ages of mice in all the salt-depleted groups, irrespective of genotype or amiloride treatment (compare Table 1 and Table 2).
Plasma composition and haemodynamic variables
Table 1 shows that plasma [Na+] and [Cl−] were in the range reported numerous times for rodents under similar experimental conditions. Comparing vehicle-infused animals in Table 2, it was apparent that both wild-type and Cftrtm2camΔF508 mice were able to defend normal plasma NaCl concentrations in response to dietary salt depletion. Only in amiloride-treated Cftrtm2camΔF508 mice subjected to salt depletion was the plasma [NaCl] reduced, but this was presumably related to the enhanced urinary NaCl excretion observed in this group (see below). Similarly no differences in mean arterial blood pressure or haematocrit were observed as a consequence of altered diet, difference in genotype or due to amiloride treatment.
Basal renal function
Altered GFR in CF patients is an inconsistent finding in the literature (Robson et al. 1971; Stenvinkel et al. 1991). In this study, using animals under more controlled conditions than readily achieved clinically, no differences in GFR were noted as a consequence of genotype, or indeed due to salt depletion or amiloride treatment. Comparing data for urine flow rate, and urinary Na+ and Cl− clearances between wild-type and Cftrtm2camΔF508 mice for salt-replete animals (Table 1), no significant differences were observed, such that no primary salt wastage was evident in the urine of CF mice.
Effect of dietary salt depletion on renal salt excretion
The effects of salt restriction may be seen by comparing equivalent groups of animals in Tables 1 and 2. Salt restriction caused a marked reduction in urinary Na+ excretion in both wild-type and Cftrtm2camΔF508 mice. Comparing the open bars in the lower panel of Fig. 1 shows that that fractional sodium excretion (FENa) was almost identical for each genotype, indicating that the ability to adapt to low salt conditions was preserved in Cftrtm2camΔF508 mice. The effect of salt restriction on urinary Cl− excretion followed a similar pattern to that of Na+, though differences between groups were more modest.
Figure 1. Effect of amiloride infusion on fractional Na+ excretion.
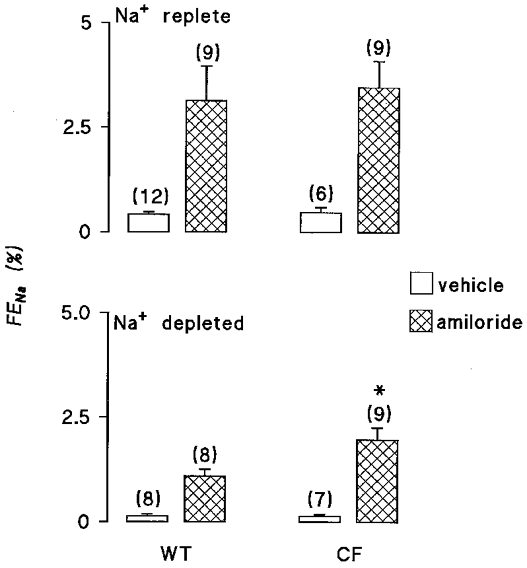
Fractional Na+ excretion (FENa) is shown for wild-type (WT) mice and Cftrtm2camΔF508 (CF) mice. The upper panel shows data for salt-replete animals, the lower panel for mice subjected to 14 days of dietary salt restriction prior to experimentation. Open bars indicate animals receiving a DMSO vehicle infusion, hatched bars represent i.v. infusion with 2 mg kg−1 h−1 amiloride. The number of animals in each group is shown in parentheses. * Significant difference between WT and CF mice receiving like treatments.
Comparing vehicle-infused animals, no significant effects of salt depletion on urine flow rate were observed in either genotype, though amiloride produced a significantly lower urine flow rate in wild-type animals subjected to salt depletion compared with salt-replete controls.
Effect of amiloride
In both salt-replete and salt-restricted animals, amiloride was only weakly diuretic. In salt-replete animals a significant increase in urine flow rate was observed for Cftrtm2camΔF508 mice, though the increment for wild-type animals just failed to reach statistical significance (P value prior to Bonferroni modification = 0.04). In salt-depleted mice no significant change in urine flow rate was observed in either wild-type or Cftrtm2camΔF508 mice in response to amiloride treatment. Small sample volumes prevented analysis of urine osmolality, such that fractional water excretion could not be calculated.
In salt-replete animals, amiloride produced a marked increase in urinary Na+ excretion, with FENa increased by around 3 %, in line with data previously reported for the rat (Shalmi et al. 1998). Inspection of Fig. 1 and Table 1 shows that the extent of this natriuresis was not different between wild-type and Cftrtm2camΔF508 mice. The response to amiloride in terms of Cl− excretion was qualitatively similar, though less marked than for Na+. For example, it can be seen in Fig. 2 that a significant chloruresis was observed in Cftrtm2camΔF508 mice, whilst the response in wild-type animals just failed to reach statistical significance (P value before Bonferroni modification = 0.03). Nevertheless, values for fractional chloride excretion (FECl) comparing the two genotypes in the presence of amiloride were virtually identical.
Figure 2. Effect of amiloride infusion on fractional Cl− excretion.
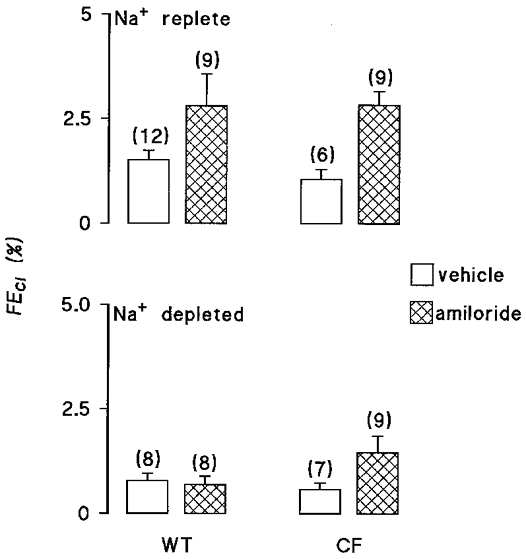
Fractional Cl− excretion (FECl) is shown for wild-type (WT) mice and Cftrtm2camΔF508 (CF) mice. The upper panel shows data for salt-replete animals, the lower panel for mice subjected to 14 days of dietary salt restriction prior to experimentation. Open bars indicate animals receiving a DMSO vehicle infusion, hatched bars represent i.v. infusion with 2 mg kg−1 h−1 amiloride. The number of animals in each group is shown in parentheses.
The pattern of response to amiloride was different in salt-depleted animals. It is apparent from Fig. 1 that amiloride again caused a significant natriuresis in both wild-type and Cftrtm2camΔF508 mice, though the size of the natriuresis was significantly smaller than observed in salt-replete animals. Perhaps the most striking difference with salt-restricted animals was that amiloride had a greater effect in Cftrtm2camΔF508 mice with a FENa almost twice that of wild-type animals. With respect to fractional Cl− excretion, no significant differences between genotypes were observed (Fig. 2).
DISCUSSION
Summary
The aims of the present study were: (1) to determine if renal salt handling was disturbed in Cftrtm2camΔF508 mice, (2) to assess whether renal adaptation to sustained salt depletion was preserved in Cftrtm2camΔF508 and (3) to determine whether there is any evidence for a functional interaction between CFTR and ENaC in the kidney. Comparing renal NaCl excretion in vehicle-infused animals revealed that Cftrtm2camΔF508 mice and wild-type controls have very similar renal salt excretion rates, plasma [Na+] and [Cl−], haematocrit and mean arterial blood pressures. Thus, insofar as we are able to determine renal handling using clearance methods, there was no evidence of a primary defect in renal salt handling. If the rates of fractional salt excretion are compared after 2 weeks of sustained dietary salt restriction, it is apparent that both Cftrtm2camΔF508 mice and wild-type mice are able to reduce renal Na+ excretion to almost exactly the same degree. Therefore, the data do not reveal any defect in the ability to adapt to low salt conditions. The infusion of the diuretic amiloride at doses designed to target the function of ENaC in the collecting duct, caused the expected significant natriuresis in all cases. However, during salt depletion, FENa was almost doubled in Cftrtm2camΔF508 mice. These data are consistent with the generalised augmentation in Na+ flux via ENaC observed in CF tissues, though the effect was only apparent during salt depletion.
Experimental model
To our knowledge, this is the first study to investigate renal function in vivo using a transgenic cystic fibrosis mouse model. To date there are still very few studies characterising renal function in the mouse in vivo. In preliminary studies we were unable to consistently maintain stable anaesthesia and renal function for periods exceeding 4 h, using a variety of anaesthetic agents. As a result a shortened clearance protocol was adopted to include a single 1 h clearance period. Values for mean arterial blood pressure (MAP) were slightly lower than observed in some other mouse strains, though GFR and urine flow rate were very similar (e.g. Schnermann et al. 1998; Lorenz et al. 1999). In terms of the CF phenotype of the mice, in addition to confirming the genotype by PCR, samples of intestine from littermate animals were used in electrophysiology experiments, which confirmed the absence of cAMP-mediated Cl− secretion in the CF tissue (Hardcastle et al. 1999). Previous studies have also shown that the nasal epithelium of Cftrtm2Cam mice exhibits enhanced amiloride-sensitive Na+ absorption (MacVinish et al. 1997), as expected for CF airway.
Effects of salt depletion
Physiological salt depletion has been extensively studied in rats and humans and it results in a fall in extracellular fluid volume (Dubose, 1990). A fall in arterial blood pressure does not generally occur due to a battery of compensatory mechanisms. These include an increase in peripheral vascular resistance, mediated by the renin-angiotensin system, autonomic nervous system and by release of vasopressin (Dubose, 1990). GFR is typically preserved due to an increased filtration fraction, and several tubular Na+ and Cl− transport processes are up-regulated to promote renal salt retention. These include enhanced proximal tubule reabsorption mediated by renal nerve stimulation and also the greater physical forces which promote reabsorption when the filtration fraction is increased (i.e. glomerulotubular balance) (Gonzalez-Campoy & Knox, 1992). Vasopressin is expected to promote salt reabsorption in the thick ascending limb (Hall & Vannay, 1980) and the release of aldosterone following stimulation of the renin-angiotensin system upregulates salt reabsorption in the cortical collecting duct (see below). In the present study, mice were apparently able to adapt to salt depletion quite adequately, since they did not show a reduced mean arterial blood pressure or a fall in plasma Na+ or Cl− concentration. It should be noted, however, that experimental success rate was reduced during sodium depletion for all treatment groups. The reason for this was not clear, though these animals appeared to be more susceptible to cardiorespiratory depression during anaesthesia. In terms of renal adaptation there was a significant and equal reduction in FENa in both wild-type and Cftrtm2camΔF508 mice. It was also interesting to note that the response to amiloride infusion in terms of FENa was blunted in both genotypes compared with salt-replete animals. This is consistent with increased salt reabsorption in nephron segments upstream of the collecting duct.
Psuedo-Bartter's Syndrome
Bartter's Syndrome is a salt-losing nephropathy caused by mutations in transport proteins in the thick ascending limb of Henle's loop (Simon & Lifton, 1998). The phenotype is characterised by metabolic alkalosis, with wasting of Na+, Cl−, K+ and divalent cations. It has been recognised for many years that a subset of CF children present with a similar condition, with potential causes including excessive sweating, vomiting or renal salt wasting (Mauri et al. 1997). In the present study we observed no difference in basal Na+ or Cl− excretion in salt-replete animals (Table 1), indicating that, at least in this CF mouse model, no primary renal salt wastage occurs. In addition, when salt depletion was applied, the Cftrtm2camΔF508 mice were equally good at reducing renal salt excretion (Table 2). That CF kidney might have a reduced ability to cope with low salt conditions, thereby compounding the problem of salt depletion from excessive sweating, is also not supported by the present study. These data are consistent with those reports of human cases of pseudo-Bartter's patients where renal Cl− excretion has been analysed and found to be low (Mauri et al. 1997).
Effect of amiloride
The dose of amiloride used (2 mg kg−1 h−1) was designed to target ENaC function in the collecting duct (Shalmi et al. 1998). The data are consistent with this, since an FENa of around 3 % in response to amiloride is expected in both human and rat (Shalmi et al. 1998) during normal dietary salt intakes. Similar levels of FENa were also reported in salt-depleted rats (Shalmi et al. 1998) as those observed in the current study in mice. The major finding of this study is that Cftrtm2camΔF508 mice exhibit a significantly enhanced natriuretic response to amiloride, but this is only apparent during salt depletion. It has been recognised for several years that Na+ absorption is enhanced in CF airway (Boucher et al. 1986) and more recently this has been shown in several laboratories to be due to loss of the normal inhibition of the amiloride-sensitive Na+ channel ENaC by CFTR (e.g. Mall et al. 1998; Schreiber et al. 1999). Other tissues which co-express ENaC and CFTR in the apical membrane include the intestinal epithelia (Mall et al. 1999) and renal principal cells (Letz & Korbmacher, 1997). It is now apparent that human CF intestine shows the same enhancement in ENaC function as in the CF airway (Mall et al. 1999). A similar effect has also been reported in the intestine of CF mouse but only during dietary salt restriction (Grubb & Boucher, 1997). Recent data from the sweat gland have challenged the idea that reciprocal activity of CFTR and ENaC is the only type of interaction that can occur. Reddy et al. (1999) have now shown that in the sweat duct CFTR expression is required for normal ENaC activity. The nature of CFTR-ENaC interaction appears, therefore, to be tissue specific.
In murine renal collecting duct cells, Letz & Korbmacher (1997) showed a reciprocal regulation of ENaC and CFTR, though to date no studies have tested whether ENaC function is enhanced in the CF kidney. Our findings show that whilst amiloride is effective at producing a natriuresis in salt-replete animals, indicating that ENaC is active, no difference between wild-type and CF animals exists (or if there is a difference, it is fully compensated). During salt depletion, a substantial increase in ENaC expression at the apical membrane is expected to occur in response to elevated plasma aldosterone (Frindt et al. 1990; Dijkink et al. 1999). The levels of FENa were blunted in response to amiloride in salt-restricted animals, despite this expected increase in transport through ENaC. This is expected, however, since the delivered load of Na+ to the collecting duct will be markedly reduced under these conditions (Shalmi et al. 1998). Thus there is avid absorption of a reduced Na+ load in the cortical collecting duct. Under these conditions CF mice showed enhanced amiloride-sensitive FENa. Although the molecular mechanism responsible for this effect cannot be studied directly in vivo, the data are consistent with reduced inhibition of ENaC in the collecting duct epithelium of CF animals. An alternative explanation could be that enhanced secretion of aldosterone previously observed in CF mice exposed to identical dietary salt restriction (Grubb & Boucher, 1997) is responsible. Accordingly there would be more active ENaC channels present in the collecting duct of CF mice and thus a greater effect of amiloride. However, as Grubb & Boucher (1997) pointed out, the levels of aldosterone in both wild-type and CF animals were already 3-4 times higher than that expected to give maximal effects of the hormone. Thus, the differences observed in the effect of amiloride are unlikely to be a consequence of different rates of aldosterone production.
Physiological significance
As with CF patients it is apparent that CF mice do not exhibit primary changes in renal function. Indeed, when experimental conditions are tightly controlled, the tendency to salt retention in CF patients (Stenvinkel et al. 1991) is not observed in the mouse. Further work is indicated in CF patients, to determine if this reduced Na+ clearance is secondary to contraction of the extracellular fluid volume, rather than due to altered tubular transport. Since the enhanced effect of amiloride was not present during salt-replete conditions, problems of renal salt retention, which might lead to hypertension are not expected. Paradoxically the ‘defect’ during salt restriction will, in fact, lead to an enhanced overall capacity for renal salt retention, which could conceivably provide an advantage during states of salt deprivation.
Acknowledgments
We thank the Wellcome Trust, Royal Society and Sheffield Childrens’ Appeal for financial support. The technical assistance of Sarah Jennings is gratefully acknowledged.
References
- Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;251:679–681. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Boucher RC, Stutts MJ, Knowles MR, Cantley L, Gatzy JT. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. Journal of Clinical Investigation. 1986;78:1245–1252. doi: 10.1172/JCI112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge WH, Abella BS, Southern KW, Ratcliff R, Jiang C, Cheng SH, Macvinish LJ, Anderson JR, Cuthbert AW, Evans MJ. Generation and characterization of a ΔF508 cystic fibrosis mouse model. Nature Genetics. 1995;10:445–452. doi: 10.1038/ng0895-445. [DOI] [PubMed] [Google Scholar]
- Crawford I, Maloney PC, Zeitlin PL, Guggino WB, Hyde SC, Turley H, Gatter KC, Harris A, Higgins CF. Immunocytochemical localization of the cystic fibrosis gene product CFTR. Proceedings of the National Academy of Sciences of the USA. 1991;88:9262–9266. doi: 10.1073/pnas.88.20.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devuyst O, Burrow CR, Schwiebert EM, Guggino WB, Wilson PD. Developmental regulation of CFTR expression during human nephrogenesis. American Journal of Physiology. 1996;271:F723–735. doi: 10.1152/ajprenal.1996.271.3.F723. [DOI] [PubMed] [Google Scholar]
- Dijkink L, Hartog A, Deen PM T, Os van CH, Bindels RJ M. Time-dependent regulation by aldosterone of the amiloride-sensitive Na+ channel in rabbit kidney. Pflügers Archiv. 1999;438:354–360. doi: 10.1007/s004240050920. [DOI] [PubMed] [Google Scholar]
- Dubose TD. Salt wastage and salt depletion. In: Seldin DW, Giebisch G, editors. The Regulation of Sodium Chloride Balance. New York: Raven Press; 1990. pp. 419–432. [Google Scholar]
- Frindt G, Sackin H, Palmer LG. Whole-cell currents in rat cortical collecting tubule: low-Na diet increases amiloride-sensitive conductance. American Journal of Physiology. 1990;258:F562–567. doi: 10.1152/ajprenal.1990.258.3.F562. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Campoy JM, Knox FG. Integrated responses of the kidney to alterations in extracellular fluid volume. In: Seldin DW, Giebisch G, editors. The Kidney: Physiology and Pathophysiology. 2. New York: Raven Press; 1992. pp. 2041–2097. [Google Scholar]
- Grubb BR, Boucher RC. Enhanced colonic Na+ absorption in cystic fibrosis mice versus normal mice. American Journal of Physiology. 1997;272:G393–400. doi: 10.1152/ajpgi.1997.272.2.G393. [DOI] [PubMed] [Google Scholar]
- Hall JE, Vannay DM. Effect of vasopressin on electrical potential difference and chloride transport in mouse medullary thick ascending limb of Henle's loop. Journal of Clinical Investigation. 1980;66:792–802. doi: 10.1172/JCI109917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle J, Hardcastle PT, Klaren PH M, Taylor CJ, White SJ. The action of 5-hydroxytryptamine on normal and cystic fibrosis mouse colon: effects on secretion and intracellular calcium. Journal of Pharmacy and Pharmacology. 1999;51:449–456. doi: 10.1211/0022357991772501. [DOI] [PubMed] [Google Scholar]
- Ho K. The ROMK-cystic fibrosis transmembrane conductance regulator connection: new insights into the relationship between ROMK and the cystic fibrosis transmembrane conductance regulator. Current Opinions in Nephrology and Hypertension. 1998;7:49–58. doi: 10.1097/00041552-199801000-00009. [DOI] [PubMed] [Google Scholar]
- Husted RF, Volk KA, Sigmund RD, Stokes JB. Anion secretion by the inner medullary collecting duct: Evidence for involvement of the cystic fibrosis transmembrane conductance regulator. Journal of Clinical Investigation. 1995;95:644–650. doi: 10.1172/JCI117709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letz B, Korbmacher C. cAMP stimulates CFTR-like Cl− channels and inhibits amiloride-sensitive Na+ channels in mouse CCD cells. American Journal of Physiology. 1997;272:C657–666. doi: 10.1152/ajpcell.1997.272.2.C657. [DOI] [PubMed] [Google Scholar]
- Ling BN, Kokko KE, Eaton DC. Prostaglandin E2 activates clusters of apical Cl− channels in principal cells via a cyclic adenosine monophosphate-dependent pathway. Journal of Clinical Investigation. 1994;93:829–837. doi: 10.1172/JCI117037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz JN, Schultheis PJ, Traynor T, Shull GE, Schnermann J. Micropuncture analysis of single-nephron function in NHE3-deficient mice. American Journal of Physiology. 1999;277:F447–453. doi: 10.1152/ajprenal.1999.277.3.F447. [DOI] [PubMed] [Google Scholar]
- MacVinish LJ, Goddard C, Colledge WH, Higgins CF, Evans MJ, Cuthbert AW. Normalization of ion transport in murine cystic fibrosis nasal epithelium using gene transfer. American Journal of Physiology. 1997;273:C734–740. doi: 10.1152/ajpcell.1997.273.2.C734. [DOI] [PubMed] [Google Scholar]
- Mall M, Bleich M, Greger R, Schreiber R, Kunzelmann K. The amiloride-inhibitable Na+ conductance is reduced by the cystic fibrosis transmembrane conductance regulator in normal but not in cystic fibrosis airways. Journal of Clinical Investigation. 1998;102:15–21. doi: 10.1172/JCI2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall M, Bleich M, Kuehr J, Brandis M, Greger R, Kunzelmann K. CFTR-mediated inhibition of epithelial Na+ conductance in human colon is defective in cystic fibrosis. American Journal of Physiology. 1999;277:G709–716. doi: 10.1152/ajpgi.1999.277.3.G709. [DOI] [PubMed] [Google Scholar]
- Mauri S, Pedroli G, Ròdeberg A, Laux-Eend R, Monotti R, Bianchetti MG. Acute metabolic alkalosis in cystic fibrosis: prospective study and review of the literature. Mineral and Electrolyte Metabolism. 1997;23:33–37. [PubMed] [Google Scholar]
- Morales MM, Carroll TP, Morita T, Schweibert EM, Devuyst O, Wilson PD, Lopes AG, Stanton BA, Dietz HC, Cutting GR, Guggino WB. Both wild-type and a functional isoform of CFTR are expressed in kidney. American Journal of Physiology. 1996;270:F1038–1048. doi: 10.1152/ajprenal.1996.270.6.F1038. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Light MJ, Quinton PM. Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl− channel function. Nature. 1999;402:301–304. doi: 10.1038/46297. [DOI] [PubMed] [Google Scholar]
- Robson AM, Tateishi S, Ingelfinger JR, Strominger DB, Klahr S. Renal function in patients with cystic fibrosis. Journal of Paediatrics. 1971;79:42–50. doi: 10.1016/s0022-3476(71)80056-2. [DOI] [PubMed] [Google Scholar]
- Rommens J, Kerem B-S, Greer W, Chang P, Tsui L-C, Ray P. Rapid non-radioactive detection of the major cystic fibrosis mutation. American Journal of Human Genetics. 1990;46:395–396. [PMC free article] [PubMed] [Google Scholar]
- Rubera I, Tauc M, Bidet M, Poujeol C, Cuiller B, Watrin A, Touret N, Poujeol P. Chloride currents in primary cultures of rabbit proximal and distal convoluted tubules. American Journal of Physiology. 1998;275:F651–663. doi: 10.1152/ajprenal.1998.275.5.F651. [DOI] [PubMed] [Google Scholar]
- Schnermann J, Chou C-L, Ma T, Traynor T, Knepper MA, Verkman AS. Defective proximal tubular reabsorption in transgenic aquaporin-1 null mice. Proceedings of the National Academy of Sciences of the USA. 1998;95:9660–9664. doi: 10.1073/pnas.95.16.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Hopf A, Mall M, Greger R, Kunzelmann K. The first nucleotide binding domain of the cystic fibrosis transmembrane conductance regulator is important for inhibition of the epithelial Na+ channel. Proceedings of the National Academy of Sciences of the USA. 1999;96:5310–5315. doi: 10.1073/pnas.96.9.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal AS, Boulpaep EL. cAMP-activated chloride channel on the basolateral membrane of renal proximal tubule. Journal of the American Society of Nephrology. 1992;3:819. [Google Scholar]
- Segal AS, Geibel J, Boulpaep EL. A chloride channel in the basolateral membrane of rabbit proximal tubule. Journal of the American Society of Nephrology. 1993;4:879. [Google Scholar]
- Shalmi M, Jonassen T, Thomsen K, Kibble JD, Bie P, Christensen S. Model explaining the relation between distal nephron Li+ reabsorption and urinary Na+ excretion in rats. American Journal of Physiology. 1998;274:F445–452. doi: 10.1152/ajprenal.1998.274.3.F445. [DOI] [PubMed] [Google Scholar]
- Simon DB, Lifton RP. Ion transporter mutations in Gitelman's and Bartter's syndromes. Current Opinion in Nephrology and Hypertension. 1998;7:43–47. doi: 10.1097/00041552-199801000-00008. [DOI] [PubMed] [Google Scholar]
- Stenvinkel P, Hjelte GA, Hedman A, Hultman E, Strandvik B. Decreased renal clearance of sodium in cystic fibrosis. Acta Paediatrica Scandinavica. 1991;80:194–198. doi: 10.1111/j.1651-2227.1991.tb11833.x. [DOI] [PubMed] [Google Scholar]
- Tauc M, Bidet M, Poujeol P. Chloride currents activated by calcitonin and cAMP in primary cultures of rabbit distal convoluted tubule. Journal of Membrane Biology. 1996;150:255–273. doi: 10.1007/s002329900049. [DOI] [PubMed] [Google Scholar]
- Vandorpe D, Kizer N, Ciampollilo F, Moyer B, Karlson K, Guggino WB, Stanton BA. CFTR mediates electrogenic chloride secretion in mouse inner medullary collecting duct (mIMCD-K2) cells. American Journal of Physiology. 1995;269:C683–689. doi: 10.1152/ajpcell.1995.269.3.C683. [DOI] [PubMed] [Google Scholar]


