Abstract
Using patch-clamp techniques, a hyperpolarization-activated current (Ih) was recorded from synaptic terminals of mouse cerebellar basket cells.
Ih was blocked quickly and reversibly by 2 mM Cs+, and subtraction revealed a rapidly activating and deactivating Ih current. Similar gating and block of presynaptic Ih were also seen with the more selective inhibitor ZD 7288 (10 μM).
The time constant of activation (τa) of presynaptic Ih current became faster with membrane hyperpolarization, being ≈74 ms at -130 mV, changing e-fold for a 33 mV change in membrane potential.
Whole-cell recordings from basket cell somata also revealed an Ih current, which was similarly sensitive to block by ZD 7288.
Inhibition of Ih by 10 μM ZD 7288 reduced the frequency (≈34 %) and amplitude (≈26 %) of spontaneous IPSCs (sIPSCs) recorded in Purkinje cells, one of the principal synaptic targets of basket neurones.
This is the first report of an Ih current in mammalian inhibitory presynaptic terminals, which may be an important target for neuromodulation in the cerebellum. Comparing the biophysical properties and distribution of cloned hyperpolarization-activated cation channels, we also suggest a molecular candidate underlying Ih at these synapses.
A key goal in physiology is understanding the molecular processes associated with fast synaptic transmission between CNS neurones. Whilst we have a wealth of molecular detail about many voltage-gated ion channels, and are beginning to determine their precise cellular location, there is still a big gap in our knowledge about the ion channels located in presynaptic terminals in the brain. Direct electrophysiological studies of the giant glutamatergic synapse at the calyx of Held have provided us with considerable information about the properties of Ca2+ channels involved in transmitter release (e.g. Forsythe et al. 1998). We have recently developed a novel presynaptic preparation for electrophysiological studies, namely the distinctive and influential inhibitory synaptic terminals formed by cerebellar basket cells on Purkinje neurones, and have begun to dissect out the types of voltage-gated K+ channels located at this important synapse (Southan & Robertson, 1998, 2000). In the present study, we describe some of the properties and roles for a hyperpolarization-activated current (Ih) in basket cell presynaptic terminals.
Since Ih is a mixed inward Na+/K+ current, with an equilibrium potential of ∼-30 mV, once activated, it causes a slow depolarization leading the cell to threshold; in the heart, such currents play a crucial role in pacemaking activity (DiFrancesco, 1993). The role of Ih in the CNS is becoming increasingly more complex however (see Pape, 1996). In thalamic relay neurones (McCormick & Pape, 1990) and in hippocampal interneurones (Maccaferri & McBain, 1996) Ih can generate pacemaking currents. Additionally, Ih currents play a significant part in determining the resting membrane potential and conductance of neurones (Pape, 1996), and in synaptic integration in dendrites (Magee, 1998, 1999). Ih channels are also found in axons (Grafe et al. 1997) and in the presynaptic calyx of chick ciliary ganglion neurones (Fletcher & Chiappinelli, 1992), but their precise function in such processes is unresolved. Beaumont & Zucker (2000) have recently reported a functional role for Ih channels in regulating synaptic plasticity at the presynapse of crayfish neuromuscular junctions. Here, we demonstrate that Ih is present in the terminals and somata of basket interneurones in the mammalian CNS, and may be functionally active in regulating GABA release at this synapse.
METHODS
Our methods have been previously described in detail (see Southan & Robertson, 1998). Male TO (Charles River) mice, 3-5 weeks old, were killed by cervical dislocation and decapitated. Parasaggital cerebellar slices (250 μm thick) were prepared in chilled sucrose-based artificial cerebrospinal fluid (ACSF) using a Vibroslice (Campden Instruments). The standard ACSF contained (mM): NaCl 124, KCl 3, NaHCO3 26, NaH2PO4 2.5, MgSO4 2, CaCl2 2, D-glucose 10; bubbled with 95 % O2-5 % CO2.
Individual neurones and nerve terminals were visualized with differential interference contrast (DIC) optics using an Axioskop FS microscope (Zeiss, Germany). Basket cell nerve terminals are fine processes surrounding the Purkinje cell soma, descending to the axon initial segment region (Ramón y Cajal, 1911), and were unambiguously identified using Lucifer Yellow fluorescence microscopy (Southan & Robertson, 1998).
Recordings were made at room temperature, using patch-clamp techniques with electrodes of resistance between 3 and 7 MΩ for somatic recording and 10-15 MΩ for basket cell nerve terminal recording. Series resistance in terminals was 14-30 MΩ, and compensated for (65-95 %) throughout. Electrodes (GC150-F10, Clark Electromedical Instruments) were filled with an intracellular solution containing (mM):- KCl 140, MgCl2 1, CaCl2 1, EGTA 10, Hepes 10; pH 7.3. Electrophysiological recordings were made using an EPC-9 amplifier (HEKA), controlled by Pulse software (HEKA) with a Macintosh (7500/100) computer. Data were filtered at one-third of the appropriate sampling frequency. IPSCs were individually identified before measurement. Data analysis was carried out using Axograph (Axon Instruments), Pulsefit (HEKA), Igor (Wavemetrics) and Kaleidagraph software. Data are presented as mean values ±s.e.m., where n = number of cells. Statistical significance was determined using Student's t test or a Wilcoxon signed rank test (Statview II, Abacus Concepts). All drugs (Sigma, Tocris) were bath applied.
RESULTS
We have made direct patch-clamp recordings from the synaptic terminals of cerebellar basket cells (Southan & Robertson, 1998). These terminals wrap around the somata and axon initial segment of Purkinje cells, and by using moderately high resistance patch electrodes, we are able to record voltage-gated currents in ‘whole-cell’ mode. It is important to emphasize that all such recordings were anatomically confirmed as basket terminals using Lucifer Yellow; this avoids possible confusion with other cellular processes on, or near, Purkinje cells. Figure 1 shows one such retrograde Lucifer Yellow fill. The terminal recording site in this case was ∼125 μm from the basket cell soma, and the fluorescent dye has back-filled a substantial part of the neurone during this 25 min recording. Here, there are at least four pericellular baskets and pinceau structures around adjacent Purkinje cells (Ramón y Cajal, 1911), and recordings from this most distal site revealed spontaneous action currents with Na+ and mixed K+ currents. Whilst making such presynaptic terminal recordings, we sought to examine whether hyperpolarization-activated currents were also present.
Figure 1. Retrograde labelling of basket cell during terminal recording.
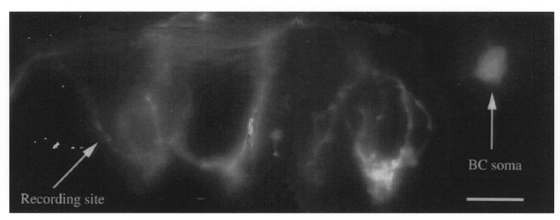
Montage reconstruction of a basket cell (BC) filled with Lucifer Yellow during an electrophysiological recording from a distal nerve terminal. The connecting axon, which was clearly observed down the microscope, is too faint to be seen here. Arrows indicate recording site, and back-filled soma. Scale bar is 20 μm.
Properties of Ih in presynaptic terminals
Figure 2A shows inward currents evoked by 500 ms hyperpolarizing steps from -70 to -160 mV (in -10 mV steps), and outward K+ currents on the return step to +30 mV. The inward, hyperpolarization-activated current is non-inactivating over 500 ms and bears many similarities to previously described Ih currents in CNS neurones. Similar currents were observed in 14/14 unambiguously identified, stable, basket cell terminal recordings. The threshold for this current is slightly more negative (∼-80 mV) than the holding potential of -70 mV (Fig. 2B). There was some variability in Ih amplitude between terminals, ranging from -38 to -129 pA (mean -69 ± 16.7 pA, at -130 mV; n = 5). A hallmark property of Ih currents is their sensitivity to external Cs+, which blocks in the low millimolar range. CsCl (2-6 mM) completely blocked the Ih current in all five terminals tested. Block by 2 mM Cs+ was extremely rapid in onset (within 30 s of solution change) and could be readily reversed (within 40 s of washout). Digital subtraction of the Cs+-sensitive current (Fig. 2C) reveals the ‘pure’Ih current, which activates and deactivates rapidly. (The currents shown in Fig. 2C were obtained from the recording site shown in Fig. 1.)
Figure 2. Electrophysiological characterization of Ih.
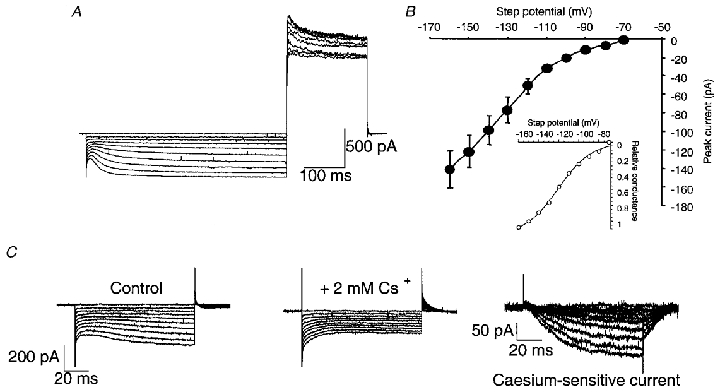
A, hyperpolarizing steps from a holding potential of -70 mV in a basket cell nerve terminal reveal an Ih current. Steps were 500 ms long from -70 to -160 mV, in -10 mV increments, followed by a return step to +30 mV. The return step shows the voltage-activated outward K+ current. No leak subtraction was used. B, current-voltage plot for Ih current in basket cell terminals. Peak inward current was measured (at the end of the voltage step), from 5 terminals from 5 mice, by subtracting the current remaining after caesium block from the control current (see C, and Results). Inset shows a Boltzmann curve fitted to these data, which has a V½ of -120 mV and k value of 16 mV. C, voltage steps from -70 to -160 mV in control, and in 2 mM CsCl, and the resulting Ih current revealed by subtraction.
However, certain inwardly rectifying K+ channels are also blocked by external Cs+, so we employed a more specific blocker of Ih, ZD 7288 (4-N-ethyl-N-phenylamino)-1,2-dimethyl-6-(methylamino)pyrimidinium chloride). First developed as a bradycardic compound, ZD 7288 has proved a useful tool with which to inhibit Ih in a number of central and peripheral neurones. Preliminary experiments revealed that little or no block of basket terminal Ih was obtained with concentrations of ZD 7288 below 1 μM, at least during the time course of our terminal recordings (maximal 53 min, generally less than 20 min), so 10 μM was used as the test concentration thereafter. At this concentration ZD 7288 completely blocked Ih, revealing an identical current in both amplitude and kinetics to that seen with Cs+ block (Fig. 3Aa and b). (In two experiments Cs+ was applied before ZD 7288 and full Cs+ reversal allowed direct comparison.) In common with other investigators, we find that ZD 7288 block requires several minutes to reach steady state, consistent with a proposed intracellular site of action (Harris & Constanti, 1995).
Figure 3. Sensitivity of terminal Ih to ZD 7288 and voltage dependence of activation.

A, the Ih current in basket cell nerve terminals is blocked by 10 μM ZD 7288, and the drug-sensitive component may be revealed by subtraction. Aa, voltage steps from -70 to -130 mV, for 100 ms, before and after 75 s 10 μM ZD 7288. Ab, subtracted trace showing ZD 7288-sensitive current in another terminal (step to -160 mV). Ba, single exponential fit superimposed on ZD 7288-subtracted Ih gives a τa of 26 ms (-160 mV) in this example. Bb, mean data for dependence of τa on step potential are shown graphically, with an exponential least-squares fit showing an e-fold change for 33 mV. n values are shown in parentheses. Data points are means ±s.e.m. of 5-12 separate basket cell terminals, held at -70 mV.
Figure 3B shows the results of experiments to measure the rate of activation of Ih, in order to compare the current in basket cell terminals with other Ih currents in the CNS, and more specifically, with some recently cloned Ih channels. Single exponential fits, such as that shown in Fig. 3Ba, were used to obtain activation time constants (τa), and mean data from a number of cells (n = 5-12) at a range of step potentials (Fig. 3Bb). The activation time constant became faster with more hyperpolarized steps, with a mean value of 73.5 ± 6.7 ms (n = 12 terminals) at -130 mV. The fitted line in Fig. 3Bb gives a measure of the voltage sensitivity of τa, which between -120 and -160 mV changes e-fold for a 33 mV change in membrane potential. The deactivation time constant was 13.5 ± 1.9 ms (n = 4) at -70 mV. It is worth noting that in some cells current deactivation showed a ‘hook’, or short plateau phase immediately following repolarization; such a phenomenon is also observed in both cloned and ‘native’Ih channels (Magee, 1998; Santoro et al. 1998).
Ih currents in basket cell somata
An Ih current was also present in 10 out of 12 recordings from basket cell somata. This current activated at potentials below -90 mV, and was totally blocked by 10 μM ZD 7288 (n = 8; complete block in 12-15 min). The mean amplitude of basket cell somatic Ih was -79.4 ± 9.9 pA at -130 mV (n = 5), and current activation was described by a single exponential with a time constant (τa) of 63.2 ± 14 ms (at -130 mV, n = 5).
Application of 10 μM ZD 7288 to basket cells reduced the holding current at -70 mV by 28.4 ± 5.1 % (n = 4). An identical effect was observed following application of Cs+ and ZD 7288 during terminal recordings (29.7 ± 8.0 %; n = 5).
Role of Ih in GABA release
We were next interested in determining if the Ih current in the basket cells plays a role in GABA release. Using the frequency and amplitude of rapidly rising sIPSCs as an index of presynaptic excitability (Southan & Robertson, 1998), we found that in five Purkinje cells, 10 μM ZD 7288 caused a mean 26.2 % reduction in control amplitude (-74.5 ± 0.6 pA; P = 0.01), and a 34.4 % reduction in mean control frequency (12.2 ± 2 Hz, P = 0.01). Example IPSCs from one Purkinje cell in control and ZD 7288 are shown in Fig. 4. With higher concentrations of ZD 7288 (50 and 100 μM, 5 cells each), control sIPSC amplitude was reduced by 29.8 % and 54 %, respectively, whilst frequency was reduced by 35 % and 27 %, respectively. ZD 7288 effects were, once again, slow to reach steady state (full block in 15 min), and washed off only partially within 25 min. sIPSCs are a mixture of both miniature events (mIPSCs, Llano & Gerschenfeld, 1993), and currents evoked by spontaneous action potentials in presynaptic cells. In the presence of 1 μM TTX to abolish voltage-gated Na+ currents, ZD 7288 (10 μM) had little effect (15.3 % increase) on control mIPSC amplitude (-27.7 ± 0.1 pA; n = 3; P = 0.05), whilst mIPSC frequency was unaffected (control 4.9 ± 2.1 Hz, increasing by 6.7 %, P = 0.05). At higher concentrations of ZD 7288 (100 μM), mIPSC frequency was increased by 23.5 %, with an ∼10 % reduction in amplitude (n = 3). We also wished to exclude indirect effects of ZD 7288 via changes in excitatory drive that may be influencing IPSC release from basket cells. In three experiments conducted with the AMPA receptor blocker CNQX (6-cyano-7-nitroquinoxaline-2,3-dione; 5 μM), ZD 7288 still reduced sIPSC amplitude and frequency (data not shown), suggesting that the Ih blocker is directly affecting GABA release from interneurones. Interestingly, CNQX alone increased the amplitude of sIPSCs.
Figure 4. Effects of Ih blocker on GABA release.
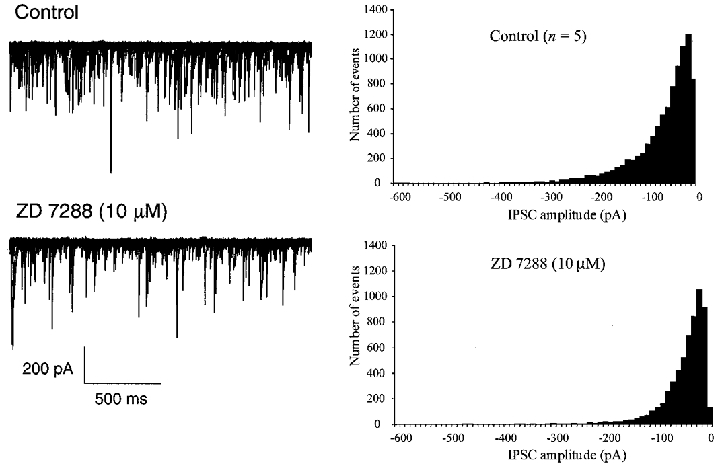
The Ih blocker ZD 7288 (10 μM) reduces the amplitude and frequency of spontaneous IPSCs recorded in cerebellar Purkinje cells. Left-hand traces show control and drug data, with twenty-five 2 s sweeps superimposed. Histograms show combined data from 5 different Purkinje cells, showing sIPSC amplitude histograms before and after 10 μM ZD 7288. Bin width 10 pA, 750 s sampling.
DISCUSSION
The findings presented here are the first direct demonstration of a hyperpolarization-activated current in a mammalian inhibitory CNS presynaptic terminal. This current activates at potentials below -80 mV and is blocked by low concentrations of caesium (Halliwell & Adams, 1982) and the more selective agent ZD 7288 (BoSmith et al. 1993). These characteristics would suggest that basket cell terminals have a similar Ih current to that found in the sino-atrial node in the heart (see DiFrancesco, 1993, for review), and in several other types of CNS neurone (see Pape, 1996). We also report that cerebellar basket cells have a comparable somatic Ih current, and that pharmacological tools which block Ih lead to decreased GABA release. We further suggest a likely candidate for the molecular identity of basket cell terminal Ih.
In other CNS neurones, Ih currents activate at more positive potentials (e.g. V½ values of -84 to -90 mV, Maccaferri & McBain, 1996; Magee, 1998) than those observed here in basket cell synaptic terminals (V½∼-120 mV). Basket cell terminals have a very negative membrane potential under our recording conditions, and a substantial voltage-gated K+ current is active near rest (Southan & Robertson, 1998, 2000), which would oppose the depolarizing influence of Ih currents. It is possible, however, that we are rapidly dialysing out key components from these tiny terminals, which influence the gating and threshold of Ih. For instance, Raes et al. (1997) have shown that addition of ATP and cAMP to their pipette solutions caused substantial depolarizing shifts in the activation curve of Ih in dorsal root ganglion neurones. Additionally, both native (DiFrancesco & Mangoni, 1994) and cloned Ih channels (Ludwig et al. 1998, 1999) suffer 20-30 mV hyperpolarizing shifts in their activation curve when patches are excised.
ZD 7288 dramatically decreased the amplitude and frequency of spontaneous IPSCs recorded in the soma of Purkinje cells, many of which would arise from neighbouring basket interneurones. The reduction in GABA release was not dependent on excitatory synaptic activity and mIPSC release was minimally affected by the Ih blocker. One economical explanation for these effects is prompted by the comprehensive study of Maccaferri & McBain (1996), who showed that ZD 7288 (albeit at higher concentrations than those used here) hyperpolarized hippocampal interneurones, producing a decrease in their spontaneous firing rate; such an action would lead to a decrease in sIPSCs postsynaptically, as observed here. We suggest that block of Ih leads to an overall reduction in the number of synaptic release sites activated by basket cell action potentials, although we cannot determine whether Ih channels in somatic or axonal (or both) compartments underlie this. Additionally, Ih channels may play even more complex roles in basket cell neurotransmission, perhaps by producing rebound after-depolarizations or direct interactions with the synaptic release machinery (e.g. Beaumont & Zucker, 2000).
This highly modulatable Ih current may be an important target for neurotransmitters, such as noradrenaline (NA) in the cerebellar cortex (Llano & Gerschenfeld, 1993). Indeed, NA increases Ih in a variety of central neurones (McCormick & Pape, 1990; Banks et al. 1993; Pedarzani & Storm, 1995; Maccaferri & McBain, 1996). In contrast, opioid neurotransmitter agonists reduce Ih in hippocampal interneurones, decreasing their excitability (Svoboda & Lupica, 1998).
Kinetics of native Ih currents vary markedly within the CNS. For instance, the fast time constant of activation is ∼120 ms (at -120 mV) in substantia nigra neurones (Harris & Constanti, 1995) and ∼120-190 ms at similar potentials in the soma of hippocampal interneurones (Maccaferri & McBain, 1996; Svoboda & Lupica, 1998). In contrast, in an exhaustive study of Ih in the dendrites of CA1 pyramidal neurones (Magee, 1998), τa is ∼80 ms (at -120 mV) at room temperature, close to the values obtained here in basket cells, although deactivation rates in basket cells are ∼10 times faster (at -70 mV) than dendritic Ih channels. However, we must exercise caution when comparing time constants between CNS preparations (and cloned channels, see below), since these will depend on not only the precise ionic composition of the recording solutions (Maruoka et al. 1994; Magee, 1998), but also on the second messenger status of the cell. Nevertheless, the Ih current in the basket cell terminals is rapidly activating, and this information, coupled with clues from histochemical mapping studies, allows us to speculate as to the molecular identity of Ih currents in basket cell terminals.
Of the four HCN (hyperpolarization-activated, cyclic nucleotide-gated, see Biel et al. 1999 for review) channels cloned and expressed thus far, mRNA for HCN1 is prominently expressed in basket and stellate cells of the mouse cerebellar cortex (Moosmang et al. 1999). This correlates extremely well with the detailed antibody mapping work of Santoro et al. (1997), who showed intense labelling of mouse basket cell terminals (but not somatic regions) with HCN1-selective antibodies. Subsequent electrophysiological characterization of HCN1 in oocytes (Santoro et al. 1998) reveals that this channel activates rapidly (τa∼98 ms at -130 mV, room temperature), which compares favourably with Ih in basket terminals (τa∼74 ms at -130 mV). Calculation of the voltage dependence of τa for HCN1 yields an e-fold change for 23 mV, compared to 33 mV in the present study. Interestingly, HCN1 is also found at high levels in the apical dendrites of CA1 pyramidal cells (Moosmang et al. 1999), perhaps underlying the fast Ih there (Magee, 1998). The two other well-characterized HCN channels, HCN2 (Ludwig et al. 1998) and HCN4 (Ludwig et al. 1999; Seifert et al. 1999) activate with time constants at least 2 times, and up to 9 times more slowly than HCN1 or the basket cell Ih. Drawing these threads together leads us to suggest that the hyperpolarization-activated channels in mouse basket cell terminals are composed of HCN1 channel subunits.
We know little of the properties or complement of ion channels at synaptic terminals. Here, we demonstrate, for the first time, that hyperpolarization-activated channels are functionally active in presynaptic terminals of mammalian CNS neurones. These key channels may provide a significant route for modulating central synaptic transmission.
Acknowledgments
We thank the Wellcome Trust for supporting this project and Alistair Mathie for helpful discussion.
References
- Banks MI, Pearce RA, Smith PH. Hyperpolarization-activated cation current (Ih) in neurons of the medial nucleus of the trapezoid body: Voltage-clamp analysis and enhancement by norepinephrine and cAMP suggest a modulatory mechanism in the auditory brainstem. Journal of Neurophysiology. 1993;70:1420–1432. doi: 10.1152/jn.1993.70.4.1420. [DOI] [PubMed] [Google Scholar]
- Beaumont V, Zucker RS. Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nature Neuroscience. 2000;3:133–141. doi: 10.1038/72072. [DOI] [PubMed] [Google Scholar]
- Biel M, Ludwig A, Zong X, Hofmann F. Hyperpolarization-activated cation channels: a multi-gene family. Reviews of Physiology, Biochemistry and Pharmacology. 1999;136:165–181. doi: 10.1007/BFb0032324. [DOI] [PubMed] [Google Scholar]
- BoSmith RE, Briggs I, Sturgess NC. Inhibitory actions of ZENECA ZD 7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. British Journal of Pharmacology. 1993;110:343–349. doi: 10.1111/j.1476-5381.1993.tb13815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annual Review of Physiology. 1993;55:455–472. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Mangoni M. Modulation of single hyperpolarization-activated channels (if) by cAMP in the rabbit sino-atrial node. The Journal of Physiology. 1994;474:473–482. doi: 10.1113/jphysiol.1994.sp020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher GH, Chiappinelli VA. An inward rectifier is present in presynaptic nerve terminals in the chick ciliary ganglion. Brain Research. 1992;575:103–112. doi: 10.1016/0006-8993(92)90429-d. [DOI] [PubMed] [Google Scholar]
- Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron. 1998;20:797–807. doi: 10.1016/s0896-6273(00)81017-x. [DOI] [PubMed] [Google Scholar]
- Grafe P, Quasthoff S, Grosskreutz J, Alzheimer C. Function of the hyperpolarization-activated inward rectification in non-myelinated peripheral rat and human axons. Journal of Neurophysiology. 1997;77:421–426. doi: 10.1152/jn.1997.77.1.421. [DOI] [PubMed] [Google Scholar]
- Halliwell JV, Adams PR. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Research. 1982;250:71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Harris NC, Constanti A. Mechanism of block by ZD 7288 of the hyperpolarization-activated inward rectifying current in guinea pig substantia nigra neurons in vitro. Journal of Neurophysiology. 1995;74:2366–2378. doi: 10.1152/jn.1995.74.6.2366. [DOI] [PubMed] [Google Scholar]
- Llano I, Gerschenfeld HM. β-Adrenergic enhancement of inhibitory synaptic activity in rat cerebellar stellate and Purkinje cells. The Journal of Physiology. 1993;468:201–224. doi: 10.1113/jphysiol.1993.sp019767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Stieber J, Hullin R, Hofmann F, Biel M. Two pacemaker channels from human heart with profoundly different activation kinetics. EMBO Journal. 1999;18:2323–2329. doi: 10.1093/emboj/18.9.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. The Journal of Physiology. 1996;497:119–130. doi: 10.1113/jphysiol.1996.sp021754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape H-C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation of thalamic relay neurones. The Journal of Physiology. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurones. Journal of Neuroscience. 1998;18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nature Neuroscience. 1999;2:508–514. doi: 10.1038/12229. [DOI] [PubMed] [Google Scholar]
- Maruoka F, Nakashima Y, Takano M, Ono K, Noma A. Cation-dependent gating of the hyperpolarization-activated cation current in the rabbit sino-atrial node cells. The Journal of Physiology. 1994;477:423–435. doi: 10.1113/jphysiol.1994.sp020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmang S, Biel M, Hofmann F, Ludwig A. Differential distribution of four hyperpolarization-activated cation channels in mouse brain. Biological Chemistry. 1999;380:975–980. doi: 10.1515/BC.1999.121. [DOI] [PubMed] [Google Scholar]
- Pape H-C. Queer current and pacemaker: The hyperpolarization-activated cation current in neurones. Annual Review of Physiology. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Storm JF. Protein-kinase A-independent modulation of ion channels in the brain by cyclic AMP. Proceedings of the National Academy of Sciences of the USA. 1995;92:11716–11720. doi: 10.1073/pnas.92.25.11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes A, Wang Z, van den Berg RJ, Goethals M, Van de Vijver G, van Bogaert PP. Effect of cAMP and ATP on the hyperpolarization-activated current in mouse dorsal root ganglion neurons. Pflügers Archiv. 1997;434:543–550. doi: 10.1007/s004240050434. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. In: Histology of the Nervous System of Man and Vertebrates. Swanson N, Swanson L, editors. Oxford: Oxford University Press; 1911. [Google Scholar]
- Santoro B, Grant SGN, Bartsch D, Kandel ER. Interactive cloning with the SH3 domain of N-src identifies a new brain specific ion channel protein, with homology to Eag and cyclic nucleotide-gated channels. Proceedings of the National Academy of Sciences of the USA. 1997;94:14815–14820. doi: 10.1073/pnas.94.26.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell. 1998;93:717–729. doi: 10.1016/s0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- Seifert R, Scholten A, Gauss R, Mincheva A, Lichter P, Kaupp UB. Molecular characterization of a slowly gating human hyperpolarization-activated channel predominantly expressed in thalamus, heart, and testis. Proceedings of the National Academy of Sciences of the USA. 1999;96:9391–9396. doi: 10.1073/pnas.96.16.9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan AP, Robertson B. Patch-clamp recordings from cerebellar basket cell bodies and their presynaptic terminals reveal an asymmetric distribution of voltage-gated potassium channels. Journal of Neuroscience. 1998;18:948–955. doi: 10.1523/JNEUROSCI.18-03-00948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan AP, Robertson B. Electrophysiological characterization of voltage-gated K+ currents in cerebellar basket and Purkinje cells: Kv1 and Kv3 channel subfamilies are present in basket cell nerve terminals. Journal of Neuroscience. 2000;20:114–122. doi: 10.1523/JNEUROSCI.20-01-00114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda KR, Lupica CR. Opioid inhibition of hippocampal interneurones via modulation of potassium and hyperpolarization-activated cation (Ih) currents. Journal of Neuroscience. 1998;18:7084–7098. doi: 10.1523/JNEUROSCI.18-18-07084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


