Abstract
The influx of Zn2+ through the channels of fetal and adult mouse muscle nicotinic acetylcholine receptors (γ- and ε-AChRs) and its effects on receptor function were studied in transiently transfected human BOSC 23 cells, by combining patch-clamp recordings with digital fluorescence microscopy.
ACh-induced whole-cell currents were reversibly reduced by external ZnCl2, with half-maximal inhibitory concentrations of 3 and 1 mM for γ- and ε-AChRs, respectively.
Both γ- and ε-AChR channels were permeable to Zn2+, as shown by fluorescence measurements using Zn2+-sensitive dyes. The fractional current carried by Zn2+ (Pf,Zn; 0.5 mM Zn2+ in Ca2+- and Mg2+-free medium) through γ- and ε-AChR channels was 1.7 and 4 %, respectively.
Pf,Zn increased with the concentration of ZnCl2, but was little affected by physiological concentrations of Ca2+ and Mg2+ in the external medium.
The conductance of ACh-evoked unitary events, measured by cell-attached or outside-out recordings, decreased when the patched membrane was exposed to ZnCl2 (1 or 3 mM). Simultaneous application of ACh and Zn2+ to the extra-patch membrane lengthened channel open duration (τop) by 50%. No obvious increment of τop was observed following exposure of inside-out patches to Zn2+.
The possible physiological relevance of zinc-induced modulation of AChR channels is discussed.
Considerable quantities of Zn2+ are present in mammalian nerve cells, about 90% of it bound to zinc-fingers of regulatory proteins (Huang, 1997), the remainder stored in synaptic vesicles and released during synaptic activity, reaching extracellular concentrations estimated to be in the range of hundreds of micromolar at the synapses (Assaf & Chung, 1984). At these concentrations, Zn2+ influences transmitter release and modulates postsynaptic receptors for glutamate, GABA and glycine (as reviewed by Huang, 1997; Choi & Koh, 1998), with possible consequences for synaptic transmission. Outside the physiological range, both the excess and deficiency of Zn2+ affect cellular metabolic processes, inducing neurotoxic symptoms (Choi & Koh, 1998). Intracellular Zn2+ excess, which may result in cell death, can be attained during intense synaptic activity, when Zn2+ enters postsynaptic cells through a variety of pathways, such as voltage-activated Ca2+ channels, Zn2+-permeable receptors or the Na+-Ca2+ exchanger (Koh & Choi, 1994; Yin & Weiss, 1995; Yu & Choi, 1997; Sensi et al. 1997).
At the neuromuscular junction, Zn2+ inhibits the spontaneous transmitter release in the presence of Ca2+, while transiently increasing the spontaneous transmitter release in the absence of Ca2+ (Benoit & Mambrini, 1970; Nishimura, 1987; Wang & Quastel, 1990). Frog endplate AChRs are permeable to Zn2+, as estimated from reversal potential measurements (Adams et al. 1980). Thus, during synaptic activity, Zn2+ may enter into muscle cells, possibly modulating AChR function. However, it is not known whether muscle AChRs are directly modulated by Zn2+. The aim of the present study was to measure the Zn2+ permeability of muscle AChR channels, by combining electrophysiological and fluorescence techniques, and to investigate the influence of Zn2+ on AChR function.
METHODS
Expression of AChR subunits in BOSC 23 cells
Full-length cDNAs encoding α (P04756), β (P09690), γ (P04760), ε (P20782) and δ (P02716) subunits of mouse muscle nicotinic AChR in the SV40-based pSM expression vector were kindly provided by Dr J. Patrick (Baylor College of Medicine, Houston, TX, USA). The cDNAs coding for the α, β, γ and δ (γ-AChR) or the α, β, ε and δ (ε-AChR) subunits (0.2 μg each per 35 mm dish) were transiently transfected into the human cell line BOSC 23 using a Ca2+ phosphate method, as previously described (Fucile et al. 1996). Cells were washed twice 8-12 h after the start of transfection and used for experiments 36-48 h after transfection. The culture medium consisted of Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA), supplemented with 10% calf serum (Hyclone, USA).
Solutions
The standard external solution had the following composition (mm): NaCl 140, KCl 2.5, CaCl2 2, MgCl2 2, Hepes-NaOH 10 and glucose 10, pH 7.3. Fluorescence determination of Zn2+ influx through AChRs was performed using an external solution containing (mm): NaCl 140, KCl 2.5, Hepes-NaOH 10 and glucose 10, pH 7.3, plus various concentrations of Zn2+. Patch pipettes were filled with internal solution containing (mm): CsCl 150 and Hepes-CsOH 10, pH 7.3, for combined fluorescence and whole-cell current measurements; or CsCl 140, MgCl2 2, Hepes-CsOH 10, EGTA 0.5 and Na-ATP 4, pH 7.3, for whole-cell recordings. For calibration of fluorescence measurements, intra- and extracellular solutions were prepared by adding 50 μm Mg2+-Green or 2 mm ZnCl2, respectively, to a solution composed of (mm): N-methyl-d-glucamine (NMGA) 140 and Hepes-HCl 10, pH 7.3. For outside-out recordings, patch pipettes were filled with the following solution (mm): KCl 140, CaCl2 1, MgCl2 2, EGTA 11, Mg-ATP 2 and Hepes-KOH 10, pH 7.3. Inside-out patches were bathed with the following solution (mm): KCl 155, MgCl2 1 and Hepes-KOH 10, pH 7.3, supplemented with 0.2 mm ZnCl2 where indicated. Mg2+-Green and Newport Green were purchased from Molecular Probes, TPEN from Sigma and ZnCl2 from Fluka or Sigma. All other chemicals were of analytical grade.
Whole-cell recordings
Whole-cell recordings of ACh- and nicotine-induced currents were performed at room temperature (24-26°C) using borosilicate glass patch pipettes (2-4 MΩ tip resistance) connected to an Axopatch 200A amplifier (Axon Instruments). The series resistance, estimated from slow transient cancellation, was compensated by 80-90%. Currents were digitized at 500 Hz and analysed with pCLAMP programs (pCLAMP 6, Axon Instruments). Unless otherwise indicated, recordings were performed at a membrane holding potential of -50 mV. Agonists were applied by a gravity-driven perfusion system (for details see Ragozzino et al. 1998). The concentration of Zn2+ yielding half-maximal inhibition (x0) was obtained by non-linear fitting of the data to eqn (1):
| (1) |
where [Zn2+] represents the concentration of Zn2+, nH is the Hill coefficient, Imax is the maximum current response and K is a constant that takes into account the residual, non-blocked current observed at high concentrations of Zn2+.
Single-channel recordings
Single-channel currents were recorded at room temperature (23-26°C) in the cell-attached, outside-out or inside-out configuration using a low-noise Axopatch 200B amplifier (Axon Instruments). For cell-attached recordings, Sylgard-coated borosilicate patch pipettes (3-5 MΩ resistance) were filled with standard external solution plus ACh (100 nm). In one set of experiments, ZnCl2 (0.05 to 0.1 or 1 mm) was included in the patch pipette. Since control channel open duration (τop) varied in different cell preparations, ranging from 2.8 to 5.5 ms, the values of τop in the presence of Zn2+ were compared to control values obtained in the same experimental session. In another set of experiments, the effects on single-channel activity of ACh (20 μm), ACh + ZnCl2 (200 μm) and TPEN (50 μm) applied to the extra-patch membrane, using a gravity-driven fast perfusion system (RSC-200, Biologic, France), were studied. Control ACh-evoked unitary events were recorded in each cell at the beginning of the experiment, while superfusing with standard external solution, then test solutions were applied for 2-4 min, washed out and channel activity recorded again. This protocol ensured that recordings were performed at stable membrane potentials, since during ACh application cells depolarized, then repolarized due to AChR desensitization, as revealed by changes in channel amplitude. Once exposed to ZnCl2, Petri dishes were discarded. Data were sampled at 10 kHz and analysed after Gaussian digital filtering at 2 kHz, using a threshold-crossing method by pCLAMP 6 software (Axon Instruments), as previously detailed (Fucile et al. 1996). Channel slope conductance was calculated by least-squares linear fitting of unitary i–V relations. Cell resting potential was estimated from these fits, assuming a reversal potential of 0 mV (see Fucile et al. 1996) and the kinetic properties of ACh-evoked events were compared at an estimated membrane potential of about -60 mV. Results are given as means ±s.e.m. To ascertain that the lengthening of τop caused by extra-patch ZnCl2 was significant, in each patch τop values recorded under control conditions and after application of ACh plus Zn2+ were compared by means of a one-way ANOVA, using Origin 4 (Microcal, USA). Statistical significance was accepted for P < 0.05. For outside-out recordings, ACh (200 nm) or ACh plus ZnCl2 (3 mm) was applied to the excised membrane patch using the fast perfusion system as above. For inside-out recordings, patch pipettes were filled with normal external solution plus ACh (100 nm), while cells were equilibrated in a nominally Ca2+-free, KCl-containing solution. After patch excision, channel activity was recorded for 2-20 min, Zn2+-containing solution was then added and channel activity recorded for 2-5 min. For all the excised patches, recordings were performed at potentials ranging between -50 and -90 mV. Data were collected and analysed as above.
Fluorescence measurements
Fluorescence determinations were made using real-time confocal laser microscopy (Odyssey, Noran Instruments, CA, USA), as previously described in detail (Ragozzino et al. 1998). In these experiments, ACh was routinely replaced by nicotine (except where otherwise indicated), to avoid possible artefacts caused by the stimulation of muscarinic receptors. The nicotine-induced rise of cytosolic Zn2+ concentration was expressed as the ratio of fluorescence increase over basal fluorescence (ΔF/F0). Fluorescence signals were measured as averages over square domains approximating cell shape, assuming a homogeneous receptor density. All determinations were performed with the confocal slit set at 100 μm, to detect fluorescence changes over the entire cell depth. Identical conditions of illumination and detection were maintained, taking care that the basal fluorescence of each cell was similar. Loading with cell-permeant dyes (Newport Green diacetate, 5 μm, or Mg2+-Green acetoxymethyl ester, 4 μm) was achieved by incubating transfected cells with the dye at 37°C for 20-40 min in serum-free DMEM, then extensively washing with standard medium. When fluorescence and current responses were to be measured simultaneously, cells responding with a large fluorescence increase to a preliminary nicotine application were selected. After rupture of the membrane patch and establishment of the whole-cell configuration, the cell-impermeant dye (Mg2+-Green, 50 μm; Newport Green, 10 μm; Fluo-3, 250 μm) contained in the patch pipette diffused into the cell, leading to a slow increase in the basal fluorescence that reached a stable level (F0) within 5-6 min. Only cells that exhibited stable F0 during experiments were considered for analysis. To rule out artefacts due to fluorescence run-up (see Sensi et al. 1997), F0 was measured immediately before agonist application. The permeability of γ- and ε-AChR channels to Zn2+ was evaluated by estimating the fractional Zn2+ current (Pf,Zn) of AChR subtypes using the procedure previously described for Ca2+ (Schneggenburger et al. 1993; Zhou & Neher, 1993; Ragozzino et al. 1998). Briefly, during the first 1-3 s of agonist application, the fluorescence increase (F =ΔF/F0) and the current integral (Q) were measured at 50 ms intervals and their point ratio (F/Q) calculated. To determine Pf,Zn, the F/Q ratio at a given Zn2+ concentration was normalized to the F/Q ratio of a pure Zn2+ current (5-50 pA peak amplitude), obtained using an extracellular solution with Zn2+ as the only charge carrier (see ‘Solutions’). Measurements performed using patch pipettes filled with CsCl or NMGA solutions yielded comparable values for Pf,Zn. All the results are expressed as means ±s.e.m.
RESULTS
Mouse muscle AChRs are permeable to Zn2+
To determine the optimal Zn2+ concentration for fluorescence measurements, we tested the effects of Zn2+ on the amplitude of whole-cell currents evoked by ACh (10 μm; IACh) at the γ- and ε-AChRs. Zn2+ reduced IACh amplitude at both receptors in a concentration-dependent manner (Fig. 1A and B). This effect was fully reversible by wash in Zn2+-free medium (2 min if Zn2+ concentration was < 1 mm; 8-10 min at higher concentrations). Fitting the relationship between IACh amplitude and Zn2+ dose with eqn (1) (Fig. 1C) showed that the ε-AChR was blocked by Zn2+ with higher potency than the γ-AChR. However, even at high Zn2+ concentrations the block was not complete. For both the γ- and ε-AChRs, the block of IACh by Zn2+ was voltage independent for membrane potentials ranging from -150 to +50 mV (not shown). Thus, we studied the Zn2+ permeability of the AChR channels using a Zn2+ concentration of 0.5 mm, which induces only a minor block of the γ- and ε-AChRs. The Zn2+-induced block of IACh was investigated in more detail by single-channel recordings (see below).
Figure 1. Zn2+ reversibly blocks nicotine-evoked currents in BOSC 23 cells expressing muscle AChRs.
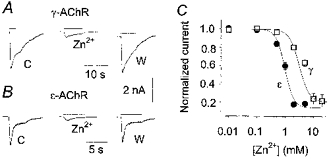
A, typical inward whole-cell currents (downward deflections) evoked by ACh (10 μm, indicated by bars) before (control, C), during (Zn2+) and after (wash, W) the simultaneous application of ZnCl2 (10 mm) to a cell expressing γ-AChR. Holding potential, -50 mV. Note the full reversibility of the block with wash (8 min). B, in a cell expressing ε-AChR, 2 mm ZnCl2 produced a comparable block of the ACh-evoked current. C, inhibition curves at a holding potential of -50 mV. The amplitude of the current recorded in the presence of various concentrations of Zn2+ was normalized to the control response in each cell and plotted vs. Zn2+ dose. Each point represents the mean of 4-8 values, with error bars (representing s.e.m.) omitted when smaller than the symbols (□ for γ-AChR, • for ε-AChR). The continuous lines represent the best fit of the data with eqn (1), yielding: x0 = 3.1 mm, nH = 2.46 and K = 0.2 for γ-AChR; x0 = 0.97 mm, nH = 2.84 and K = 0.14 for ε-AChR.
The Zn2+ influx in standard medium, containing Ca2+ and Mg2+, was studied using the specific low-sensitivity dye Newport Green diacetate (Kd = 1.5 μm, as indicated by the manufacturer). When ZnCl2 was added to the standard external solution, prolonged application of nicotine (100 μm) evoked tiny but sustained fluorescence changes (ΔF/F0 = 0.16 ± 0.03 for γ-AChR, n = 23), which did not recover to basal even 3-4 min after nicotine and/or ZnCl2 washout, unless the cell-permeant Zn2+ chelator TPEN (40 μm) was added to the standard solution (Fig. 2A). The fluorescence change developed very slowly, presumably as Zn2+ accumulated within the cell to a level detectable by this low-affinity dye. No fluorescence increase was elicited by nicotine in the absence of Zn2+, confirming that Newport Green specifically recognizes Zn2+ (Canzoniero et al. 1999), but the small size and slow rise of the responses observed limited the reliability of the Zn2+ influx measurements performed with this fluorescent dye. We therefore turned to Mg2+-Green, a dye with a higher affinity for Zn2+ but which is sensitive to other divalent cations. In a Ca2+-, Mg2+-free solution containing ZnCl2, nicotine promptly elicited a long-lasting increase of fluorescence (ΔF/F0 = 0.46 ± 0.06 for γ-AChR, n = 20; 0.35 ± 0.05 for ε-AChR, n = 36), which was insensitive to the removal of the transmitter or external Zn2+, but rapidly recovered to basal level (time for half-recovery, T0.5 = 10 ± 5 s) after application of TPEN (Fig. 2B). After fluorescence recovery and TPEN washout, a second nicotine application elicited a comparable response (data not shown). Thus, Mg2+-Green appeared to be sensitive enough to measure Zn2+ influx in our cells. We also compared the results obtained in the absence of external Ca2+ and Mg2+ to those obtained under physiological conditions as, in neurones, Zn2+ influx is reduced by the addition of Ca2+ and Mg2+ to the external medium (Sensi et al. 1997). Using standard external solution plus ZnCl2, the response to nicotine showed an initial transient followed by a sustained plateau, terminated by TPEN addition but not by agonist or Zn2+ withdrawal (Fig. 2Ca). In each of the eight cells tested (four loaded with cell-impermeant Mg2+-Green under the whole-cell recording configuration, the others with the cell-permeant dye), this response virtually overlapped (e.g. Fig. 2Ca) the sum of the transient fluorescence increase (Fig. 2Cb) recorded in standard external medium (no ZnCl2 added) and the sustained fluorescence signal due to the influx of Zn2+ in Ca2+-, Mg2+-free medium (Fig. 2Cc). No fluorescence increase was detected when nicotine was applied in the absence of Ca2+, Mg2+ and Zn2+ (n = 21), or when ZnCl2 (0.1-5 mm) was applied in the absence of nicotine (data not shown).
Figure 2. Zn2+ influx through mouse muscle AChR channels is independent of Ca2+ and Mg2+.
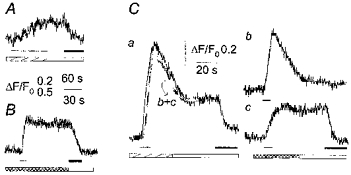
Representative examples of fluorescence responses to nicotine (100 μm, indicated by the thin bar) obtained, under different conditions, in three cells expressing γ-AChR. A, the small and slow fluorescence increase in a cell loaded with cell-permeant Newport Green diacetate and equilibrated in standard external solution plus ZnCl2 (0.5 mm, hatched bar) was unaffected by Zn2+ washout with a Ca2+-, Mg2+-free solution (open bar), but recovered to basal when TPEN (40 μm, filled bar) was applied. B, in cells loaded with Mg2+-Green acetate and equilibrated in Ca2+-, Mg2+-free solution containing Zn2+ (cross-hatched bar), the sustained fluorescence response was prompt and larger than those observed using Newport Green, and recovered to basal only upon TPEN application (in Ca2+-, Mg2+-free solution). C, in a cell loaded with Mg2+-Green via the patch pipette, the fluorescence response in standard external solution plus ZnCl2 (a) was matched by the sum (trace labelled b+c) of the transient responses obtained in standard external solution in the absence of Zn2+(b), and the sustained response observed in Ca2+-, Mg2+-free solution containing Zn2+ (c). TPEN application caused recovery in both a and c. Membrane holding potential, -50 mV.
Thus, under our experimental conditions, Zn2+ influx appeared to be little influenced by Ca2+ and Mg2+ at physiological concentrations. These observations indicate that Zn2+ permeation through γ- and ε-AChR channels may be adequately studied in BOSC 23 cells loaded with Mg2+-Green using a Ca2+-, Mg2+-free medium.
Fractional Zn2+ current through γ- and ε-AChRs
The fractional current carried by Zn2+ (Pf,Zn) through γ- and ε-AChR channels was determined by simultaneously recording, in the presence of Zn2+ (0.5 mm), agonist-evoked fluorescence and whole-cell current responses, in cells loaded with the cell-impermeant dye Mg2+-Green (50 μm) via the patch pipette (e.g. Fig. 3A). Application of nicotine elicited a current that was terminated by agonist washout, whereas the parallel fluorescence increase was maintained until TPEN was added to the external medium (see Fig. 3A). To estimate Pf,Zn, we determined the ratio of nicotine-elicited fluorescence to charge responses (F/Q) and normalized this to the F/Q ratio obtained when Zn2+ was the only charge carrier (Fig. 3B), as described previously (Vernino et al. 1994; Ragozzino et al. 1998). The fetal and adult AChR channels showed a significant difference in their permeability to Zn2+, as Pf,Zn was 1.7 ± 0.3% for γ-AChR (n = 15) and 3.9 ± 0.9% (n = 11) for ε-AChR (one-way ANOVA, P = 0.014). The same Pf,Zn (1.7 ± 0.4%, n = 11 for γ-AChR) was obtained for ACh-induced responses, indicating that the agonist used did not influence Zn2+ influx. Similar values for Pf,Zn of γ-AChR were obtained when the intracellular solution contained NMGA instead of CsCl.
Figure 3. Fractional Zn2+ current through muscle AChRs.
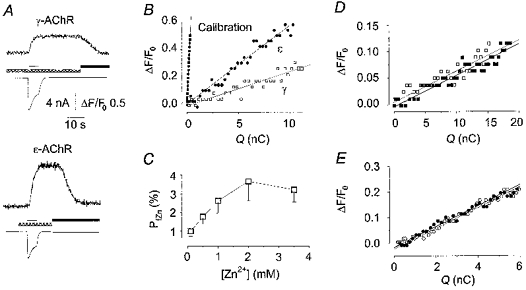
A, typical fluorescence and current responses evoked by nicotine (thin bar), simultaneously recorded in two cells expressing γ-AChR (top) and ε-AChR (bottom), equilibrated in Ca2+-, Mg2+-free solution containing Zn2+ (0.5 mm, cross-hatched bar). Note that the current response was terminated by nicotine washout, while fluorescence recovered to basal only upon TPEN application (filled bar). Membrane holding potential, -50 mV. B, plot of ΔF/F0vs. Q in the same cells as in A (□, γ-AChR; •, ε-AChR) and in a third cell used for calibration measurements (▪). Data points were calculated every 50 ms. The slopes of the straight lines, obtained by best fitting the data, represent the F/Q ratios of the responses. C, in cells expressing γ-AChR, Pf,Zn increased with Zn2+ concentration in the extracellular medium. Each point represents the mean ±s.e.m. of 6-11 cells. D, plot of ΔF/F0vs. Q in a cell loaded with Newport Green, with nicotine applied in the presence of ZnCl2 (0.5 mm, □), or ZnCl2 plus CaCl2 and MgCl2 (2 mm each, ▪). Note that the values virtually overlap. E, plot of ΔF/F0vs. Q in a cell loaded with Fluo-3, and superfused with nicotine in standard external solution (○) and in the presence of ZnCl2 (0.5 mm, •). Data points were obtained and fitted as in B.
The voltage dependence of Zn2+ influx was studied for membrane potentials ranging between -80 and +50 mV. Current-voltage relations were linear for γ- and ε-AChRs, both reversing close to 0 mV (not shown). At hyperpolarized potentials (-80 to -30 mV), Pf,Zn was voltage independent, but it abruptly rose when the membrane potential approached the equilibrium potential of monovalent cations (0 mV) and most of the current was carried by Zn2+ ions (not shown). At positive membrane potentials, fluorescence increases were observed (not shown), indicating that Zn2+ enters the cell even when the net current is outward.
Since the synaptic Zn2+ concentration may increase up to 100-fold during intense synaptic activity (Assaf & Chung, 1984), we determined Pf,Zn for Zn2+ concentrations between 0.1 and 3.5 mm. In cells expressing the γ-AChR, Pf,Zn became larger with increasing Zn2+ concentration, reaching a plateau at millimolar concentrations (Fig. 3C).
It remains to be confirmed whether the measurements of Zn2+ influx performed in the absence of Ca2+ and Mg2+ are representative of more physiological conditions. As shown in Fig. 2C, the late phase of the nicotine-evoked fluorescence response in Mg2+-Green-loaded cells (attributable to Zn2+) was not influenced by the addition of Ca2+ and Mg2+ at physiological concentrations. The observation that Pf,Zn increased about 2-fold when the concentration of external Zn2+ was raised from 0.1 to 0.5 mm (Fig. 3C), which causes only a small (if any) reduction of current amplitude (see Fig. 1C), supports the notion that fluorescence signals are adequately sensitive to changes in Zn2+ influx. This point was further investigated by directly measuring the F/Q ratio of nicotine-induced responses in cells loaded with the Zn2+-specific dye Newport Green (Fig. 3D). As tested in cells expressing the γ-AChR at an extracellular Zn2+ concentration of 0.5 mm, the F/Q ratio did not change when Ca2+ and Mg2+ (2 mm each) were added to the external medium (0.009 ± 0.002 vs. 0.008 ± 0.002, n = 7). Given the low sensitivity of Newport Green, we also examined the effect of Zn2+ on the nicotine-evoked Ca2+ responses of five cells loaded with the Ca2+-sensitive, Zn2+-insensitive dye Fluo-3 (Fig. 3E). The F/Q ratio was unaffected (ANOVA test, P > 0.75) by addition to the standard external medium of 0.5 or 5 mm ZnCl2, the values being 0.0354 ± 0.006 (normal external solution), 0.0322 ± 0.007 (0.5 mm Zn2+) and 0.034 ± 0.009 (5 mm Zn2+). These data confirm that divalent cations at physiological concentrations interfere little with each other’s influx through AChR channels.
Effects of Zn2+ on unitary γ-AChR channel function
To analyse the effects of Zn2+ on AChR channel behaviour, cell-attached and outside-out recordings of unitary events evoked by ACh were performed in cells expressing the γ-AChR. Under control conditions, cell-attached channel conductance and mean open time were 34.7 ± 0.6 pS and 4.4 ± 0.2 ms (n = 5), respectively, in line with previous observations in the same cell system (Fucile et al. 1996). When ZnCl2 (50 or 100 μm) was included in the pipette-filling solution (3 cells), neither the single-channel slope conductance (35.6 ± 0.7 pS), nor the channel open time (3.8 ± 0.4 ms) was different from the control values. At millimolar concentrations, ZnCl2 clearly reduced channel conductance, with no significant effect on mean channel open time, the values being 24.9 ± 1.4 pS and 4.8 ± 0.5 ms (n = 6) at 1 mm. However, patches were very unstable and recordings using ZnCl2 at its half-maximal inhibitory concentration (3 mm for γ-AChR) could only be performed in the outside-out configuration (Fig. 4A). In the three patches examined, the unitary conductance of ACh-evoked channels (at -70 mV) in the presence of ZnCl2 was 65-76% (mean, 70.4%) of the control conductance (26.5 vs. 37.4 pS). In contrast, channel open duration was essentially unaltered in the presence of Zn2+ (4.3 ms for the control, 3.9 ms in the presence of Zn2+). Following patch excision, a slight decrease in channel opening frequency was observed, independent of Zn2+ addition. Together, these data show that in the presence of 1-3 mm ZnCl2, unitary channel conductance was reduced by about 70%, in agreement with the whole-cell data. No flickering behaviour was ever observed in the presence or absence of Zn2+ at these low ACh concentrations (data not shown).
Figure 4. Effect of ZnCl2 on γ-AChR channel behaviour.
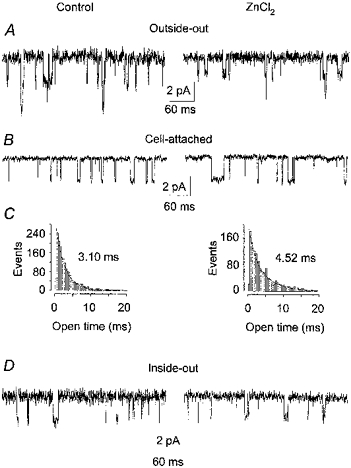
A, typical traces recorded in an outside-out patch (pipette potential, -70 mV), superfused with ACh (200 nm, left), then with ACh plus ZnCl2 (3 mm, right). Downward deflections represent inward currents. Conductances and mean open times were: Control, 37.5 pS and 3.9 ms; ZnCl2, 26.6 pS and 3.9 ms. Note the slight reduction in opening frequency, due to channel run-down. B, representative examples of cell-attached currents recorded in a BOSC 23 cell before (Control) and after a 4 min application to the extra-patch membrane of ACh (20 μm) plus ZnCl2 (200 μm), as indicated. Unitary slope conductances were 38.4 pS (Control) and 37.9 pS (ZnCl2). Traces were recorded at an estimated transmembrane potential of about -60 mV, with 100 nm ACh in the patch pipette. C, histograms of the channel open times for the recordings shown in B, with the indicated mean open times. Superimposed are the best fits of the data with the sum of two exponentials, with the following time constants (weight): Control, τ1 = 2.1 ms (66%), τ2 = 3.9 ms (34%); ZnCl2, τ1 = 1.7 ms (24%), τ2 = 4.9 ms (76%). Note the increase of τop following administration of ZnCl2. D, typical events recorded in an inside-out patch before (Control) and 1 min after patch exposure to ZnCl2 (200 μm), as indicated. Recordings were performed with a pipette potential of -80 mV. Conductance and mean open time were: Control, 31 pS and 2.97 ms; ZnCl2, 29 pS and 3.01 ms. Note the ineffectiveness of Zn2+ application.
To examine whether the increase of cytosolic Zn2+ concentration caused by its influx through AChR channels influences channel activity, cell-attached recordings were performed before and after (or during) application of ACh (20 μm) plus ZnCl2 (200 μm) to the extra-patch membrane (Fig. 4B). Under control conditions, unitary events showed a conductance of 39 ± 1 pS, slightly higher than in the absence of continuous superfusion, and a mean τop of 3.2 ± 0.3 ms (n = 9). After exposure of the extra-patch membrane to ACh plus ZnCl2 for 2-4 min, τop significantly increased in seven out of nine cells tested (Fig. 4B), with increments ranging from 20% (significant, one-way ANOVA, P < 0.001) to 100% (mean, 50 ± 10%). Open time distributions were well fitted by two exponential components before and after Zn2+ application (e.g. Fig. 4C). Neither channel conductance (38.9 ± 1.5 pS, n = 9) nor closed duration (not shown) was affected by Zn2+ application. τop did not decrease even 15 min after washout of ACh and ZnCl2. No significant change of τop was observed when ACh alone was applied to the cells prior to Zn2+ application (n = 3, data not shown). We also tested whether the Zn2+ chelator TPEN was able to revert the Zn2+-induced increase of τop. To this purpose, ACh-evoked events were recorded in cells treated for 2-4 min with TPEN (50 μm), after the effect of Zn2+ had been assessed. τop was reduced to control values in only one out of four cells tested (not shown), whereas in the other three cells the mean τop remained elevated. The scattering in the extent of the extra-patch Zn2+ effect and the lack of reversibility by TPEN suggest that the action of Zn2+ was mediated by some intracellular effector. To test this hypothesis, inside-out patches were exposed (1-5 min) to Zn2+ (200 μm, as in cell-attached experiments). Since in a different cell line (BC3H1), a 3-fold decrease of τop was observed within 20 min of patch excision (Covarrubias & Steinbach, 1990), we monitored the τop of AChR channels in transfected BOSC 23 cells. In recordings from seven inside-out patches lasting 10-20 min, no significant change of τop was observed (4.0 ± 0.5 ms within 60 s of patch excision, 4.3 ± 0.5 ms 10-20 min later, P = 0.6, one-way ANOVA). At the end of control recordings, three of these patches were exposed to Zn2+, which was unable to modify τop. Accordingly, these data were pooled with others obtained at earlier times (3-10 min) after patch excision. In none of the eight inside-out patches examined did Zn2+ modify τop, which, on average, was 3.9 ± 0.4 ms (n = 8) under control conditions and 3.8 ± 0.4 ms in the presence of Zn2+ (Fig. 4D). The discrepancy between this study and that of Covarrubias & Steinbach (1990) might be due to differences in the cell type used (BOSC 23 vs. BC3H1) and/or in the recording conditions (standard external solution in the patch pipette, nominally Ca2+-free solution in the bath vs. EGTA-containing solution in the bath and in the patch pipette). However, a detailed investigation of this point is beyond the purposes of the present study.
DISCUSSION
Zinc is a trace element crucial for embryonic development and animal growth and survival (for review see Smart et al. 1994). Functionally, two distinct pools of zinc exist: one is tightly bound to proteins, the other is stored in synaptic vesicles and released upon synaptic activity. In the brain, releasable Zn2+ mediates toxic responses (as reviewed by Choi & Koh, 1998) and modulates the function of several receptors, including excitatory and inhibitory amino acid receptors (Smart et al. 1994) and a neuronal nicotinic receptor (Palma et al. 1998). In addition, it controls nociceptive pathways in the spinal cord (Larson & Kitto, 1997). As for the neuromuscular junction, an increasing concentration of Zn2+ is observed in rat motoneurones in the first postnatal days (Kozma & Ferke, 1979) and exogenous Zn2+ influences spontaneous and evoked ACh release (Benoit & Mambrini, 1970; Nishimura, 1987; Wang & Quastel, 1990). Furthermore, the frog end-plate channel is permeable to Zn2+, as demonstrated by the shift of the IACh reversal potential upon changes of the extracellular Zn2+ concentration (Adams et al. 1980). This paper provides a direct measurement of the fraction of current carried by Zn2+ ions through the channels of the fetal and adult types of mouse muscle nicotinic AChR expressed in transiently transfected BOSC 23 cells, as well as demonstrating that cytosolic Zn2+ accumulation influences AChR function. We show that γ- and ε-AChR channels are permeable to Zn2+ ions, both in the presence and in the absence of Ca2+ and Mg2+. At 0.5 mm, i.e. a concentration possibly attained at active synapses (see Assaf & Chung, 1984), the value of Pf,Zn for ε-AChRs is almost double that for γ-AChRs. A 2-fold difference has also been observed for the Ca2+ fractional permeability (Pf,Ca) of the two AChR types measured in BOSC 23 cells (Ragozzino et al. 1998) and at developing mouse synapses (Villaroel & Sakmann, 1996), suggesting that γ- and ε-AChRs have a differential permeability to divalent cations. At the frog end-plate, the permeability to Zn2+ is about 10% higher than to Ca2+ (Adams et al. 1980), in line with the observation that, for divalent cations, the smaller the size, the greater the permeability through the ε-AChR channels (Lewis & Stevens, 1983). Our data indicate that, for γ-AChRs, Pf,Zn increases with the concentration of Zn2+, so that at a concentration of 2 mm, the fractional permeability for Zn2+ (in a Ca2+-, Mg2+-free solution) is double that for Ca2+. For ε-AChRs, the strong block of IACh hinders the reliable measurement of Pf,Zn at millimolar Zn2+ concentrations. We further show that Ca2+ and Mg2+ at physiological concentrations do not appreciably alter the amplitude of the long-lasting increase of cytosolic Zn2+ concentration evoked by nicotine, and conversely Zn2+ up to 5 mm does not influence fractional Ca2+ influx. These data indicate that, at these concentrations, the permeation of each divalent cation through the AChR channels is little influenced by the presence of others.
As for other divalent cations, Zn2+ permeates through the receptor channel, but, at millimolar concentrations, it reversibly reduces the amplitude of IACh. For Ca2+ and Mg2+, the block has been ascribed to the high-affinity interaction of divalent cations with negative residues in the channel pore, which decreases channel conductance (Imoto et al. 1988). We show here that Zn2+ acts by a similar mechanism, as the addition of 1-3 mm ZnCl2 to the external medium comparably reduced both the unitary conductance and the amplitude of whole-cell currents to about 70% of control values. Furthermore, the voltage independence of Zn2+ block and the absence of flickering behaviour in single-channel openings recorded in the presence of Zn2+ indicate that Zn2+ is not an open-channel blocker. With respect to the γ-AChR, the ε-AChR has one more net negative charge in both the cytoplasmic and extracellular ring close to the membrane-spanning segment M2 (Imoto et al. 1988), so that it might be more sensitive to divalent cations than the γ-AChR. This appears to be the case for Zn2+-induced block, which is 3-fold stronger for the ε- than the γ-AChR.
Interestingly, both in the presence and in the absence of Ca2+ and Mg2+, the nicotine-induced increase of the cytosolic Zn2+ concentration did not recover until the specific Zn2+ chelator TPEN was applied. This also happens in cultured cortical neurones, where Zn2+ is believed to mediate important physio-pathological processes (Sensi et al. 1997; Canzoniero et al. 1999), and indicates that Zn2+ is poorly buffered and/or extruded by cells, suggesting that Zn2+ may accumulate within the cells and mediate long-lasting effects. Indeed, we demonstrate that, under conditions leading to an increase of the cytosolic Zn2+ concentration, the open duration of the γ-AChR channel is lengthened. This effect requires the influx of a substantial amount of Zn2+, as no channel modulation was observed when Zn2+ was included in the patch pipette. In addition, the lengthening of τop is not caused by Ca2+ influx through the γ-AChR channel, as there was no change of τop when cells were exposed to ACh in the absence of Zn2+, while Ca2+ influx was the same as in the presence of Zn2+. Thus, the reported increase of τop appears to be specifically related to Zn2+ influx. One might imagine that an increase of τop would lead to a potentiation of IACh in the presence of similar concentrations of extracellular Zn2+, which was not observed. However, the effect of Zn2+ on single-channel open duration requires Zn2+ accumulation, which, under whole-cell recording conditions, is probably prevented by chelation by the EGTA contained in the patch pipette. The Zn2+-induced lengthening of channel open time is quite unusual, as Zn2+ has been described to reduce the opening frequency of GABA-gated receptor channels without altering their open duration, or to reduce the open duration of NMDA-gated channels (as reviewed by Smart et al. 1994). It has been known for many years that Ca2+ entry favours AChR desensitization at the frog end-plate (Miledi, 1980). The data reported here suggest that the influx through the AChR channel of Zn2+, another divalent cation, affects channel function (namely, its open duration) by an analogous mechanism, possibly acting through intracellular mediators (Miledi et al. 1989).
All these data suggest that Zn2+ might play a physiological role in neuromuscular transmission, as is the case for central synapses (for review see Choi & Koh, 1998). Though it has not yet been demonstrated that Zn2+ is released at the neuromuscular junction, Zn2+ is present in plasma at a concentration of about 10 μm in adult humans (Vallee & Falchuk, 1993). Thus, it might accumulate in muscle fibres during periods of intense activity, modulating AChR channel function. In conclusion, Zn2+ permeates through AChR channels and reduces their conductance, probably acting on extracellular regulatory sites (Lena & Changeux, 1993). Furthermore, Zn2+ induces the lengthening of channel open durations, possibly through cytosolic mechanisms, deserving further investigation.
Acknowledgments
We thank Dr R. Miledi and Dr P. Bregestovski for helpful suggestions. This work was supported in part by MURST grants to F.E. and F.G.
References
- Adams DJ, Dwyer TM, Hille B. The permeability of endplate channels to monovalent and divalent metal cations. Journal of General Physiology. 1980;75:493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf SY, Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Benoit PR, Mambrini J. Modification of the transmitter release by ions which prolong the presynaptic action potential. Journal of Physiology. 1970;210:681–695. doi: 10.1113/jphysiol.1970.sp009235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canzoniero LMT, Turetsky DM, Choy DW. Measurement of intracellular free zinc concentrations accompanying zinc-induced neuronal death. Journal of Neuroscience. 1999;19(RC31):1–6. doi: 10.1523/JNEUROSCI.19-19-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Koh JY. Zinc and brain injury. Annual Review of Neuroscience. 1998;21:347–375. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- Covarrubias M, Steinbach JH. Excision of membrane patches reduces the mean open time of nicotinic acetylcholine receptors. Pflügers Archiv. 1990;416:385–392. doi: 10.1007/BF00370744. [DOI] [PubMed] [Google Scholar]
- Fucile S, Mileo AM, Grassi F, Salvatore AM, Alemë S, Eusebi F. Identification of a determinant of acetylcholine receptor kinetics in the extracellular portion of the γ subunit. European Journal of Neuroscience. 1996;8:2564–2570. doi: 10.1111/j.1460-9568.1996.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Huang EP. Metal ions and synaptic transmission: Think zinc. Proceedings of the National Academy of Sciences of the USA. 1997;94:13386–13387. doi: 10.1073/pnas.94.25.13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto K, Busch C, Sakmann B, Mishina M, Konno T, Nakai J, Bujo H, Mori Y, Fukuda K, Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988;335:645–651. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Koh JY, Choi DW. Zinc toxicity on cultured cortical neurons: involvement on N-methyl-D-aspartate receptors. Neuroscience. 1994;60:1049–1057. doi: 10.1016/0306-4522(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Kozma M, Ferke A. Trace element localization and changes in zinc and copper concentrations during postnatal development of the rat CNS. Acta Histochemica. 1979;65:219–227. doi: 10.1016/S0065-1281(79)80010-0. [DOI] [PubMed] [Google Scholar]
- Larson AA, Kitto KF. Manipulations of zinc in the spinal cord, by intrathecal injection of zinc chloride, disodium-calcium-EDTA, or dipicolinic acid, alter nociceptive activity in mice. Journal of Pharmacology and Experimental Therapeutics. 1997;282:1319–1325. [PubMed] [Google Scholar]
- Lena C, Changeux J-P. Allosteric modulation of the nicotinic acetylcholine receptor. Trends in Neurosciences. 1993;16:181–186. doi: 10.1016/0166-2236(93)90150-k. [DOI] [PubMed] [Google Scholar]
- Lewis CA, Stevens CF. Acetylcholine receptor channel ionic selectivity: Ions experience an aqueous environment. Proceedings of the National Academy of Sciences of the USA. 1983;80:6110–6113. doi: 10.1073/pnas.80.19.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Intracellular calcium and desensitization of acetylcholine receptors. Proceedings of the Royal Society. 1980;209:447–452. doi: 10.1098/rspb.1980.0106. B. [DOI] [PubMed] [Google Scholar]
- Miledi R, Parker I, Woodward RM. Membrane currents elicited by divalent cations in Xenopus oocytes. Journal of Physiology. 1989;417:173–195. doi: 10.1113/jphysiol.1989.sp017796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M. Zinc competitively inhibits calcium-dependent release of transmitter at the mouse neuromuscular junction. Pflügers Archiv. 1987;410:623–626. doi: 10.1007/BF00581322. [DOI] [PubMed] [Google Scholar]
- Palma E, Maggi L, Miledi R, Eusebi F. Effects of Zn2+ on wild and mutant neuronal α7 nicotinic receptors. Proceedings of the National Academy of Sciences of the USA. 1998;95:10246–10250. doi: 10.1073/pnas.95.17.10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino D, Barabino B, Fucile S, Eusebi F. Ca2+ permeability of mouse and chick nicotinic acetylcholine receptors expressed in transiently transfected human cells. Journal of Physiology. 1998;507:749–757. doi: 10.1111/j.1469-7793.1998.749bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Zhou Z, Konnerth A, Neher E. Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron. 1993;11:133–143. doi: 10.1016/0896-6273(93)90277-x. [DOI] [PubMed] [Google Scholar]
- Sensi SL, Canzoniero LMT, Yu SP, Ying HS, Koh J-Y, Kerchner GA, Choi DW. Measurements of intracellular free zinc in living cortical neurons: routes of entry. Journal of Neuroscience. 1997;17:9554–9564. doi: 10.1523/JNEUROSCI.17-24-09554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG, Xie X, Krishek BJ. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Progress in Neurobiology. 1994;42:393–441. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiological Reviews. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- Vernino S, Rogers M, Radcliffe KA, Dani JA. Quantitative measurements of calcium flux through muscle and neuronal nicotinic acetylcholine receptors. Journal of Neuroscience. 1994;14:5514–5524. doi: 10.1523/JNEUROSCI.14-09-05514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaroel A, Sakmann B. Calcium permeability increase of endplate channels in rat muscle during postnatal development. Journal of Physiology. 1996;496:331–338. doi: 10.1113/jphysiol.1996.sp021688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Quastel DM. Multiple actions of zinc on transmitter release at mouse end-plates. Pflügers Archiv. 1990;415:582–587. doi: 10.1007/BF02583509. [DOI] [PubMed] [Google Scholar]
- Yin HZ, Weiss JH. Zn2+ permeates Ca2+ permeable AMPA/kainate channels and triggers selective neuronal injury. NeuroReport. 1995;6:2553–2556. doi: 10.1097/00001756-199512150-00025. [DOI] [PubMed] [Google Scholar]
- Yu SP, Choi DW. Na+-Ca2+ exchange currents in cortical neurons: concomitant forward and reverse operation and effect of glutamate. European Journal of Neuroscience. 1997;9:1273–1281. doi: 10.1111/j.1460-9568.1997.tb01482.x. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Neher E. Calcium permeability of nicotinic acetylcholine receptor channels in bovine adrenal chromaffin cells. Pflügers Archiv. 1993;425:511–517. doi: 10.1007/BF00374879. [DOI] [PubMed] [Google Scholar]


