Abstract
We examined the pattern of activation and deactivation of the stress-activated protein kinase signalling molecules c-Jun NH2-terminal kinase (JNK) and p38 kinase in skeletal muscle in response to prolonged strenuous running exercise in human subjects.
Male subjects (n = 14; age 32 ± 2 years; VO2,max 60 ± 2 ml kg−1 min−1) completed a 42.2 km marathon (mean race time 3 h 35 min). Muscle biopsies were obtained 10 days prior to the marathon, immediately following the race, and 1, 3 and 5 days after the race. The activation of JNK and p38, including both p38α and p38γ, was measured with immune complex assays. The phosphorylation state of p38 (α and γ) and the upstream regulators of JNK and p38, mitogen-activated protein kinase kinase 4 (MKK4) and mitogen-activated protein kinase kinase 6 (MKK6), were assessed using phosphospecific antibodies.
JNK activity increased 7-fold over basal level immediately post-exercise, but decreased back to basal levels 1, 3 and 5 days after the exercise. p38γ phosphorylation (4-fold) and activity (1.5-fold) increased immediately post-exercise and returned to basal levels at 1, 3 and 5 days following exercise. In contrast, p38α phosphorylation and activity did not change over the time course studied. MKK4 and MKK6 phosphorylation increased and decreased in a trend similar to that observed with JNK activity and p38γ phosphorylation. Prolonged running exercise did not affect JNK, p38α, or p38γ protein expression in the days following the race.
This study demonstrates that both JNK and p38 intracellular signalling cascades are robustly, yet transiently increased following prolonged running exercise. The differential activation of the p38 isoforms with exercise in human skeletal muscle indicates that these proteins may have distinct functions in vivo.
The c-Jun NH2-terminal kinase (JNK) and p38 kinase are ubiquitously expressed intracellular signalling molecules that can be activated by stressors, such as proinflammatory cytokines (Han et al. 1994; Kyriakis et al. 1994; Raingeaud et al. 1995), osmotic shock (Galcheva-Gargova et al. 1994; Han et al. 1994), shear stress (Li, Y. S. et al. 1996) and stretch (Seko et al. 1999). During the last few years, our group and others have demonstrated that physical exercise can increase JNK and p38 activities in human skeletal muscle in vivo (Aronson et al. 1998; Widegren et al. 1998). Consistent with the classification of JNK as a ‘stress-activated protein kinase’, we also have demonstrated that injury-producing exercise such as maximal eccentric muscle contractions increase JNK activity to a much greater extent (15-fold) than less damaging maximal concentric muscle contractions (4-fold) in human skeletal muscle (Boppart et al. 1999).
Since the p38 kinase is activated by several of the same stressors that activate JNK (Han et al. 1994; Raingeaud et al. 1995), p38 activity also may be markedly increased in response to injury-producing exercise in skeletal muscle. Four isoforms of p38 have been identified in mammalian tissues, including p38α, β, γ and δ (Han et al. 1994; Jiang et al. 1996, 1997; Li, Z. et al. 1996). p38α and p38β mRNA are ubiquitously expressed in mammalian tissues, with highest expression in cardiac, brain and skeletal muscle (Han et al. 1994; Jiang et al. 1996), whereas p38γ mRNA is almost exclusively expressed in skeletal muscle (Li, Z. et al. 1996). Despite our current knowledge about the high expression of p38 in muscle, no information exists regarding the differential regulation of the specific p38 isoforms in skeletal muscle in vivo.
Marathon running requires both eccentric and concentric contractile activity and is known to induce muscle damage (Siegel et al. 1980; Warhol et al. 1985) and inflammation (Camus et al. 1997). In the current study we tested the hypothesis that marathon running markedly increases the activation of the stress-activated protein kinases JNK and p38. For this purpose, we determined the acute and prolonged effects of marathon running on JNK activity, the phosphorylation and activation of two isoforms of p38 (p38α and p38γ), and the phosphorylation of two upstream regulators of JNK and p38 (mitogen-activated protein kinase kinase 4 (MKK4) and mitogen-activated protein kinase kinase 6 (MKK6), respectively) immediately after and in the days following marathon running.
METHODS
Subjects
This study was approved by the Copenhagen Ethics Committee and conforms with the code of Ethics of the World Medical Association (Declaration of Helsinki). Fourteen male subjects, aged 23-48, were screened by a medical and fitness history questionnaire and physical examination. Exclusion criteria included any clinical evidence of cardiac, pulmonary, or endocrine abnormalities. Subjects were recruited by advertisement at the local club for distance runners and the volunteers were fully informed of any risks and discomfort associated with these experiments. Subject characteristics are listed in Table 1. Maximal oxygen consumption (VO2,max) was determined for each subject on a treadmill 2 weeks prior to the marathon, using procedures previously described (Asp et al. 1997). All subjects consumed a carbohydrate rich diet 2 days prior to the marathon (containing at least 8 g of carbohydrate per kg body weight per day). Subjects consumed the same breakfast on each day muscle biopsies were obtained, and arrived at the laboratory daily for muscle biopsy and blood collection after a minimum 2 h fast. Subjects maintained a constant activity level 2 days before biopsies were taken. Light walking and bicycling were allowed, but subjects abstained from strenuous or prolonged running.
Table 1.
Subject characteristics
| Characteristics | |
|---|---|
| Age (years) | 32 ± 2 |
| Sex | Male, n = 14 |
| Weight (kg) | 80 ± 2 |
| Height (cm) | 183 ± 2 |
| Body mass index (kg m−2) | 24.1 ± 0.01 |
| VO2,max (ml kg−1 min−1) | 60.2 ± 1.5 |
| VO2,max range (ml kg−1 min−1) | 50.5–70.6 |
| Race time (h:min) | 3:35 ± 0:11 |
| Race time, range (h:min) | 2:55–4:21 |
Values are means ±s.e.m.
Experimental protocols
Percutaneous needle biopsies were obtained from the vastus lateralis muscle under local anaesthesia (xylocaine; 20 mg ml−1, Astra, Sweden) using a 5 mm diameter side-cutting Bergstrom needle with applied suction. To avoid collecting biopsies just prior to the marathon, the first biopsy was obtained from each subject 10 days before the marathon. The race began at 09.30 h, and post-race biopsies were taken between 12.30 and 14.00 h, within 23 min (mean 11.1 ± 2.0 min, range 4-23 min) after the completion of the marathon. Subjects returned to the laboratory 1, 3 and 5 days following the marathon, and needle biopsies were obtained. Basal muscle biopsies and biopsies on days 1, 3 and 5 were collected at the same time in the morning, between 07.00 and 11.00 h, after the subjects had rested supine for approximately 20 min. Samples were obtained in a random manner from the non-dominant and dominant legs.
Muscle processing
Approximately 50 mg of the vastus lateralis muscle obtained from muscle biopsies was homogenized (Polytron; Brinkman Instruments, Inc., Westbury, NY, USA) in ice-cold lysis buffer containing 20 mM Hepes, pH 7.4, 2 mM EGTA, 50 mM β-glycerophosphate, 1 mM DTT, 1 mM Na3VO4, 1 % Triton X-100, 10 % glycerol, 10 mM leupeptin, 3 mM benzamidine, 5 mM pepstatin A, 10 mg ml−1 aprotinin and 1 mM phenylmethylsulphonyl fluoride (lysis buffer). Homogenates were rotated for 1 h at 4°C and centrifuged at 13000 g for 68 min at 4°C. Samples were quickly frozen in liquid nitrogen and stored at -80°C. Protein concentrations of the muscle lysates were measured using the Bradford method (Bradford, 1976).
Activity assays
For JNK activity, muscle lysates (250 μg protein) were immunoprecipitated with 1.0 μg of anti-JNK1 antibody and 50 μl of prewashed protein A beads. Following immunoprecipitation, the JNK immune complexes were washed, resuspended in 30 μl kinase assay buffer, and kinase reactions were carried out in a reaction mixture containing 3 μg inactive glutathione S-transferase (GST)-c-Jun as substrate, 3.75 mM MgCl2, 50 μm ATP and 10 μCi [γ-32P]ATP, for 30 min at 30°C, as described previously (Aronson et al. 1997). For p38 activity, muscle lysates (500 μg protein) were immunoprecipitated with 3-5 μg of anti-p38 antibody (α and γ) and 50 μl of prewashed protein A beads. Following immunoprecipitation, the p38 immune complexes were washed twice in lysis buffer and four times in p38 kinase buffer (20 mM Hepes, pH 7.6, 20 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM DTT and 10 mM MgCl2). Immune complexes were resuspended in 30 μl p38 kinase buffer containing 2 μg of activating transcription factor-2 (ATF-2) (substrate), 20 μm ATP and 10 μCi [γ-32P]ATP, and reactions were carried out for 30 min at 30°C. For both JNK and p38 activity assays, reactions were terminated with Laemmli sample buffer, samples were heated to 60°C, and labelled reaction products were resolved on 10 % SDS polyacrylamide gels. Gels were stained in fast green stain diluted 1:1, destained in 30 % ethanol and 10 % glacial acetic acid, dried, and exposed to a PhosphorImager screen. Specific bands were quantified using a PhosphorImager analysis system (Molecular Dynamics, Inc., Sunnyvale, CA, USA).
Immunoblotting
To determine p38 phosphorylation, muscle lysates were resolved on 10 % SDS-polyacrylamide gels, transferred to nitrocellulose paper, blocked with 5 % bovine serum albumin (BSA) diluted in TBS-Tween and immunoblotted with a phosphospecific p38 antibody which recognizes p38 phosphorylated at Tyr 182. Similar procedures were used to determine MKK4 and MKK6 phosphorylation using phosphospecific antibodies that recognize MKK4 phosphorylated on Thr 223 and MKK6 phosphorylated on Ser 207. All phosphospecific antibodies were diluted 1:1000. Following determination of p38 phosphorylation, membranes were incubated in stripping buffer (62.5 mM Tris, pH 6.7, 2 % SDS and 100 mM 2-mercaptoethanol) for 30 min at 50°C, washed extensively, and used to determine p38 protein expression using a p38 antibody (1:2000). Additional immunoblotting was performed to determine JNK protein expression using a JNK1 (1:2000) antibody. For all immunoblotting experiments, membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (1:2000) and immunoreactive proteins were detected using enhanced chemiluminescence.
Materials
Anti-p38γ was kindly provided by J. Han (Scripps Research Institute, La Jolla, CA, USA); anti-JNK1, anti-p38, and ATF-2 (1-96) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA); phosphospecific p38, MKK4 and MKK6 antibodies were from New England Biolabs (Beverly, MA, USA). Protein A-agarose was purchased from Pierce Chemical Co. (Rockford, IL, USA), and [γ-32P]ATP was from DuPont-New England Nuclear (Boston, MA, USA). The enhanced chemiluminescence kit was purchased from Amersham Life Sciences (Arlington Heights, IL, USA) and NEN Life Science Products (Boston, MA, USA), dye reagent for determination of protein concentrations was from Bio-Rad Laboratories (Hercules, CA, USA), and all other chemicals were purchased from Sigma Chemical Co. (St Louis, MO, USA).
Statistical analysis
All data are expressed as means ±s.e.m. Data were compared using one-way repeated measures analysis of variance (ANOVA) (SPSS, SigmaStat, Chicago, IL, USA). If significance was detected, Student-Newman-Keuls post-hoc test was used to determine where the significance occurred. Pearson's correlation analysis also was used to determine associations between variables. The differences between groups were accepted to be significant at P < 0.05.
RESULTS
JNK activity and protein expression
Figure 1A shows the reaction products generated from the JNK activity assay for one representative subject and demonstrates that JNK activity is markedly increased immediately post-exercise compared with basal levels. In examining all subjects, the magnitude of change in JNK activity immediately post-exercise ranged from virtually no increase (9 % increase) to 16-fold above basal values, with the average increase 7-fold above basal values (Fig. 1B). The peak increases in JNK activity occurred immediately post-exercise for all subjects. Twenty-four hours following the marathon, there was no longer a significant increase in JNK activity. Variation in JNK activity existed among subjects 1 day following exercise, with enzyme activity below basal values in some subjects and activity remaining as high as 5-fold above basal values in others. JNK activity was at basal values 3 and 5 days following the marathon. To test the possibility that protein expression is altered in the days following a single bout of prolonged running exercise, we immunoblotted muscle lysates using a JNK antibody. The representative immunoblot in Fig. 1C shows that the single bout of prolonged exercise had no effect on JNK protein expression in the muscle.
Figure 1. Effect of marathon running on JNK activity.
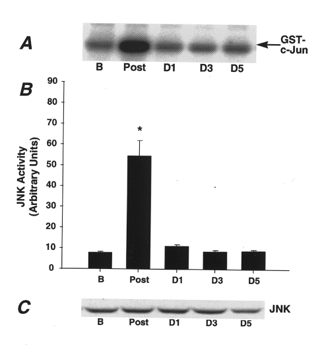
A, reaction products from the JNK activity assay from one representative subject in the basal state (B), immediately post-exercise (Post), and days 1, 3 and 5 post-exercise (D1, D3, D5). B, JNK activity for all subjects completing the marathon race. Data are means ±s.e.m., *P < 0.05vs. basal state and days 1, 3 and 5. C, representative immunoblot of JNK protein expression in muscle lysates from one representative subject.
p38 phosphorylation, activity and protein expression
The phosphospecific antibody used in our study recognized two phosphorylated proteins (Fig. 2A). By immunoprecipitation and immunoblotting using antibodies to p38α and p38γ, we determined that the lower molecular weight band is p38α and the higher molecular weight band is p38γ. Figure 2A shows a representative immunoblot for one subject and demonstrates that p38γ phosphorylation was greatly increased after prolonged running, whereas the phosphorylation of p38α does not change. The magnitude of change in p38γ phosphorylation ranged from no increase to 34-fold above basal levels, with the average increase 4-fold above basal levels (Fig. 2B). p38γ phosphorylation decreased to basal levels 1 day post-exercise, and remained decreased 3 and 5 days post-exercise. Quantification of p38α phosphorylation in muscle lysate from all subjects demonstrated that there was no change in the phosphorylation state of this enzyme over the time course studied (data not shown). p38γ (Fig. 2C) or p38α (data not shown) protein expression also were not altered.
Figure 2. Differential regulation of p38α and p38γ phosphorylation immediately following a marathon race.
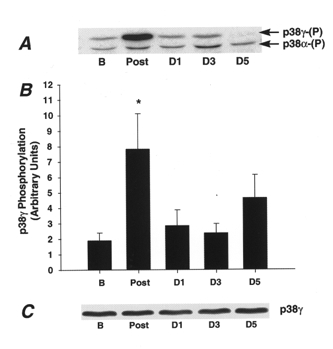
A, immunoblot of p38α and p38γ phosphorylation from one representative subject in the basal state (B), immediately post-exercise (Post), and days 1, 3 and 5 (D1, D3, D5) post-exercise. B, p38γ phosphorylation for all subjects completing the marathon race. Data are means ±s.e.m., *P < 0.05vs. basal value and days 1, 3 and 5. C, representative immunoblot of p38γ protein expression in muscle lysates from one representative subject.
We also tested the activation of p38 (γ and α) using an in vitro immune complex assay. Figure 3A shows the reaction products generated from the p38γ and p38α activity assays for four representative subjects in the basal state and immediately post-exercise. Consistent with p38 phosphorylation, p38γ activity was increased (Fig. 3B) and p38α activity did not change (data not shown) immediately post-exercise compared with basal values.
Figure 3. Effect of marathon running on p38α and p38γ activity.
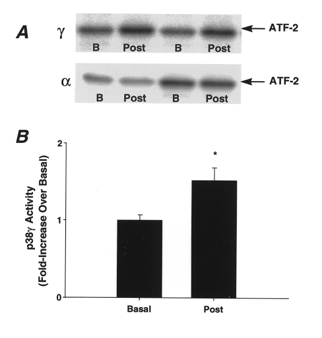
A, reaction products from the p38α and p38γ activity assays from four representative subjects in the basal state (B) and immediately post-exercise (Post). B, p38γ activity for all but one subject completing the marathon race (n = 13). Data are means ±s.e.m., *P < 0.05vs. basal values.
MKK4 and MKK6 phosphorylation
MKK4 can phosphorylate and activate both JNK and p38 (Derijard et al. 1995; Moriguchi et al. 1997), whereas MKK3 and MKK6 can phosphorylate and activate only the p38 stress-activated kinase (Cuenda et al. 1997; Enslen et al. 1998; Keesler et al. 1998). Figure 4A shows that MKK4 phosphorylation was increased 4-fold above basal levels immediately following prolonged exercise, and was back to baseline 24 h later and remained at this level 3 and 5 days post-exercise. Figure 4B shows that MKK6 phosphorylation was increased 1.5-fold immediately post-exercise. It then decreased back to basal values 1 day post-exercise, and remained decreased 3 and 5 days post-exercise.
Figure 4. Effect of marathon running on MKK4 and MKK6 phosphorylation.
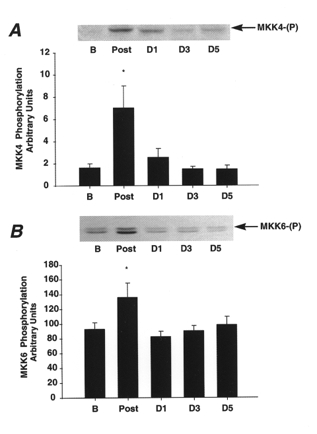
Representative immunoblots of MKK4 and MKK6 phosphorylation are shown at the top of each figure. A, MKK4 phosphorylation under basal conditions (B), immediately post-exercise (Post), and days 1, 3 and 5 (D1, D3, D5) post-exercise. B, MKK6 phosphorylation under basal conditions (B), immediately post-exercise (Post), and days 1, 3 and 5 (D1, D3, D5) post-exercise. Data are means ±s.e.m., *P < 0.05vs. basal levels and days 1, 3 and 5.
Correlations
The subjects with the highest JNK activity levels also had the highest levels of p38γ phosphorylation. Using correlation analysis, a strong positive association was detected between JNK activity and p38γ phosphorylation immediately post-exercise (r = 0.84, P < 0.001; Fig. 5). The magnitude of increase in JNK activity was not significantly correlated with fitness level (VO2,max; r = 0.04, n.s.) or race time (r = 0.32, n.s.).
Figure 5. Correlation between JNK activity and p38γ phosphorylation.
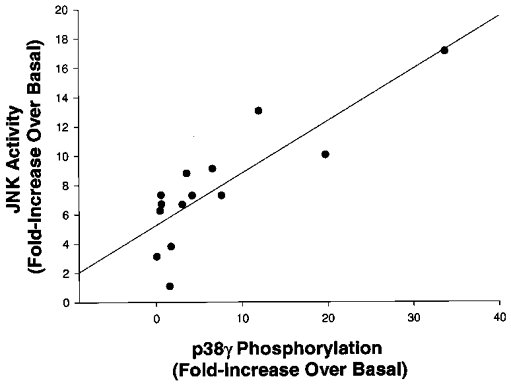
Correlation analysis was determined on the fold-increases in JNK activity and p38γ phosphorylation immediately post-exercise for all subjects (n = 14).
DISCUSSION
Injury-producing exercise such as marathon running, places tremendous metabolic and mechanical stresses on the skeletal muscle fibres. We hypothesized that the stress-activated protein kinase signalling cascades would be highly activated in response to this type of cellular stress. JNK and p38, and their upstream regulators MKK4 and MKK6, were consistently activated immediately following marathon running in human skeletal muscle. Interestingly, p38γ, and not p38α, was significantly phosphorylated and activated immediately following the race. Prolonged activation or elevated expression of the stress-activated protein kinases was not observed in the days following exercise. The preferential activation of p38γ compared with p38α demonstrates that differential regulation of p38 isoforms exists in skeletal muscle in response to exercise.
The increase in JNK activity immediately following prolonged running in human skeletal muscle (7-fold) was slightly greater than the degree of activation we have previously observed with 60 min of submaximal cycling exercise (6-fold) (Aronson et al. 1998), yet lower than the increase observed following maximal eccentric exercise (15-fold) (Boppart et al. 1999). The increase in p38γ phosphorylation (4-fold) immediately following marathon running also was greater than the increase in total p38 phosphorylation previously observed with 60 min of submaximal cycling exercise in human skeletal muscle (2-fold) (Widegren et al. 1998). Therefore, the degree of JNK and p38 activation and/or phosphorylation following exercise is probably dependent on the type of exercise (eccentric, high intensity vs. concentric, low intensity) and the extent of muscle injury.
Both JNK activity and p38γ phosphorylation were markedly elevated immediately post-exercise despite the fact that exercise persisted for an extended period of time. The decrease in JNK activity and p38 phosphorylation observed 1 day following the race is consistent with results from our previous study showing that JNK activity decreases back to basal levels 6 h following maximal eccentric exercise (Boppart et al. 1999). The phosphorylation of p38 also has been shown to decrease to basal levels 60 min following submaximal cycling exercise (Widegren et al. 1998). Together, these data suggest that prolonged contractile activity can sustain the activation of JNK and p38 and that the deactivation of these kinases occurs quickly upon cessation of exercise.
One isoform of p38, p38γ, was preferentially phosphorylated and activated immediately following prolonged running exercise, whereas another isoform, p38α, was not regulated. The differences in p38γ activity (1.5-fold) and p38γ phosphorylation (4-fold) may be due to the fact that ATF-2 is not the optimal substrate for p38γin vitro (Cuenda et al. 1997; Li, Z. et al. 1996). Nonetheless, the differential regulation of the p38 isoforms with exercise and contraction in human and rat skeletal muscle may indicate that these proteins have specific functions in muscle in vivo. Experiments using adenoviruses which overexpress either p38α or p38β in cardiomyocytes suggest that p38β is involved in promoting a hypertrophic response, whereas p38α is involved in mediating apoptosis (Wang et al. 1998). These findings and the results from our study highlight the need to examine the activation of the different isoforms of p38 in future studies on skeletal muscle. Inhibitors of p38 exist, which can be used to determine the consequence of p38 activation in skeletal muscle; however, the most commonly used p38 inhibitors, SB203580 and SB202190, are only effective on α and β isoforms, and not γ.
The significant increases in JNK activity and p38γ phosphorylation prompted us to investigate the activation of the upstream regulators of JNK and p38 following marathon running. MKK4, which is the upstream regulator of JNK, was increased 4-fold immediately following the race. This finding demonstrates that MKK4 activity is increased in response to injury-producing exercise. Although MKK4 also can phosphorylate p38, MKK3 and MKK6 are the primary regulators of p38 phosphorylation (Keesler et al. 1998). Consistent with the evidence that MKK6 is a strong activator of p38γ phosphorylation and activity (Keesler et al. 1998), we found that MKK6 phosphorylation was increased in the immediately post-exercise state, and that a strong positive correlation existed between these two molecules (r = 0.69, P < 0.01).
In summary, JNK and p38γ activities are highly, yet transiently activated in skeletal muscle immediately following prolonged running exercise. This study also provides the first evidence of p38γ-specific regulation in skeletal muscle in response to exercise. It will be important to identify the specific substrates and transcription factors activated by JNK and p38γ in response to exercise. Although the immediate and prolonged consequences of increased JNK and p38 activities following exercise are not known, these stress-activated protein kinases may be responsible for skeletal muscle adaptations to exercise.
Acknowledgments
This work was supported by the National Institutes of Arthritis, Musculoskeletal and Skin Diseases Grant AR-42238 (L.J.G.), the American College of Sports Medicine Foundation (M.D.B.), the Danish Sport Research Council Grant (S.A.), and the Danish National Research Foundation Grant (S.A.). M.D.B. was supported by an Institutional National Research Service Award from NIH (T32 DK07260-22, Joslin Diabetes Center), S.A. was supported by the Weimann Foundation, Denmark, and J.F.P.W. was supported by a post-doctoral fellowship from the Alfred Benzon Foundation, Denmark. R.A.F. is a Brookdale National Fellow at Boston University.
References
- Aronson D, Boppart MD, Dufresne SD, Fielding RA, Goodyear LJ. Exercise stimulates c-jun NH2 kinase activity in human skeletal muscle. Biochemical and Biophysical Research Communications. 1998;251:106–110. doi: 10.1006/bbrc.1998.9435. [DOI] [PubMed] [Google Scholar]
- Aronson D, Dufresne SD, Goodyear LJ. Contractile activity stimulates the c-Jun NH2-terminal kinase pathway in rat skeletal muscle. Journal of Biological Chemistry. 1997;272:25636–25640. doi: 10.1074/jbc.272.41.25636. [DOI] [PubMed] [Google Scholar]
- Asp S, Rohde T, Richter EA. Impaired muscle glycogen resynthesis after a marathon is not caused by decreased muscle GLUT-4 content. Journal of Applied Physiology. 1997;83:1482–1485. doi: 10.1152/jappl.1997.83.5.1482. [DOI] [PubMed] [Google Scholar]
- Boppart MD, Aronson D, Gibson L, Roubenoff R, Abad LW, Bean J, Goodyear LJ, Fielding RA. Eccentric exercise markedly increases c-Jun NH2 terminal kinase activity in human skeletal muscle. Journal of Applied Physiology. 1999;87:1668–1673. doi: 10.1152/jappl.1999.87.5.1668. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein, utilizing the principal of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Camus G, Poortmans J, Nys M, Deby-Dupont G, Duchateau J, Deby C, Lamy M. Mild endotoxaemia and the inflammatory response induced by a marathon race. Clinical Science. 1997;92:415–422. doi: 10.1042/cs0920415. [DOI] [PubMed] [Google Scholar]
- Cuenda A, Cohen P, Buee-Scherrer V, Goedert M. Activation of stress-activated protein kinase-3 (SAPK3) by cytokines and cellular stresses is mediated via SAPKK3 (MKK6); comparison of the specificities of SAPK3 and SAPK2 (RK/p38) EMBO Journal. 1997;16:295–305. doi: 10.1093/emboj/16.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derijard B, Raingeaud J, Barrett T, Wu T, Han I-H, Ulevitch J, Davis RJ. Independent human MAP kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. Journal of Biological Chemistry. 1998;273:1741–1748. doi: 10.1074/jbc.273.3.1741. [DOI] [PubMed] [Google Scholar]
- Galcheva-Gargova Z, Derijard B, Wu I-H, Davis RJ. An osmosensing signal transduction pathway in mammalian cells. Science. 1994;265:806–811. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Chen C, Li Z, Guo W, Gegner JA, Lin S, Han J. Characterization of the structure and function of a new mitogen-activated protein kinase (p38beta) Journal of Biological Chemistry. 1996;271:17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Gram H, Zhao M, New L, Gu J, Feng L, Padova FD, Ulevitch R, Han J. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38δ. Journal of Biological Chemistry. 1997;272:30122–30128. doi: 10.1074/jbc.272.48.30122. [DOI] [PubMed] [Google Scholar]
- Keesler GA, Bray J, Hunt J, Johnson DA, Gleason T, Yao Z, Wang SW, Parker C, Yamane H, Cole C, Lichenstein HS. Purification and activation of recombinant p38 isoforms α, β, γ, and δ. Protein Expression and Purification. 1998;14:221–228. doi: 10.1006/prep.1998.0947. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Avruch J, Woodgett JR. A MAP kinase subfamily activated by cellular stress and tumour necrosis factor. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Li YS, Shyy JY, Li S, Lee J, Su B, Karin M, Chien S. The Ras-JNK pathway is involved in shear-induced gene expression. Molecular and Cellular Biology. 1996;16:5947–5954. doi: 10.1128/mcb.16.11.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Jiang Y, Ulevitch RJ, Han J. The primary structure of p38γ: a new member of p38 group of MAP kinases. Biochemical and Biophysical Research Communications. 1996;228:334–340. doi: 10.1006/bbrc.1996.1662. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Toyoshima F, Masuyama N, Hanafusa H, Gotoh Y, Nishida E. A novel SAPK/JNK kinase, MKK7, stimulated by TNFα and cellular stress. EMBO. 1997;16:7045–7053. doi: 10.1093/emboj/16.23.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. Journal of Biological Chemistry. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Seko Y, Takahashi N, Tobe K, Kadowaki T, Yazaki Y. Pulsatile stretch activates mitogen-activated prtoein kinase (MAPK) family members and focal adhesion kinase (p125FAK) in cultured rat cardiac myocytes. Biochemical and Biophysical Research Communications. 1999;259:8–14. doi: 10.1006/bbrc.1999.0720. [DOI] [PubMed] [Google Scholar]
- Siegel AJ, Silverman LM, Lopez RE. Creatine kinase elevations in marathon runners: relationship to training and competition. Yale Journal of Biological Medicine. 1980;53:275–279. [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huang S, Sah VP, Ross J, Jr, Brown JH, Han J, Chien K. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. Journal of Biological Chemistry. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- Warhol MJ, Siegel AJ, Evans WJ, Silverman SM. Skeletal muscle injury and repair in marathon runners after competition. American Journal of Pathology. 1985;118:331–339. [PMC free article] [PubMed] [Google Scholar]
- Widegren U, Jiang XJ, Krook A, Chibalin A, Bjornholm M, Tally M, Roth RA, Henriksson J, Wallberg-Henriksson H, Zierath JR. Divergent effects of exercise on metabolic and mitogenic signaling pathways in human skeletal muscle. FASEB Journal. 1998;12:1379–1389. doi: 10.1096/fasebj.12.13.1379. [DOI] [PubMed] [Google Scholar]


