Abstract
The mechanisms underlying the dorsal root potential (DRP) were studied in transverse slices of turtle spinal cord. DRPs were evoked by stimulating one filament in a dorsal root and were recorded from another such filament.
The DRP evoked at supramaximal stimulus intensity was reduced but not eliminated after blockade of GABAA receptors. The remaining component was eliminated by blocking NMDA and AMPA receptors.
The DRP was reduced but not eliminated after blockade of AMPA receptors. The early component of the remaining DRP was dependent on GABAA receptors and the residual component on NMDA receptors.
The DRP was reduced but not eliminated by TTX. GABAA, NMDA and AMPA receptors contributed to the generation of the TTX-insensitive DRP. The early component of the DRP in the presence of TTX depended on GABAA receptor activation, and the late component mainly on the activation of NMDA receptors.
Our results show that part of the DRP is generated by a TTX-resistant, probably non-spiking micro-circuit with separate components mediated by GABA and glutamate.
Primary afferent fibres enter the spinal cord via dorsal roots and establish synaptic contacts with a wide variety of neurones. Some of these contacts occur in synaptic arrangements wherein primary afferent terminals are both pre- and postsynaptic to axon terminals and dendrites (see Willis & Coggeshall, 1991, for review). This suggests a complex regulation of information transfer at the early stages of somatosensory processing. A broad repertoire of ionotropic and metabotropic receptors in primary afferent terminals (Coggeshall & Carlton, 1997) might contribute to this regulation. Among ionotropic receptors, GABAA receptors are thought to mediate presynaptic inhibition and to be the main generators of synaptically evoked primary afferent depolarization (Rudom’n & Schmidt, 1999; Willis, 1999). Primary afferent terminals also contain ionotropic receptors for glutamate (Liu et al. 1994; Coggeshall & Carlton, 1997) but their function is not well known. In the present study, we used the dorsal root potential (DRP) in a slice preparation of the turtle spinal cord to monitor the voltage response in primary afferent terminals evoked by activation of dorsal root fibres. We found that a component of the evoked DRP was insensitive to the blocking of GABAA receptors. Rather, it depended on the activation of AMPA and NMDA receptors. A DRP with GABA- and glutamate-mediated components was still recorded in the presence of TTX. These results suggest that interactions between primary afferent terminals can be mediated by a TTX-insensitive, probably non-spiking micro-circuit.
METHODS
Preparation
Adult turtles (Pseudemys scripta, 15–20 cm carapace length) were anaesthetized with pentobarbitone (100 mg kg−1, i.p.). The plastron was opened and the blood removed by intraventricular perfusion with Ringer solution (6–10°C) of the following composition (mm): 120 NaCl, 5 KCl, 15 NaHCO3, 3 CaCl2, 2 MgCl2 and 20 glucose (Russo & Hounsgaard, 1996). The animal was then killed by decapitation. The surgical procedures complied with Danish legislation and were approved by the controlling body under the Ministry of Justice. Transverse slices comprising one or two segments were obtained from the lumbar and cervical enlargements. For experiments, the preparation was placed in a chamber (2 ml volume) and superfused at room temperature (20–22°C) at a rate of 1 ml min−1 with Ringer solution saturated with 2% CO2 and 98% O2 to attain pH 7.6.
Electrophysiological recording and stimulation
A dorsal root was split into two bundles of fibres mounted in suction electrodes for stimulation and for recording the DRP. When using two-segment slices, stimulation was applied on an adjacent dorsal root. Simultaneous spinal cord dorsum potential (CDP) recordings were obtained with a suction electrode on the dorsal surface of the spinal cord near to the dorsal root entry. DRP and CDP recordings were performed with differential amplifiers. The bandwidth was 0.1 Hz to 1 kHz or DC to 1 kHz for DRP and 0.1 Hz to 1 kHz for CDP recordings. Stimulation was achieved by applying brief (0.5 ms) constant current pulses. The stimulus threshold was defined for the DRP as the minimum intensity giving rise to a measurable response. Unless otherwise stated the responses to eight stimuli applied every 20 s or slower were averaged. Values are expressed as the mean ± s.e.m.
Drugs
In some experiments the following drugs were added to the normal Ringer solution: 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20–50 μm), strychnine (5–10 μm), bicuculline (20–40 μm), picrotoxin (100 μm), (+)-2-amino-5-phosphonopentanoic acid (AP-5, 50–100 μm), ifenprodil (5–10 μm) and tetrodotoxin (TTX, 150–200 nm).
RESULTS
Typical recordings of the DRP and CDP in the turtle spinal cord (see Delgado-Lezama et al. 1999) are illustrated in Fig. 1A. They show the increase in response amplitude with stimulus intensities ranging from 1.5 times threshold to supramaximal intensity. The early transient in the DRP at 2 and 50 times threshold represents a dorsal root reflex (Willis, 1999). At high stimulus intensities, the DRP peaked approximately 35 ms after the stimulus and lasted for more than 1 s. The CDP had an early negative- and a late positive-going component (Fig. 1A). The negative component, which represents the early postsynaptic response in spinal neurones, preceded the DRP by 6 ms, whereas the positive component occurred simultaneously with the negative DRP. In all preparations, the DRP and CDP recordings were stereotyped and robust over many hours (n = 45).
Figure 1.
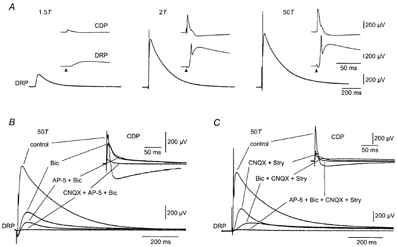
DRP and CDP sensitivity to stimulus intensity and GABAA, AMPA and NMDA receptor antagonists
A, DRP evoked by dorsal root stimulation at 1.5, 2 and 50 times threshold (T). Insets show simultaneously recorded CDP and DRP at a faster sweep speed. Arrows indicate the time of stimulus application B, DRP at 50T in normal medium (control) and after sequential application of 40 μm bicuculline (Bic), 100 μm AP-5 (AP-5 + Bic) and 25 μm CNQX (CNQX + AP-5 + Bic). The simultaneously recorded CDP is shown in the insets at a faster sweep speed. C, DRP at 50T, in normal medium (control) and after sequential addition of 25 μm CNQX and 10 μm strychnine (CNQX + Stry), 40 μm bicuculline (Bic + CNQX + Stry), and finally 100 μm AP-5 (AP-5 + Bic + CNQX + Stry). Inset, simultaneously recorded CDP at a faster sweep speed. In this and subsequent figures, negative polarity is up and the DRP was recorded in DC mode. A and C are from the same preparation.
We first investigated the sensitivity of the DRP and CDP evoked by supramaximal dorsal root stimulation to the blocking of different types of ionotropic receptor (Fig. 1B and C). As illustrated in Fig. 1B, bicuculline (an antagonist of GABAA receptors) abolished the dorsal root reflex and reduced the amplitude of the DRP. Surprisingly, a substantial component of the DRP was always retained (46.8 ± 18.9 % of control amplitude, n = 8). In parallel, the early negative-going component of the CDP was reduced in amplitude and prolonged, whereas the later positive-going component disappeared (Fig. 1B). Picrotoxin, another GABAA receptor antagonist, produced similar results (4/4 experiments; data not shown).
Part of the bicuculline-insensitive component of the DRP depended on the activation of NMDA receptors since it was reduced by addition of the selective antagonist AP-5 (Fig. 1B). In eight experiments, AP-5 reduced the bicuculline-insensitive DRP by 54.1 ± 5%. In contrast, the residual CDP response was insensitive to AP-5 (Fig. 1B). The bicuculline- and AP-5-insensitive components of the DRP and CDP were largely abolished by CNQX (Fig. 1B, n = 3).
To further differentiate between these GABAergic and glutamatergic pathways we tested the role of AMPA receptor activation in the generation of the DRP and CDP in the control state (normal Ringer solution in the bath). Addition of CNQX abolished the dorsal root reflex and reduced the amplitude of the DRP. In 14 experiments, CNQX reduced the DRP amplitude to 39.9 ± 4.2 % of its control value. Both the early negative- and the late positive-going components of the CDP were also strongly reduced. In some experiments (n = 7) we also applied strychnine to rule out a contribution of glycine receptors to the CNQX-resistant response (Fig. 1C). No differences were observed compared to experiments where only CNQX was used. The DRP remaining after block of AMPA receptors was composed of an early bicuculline-sensitive component and a residual component with a slower time course that was removed by AP-5 (Fig. 1C). The CNQX- and bicuculline-resistant DRP was also reduced by the NMDA receptor antagonist ifenprodil (5/6 experiments; data not shown). The small CDP remaining after block of AMPA receptors was only slightly reduced by bicuculline and AP-5 (Fig. 1C).
The results illustrated in Fig. 1 suggested that a DRP could be activated through pathways not involving GABAergic interneurones. Identification of these pathways was complicated, however, by the possible contribution of complex polysynaptic pathways to DRP generation (Wall & Lidierth, 1997; Rudom’n & Schmidt, 1999; Willis, 1999). For this reason we reduced the circuitry involved in the generation of the DRP by adding low concentrations of TTX to the control bath solution. Under these conditions, spike generation is abolished in all axons except unmyelinated C fibres and Ad fibres (Yoshida et al. 1978; Kobayashi et al. 1993). Figure 2 shows the control DRP and CDP responses to stimuli at 1.5, 10 and 50 times threshold, and responses 2 h after addition of TTX to the control bath solution. TTX abolished the low threshold DRP and CDP (Fig. 2A) and greatly reduced the high threshold responses (Fig. 2B and C). The absence of low threshold responses and the longer latency of the DRP and CDP in the presence of TTX are consistent with the high threshold and slow conduction velocity of fibres bearing TTX-insensitive Na+ channels. TTX reduced the peak amplitude of the DRP at supramaximal stimulus intensity to 22.4 ± 7 % of control when stimulating within the same segment (n = 4) and to 23.9 ± 11 % when stimulating in an adjacent segment (n = 5) to the recorded dorsal root filament. The DRP was never fully abolished by TTX even after incubating the slices in TTX-containing medium for 12–48 h (n = 5). Therefore, assuming that spike activity dependent on TTX-sensitive channels was eliminated, the TTX-resistant DRP must have been generated by a micro-circuitry with Ad and C fibres as the only spike-generating elements.
Figure 2.
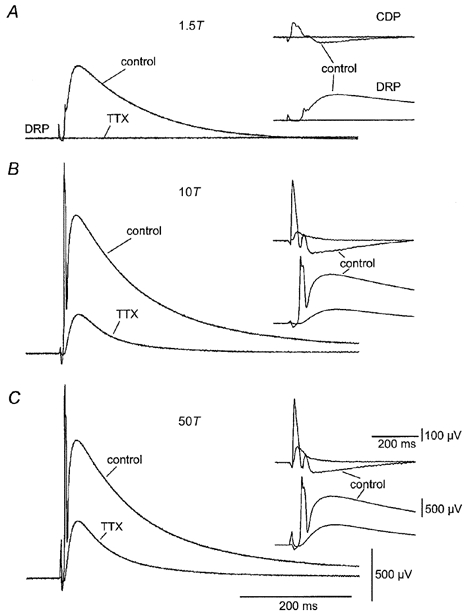
DRP and CDP sensitivity to TTX
DRP and CDP at 1.5T (A), 10T (B) and 50T (C). Insets show simultaneously recorded DRP and CDP at a faster sweep speed. Each set of recordings shows superimposed sweeps in normal medium (control) and in medium containing 150 nm TTX (TTX).
Finally, we tested the sensitivity of the TTX resistant DRP to the blocking of ionotropic receptors for GABA and glutamate (Fig. 3). In the presence of TTX there was still a sizeable bicuculline-resistant component of the DRP (Fig. 3A). Bicuculline reduced the amplitude of the DRP to 28.7 ± 6 % of control (n = 6). Figure 3A shows that most of the bicuculline-resistant DRP was eliminated by AP-5. A smaller component was mediated by AMPA receptors since it disappeared after addition of CNQX (Fig. 3A). Note the longer latency and slower rise time of the NMDA and AMPA receptor-mediated components. Despite a clear effect on the bicuculline-resistant DRP, AP-5 had little effect on the CDP (Fig. 3A). The same sequential reduction in amplitude of the DRP in response to sequential block of GABAA, NMDA and AMPA receptors was seen in 12 experiments.
Figure 3.
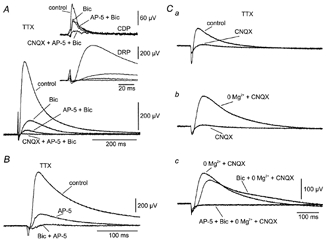
Effect of ionotropic receptor blockade on TTX-insensitive DRP
A, DRP at 50T. Inset, simultaneously recorded CDP and DRP at a faster sweep speed. Each set of recordings shows superimposed sweeps in medium containing 150 nm TTX (control) and after sequential addition of 40 μm bicuculline (Bic), 100 μm AP-5 (AP-5 + Bic) and 50 μm CNQX (CNQX + AP-5 + Bic). B, effect of sequential application of 100 μm AP-5 (AP-5) and 40 μm bicuculline (Bic + AP-5) on TTX-resistant DRP at 50T. C, Mg2+ sensitivity of TTX- and CNQX-resistant DRP at 50T. Ca, reduction of TTX-resistant DRP (control) by 25 μm CNQX (CNQX). Cb, recovery of TTX- and CNQX-resistant DRP by eliminating Mg2+ (0 Mg2+ + CNQX). Cc, 40 μm bicuculline (Bic + 0 Mg2+ + CNQX) blocks the early component of the CNQX-resistant DRP in the absence of Mg2+ while the remaining DRP is blocked by 100 μm AP-5 (AP-5 + Bic + 0 Mg2+ + CNQX). A, B and C are from different preparations. All recordings were obtained in the presence of 150 nm TTX.
The prominent role of NMDA receptors in the generation of the TTX-resistant DRP is illustrated in Fig. 3B and C. Addition of AP-5 strongly reduced the DRP recorded in normal medium containing TTX (Fig. 3B). The remaining response was virtually eliminated by bicuculline.
The relative importance of AMPA, NMDA and GABAA receptors in the TTX-resistant DRP was explored further (Fig. 3C). First it was shown that the DRP in the presence of TTX was nearly eliminated by CNQX (Fig. 3Ca). A substantial DRP was recovered, however, by excluding Mg2+ from the medium (Fig. 3Cb; 3/3 experiments). Only the earliest part of this DRP was mediated by the activation of GABAA receptors: i.e. the bicuculline-influenced response was shifted to the right, but was only slightly reduced in amplitude (Fig. 3Cb). The remaining response was eliminated by application of AP-5 (Fig. 3Cc).
DISCUSSION
The negative DRP reflects the primary afferent depolarization (PAD) classically associated with presynaptic inhibition in the mammalian spinal cord (Rudom’n & Schmidt, 1999). PAD is thought to be generated by activation of GABAA receptors in primary afferent terminals at axo-axonic synapses with GABAergic interneurones (Rudom’n & Schmidt, 1999). Our results show that a TTX-insensitive, probably non-spiking micro-circuit contributes to the generation of the DRP in primary afferents in the turtle.
Non-GABAergic DRP
In agreement with observations in other species (Thompson & Wall, 1996; Kremer & Lev-Tov, 1998) we found that a significant component of the DRP in the turtle was not mediated by GABAA receptors. The GABA-independent DRP had AP-5- and CNQX-sensitive parts. We suggest that these parts are generated by ionotropic glutamate receptors on primary afferent terminals. In other species AMPA and NMDA receptors are present in primary afferent terminals (Liu et al. 1994; Coggeshall & Carlton, 1997). A direct action of glutamate on primary afferent terminals is also supported by the stronger contribution of NMDA receptors to the DRP than to the CDP and by the fact that the DRP was sensitive to ifenprodil, an antagonist of the NMDA receptor containing the NR2B subunit. This subunit is predominantly located presynaptically in the spinal cord (Boyce et al. 1999).
DRP generation by a non-spiking micro-circuit
A robust DRP with both GABAA and NMDA receptor-mediated components was still observed when polysynaptic pathways were attenuated by CNQX (Fig. 1C). Because of the thickness of the preparation there is obviously slow access of drugs to the central parts by diffusion. However, insufficient block of ionotropic receptors is unlikely to account for our findings. We used saturating concentrations of antagonists and data were collected after long application times (>1 h) to ensure a steady state had been reached. In addition, the GABA and NMDA receptor-mediated DRP persisted in the presence of TTX. This also rules out mediation by conventional polysynaptic pathways. Low concentrations of TTX spare action potential generation in C and Ad primary afferents (Yoshida et al. 1978) because only these fibres express TTX-resistant PN3 and SNS2 Na+ channels (Sangameswaran et al. 1996; Dib-Hajj et al. 1998; Tate et al. 1998). These channels resist micromolar concentrations of TTX while Na+ channels in other primary afferents and in the brain and spinal cord are sensitive to nanomolar concentrations of TTX (White et al. 1993). In our previous studies, lower concentrations of TTX (50–100 nm) than used here blocked action potentials in dorsal horn neurones while synaptic inputs mediated by high threshold, slow conducting fibres remained (Russo & Hounsgaard, 1996). Inadequate block of TTX-sensitive Na+ channels in dorsal horn neurones is unlikely since a DRP could still be evoked after incubation in TTX-containing medium for more than 48 h. Therefore, under the assumption that TTX-insensitive Na+ channels are not expressed in spinal cord neurones (Sangameswaran et al. 1996; Dib-Hajj et al. 1998; Tate et al. 1998), a major implication of our results is that a non-spiking micro-circuit around primary afferent terminals contributes to the generation of PAD following activation of Ad and C fibres. The similar time course of the DRP in the presence of CNQX and TTX (Fig. 1C and 2) suggests that a non-spiking micro-circuit generates the DRP when polysynaptic pathways are attenuated. Finally, in the cat a TTX-insensitive DRP with a similar slow time course was evoked by dorsal horn stimulation (Rudom’n & Mu-oz-Mart’nez, 1969). This suggests that the findings presented here may apply to other vertebrates, including mammals.
Reciprocal synapses and glutamate spillover?
The TTX-resistant DRP had components mediated by GABAA, NMDA and AMPA receptors. Therefore, the main points to discuss are the possible substrate for the DRP generated by a TTX-insensitive micro-circuit around primary afferent terminals and how different components of the DRP are generated.
Several sources of TTX-resistant release of transmitters with an action on primary afferents can be envisioned. Primary afferents, including C and Ad fibres, are known to engage in reciprocal synapses with GABAergic dendrites in the dorsal horn (Gobell, 1976; Todd, 1996). Therefore, one possibility is that the GABA-mediated component of the TTX-resistant DRP is due to dendritic release of GABA (Fig. 4 A, left). Dendritic release of GABA is well established in other regions of the CNS (Jahr & Nicoll, 1980; Schwartz, 1987; Zilberter et al. 1999) and, in analogy with the present findings, often depends on activation of NMDA receptors (Isaacson & Strowbridge, 1998).
Figure 4.
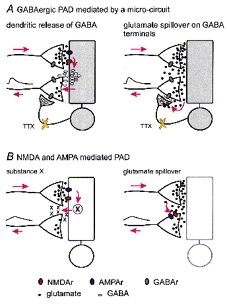
Possible mechanisms for the DRP generated by a non-spiking micro-circuit
In the four diagrams the proposed flow of signals from action potentials in stimulated primary afferents to DRPs recorded in other primary afferents is indicated by red arrows. A, the GABAergic component of the TTX-resistant DRP may be mediated by GABA released from dendrites (left) or from GABAergic terminals excited by glutamate spillover (right). B, the glutamate component may be produced indirectly by release of substance X (left) or by spillover of glutamate from neighbouring terminals (right). NMDAr, NMDA receptor; AMPAr, AMPA receptor; GABAr, GABA receptor. TTX-resistant DRP at 50T. C, Mg2+ sensitivity of TTX- and CNQX-resistant DRP at 50T. Ca, reduction of TTX-resistant DRP (control) by 25 μm CNQX (CNQX). Cb, recovery of TTX- and CNQX-resistant DRP by eliminating Mg2+ (0 Mg2+ + CNQX). Cc, 40 μm bicuculline (Bic + 0 Mg2+ + CNQX) blocks the early component of the CNQX-resistant DRP in the absence of Mg2+ while the remaining DRP is blocked by 100 μm AP-5 (AP-5 + Bic + 0 Mg2+ + CNQX). A, B and C are from different preparations. All recordings were obtained in the presence of 150 nm TTX.
An alternative mechanism for the GABA component of the TTX-resistant DRP is that glutamate released from Ad and C fibres acts on GABAergic terminals on primary afferents and causes GABA release (Fig. 4A, right). Our data do not support this mechanism because the GABAergic component of the DRP always preceded the NMDA component, even in Mg2+-free Ringer solution. If glutamate spills over (see below) to excite GABAergic terminals, glutamate should reach neighbouring primary afferent terminals at the same time, and therefore the NMDA component should not appear later than the GABA component. Finally, it is possible that GABAergic interneurones express an as yet unknown TTX-resistant Na+ channel. These hypotheses, however, have not yet been tested directly.
Regarding the NMDA and AMPA receptor-mediated DRP, it is possible that glutamate acts on dendrites or axon terminals as just discussed for GABA, but to promote the release of another substance that generates a PAD (substance X in Fig. 4B, left). A simpler alternative is that glutamate released from primary afferents spills over and generates a DRP by acting on NMDA and AMPA receptors on presynaptic terminals (Fig. 4B, right). This possibility, analogous to findings elsewhere in the CNS (Isaacson, 1999), is compatible with several observations. The slow time course of the DRP mediated by AMPA and NMDA receptors under all experimental conditions is easily accounted for by spillover but rather incompatible with the duration being determined by a synaptic network of spiking interneurones. Note also that the bicuculline-sensitive component, in the absence of polysynaptic pathways, mainly contributes to the DRP during the first 200 ms (Figs 1C and 3C). In the presence of TTX, the GABA-independent DRP depends more on NMDA receptors than on AMPA receptors. This is also in agreement with mediation by glutamate spillover since the low concentration of extrasynaptic glutamate attained by diffusion from synaptic clefts favours activation of high affinity NMDA receptors over low affinity AMPA receptors (Rusakov et al. 1999). As a final point in support of glutamate spillover, the latency and time to peak of the NMDA receptor-mediated DRP in the presence of TTX were much longer than those of the NMDA-mediated component of the CDP and the GABA-mediated DRP (Fig. 3).
Conclusions
Although the cellular mechanisms remain to be explored more directly, our results provide evidence for novel mechanisms for PAD. The prolonged activation of NMDA receptors suggested by our study may have important functional implications since NMDA receptors in primary afferents have been implicated in pain processing (Urban & Nagy, 1997) and are thought to mediate substance P release (Liu et al. 1997). Our findings provide the first electrophysiological evidence suggesting that a substantial component of the DRP can be generated by a mechanism not shaped by a synaptic network of spiking neurones, a possibility that has long been hypothesized based on anatomical evidence (Willis & Coggeshall, 1991). This non-spiking circuit could represent an effective mechanism for the local regulation of transmitter release around active primary afferents. Finally, volume conduction and non-spiking neuronal interactions have precedents in other neural networks devoted to the analysis of sensory information such as in the olfactory bulb (Jahr & Nicoll, 1980; Nowycky et al. 1981) and the retina (Werblin, 1979), suggesting a common functional principle in early sensory processing.
Acknowledgments
This work was partly supported by CSIC (R.E.R.), CONACYT (R.D.L.) and the Lundbeck Foundation and The Danish MRC (J.H.).
References
- Boyce S, Wyatt A, Webb JK, O’Donnell R, Mason G, Rigby M, Sirinathsinghji D, Hill RG, Rupniak NM. Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology. 1999;38:611–623. doi: 10.1016/s0028-3908(98)00218-4. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Carlton SM. Receptor localization in the mammalian dorsal horn and primary afferent neurons. Brain Research Reviews. 1997;24:28–66. doi: 10.1016/s0165-0173(97)00010-6. [DOI] [PubMed] [Google Scholar]
- Delgado-Lezama R, Perrier J-F, Hounsgaard J. Oscillatory interaction between dorsal root excitability and dorsal root potentials in the spinal cord of the turtle. Neuroscience. 1999;93:731–739. doi: 10.1016/s0306-4522(99)00187-6. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Tyrrell L, Black JA, Waxman SG. NaNa novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proceedings of the National Academy of Sciences of the USA. 1998;95:8963–8968. doi: 10.1073/pnas.95.15.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobell S. Dendroaxonic synapses in the substantia gelatinosa glomeruli of the spinal trigeminal nucleus of the cat. Journal of Comparative Neurology. 1976;167:165–176. doi: 10.1002/cne.901670204. [DOI] [PubMed] [Google Scholar]
- Isaacson JS. Glutamate spillover mediates excitatory transmission in the rat olfactory bulb. Neuron. 1999;23:377–384. doi: 10.1016/s0896-6273(00)80787-4. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Strowbridge BW. Olfactory reciprocal synapses: dendritic signaling in the CNS. Neuron. 1998;20:749–761. doi: 10.1016/s0896-6273(00)81013-2. [DOI] [PubMed] [Google Scholar]
- Jahr CE, Nicoll RA. Dendrodendritic inhibition: demonstration with intracellular recording. Science. 1980;207:1473–1475. doi: 10.1126/science.7361098. [DOI] [PubMed] [Google Scholar]
- Kobayashi J, Otha M, Terada Y. C fiber generates a slow Na+ spike in the frog sciatic nerve. Neuroscience Letters. 1993;162:93–96. doi: 10.1016/0304-3940(93)90568-6. [DOI] [PubMed] [Google Scholar]
- Kremer E, Lev-Tov A. GABA-receptor-independent dorsal root afferents depolarization in the neonatal rat spinal cord. Journal of Neurophysiology. 1998;79:2581–2592. doi: 10.1152/jn.1998.79.5.2581. [DOI] [PubMed] [Google Scholar]
- Liu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang H, Sheng M, Jan LY, Jan YN, Bausbaum AL. Evidence for presynaptic N-methyl-D-aspartate autoreceptors in the spinal cord dorsal horn. Proceedings of the National Academy of Sciences of the USA. 1994;94:8383–8387. doi: 10.1073/pnas.91.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky MC, Mori K, Shepherd GM. GABAergic mechanisms of dendrodendritic synapses in isolated turtle olfactory bulb. Journal of Neurophysiology. 1981;46:639–648. doi: 10.1152/jn.1981.46.3.639. [DOI] [PubMed] [Google Scholar]
- Rudomín P, Muoz-Martênez J. A tetrodotoxin-resistant primary afferent depolarization. Experimental Neurology. 1969;25:106–115. doi: 10.1016/0014-4886(69)90074-0. [DOI] [PubMed] [Google Scholar]
- Rudomkn P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Experimental Brain Research. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Rusakov DA, Kullmann DM, Stewart MG. Hippocampal synapses: do they talk to their neighbours? Trends in Neurosciences. 1999;22:382–388. doi: 10.1016/s0166-2236(99)01425-3. [DOI] [PubMed] [Google Scholar]
- Russo RE, Hounsgaard J. Plateau-generating neurones in the dorsal horn in an in vitro preparation of the turtle spinal cord. Journal of Physiology. 1996;493:39–54. doi: 10.1113/jphysiol.1996.sp021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangameswaran L, Delgado SG, Fish LM, Koch BD, Jakeman LB, Stewart GR, Sze P, Hunter JC, Eglen RM, Herman RC. Structure and function of a novel voltage-gatedtetrodotoxin-resistant sodium channel specific to sensory neurons. Journal of Biological Chemistry. 1996;271:5953–5956. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- Schwartz EA. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science. 1987;238:350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- Tate S, Benn S, Hick C, Trezise D, John V, Mannion RJ, Costigan M, Plumpton C, Grose D, Gladwell Z, Kendall G, Dale K, Bountra C, Woolf CJ. Two sodium channels contribute to the TTX-R sodium current in primary sensory neurons. Nature Neuroscience. 1998;1:653–655. doi: 10.1038/3652. [DOI] [PubMed] [Google Scholar]
- Thompson SW, Wall PD. The effect of GABA and 5-HT receptor antagonists on rat dorsal root potentials. Neuroscience Letters. 1996;217:153–156. [PubMed] [Google Scholar]
- Todd AJ. GABA and glycine in synaptic glomeruli of the rat spinal dorsal horn. European Journal of Neuroscience. 1996;8:2492–2498. doi: 10.1111/j.1460-9568.1996.tb01543.x. [DOI] [PubMed] [Google Scholar]
- Urban L, Nagy I. Is there a nociceptive carousel? Trends in Pharmacological Sciences. 1997;18:223–224. doi: 10.1016/s0165-6147(97)01081-x. [DOI] [PubMed] [Google Scholar]
- Wall PD, Lidierth M. Five sources of a dorsal root potential: their interactions and origins in the superficial dorsal horn. Journal of Neurophysiology. 1997;78:860–871. doi: 10.1152/jn.1997.78.2.860. [DOI] [PubMed] [Google Scholar]
- Werblin FS. Integrative pathways in local circuits between slow-potential cells in the retina. In: Schmitt FO, Worden FG, editors. The Neurosciences. Fourth Study Program. Cambridge, MA, USA and London, UK: MIT Press; 1979. pp. 193–211. [Google Scholar]
- White JA, Alonso A, Kay AR. A heart-like Na+ current in the medial entorhinal cortex. Neuron. 1993;11:1037–1047. doi: 10.1016/0896-6273(93)90217-f. [DOI] [PubMed] [Google Scholar]
- Willis WD., Jr Dorsal root potentials and dorsal root reflexes: a double-edged sword. Experimental Brain Research. 1999;124:395–421. doi: 10.1007/s002210050637. [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. 2. New York: Plenum Press; 1991. [Google Scholar]
- Zilberter Y, Kaiser KM, Sakmann B. Dendritic GABA release depresses excitatory transmission between layer 2/3 pyramidal and bitufted neurons in rat neocortex. Neuron. 1999;24:979–988. doi: 10.1016/s0896-6273(00)81044-2. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Matsuda Y, Samejima A. Tetrodotoxin-resistant sodium and calcium components of action potentials in dorsal root ganglion cells of the adult mouse. Journal of Neurophysiology. 1978;41:1096–1106. doi: 10.1152/jn.1978.41.5.1096. [DOI] [PubMed] [Google Scholar]


