Abstract
Event-related potentials (31-channel ERPs) were recorded from 38 depressed, unmedicated outpatients and 26 healthy adults (all right-handed) in tonal and phonetic oddball tasks developed to exploit the perceptual challenge of a dichotic stimulation. Tonal nontargets were pairs of complex tones (corresponding to musical notes G and B above middle C) presented simultaneously to each ear (L/R) in an alternating series (G/B or B/G; 2-s fixed SOA). A target tone (note A) replaced one of the pair on 20% of the trials (A/B, G/A, B/A, A/G). Phonetic nontargets were L/R pairs of syllables (/ba/, /da/) with a short voice onset time (VOT), and targets contained a syllable (/ta/) with a long VOT. Subjects responded with a left or right button press to targets (counterbalanced across blocks). Target detection was poorer in patients than controls and for tones than syllables. Reference-free current source densities (CSDs; spherical spline Laplacian) derived from ERP waveforms were simplified and measured using temporal, covariance-based PCA followed by unrestricted Varimax rotation. Target-related N2 sinks and mid-parietal P3 sources were represented by CSD factors peaking at 245 and 440 ms. The P3 source topography included a secondary, left-lateralized temporal lobe maximum for both targets and nontargets. However, a subsequent hemispheric spatiotemporal PCA disentangled temporal lobe N1 and P3 sources as distinct factors. P3 sources were reduced in patients compared with controls, even after using performance as a covariate. Results are consistent with prior reports of P3 reduction in depression and implicate distinct parietal and temporal generators of P3 when using a dichotic oddball paradigm.
Keywords: Event-related potential (ERP), Principal components analysis (PCA), Current source density (CSD), surface Laplacian, Oddball task, Dichotic listening, P300, Depression
1. Introduction
1.1 P3 component identification
The archetype of cognitive ERPs is a positive-going deflection with a midparietal maximum at around 300 ms post-stimulus. P3 is known to be influenced by stimulus expectancy and probability, as well as response contingencies (e.g., Duncan-Johnson and Donchin, 1977; Simson et al., 1976). Although P3 has been identified using diverse paradigms, ranging from the original cued-pair paradigm (Sutton et al., 1965) to memory-related tasks (e.g., Friedman, 1990), the oddball task has become the de facto standard target-detection paradigm for producing P3. However, the impression of a ubiquitous P3 rapidly gave way to an understanding that functionally and topographically distinct components contribute to what came to be known as a late positive complex (Squires et al., 1975; Sutton and Ruchkin, 1984).
The concept of an ERP component has been formalized in terms of a characteristic waveform and topography, as well as the specific experimental manipulations required to produce it (e.g., Johnson, 1993; Kayser and Tenke, 2005; Picton et al., 2000). Of these, topography has been particularly emphasized as an essential characteristic (e.g., Spencer et al., 2001), owing to the correspondence between a characteristic topography and an underlying pattern of activation of neuroanatomical generators (e.g., Kayser and Tenke, 2005, 2006a). However, it has long been recognized that the redundancy and reference-dependency of ERP topographies may frustrate efforts at identifying their underlying neuronal generators (e.g., Nunez and Srinivasan, 2006; Tenke and Kayser, 2005). A surface Laplacian (second spatial derivative) removes much of this redundancy, yielding sharper CSD topographies that represent the underlying current sources (current flows radially from brain into skull and scalp) and sinks (into brain). CSD topographies are unaffected by the recording reference and less influenced by distant generators.
ERP components recorded in healthy adults during simple binaural oddball tasks show a topographic specificity consistent with the regional and hemispheric specialization expected for material-specific categorization and evaluation. Using Principal Components Analysis (PCA) methodology to extract components in a data-driven manner, Kayser et al. (1998) reported content-dependent (complex tones or consonant-vowel syllables) hemispheric ERP asymmetries in a binaural oddball task, with relatively larger amplitudes within the N2-P3 complex over the right frontotemporal region for a tonal task and over the left temporoparietal region for a phonetic task. The origins of these differences were subsequently explored by applying temporal PCA to montages of surface Laplacian (CSD) waveforms (Kayser and Tenke, 2006a, 2006b), thereby providing a reference-independent description of the data characterized by sharper component topographies that are more closely related to the underlying neuronal generators.
1.2 Task difficulty and P3 reductions in depression
ERP studies of patients having a depressive disorder have yielded conflicting findings, with some studies reporting decreased amplitude of the P3 potential in depressed adults when compared to healthy controls, but other studies finding no difference (Roth et al., 1986). Thus, depressed patients tested in the binaural oddball tasks of Kayser et al. (1998) displayed the expected task-related asymmetry of the N2-P3 complex, and did not differ from healthy controls in P3b amplitude (Bruder et al., 2002). However, the accuracy of responses to target stimuli was very high in both patients and controls (> 96% correct), raising the possibility that the failure to detect P3 differences between depressed patients and controls could have resulted from the use of a task that was insufficiently challenging to reliably reveal the subtle cognitive deficits in depressed patients.
In contrast to a simple binaural oddball task, dichotic listening tasks impose additional processing demands by presenting conflicting information to the two ears. In healthy controls, perceptual asymmetry during a dichotic complex tone test (behavioral left ear advantage) was associated with a hemispheric asymmetry of the late positivity (larger over the contralateral right than the ipsilateral left hemisphere; Tenke et al., 1993a), and predicted P3 source asymmetries in a binaural oddball task (Tenke et al., 1998). Moreover, ERPs of depressed patients in this cognitively demanding task (Bruder et al., 1995) showed smaller P3 amplitude when compared to healthy controls, and failed to show either the behavioral left ear (right hemisphere) advantage or the associated hemispheric asymmetry of P3 seen in healthy adults for dichotic pitch discrimination.
Dichotic listening tasks typically require numerous stimuli which are paired in a quasirandom sequence. However, it was reasoned that a minimal set of stimuli could be used in the context of a dichotic oddball paradigm, thereby yielding P3 source topographies that are directly comparable to those in a binaural oddball task (i.e., Kayser and Tenke, 2006a, 2006b). Moreover, it was anticipated that the additional challenge imposed by a dichotic task would reveal a behavioral and P3 deficit in depressed patients. The present study used unrestricted temporal-PCA of reference-free surface Laplacian waveforms to quantify and compare P3 sources in depressed patients and healthy controls during dichotic oddball tasks using complex tones or syllables.
2. Materials and Methods
2.1 Participants
Twenty-six healthy adults (10 male) with no history of psychopathology, and 38 depressed outpatients (19 male) from the Depression Evaluation Service at the New York State Psychiatric Institute were recruited from the New York metropolitan area. Participants were predominantly Caucasian (45 white, 6 Asian, 6 black, the remainder mixed or unknown). Patients were all drug-free for a period of at least 7 days. Patients were slightly older than controls (mean years ± SD, 35.0 ± 10.5 vs. 30.3 ± 6.5, t = -2.023, df = 62, p < .05), but did not significantly differ in years of education (17 ± 1.9 vs.16 ± 2.3). Participants were excluded from the study if they had a hearing loss greater than 30 dB in either ear at 500, 1,000 or 2,000 Hz, or if they had an ear difference greater than 10 dB. Participants were also excluded if they had current substance abuse, a history of head trauma, or other neurological disorder. Control subjects were screened using the Structured Clinical Interview for DSM-IV, nonpatient edition (First et al., 1996) to exclude those with current or past psychopathology. All subjects were right-handed, as indicated by their Edinburgh Inventory (Oldfield, 1971; Laterality Quotients, controls = +76.3 ± 38.3; patients = +72.1 ± 46.2). Most patients met DSM-IV criteria for major depressive disorder (MDD; n = 24), dysthymia (n = 7) or both disorders (n = 4). One patient met criteria for bipolar disorder II, the remaining two being depression NOS.
2.2 Dichotic Oddball Tasks
2.2.1 Matched dichotic oddball tasks
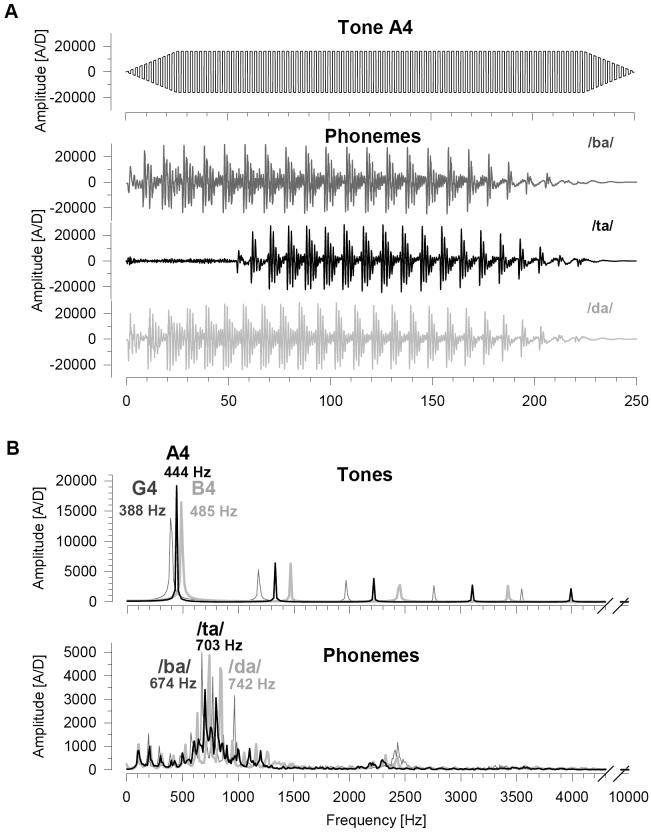
Stimuli were selected from dichotic listening tasks known to produce left ear (complex tones) and right ear (phonemes edited to equate their duration and loudness) advantages in healthy adults. As shown in Fig. 1, tonal stimuli were 250 ms square waves, linearly tapered over the first and last 20 ms, with fundamental frequencies corresponding to notes G4 (388 Hz), A4 (444 Hz), and B4 (485 Hz) on the musical scale. Phonetic stimuli were spoken consonant-vowel syllables edited to 250 ms. Pitch was not equated, thereby preserving the discriminability of the syllables by preventing complete perceptual fusion. The categorical perception of phonemes is sensitive to the vowel-onset delays (voice onset time, VOT), a property that is also observed for the neuronal response evoked in primary auditory cortex (Steinschneider et al., 1995, 1999). In order to reduce the disparity between ERPs produced for tonal and phonetic stimuli, nontarget phonemes were /ba/ (maximum spectral amplitude at 674 Hz) and /da/ (742 Hz), both of which consist of voiced consonants and short VOTs. The target phoneme /ta/ (703 Hz) consisted of an unvoiced consonant and a long VOT.
Fig. 1.
Waveforms (A) and corresponding amplitude spectra (B) of the tonal and phonetic stimuli used in the dichotic oddball task. (A) Because all three tonal stimuli had the same envelope, only the target waveform (A4) is shown. For the phonetic stimuli, the target waveform (/ta/) was unique in having an unvoiced consonant and a 57 ms voice onset time, in contrast to the nontargets (/ba/ and /da/; voiced consonants and immediate onset. All stimuli were 250 ms in duration and matched for sound pressure level. (B) Tonal and phonetic targets (black lines) were intermediate in frequency with respect to their two nontargets (gray lines).
Pairs of either nontarget tones [G4: B4] or syllables [/da/: /ba/] were presented simultaneously to each ear [L:R] in an alternating series: [G4:B4], [B4:G4], [G4:B4], [B4:G4], [G4:A4] ... or [/ba/:/da/], [/da/:/ba/], [/ba/:/da/], [/da/:/ba/], [/ta/:/da/] ... with a constant 2-s SOA. A target tone (A4) or syllable (/ta/) replaced one stimulus of the dichotic pair (e.g., [G4:A4] or [/ta/:/da/] on 20% of the trials, while maintaining the alternating stimulus sequence for the other ear. To further increase the comparability of phonetic and tonal stimuli, the nontarget syllable pair had no voice onset delay (/ba/ and /da/). The target syllable (/ta/) had a voice onset delay of 57 ms, and was, like its tonal counterpart, intermediate in pitch. Each subject received two consecutive tonal (T) and phonetic (P) task blocks. Each of the four blocks consisted of a total of 120 stimuli, including 24 target stimuli (6-each of the 4 possible target/nontarget combinations). Participants responded as rapidly and accurately as possible to targets with a button press. Response hand and task order was counterbalanced across participants (e.g., TR-TL-PL-PR or PL-PR-TR-TL).
2.2.2 Behavioral performance and asymmetry
Percent correct responses to targets (i.e., hits) and nonresponses to nontargets (percent correct rejection) were analyzed using a repeated measures ANOVA, with Group (Control, Patient) and Gender (Male, Female) as between-subjects factors and Task (tonal/phonetic) as a within-subjects factor. Gender served only as a control factor and will not be considered further in this report. Measures of perceptual asymmetry were computed as Laterality Quotient derived from correct target detection (hit rate) in right or left ears: LQ = 100 * (R - L) / (R + L). The association between performance and asymmetry measures with physiological component amplitudes for sites or regions of interest were evaluated using Pearson correlations.
2.3 ERP methods
2.3.1 Recording and preliminary processing
Scalp EEG was recorded from 13 lateral, homologous pairs of electrode sites (FP1/2, F3/4, F7/8, FC5/6, FT9/10, C3/4, T7/8, CP5/6, TP9/10, P3/4, P7/8, P9/10, O1/2) and from four midline electrode sites (Fz, Cz, Pz, Oz) using extended 10-20-system placements with an electrode cap (Electro Cap International, Inc.) and a nose reference. Electrodes at supra- and infra-orbital sites surrounding the right eye recorded blinks and vertical eye movements (bipolar), while electrodes at right and left outer canthi recorded horizontal eye movements (bipolar). All electrodes were tin, with impedances below 5 kOhm. EEG was recorded using a Grass Neurodata system at a gain of 10 k (5 k and 2.5 k for horizontal and vertical eye channels, respectively), with a bandpass of 0.1-30 Hz using a NeuroScan recording system (200 samples/s). Only recordings which were free of electrolyte bridges between electrodes were included (Tenke and Kayser, 2001). Horizontal and vertical EOGs were used for eye movement rejection and blink correction (linear regression; Semlitsch et al., 1986), after which artifactual EEG epochs were eliminated using a ±100 μV criterion, and subsequently under visual guidance using a semi-automated procedure.
2.3.2 ERP averages and CSD
In the previous binaural oddball studies (Kayser and Tenke, 2006a, 2006b), ERP averages were computed for correct responses corresponding to two tasks (tonal, phonetic), three response modes (silent count, left and right button press), and two conditions (target, nontarget). In the present dichotic oddball task, there are two tasks (tonal, phonetic), two response modes (left button press, right button press), and three conditions (target left ear, target right ear, nontarget). Due to the greater difficulty of the dichotic oddball, correct target averages were pooled across response hand and condition to assure a sufficient number of trials.1 Although this resulted in reasonably high means for number of target trials (Mean trials ± SD, tonal = 28 ± 11.2, range = 5-44; phonetic = 32 ± 11.0, range = 6-41), ERP target averages in four subjects (1 control, 3 patients) were based on fewer than nine trials. However, exclusion of these subjects did not affect the findings presented. Moreover, while the number of trials contributing to each average differed between groups for targets (controls = 34 ± 9.3; patients = 27 ± 10.0; F[1,60] = 7.63, p < .01), the number of trials for correct nontarget averages did not (controls = 127 ± 33.0; patients = 115 ± 35.3; F[1,60] = 2.27, p > .1).
Reference-free CSD waveforms were computed from the individual ERP waveforms to sharpen topographies, eliminate volume-conducted contributions from distant regions, and quantify underlying current generators (e.g., Tenke et al., 1998). We used the spherical spline surface Laplacian algorithm of Perrin et al. (1989; 50 iterations; m = 4; lambda = 10 -5), as detailed elsewhere (Kayser and Tenke, 2006a, 2006b; Tenke and Kayser, 2005).
2.3.3 Temporal CSD-PCA
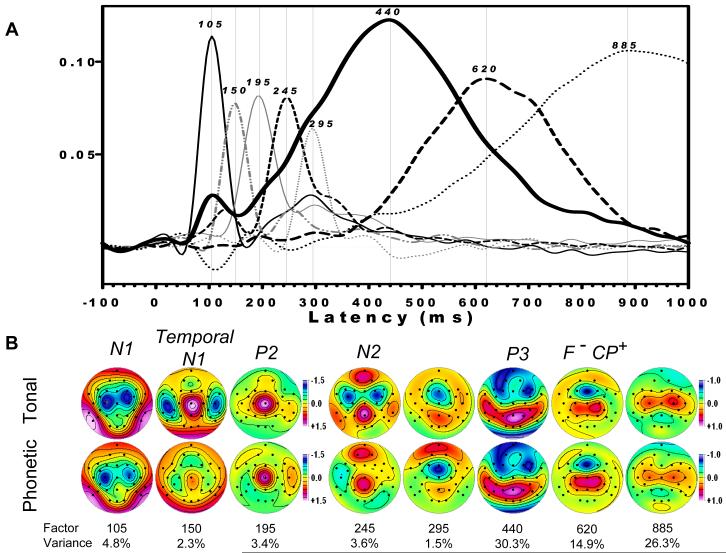
Event-related CSDs were submitted to a covariance-based PCA followed by unrestricted Varimax rotation of the covariance loadings (Matlab emulation of BMDP-4M published in appendix of Kayser and Tenke, 2003). Data consisted of 221 timepoints as variables (-100 to 1000 ms), using a total of 7936 observations consisting of 2 tasks (tonal, phonetic) × 2 conditions (target, nontarget) × 64 subjects × 31 sites (electrodes). Factors of interest, representing N1, P2, N2 and P3, were readily identifiable by loadings waveform peaks (e.g., N1 sink latency at 105 ms) and the corresponding factor score topographies (e.g., N1 sink maxima at C3/C4). These methods are presented in detail elsewhere (Kayser and Tenke, 2003, 2006a). The comparability of these factors was verified with those extracted independently for each group.
2.3.4 Hemispatial PCA
A spatiotemporal PCA approach can afford an efficient description and quantification of topographic variance (cf. Spencer et al., 2001). This representation more closely reflects the pattern of underlying neuronal generators when surface Laplacian data are used, and is thereby consistent with the identification of an ERP component with a spatially identifiable generator pattern. However, asymmetric spatial factors are unwieldy for comparing or contrasting activity in homologous anatomical regions of the two hemisphere, and paired factors representing comparable activity in each hemisphere are unlikely to be symmetric. These problems may be avoided by extracting spatial factors corresponding to hemispheres, rather than complete topographies. The application of a hemispatial PCA to dichotic oddball CSD data allows a direct comparison of factor scores in the two hemispheres, as well between conditions, tasks or groups.2
In contrast to a standard spatial PCA, for which an observation consists of a complete topography, a hemisphere serves as an observation for a hemispatial PCA. Each complete topography was therefore reduced to a pair of hemispheric topographies consisting of the 13 homologous lateral sites and the 5 midline sites (i.e., midline data repeated to map each hemisphere to the midline). Hemispheric topographies were then submitted to a spatial PCA using a covariance matrix (18 variables = sites/hemisphere; 113152 observations = 2 hemispheres × 2 conditions × 2 tasks × 64 subjects × 221 time points) followed by unrestricted Varimax rotation.
2.4 Statistical methods
The sharpened topographies of the CSDs compared to ERPs may be exploited by restricting analyses to characteristic portions of the complete montage representing generator regions underlying each unique component (e.g., sites C3/4 for N1 sink; Kayser and Tenke, 2006a; however, for an alternative see Kayser et al., 2007). By virtue of the comparability of the temporal CSD-PCA factor structure to that identified for the binaural oddball, repeated measures ANOVA were thereby computed for factor scores in the following regions previously identified by Kayser and Tenke (2006a, 2006b): 1) N1: central sites C3/C4; 2) temporal N1 (middle temporal sites T7/8); 3) N2: frontal sites (F7/8, F3/4, FC5/6, C3/4, FT9/10) for tonal task, and posterolateral sites (P7/8, P9/10, CP5/6, T7/8, TP9/10) for phonetic task; 4) P3 (detailed below); and 5) Response-related P3: midline frontal (sink) and centroparietal (C3/4, CP5/6, P3/4) sources.
In the previous study using the binaural oddball task, P3 was examined only at parietal sites (P3/4, CP5/6, P7/8), where target-related sources were largest. This approach was found to be inadequate to explore source topographies in the dichotic oddball task because of prominent secondary temporal lobe topography for both targets and nontargets (i.e., circled sites in Figs. 3 and 4). The analysis of P3 was therefore expanded to include the complete posterior topography on either hemisphere (TP9/10, CP5/6, P9/10, P7/8, P3/4, O1/2). Additional analyses were then conducted as required to identify and disentangle lateral and medial P3 sources.
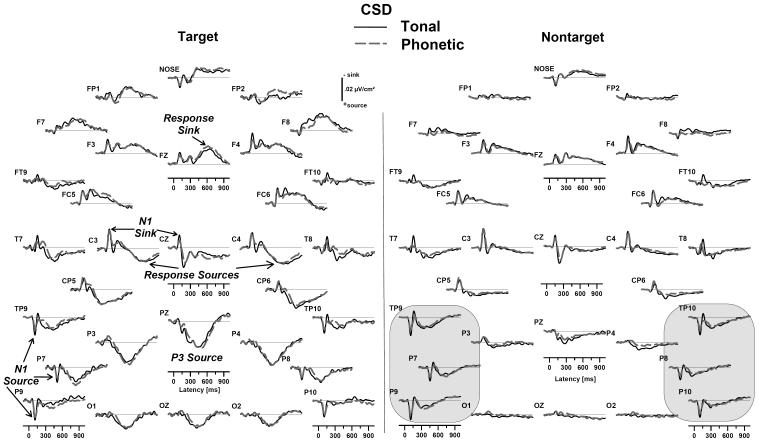
Fig. 3.
Grand mean surface Laplacian waveforms (source down) for tonal (solid black) and phonetic (dashed gray) dichotic oddball tasks (averaged across groups) indicate the (reference-independent) radial current density underlying the ERP component structure, which was highly comparable for both tasks. For targets (left panel), the topography of N1 was confined to distinct sink and source regions, consistent with generators in the Sylvian fissure, and the P3 source was maximal at the parietal midline, broadly distributed, and separable from later response-related sources. For nontargets (right panel), CSD waveforms had a noticeably greater amplitude of the “temporal N1” sink for the tonal task (solid black lines at electrodes T7/8). Although identifiable at the parietal midline (Pz), the nontarget source corresponding to P3 was most prominent at the same lateral sites where the N1 source was found (circled), including mastoid (TP9/10), posterior temporal (P7/8), and adjoining inferior temporal lobe regions (P9/10).
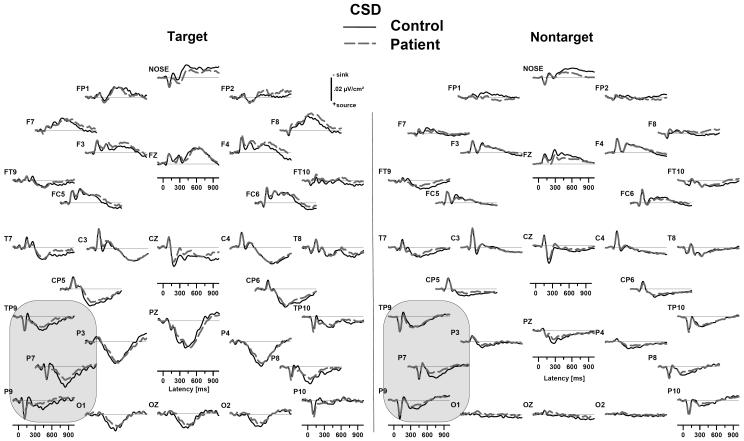
Fig. 4.
Grand mean surface Laplacian waveforms (source down) for healthy controls (solid black) and depressed patients (dashed gray), averaged across tonal and phonetic dichotic oddball tasks. Compared to controls, patients showed P3 source reductions for both targets and nontargets that were greatest over the left hemisphere at lateral sites where the N1 source was found (circled).
CSD factor scores were evaluated using repeated measures ANOVA (BMDP-4V; Dixon, 1992; MANOVA, SPSS 14.0), with Task (tonal/phonetic), Condition (nontarget/target), Hemisphere (right/left) and electrode Site as within-subject parameters (selection of electrodes varies across factors; Greenhouse-Geisser correction applied when appropriate). Group (control/depressed) and Gender (male/female) were used as a between-subjects factors. As with the performance data, gender was used only as a control factor and will not be considered further. Significant interactions were evaluated by analysis of simple effects.
3. Results
3.1 Behavioral performance
Target detection (percent correct hits) was poorer for patients than controls (Group: F[1,60] = 9.58, p = .003), and for the tonal than the phonetic task (Task: F[1,60] = 8.16, p < .01; Mean % correct ± SD, tone: control = 89.1 ± 14.9, patient = 75.8 ± 21.6; phoneme: control = 94.7 ± 6.9, patient = 83.8 ± 20.0), but there was no Task × Group interaction (F[1,60] < 1.0). Although mean reaction time was faster for the phonetic than the tonal task (Task: F[1,60] = 3.90, p = .05), it did not significantly differ across Groups (F[1,60] = 1.67, p > .1), despite a nonsignificant Group × Task trend (F[1,60] = 3.70, p > .05; Mean latency [ms] ± SD, tonal: control = 580 ± 120, patient = 630 ± 100; phonetic: control = 570 ± 90, patient = 580 ± 110). Likewise, correct rejection of nontargets was slightly better for the phonetic than the tonal task (Task: F[1,60] = 9.62, p < .005) but did not differ significantly between groups (F[1,60] < 1.0; tonal: control = 96.4 ± 5.2, patient = 97.2 ± 4.2; phonetic: control = 98.5 ± 2.1; patient = 98.5 ± 2.9).
Dichotic oddball LQs were variable, and were uncorrelated with LQs produced by standard dichotic tests (Complex Tone Test, r = -.06; Consonant-Vowel test, r = -.14). Although a phonetic-greater-than-tonal LQ difference was observed between tasks (F[1,60] = 4.55, p < .05), there was no difference between groups. These observations that were preserved after the exclusion of five outliers (all patients) with LQ>50 on one or both tasks. Surprisingly, controls showed no evidence of a perceptual asymmetry for phonemes (LQ = -.3 ± 5.5), and only a weak left-ear advantage for tones (LQ = -2.4 ± 4.8). The expected right-ear advantage for the phonetic task was observed for patients (LQ =12.5 ± 32.8), but was also seen for the tonal task (LQ = 3.7 ± 29.4), owing solely to the outliers. For these reasons, LQ will not be considered further in this report.
3.2 ERP averages and CSD
As shown in Fig. 2, nose-referenced ERP averages were quite similar for tonal and phonetic nontargets, including N1, P2 and N2 components at frontocentral sites, followed by posterior P3. The topography of P2 extended further posterior for tonal than phonetic stimuli (e.g., Pz). Targets yielded an enhanced N2, which was larger over the left hemisphere in the phonetic task, particularly at lateral sites (e.g., sites T7, CP5 vs. T8, CP6). Targets also produced a prominent P3 at the posterior midline (i.e., P3b)
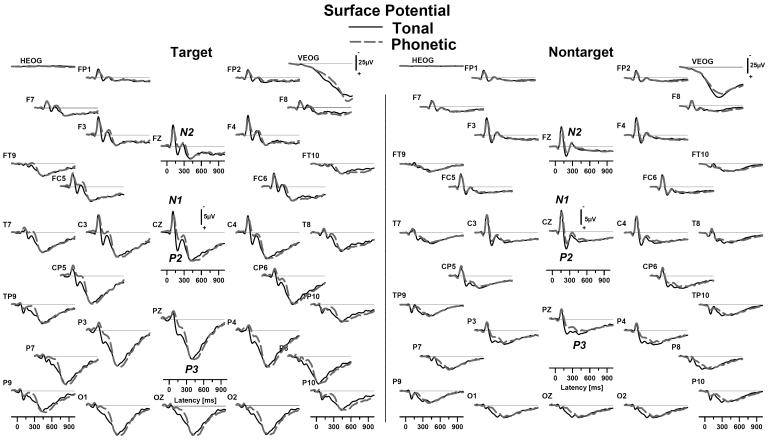
Fig 2.
Grand mean nose-referenced ERP waveforms (positive down) at each electrode for tonal (solid black) and phonetic (dashed gray) dichotic oddball tasks (averaged across groups). Waveforms for correctly identified targets (left panel) show the expected component structure for both tasks, including N1, P2, N2 and P3 (component labels in italics). Nontarget (right panel) waveforms in both tasks also showed a prominent N1/P2 complex, followed by an identifiable N2 and a shallow, broadly distributed posterior P3. Note that eye activity (HEOG, VEOG) is shown at a reduced scale.
As indicated in Fig. 3, these sharpened CSD topographies clearly represented N1 as a sink/source pair (i.e., dipole) surrounding the Sylvian fissure (i.e., primary auditory cortex on the superior temporal plane). Likewise, the peak of the P3 source was better localized to, but not restricted to, medial parietal regions, extending in lateral and anterior directions into temporal lobe sites (e.g., mastoids at TP9/10). Moreover, at sites inferior and posterior to the superior temporal plane (i.e., N1 source regions; circled regions in right panel of Fig. 3), a lateral P3 source was evident for targets and nontargets, and was larger over the left than right hemisphere. Fig. 4 shows grand average target and nontarget CSD waveforms for patients and controls, highlighting the prominent group differences at these same lateral and inferior sites (tonal and phonetic CSD averages for the two groups are available as supplementary Figs. S3-4 at http://psychophysiology.cpmc.columbia.edu/dico.htm).
3.3 Overview of target/nontarget temporal CSD-PCA solution
Factor loadings waveforms, along with their corresponding factor score topographies, are shown in Fig. 5 for the first eight CSD factors extracted from the dichotic oddball data. Table 1 directly compares these factors with those obtained for a binaural oddball task (Kayser and Tenke, 2006a; identical factors were observed for a 129-channel dense electrode array, cf. Kayser and Tenke, 2006b), based on peak latency and percent of explained variance. For example, one factor was identifiable as the N1 sink, based on its characteristic latency and sink topography (factor 105, Fig. 5). Although the ensuing characteristically endogenous components were delayed for the more difficult dichotic compared to the binaural oddball (e.g., N2 sink at 245 rather than 215 ms; P3 source at 440 rather than at 355 ms), they were also unambiguously identifiable by their temporal sequence, topography, and condition-dependence. A previously identified response-related factor, identifiable from its characteristic midline frontal sink coupled to a centroparietal source (denoted as F-CP+), was also delayed (620 rather than 560 ms). Note that the last factor (885) describes late (i.e., slow) variance per se, and may not be attributable to the condition-dependent ERP component identified as “slow wave.”
Fig. 5.
(A) Factor loadings waveforms for the first eight (by variance) Varimax-rotated PCA factors, identified by peak latency, and (B) corresponding factor score topographies for each task (averaged across groups and condition). The factor structure was consistent with that reported by Kayser and Tenke (2006a, 2006b), and included: a centrally-oriented N1 sink/source topography; a temporal N1 for the tonal task; N2, with a differential topography between tasks (frontal for tonal; left sided lateral asymmetry for phonetic); P3 source (440 ms) with a midparietal topography; and a late midline frontal sink, paired with a centroparietal source (F-CP+). In addition to the expected, but subtle, task-related asymmetries of the P3 source (greatest amplitude region extended from the midline to the right hemisphere for the tonal but to the left hemisphere for the phonetic task), the P3 source topography extended more prominently over the left than the right hemisphere for both tasks.
Table 1.
Comparison of Varimax-rotated CSD-PCA compoments observed during dichotic and binaural oddball tasks (initial factors extracted)
| Dichotic Oddball1 | Binaural Oddball2 | Previous Interpretation3 | ||
|---|---|---|---|---|
| Peak Latency [ms] | Explained Variance | Peak Latency [ms] | Explained Variance | |
| 105 | 4.8% | 105 | 4.5% | N1- |
| 150 | 2.3% | 160 | 3.9% | Temporal lobe N1- |
| 195 | 3.4% | (not observed) | P2+ | |
| 245 | 3.6% | 215 | 5.3% | N2- |
| 295 | 1.5% | 270 | 2.3% | (not interpreted) |
| 440 | 30.3% | 355 | 23.0% | P3+ |
| 620 | 14.9% | 560 | 24.0% | F-CP+ |
| 885 | 26.3% | 920 | 25.6% | SW+ |
Solution of current study
Solution of Kayser and Tenke (2006a)
Identifying sink (-) and source (+) activity
For the first eight factors, the only factor not observed in the binaural oddball task was factor 195, which represented a distinct midline P2 source waveform that was fused with N2 in the less challenging binaural oddball. As a further deviation to the binaural oddball, the topography of factor 440, corresponding to the P3 source, extended laterally from a maximum at the parietal midline into temporal lobe sites. This secondary, temporal lobe topography was left-lateralized for both tonal and phonetic tasks.
3.4 Factor 105 (N1 sink)
Repeated measures ANOVA of the C3/4 sink revealed a significant Hemisphere effect (F[1,60] = 6.95, p = .01), with larger sink amplitude over left than right hemisphere (Fig. 5b, left column). Although a prominent Condition effect was observed (F[1,60] = 13.0, p < .001), indicating larger sink for nontargets than for targets, there were no Task (F[1,60] = 1.40,p < .2) or Group effects (F[1,60] < 1.0).
3.5 Factor 245 (N2 sink)
The frontal N2 sink was larger for targets than nontargets (Condition: F[1,60] = 31.5, p < .001), larger for the tonal than the phonetic task (F[1,60] = 50.0, p < .001), and was marginally reduced in patients (Group: F[1,60] = 3.84, p = .05). The frontal sink was also asymmetric, being larger over the left hemisphere (Hemisphere: F[1,60] = 5.55, p < .05), particularly for the tonal task (Task × Hemisphere: F[1,60] = 5.80, p < .05; analysis of simple Hemisphere effects significant for phonemes, F[1,60] = 15.7, p < .001, but not for tones, F[1,60] < 1.0). The posterolateral N2 sink also showed a condition-dependency (F[1,60] = 56.0, p < .001), but was largest for phonemes (F[1,60] = 29.2, p < .001).
3.6 Factor 440 (P3 source)
3.6.1 Complete posterior topography
The complete posterior ANOVA of the P3 source showed a significant overall asymmetry, being largest over the left hemisphere (Hemisphere: F[1,60] = 4.22, p < .05; preserved using hit rates as covariates). The hemispheric asymmetry differed strongly between tasks (Task × Hemisphere: F[1,60] = 26.7, p < .001). Analysis of simple effects showed a prominent Hemisphere effect for the phonetic (F[1,60] = 16.6, p < 0.001), but not the tonal task (F[1,60] < 1.0). However, simple Task effects were prominent for the left (F[1,60] = 8.46, p = .005) but not the right hemisphere (F[1,60] = 3.74, p < .06). Moreover, the Task × Hemisphere interaction was eliminated when hit rates were used as covariates (F[1,60] < 1.0).
The condition-dependency of P3 was verified using the complete posterior ANOVA (Condition: F[1,60] = 125.3, p < .001; Condition × Site: F[5, 300] = 64.7, epsilon = .56, p < .001), and simple Condition effects were observed at most sites (at TP9/10, F[1,60] = 1.63, p > .1; at P9/10, F[1,60] = 4.23, p < .05; at all other sites, F[1,60] > 60.0, p < .001). Although target-dependent P3 showed a task-related asymmetry (Task × Condition × Hemisphere: F[1,60] = 6.20, p < .05), the asymmetry was evident for targets (Task × Hemisphere: F[1,60] = 20.8, p < .001) as well as nontargets (F[1,60] = 11.5, p < .005).
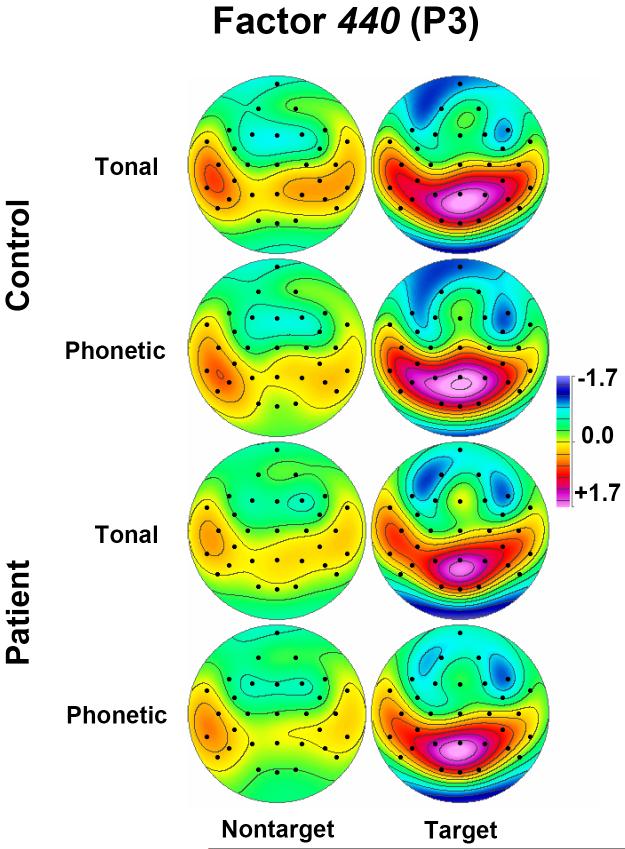
Fig. 6 details factor 440 score topographies for patients and controls.3 It is evident that the P3 source was widely distributed for targets in each task, and that prominent differences between tasks (i.e., left-lateralized asymmetry more prominent for the phonetic than tonal task) and groups (larger source for controls than patients) existed in lateral and posterior regions for nontargets as well as targets. This P3 source reduction in patients was statistically supported by the complete posterior ANOVA (F[1,60] = 7.06, p = .01), as well as for subanalyses restricted either to targets (F[1,60] = 5.11, p < .05) or nontargets (F[1,60] = 8.08, p < .01). However, when hit rates were included as covariates, the difference between groups was only preserved for nontargets (F[1,58] = 4.70, p < .05), but not for targets (F[1,58] = 2.31, p > .1).
Fig 6.
Factor score topographies of factor 440 (P3) for healthy adults (top rows) and depressed patients (bottom rows), comparing nontargets (left columns) and targets (right columns) for tonal and phonetic tasks. P3 source reductions in patients were evident at lateral sites for both targets and nontargets.
3.6.2 Correspondence between P3 source and performance
Although the use of performance measures as covariates did not eliminate nontarget P3 source reductions in patients, they did account for much of the reduction for targets. Moreover, the association between poorer target detection and P3 reductions would be expected to be clearest at medial parietal sites characteristic of P3b (i.e., P3, Pz, P4), but not necessarily at more lateral sites (P7/8; cf. Figs. 3 and 4). Consistently, the average hit rate across tasks and groups was significantly correlated with P3 source amplitudes to targets at medial sites (r = .34, p < .01; control r=.42, p<..05; patient r=.31; p>.05), but not at lateral sites (r = .21, p > .1). Nontarget sources at medial sites were also uncorrelated with performance (r = .11, p > .4). However, a significant correlation was observed between hit rates for targets and nontarget source amplitudes at lateral sites (r = .26, p < .05). This correlation did not attain significance for patients (r = .22, p>.05), and was absent in controls (r=.05), and was eliminated when poor performers(<75%) were excluded. When source activity for the same medial parietal or lateral sites was submitted to an ANOVA (Group and Gender between-subjects factors), a significant Group effect was obtained only for nontargets at lateral sites (F[1,60] = 8.06, p < .01), but not for targets at medial sites (F[1,60] < 1.0). This difference between groups for nontargets was preserved when hit rates in both tasks were used as covariates (F[1,58] = 4.81, p < .05) or when poor performers (<75%) were excluded (F[1,46]=5.24, p<.05).
3.6.3 Limitations of temporal PCA for nontargets
Because the lateral P3 source topography was present for both targets and nontargets, and group differences were observed at lateral sites for nontargets, a secondary PCA was conducted using only nontarget CSD waveforms. Although it yielded a P3 factor (22.6% variance) with a loadings peak at 320 ms and factor score topographies resembling those for nontargets in the target/nontarget PCA, the presence of a prominent secondary loadings peak (i.e., greater than half the amplitude of the primary peak) corresponding to N1 made it impossible to confidently disentangle these two components.
3.6.4 Hemispatial PCA
A spatiotemporal PCA approach was used to disentangle N1 from late source activity at temporal lobe sites. Fig. 7a shows the first six hemispatial PCA factors, ordered by explained variance. Due to the sharpened CSD topographies, five factors identified individual electrode sites (FP1/2, F7/8, T7/8, Oz, Pz). Two of these factors occupied posterior midline sites (Oz and Pz), and were associated with averaged factor score waveforms with target-related peaks corresponding to the midline P3 source; they were not considered further, because both sites are distant from the lateral P3 topography of interest (i.e., lateral parietal and temporal lobe sites). Although a factor representing anterior temporal lobe regions (T7/8) yielded factor score waveforms that included both the temporal lobe N1 sink (150 ms in the tonal task) and variable P3 peaks at 325-460 ms (across hemispheres, tasks and conditions), these sites were anterior to those identified as regions of interest (i.e., TP9/10, P7/8, P9/10; circled regions in Figs. 3 and 4).
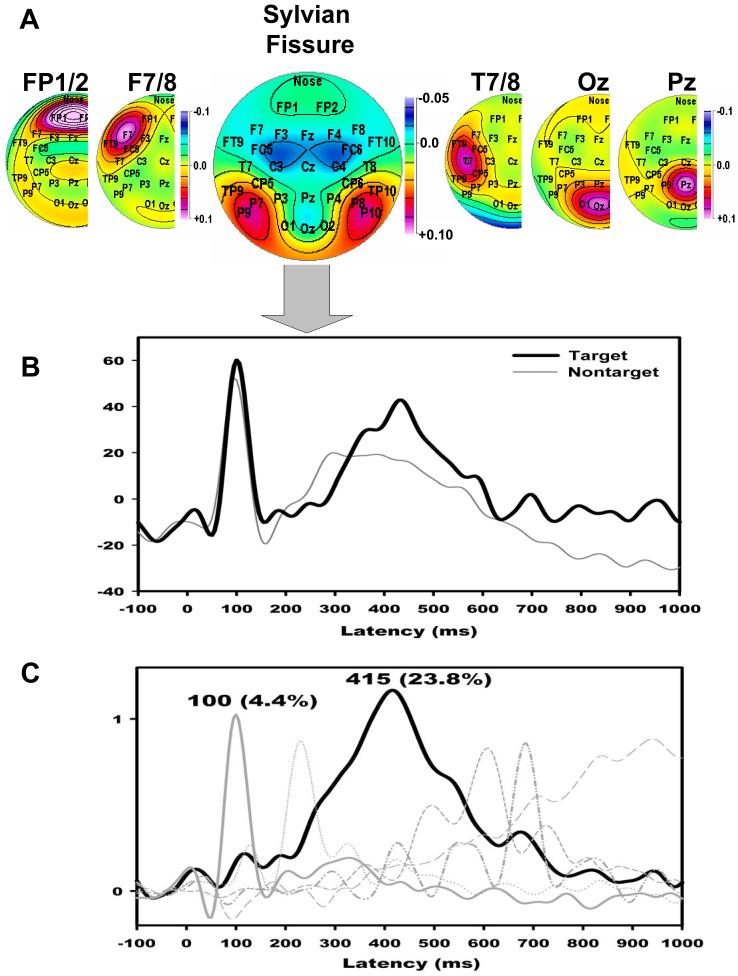
Fig. 7.
(A) Factor loadings topographies for the first six hemispatial CSD-PCA factors (by variance). CSD hemiscalp topographies are shown for left hemisphere only, except for factor 3, which is mirrored at the midline. Factor labels reflect maximal loadings site (e.g., electrodes FP1/2). The third factor is labelled Sylvian Fissure because of prominent loadings at sites where both a P3 source and N1 source/sink were observed. (B) Mean factor score waveforms corresponding to the Sylvian Fissure factor comparing targets (black) and nontargets (gray). (C) A subsequent temporal PCA restricted to the Sylvian Fissure factor score waveforms separated a temporal lobe P3 factor (415) from an N1 factor (100).
The loadings topography of the third hemispatial factor (8.9% explained variance) focused on multiple electrode sites of interest (i.e., P7/8, P9/10), and concisely represented the inversion of the N1 sink across the Sylvian fissure. Moreover, the averaged factor score waveforms for this factor included both N1 and P3 (Fig. 7b), thereby uniquely associating it with the spatial covariation of N1 and P3 observed in the nontarget PCA. Factor score waveforms for the third hemispatial factor were submitted to a covariance-based temporal PCA (221 variables = time points (-100 to 1000 ms); 512 observations = 2 Hemispheres × 2 Conditions × 2 Tasks × 64 Subjects) and subsequent Varimax rotation. As shown in Fig. 7c, this resulted in separate temporal factors corresponding to P3 (415 ms peak latency, first factor extracted, 23.8% explained variance) and N1 (100 ms, sixth factor, 4.4%).
A repeated measures ANOVA conducted for factor 415 (temporal lobe P3 source), using Task, Condition and Hemisphere as within-subject factors, indicated greater source activity for the phonetic than the tonal task (Task: F[1,60] = 10.4, p < .001), with greater source activity over the left than the right hemisphere (Hemisphere: F[1,60] = 5.65, p < .05), particularly for phonemes (Task × Hemisphere: F[1,60] = 17.1, p < .001). Simple Hemisphere effects revealed an asymmetry for the phonetic (F[1,60] = 14.7, p < .001) but not the tonal task (F[1,60] < 1.0). Moreover, simple Task effects were only observed for the left (F[1,60] = 23.7, p < .001) but not right hemisphere (F[1,60] < 1.0). The expected Condition effect was observed (F[1,60] = 9.59, p < .005), with greater source for targets than nontargets. A Task × Condition × Hemisphere interaction (F1,60] = 5.70, p < .05) originated from a symmetric source for targets, but an asymmetric source for nontargets, for the tonal task only (simple Condition × Hemisphere interaction for Tonal: F[1,60] = 3.04, p < .1; for Phonetic: F[1,60] < 1.0). Finally, a Group main effect (F[1,60] = 6.29, p < .05) confirmed a smaller temporal lobe P3 source for patients compared to controls, which was preserved when hit rates were included as covariates (F[1,58] = 4.21, p < .05).
3.7 Factor 620 (Midline frontal sink/centroparietal source)
A distinct, response-related factor was separable from factor 440, with a characteristic topography consisting of a sharply localized midline frontal sink and bilateral centroparietal sources (F-CP+; Kayser and Tenke, 2006a). The Fz sink for factor 620 showed the expected Condition effect (F[1,60] = 84.5, p < .001; larger for targets), as well as a Task × Condition × Group interaction (F[1,60] = 7.60, p < .01),4 stemming from controls (simple Task × Condition effect, F[1,60] = 7.28, p < .01; target sink larger in phonetic than tonal task), but not patients (F[1,60] = 1.09). The corresponding centroparietal source also showed a Condition effect (F[1,60] = 74.8, p < .001), a Task × Condition interaction that did not interact with Group (F[1,60] = 5.57, p < .05, greater target source for the phonetic than the tonal task), and was larger over the right than the left hemisphere (Condition × Hemisphere: F[1,60] = 3.98, p = .05; Hemisphere: F[1,60] = 7.62, p < .01).
4. Discussion
The dichotic oddball task places the perceptual and cognitive processing demands of dichotic listening tasks in the context of an oddball paradigm. The resulting CSD factor structure was closely comparable to that found for binaural oddball tasks that also use tonal and phonetic stimuli (Kayser and Tenke, 2006a,b). Notably, activity corresponding to P3 (factor 440), was dissociable from a later, response-related factor (factor 620), with a characteristic, frontally-inverting, positive slow wave topography (F-CP+), arising from a midline frontal sink that suggests a generator on the dorsal banks of the longitudinal fissure.5 The midline frontal sink is identifiable using conventional window averages (Tenke et al., 1998; local Hjorth and spherical spline methods), and is reminiscent of a spatial CSD-PCA loadings topography yielded by a novelty oddball task (Spencer et al., 1999; scores include multiple temporal components).
Previous studies in depressed patients have shown normal performance levels for binaural oddball tasks and little or no reduction in P3b amplitude (e.g., Bruder et al., 2002). Although dichotic oddball task performance was notably impaired in depressed patients, the corresponding P3 reductions in depression were preserved after performance was accounted for (as a covariate), and did not reflect a simple reduction in P3b at medial sites for targets. To the contrary, the findings suggest a dichotic processing impairment that is evidenced by an additional component: a temporal lobe source that overlaps the time course of P3b and is equally present for targets and nontargets.
4.1 Parietal and temporal contributions to P3
The factor score topography of factor 440 showed the expected maximum for targets at midline parietal sites, but extended laterally and anteriorly into the temporal lobes. This secondary topography was more prominent for nontargets, was larger over the left than the right hemisphere regardless of stimulus content, and most asymmetric for phonetic stimuli. The corresponding factor extracted using only nontarget waveforms peaked somewhat earlier and had a secondary loading on N1, suggesting that N1 generators, particularly within the superior temporal plane (Godey et al., 2001; Simson et al., 1976; Neelon et al., 2006; Vaughan and Ritter, 1970), may be reactivated during the rising phase of P3.6.The association between P3 and N1 was confirmed by hemispatial PCA and disentangled by subsequent temporal PCA (i.e., hemispatiotemporal PCA).
There is considerable evidence for the involvement of auditory cortex in the generation of endogenous ERPs. CSD analysis suggests a mismatch negativity generator in the vicinity of the primary auditory cortex (intracranial monkeys: Javitt et al., 1994; scalp recordings: Giard et al., 1990; Javitt et al., 1994; Rosburg et al., 2005). A putative P3 homolog inverts within the supratemporal plane of the monkey (Arezzo et al., 1975), and the scalp topography of human P3b in an auditory oddball task is consistent with bilateral equivalent dipoles in the posterior part of the superior temporal gyri, with the middle part of the gyrus activated during novelty (Opitz et al., 1999a). The temporoparietal junction has also been implicated as a generator of P3 (Vaughan and Ritter, 1970; Smith et al., 1990), and auditory P3 is sensitive to damage of this region (Knight et al., 1989).
Halgren et al. (1995) summarized their intracranial findings by noting that the superior temporal plane and the supramarginal gyrus are both P3 generators, and could also provide the essential processing required for P3 generation elsewhere. Although ERP morphology and characteristics vary considerably across regions, one recording from the posterior superior temporal gyrus during an auditory directed attention task is of interest because it produced a robust, but inverted, N1 followed by a P3 (cf waveform T32 in Fig. 12, Halgren et al., 1995), resembling the dichotic CSD recorded from lateral sites in the present study (Fig. 3, P7/8). The positivity was comparable for targets and distractors and was largely unchanged when the stimuli were ignored, but was somewhat reduced for nontargets. Collectively, these findings suggest that the dipole-like generator topography summarized by the third hemispatial factor (Fig. 7a) may reflect activation that is largely confined to the posterior superior temporal plane. Moreover, a case could be made for a generator in the planum temporale, which has been implicated in the processing and localization of moving or changing sounds (Barrett and Hall, 2006; Krumbholz et al., 2005), and in the analysis of sounds that are spectrally and temporally complex (Griffiths and Warren, 2002). Structurally, the planum temporale is typically larger in the left than the right hemisphere, and it is continuous with parietal cortex (Honeycutt et al., 2000).
MEG studies have reported greater-left-than-right late (>130 ms) activity in posterior superior temporal gyrus during discrimination of consonant-vowel syllables and tone patterns having variable onset times (Papanicolaou et al., 2003). N400-like MEG activity has also been localized to left primary auditory cortex in an auditory phonological mismatch paradigm (visuo-auditory priming task, Kujala et al., 2004; but also see Opitz et al., 1999b).7 A long latency (175-400 ms), intermodal (McGirk illusion) auditory mismatch negativity waveform has also been reported, having a similar sink/source surface Laplacian topography, and an inverse solution consistent with generators on the superior temporal plane, posterior to primary auditory cortex (Saint-Amour et al., 2007).
4.2 Unique properties of the dichotic oddball task
4.2.1 Cortical processing of tonal and phonetic stimuli
The topographies of N2 and P3 in a binaural oddball task vary according to stimulus type, and are consistent with a left hemisphere dominance for the phonetic stimuli, and right hemisphere dominance for the tonal stimuli (Kayser et al., 1998; Kayser and Tenke, 2006a). A key physical feature in the perception of English phonemes is voice onset time (VOT), which is reflected in the neuronal response of primary auditory cortex (monkey and human, Steinschneider et al., 1995,1999). Specifically, phonemes with short VOTs (e.g., voiced consonant /da/) only yield responses to stimulus onset, while corresponding phonemes with long VOTs (e.g., unvoiced consonant /ta/) yield an additional response to voicing onset for the vowel. Trébuchon-Da Fonseca et al. (2005) observed the second response in Heschl’s gyrus and the planum temporale, but not in anterior auditory fields. From scalp recordings, the same authors reported that the amplitudes of regional dipoles fitted to the medial portion of Heschl’s gyrus (MRI) were larger in the left than the right hemisphere for the second response, and suggested that activation of left auditory cortex reflects processing of temporal acoustic cues, which may underlie hemispheric specialization for language. This raises the possibility that left-sided temporal P3 seen for both the phonetic and tonal dichotic oddball tasks reflects the need for analysis of temporal features of these acoustic stimuli. Moreover, the rhythmic alternation of the consonant-vowel or tonal pairs in the dichotic oddball places additional temporal (contextual) processing demands, which may also contribute to the left-lateralized temporal P3 source.
It is unlikely that differences in VOT directly played a role in the present dichotic oddball findings. There was no evidence of a secondary response for the phonetic compared to the tonal task (Figs. 2-3), and the overall Hemisphere effect for N1 (left > right) was not selective for the phonetic task. Instead, Task-dependent asymmetries were observed for both N2 and P3, and the difference between groups for P3 was unrelated to Task. Another consideration is that the tonal task did not yield the expected left ear advantage, and a left-sided P3 asymmetry was observed for both phonetic and tonal dichotic oddball tasks. These findings do not concur with reports of greater P3 over the right hemisphere in the Complex Tone Test CTT (Tenke et al., 1993a), or of right hemisphere lateralization of musical syntactic violations (Koelsch et al., 2000; Patel et al., 1998), suggesting instead that the detection of discrepancies in an ongoing pattern of dichotic tones may also depend on a predominantly left-hemispheric classification processes, possibly analogous to the evaluation of prosodic context within a sentence.
The stimuli for the tonal dichotic oddball task were the same ones previously used in the CTT (Sidtis, 1981), a dichotic listening task in which a dichotic stimulus pair is compared with a subsequent binaural probe stimulus. In the CTT, both stimuli produced an identifiable P3 (Tenke et al., 1993a), but the dichotic stimulus was followed by a sustained frontal negativity. In the dichotic oddball, where the pattern of nontargets must also be held in memory to perform the task, nontargets yielded both an identifiable posterior P3 source and a prolonged midfrontal sink (positive-going slope from 300 ms to end of epoch at medial frontal sites in right panel of Fig. 3), and the amplitude of the lateral P3 source was correlated with hit rate. Collectively, these findings suggest a possible role for the lateral P3 in updating the context provided by each stimulus and in their retention over the subsequent interstimulus interval
4.2.2 Stimulus sequence
In the dichotic oddball task, nontargets are presented in a rhythmic pattern of alternating dichotic pairs that sets up a context to be retained for comparison with subsequent stimuli. If the spatial orientation of the paired dichotic stimuli is considered (left or right ear), it is clear that no stimulus pair is presented with a frequency of .5 or greater (i.e., no stimulus is frequent). Targets are also nonunique, since they may be any one of four paired stimuli: target on left or right, replacing either one of the alternating pair of standard stimuli. As such, correct performance relies more on the detection of a difference in the ongoing pattern than on target identification per se. Nontargets may also be perceived as being more “target-like,” as evidenced by the uncertainty expressed by some participants about the distinction between targets and nontargets. For some participants, this distinction may involve an implicit spatial judgement (i.e., identification of the mismatched ear), while response hand may also impose a spatial context for a given block of trials.
The present results indicate that the asymmetries identified in the dichotic oddball task reflect left hemispheric processing, regardless of stimulus content (tonal vs. phonetic). The phonetic oddball task yielded a larger P3 source over the left hemisphere than the right, as well as a significantly greater right ear advantage when compared to the tonal task. Moreover, analysis of simple effects uniquely identified the left hemisphere as the origin of the Task × Hemisphere interaction for P3. In contrast, the tonal task showed neither the expected left ear advantage nor a right- greater-than-left hemispheric asymmetry for P3.
4.3 P3 reductions in depression
Although some conflicting P3 findings for depressed patients may be due to differences in task conditions or the difficulty of comparing reference-dependent ERP topographies (Kayser and Tenke, 2006a), it has also been argued that the binaural oddball task may be too simple to consistently reveal the underlying cognitive dysfunctions in depression. More difficult dichotic listening tasks provide an appropriate cognitive load (Bruder et al., 1995; Tenke et al., 1993a), but they have the disadvantages of relying on a large number of unique stimuli, as well as a trial structure that yields ERP components with only a superficial similarity to the binaural oddball. The dichotic oddball task provides an intermediate solution to this problem. It is sufficiently difficult to reveal cognitive deficits, yet sufficiently comparable to the binaural oddball task to produce the same component structure.
Depressed patients showed P3 source reductions compared to healthy controls, in accordance with performance differences for the two groups. P3 source reductions were observed in both parietal and temporal regions and for targets and nontargets. Although reductions of parietal P3 source in depressed patients were related to their poorer performance, the temporal lobe source reductions did not reflect task performance and were most prominent for nontargets at lateral sites. Collectively, these observations suggest that the temporal lobe P3 source reductions observed for depressed patients are closely related to processing in primary and secondary auditory cortices of the superior temporal plane.
Evidence from neuroimaging studies indicate that primary auditory cortex contributes to early acoustic processing, whereas associative cortex in the superior temporal lobe is involved in higher order phonetic and tonal processing (Zatorre et al., 1992). Dichotic listening (Bruder et al., 1989; Overby et al., 1989), ERP (Bruder et al., 1995; Deldin et al., 2000; Kayser et al., 2000) and neuroimaging (Post et al., 1987) studies have reported evidence of right temporoparietal deficits in depressed patients. Although we found a reduction of the temporal lobe P3 source in depressed patients, it was not lateralized to right hemisphere. The tonal dichotic oddball task did not, however, yield the left ear (right hemisphere) advantage seen in healthy adults for the dichotic complex tone test (Bruder et al., 1995) and therefore it does not appear to probe right hemisphere dominance for tonal processing. Rather, the temporal lobe P3 showed a strong left-sided asymmetry that is presumed to reflect properties unique to the dichotic oddball tasks.
The potential clinical relevance of the temporal lobe P3 reduction in depressed patients deserves comment. In a prior study, we found preliminary evidence that reduced P3 amplitude to complex tones was associated with poorer outcome of treatment with antidepressants (Bruder et al., 1995). Inasmuch as the intensity dependence of auditory N1/P2 may index serotonergic activity in auditory cortex (Hegerl and Juckel, 1993), and may be predictive of subsequent clinical response to a selective serotonin reuptake inhibitor (Page et al., 1994; Mulert et al., 2002; 2007), these measures of temporal lobe activity deserve further study as predictors of treatment response.
Supplementary Material
Fig. S1. The impact of target ear and response hand on P3 factor score topographies, based on a subset of fifteen participants (7 male) with eight or more correct, artifact-free trials in each of the eight target averages. Averaged topographies for tonal and phonetic tasks (left two columns) are shown for each ear, as well as for differences between ears (bottom row). For the tonal task, the posterior source for left ear targets was larger and extended more prominently into the mid-parietal sites in the right hemisphere when compared to right ear targets (red and orange areas of difference map). Conversely, the source amplitude for phonetic targets was smaller for left ear stimuli, with a difference topography showing least source activity over the left hemisphere (blue and green areas of difference map). A repeated measures ANOVA produced a marginal Task × Ear effect (F[1,11] = 4.52, p = .057), evidenced by simple effects at Tonal (larger source for left than right ear; F[1,11] = 4.01, p = .07), but not Phonetic stimuli (F < 1.0).
Fig. S2. All eight target ear-by-response hand P3 target topographies for 8 controls (4 male) and 7 patients (3 male) having eight or more correct, artifact-free trials in each average. The general reduction of the posterior source topography in patients is evident, with notable exception of the parietal midline for right ear responses in the phonetic task for both hands (row 2, cols 4 and 8). However, a statistically reliable Group main effect was not observed in the corresponding posterior ANOVA. Instead, there was a Group × Ear × Hemisphere effect (F[1,11] = 5.7, p <.05; greater over left hemisphere for left targets in controls, but the opposite asymmetry for patients), a robust topographic interaction with Ear (Group × Ear × Site: F[5,55] = 4.21, p<.01, epsilon = .68; smaller sources for right than left targets at lateral sites for patients, but not controls), as well as a higher order Group × Task × Ear × Hand interaction (F[5,55] = 4.21, p < .05). In view of the reduced power available for between-subject effects, and the lack of a gender factor in the ANOVA model, it is would be difficult to justify interpretations of these differences.
Fig. S3. Grand mean surface Laplacian waveforms (source down) for tonal dichotic oddball targets (left panel) and nontargets (right panel) in healthy controls (solid black) and depressed patients (dashed gray). Compared to controls, patients showed P3 source reductions for both targets and nontargets that were greatest over the left hemisphere at lateral sites where the N1 source was found (circled for nontargets).
Fig. S4. Grand mean surface Laplacian waveforms (source down) for phonetic dichotic oddball targets (left panel) and nontargets (right panel) in healthy controls (solid black) and depressed patients (dashed gray). Compared to controls, patients showed P3 source reductions for both targets and nontargets that were greatest over the left hemisphere at lateral sites where the N1 source was found (circled for nontargets).
Acknowledgements
This work was supported in part by grant MH36295 and MH50715 from the National Institute of Mental Health (NIMH). The authors would like to acknowledge the waveform plotting software written by Charles L. Brown, III. We would also like to acknowledge the helpful comments made by two anonymous reviewers.
Footnotes
Target ear and response hand effects were systematically evaluated in a subsample restricted to fifteen participants (8 controls, 4 male; 7 patients, 3 male) who had eight or more correct, artifact-free trials in each of the eight target averages. Response hand had negligible impact on the PCA factor solution (P3 factor loadings peaks were 430 and 420 ms for separate right- and left-hand PCAs). P3 factor score topographies for target ear and response hand (supplementary Fig. S1) and group (Fig. S2) are provided at: http://psychophysiology.cpmc.columbia.edu/dico.htm
In our experience, spatial CSD-PCA factor loadings tend to isolate individual electrodes, rather than meaningful generator regions. We therefore view the hemispatial CSD-PCA merely as an additional tool to help disentangle components that have closely overlapping topographies and time courses.
The observed group main effect was preserved when age differences were eliminated by excluding the nine oldest patients. However, given that the mean age difference between groups was less than 5 years, and that the primary focus of this report was the identification of a temporal lobe ERP generator and its dissociation from concurrent parietal generators, it was concluded that equating for age throughout this report did not outweigh the loss of statistical power resulting from the elimination of subjects.
This effect was preserved when age differences were eliminated by excluding the nine oldest patients.
A bilateral pair of (dipolar) cortical generators within the fissure (e.g., supplementary motor cortex) could produce the necessary field closure to appear as a sharply isolated sink, but it would also produce artifactual field closure sources, displaced bilaterally from the midline (Tenke et al., 1993b; also see p. 2843 of Tenke and Kayser, 2005), which were not observed. By the same geometric reasoning, a deeper midline generator (e.g., anterior cingulate) would be identifiable from direct source projections onto the lateral surface.
This scenario only accounts for the shared properties of the late temporal source and N1 (i.e., temporal, spatial, and spatiotemporal covariation), but not for the complete temporal lobe source topography evident in Figs. 3, 4, 6 and 7.
N400 is paradigmatically linked to a literature that emphasizes reading and visual processing. For this reason, it is difficult to directly compare N400 findings with auditory processes, particularly when language processing is not involved.
References
- Arezzo J, Pickoff A, Vaughan HG., Jr. The sources and intracerebral distribution of auditory evoked potentials in the alert rhesus monkey. Brain Research. 1975;90:57–73. doi: 10.1016/0006-8993(75)90682-4. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Quitkin FM, Stewart JW, Martin C, Voglmaier M, Harrison WM. Cerebral laterality and depression: Differences in perceptual asymmetry among diagnostic subtypes. Journal of Abnormal Psychology. 1989;98:177–186. doi: 10.1037//0021-843x.98.2.177. [DOI] [PubMed] [Google Scholar]
- Barrett DJK, Hall DA. Response preferences for “what” and “where” in human non-primary auditory cortex. NeuroImage. 2006;32:968–977. doi: 10.1016/j.neuroimage.2006.03.050. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Stewart JW, Towey JP, Leite P, Volgmaier M, Quitkin FM. Brain event-related potentials to complex tones in depressed patients: relations to perceptual asymmetry and clinical features. Psychophysiology. 1995;32:373–381. doi: 10.1111/j.1469-8986.1995.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Kayser J, Tenke CE, Leite P, Schneier FR, Stewart JW, Quitkin FM. Cognitive ERPs in depressive and anxiety disorders during tonal and phonetic oddball tasks. Clinical Electroencephalography. 2002;33:119–124. doi: 10.1177/155005940203300308. [DOI] [PubMed] [Google Scholar]
- Deldin PJ, Keller J, Gergen JA, Miller GA. Right-posterior face processing anomaly in depression. Journal of Abnormal Psychology. 2000;109:116–121. doi: 10.1037//0021-843x.109.1.116. [DOI] [PubMed] [Google Scholar]
- Dixon WJ, editor. BMDP Statistical Software Manual to Accompany the 7.0 Software Release. University of California Press; Berkeley, CA: 1992. [Google Scholar]
- Duncan-Johnson CC, Donchin E. On quantifying surprise: the variation of event-related potentials with subjective probability. Psychophysiology. 1977;14:456–467. doi: 10.1111/j.1469-8986.1977.tb01312.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research Department. New York State Psychiatric Institute; New York, NY: 1996. Structured Clinical Interview for DSM-IV Axis-I Disorders-- Non-patient Edition (SCID-NP) [Google Scholar]
- Friedman D. ERPs during continuous recognition memory for words. Biological Psychology. 1990;30:61–87. doi: 10.1016/0301-0511(90)90091-a. [DOI] [PubMed] [Google Scholar]
- Giard MH, Perrin F, Pernier J, Bouchet P. Brain generators implicated in the processing of auditory stimulus deviance: a topographic event-related potential study. Psychophysiology. 1990;27:627–640. doi: 10.1111/j.1469-8986.1990.tb03184.x. [DOI] [PubMed] [Google Scholar]
- Godey B, Schwartz D, de Graaf JB, Chauvel P, Liegeois-Chauvel C. Neuromagnetic source localization of auditory evoked fields and intracerebral evoked potentials: a comparison of data in the same patients. Clinical Neurophysiology. 2001;112:1850–1859. doi: 10.1016/s1388-2457(01)00636-8. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Warren JD. The planum temporale as a computational hub. Trends in Neuroscience. 2002;7:348–353. doi: 10.1016/s0166-2236(02)02191-4. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Liegeois C, Chauvel P, Musolino Intracerebral potentials to rare target and distractor auditory and visual stimuli. I. Superior temporal plane and parietal lobe. Electroencephalography and Clinical Neurophysiology. 1995;94:191–220. doi: 10.1016/0013-4694(94)00259-n. [DOI] [PubMed] [Google Scholar]
- Hegerl U, Juckel G. Intensity dependence of auditory evoked potentials as an indicator of central serontonergic neurotransmission: a new hypothesis. Biological Psychiatry. 1993;33:173–187. doi: 10.1016/0006-3223(93)90137-3. [DOI] [PubMed] [Google Scholar]
- Honeycutt NA, Musick A, Barta PE, Pearlson GD. Measurement of the planum temporale (PT) on magnetic resonance imaging scans: temporal PT alone and with parietal extension. Psychiatry Research. 2000;98:103–116. doi: 10.1016/s0925-4927(00)00043-3. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Steinschneider M, Schroeder CE, Vaughan HG, Jr., Arezzo JC. Detection of stimulus deviance within primate primary auditory cortex: intracortical mechanisms of mismatch negativity (MMN) generation. Brain Research. 1994;667:192–200. doi: 10.1016/0006-8993(94)91496-6. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr. On the neural generators of the P300 component of the event-related potential. Psychophysiology. 1993;30:90–97. doi: 10.1111/j.1469-8986.1993.tb03208.x. [DOI] [PubMed] [Google Scholar]
- Kayser J, Bruder GE, Tenke CE, Stewart JW, Quitkin FM. Event-related potentials (ERPs) to hemifield presentations of emotional stimuli: Differences between depressed patients and healthy adults in P3 amplitude and asymmetry. International Journal of Psychophysiology. 2000;36:211–236. doi: 10.1016/s0167-8760(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Optimizing PCA methodology for ERP component identification and measurement: theoretical rationale and empirical evaluation. Clinical Neurophysiology. 2003;114:2307–2325. doi: 10.1016/s1388-2457(03)00241-4. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Trusting in or breaking with convention: towards a renaissance of principal components analysis in electrophysiology. Clinical Neurophysiology. 2005;116:1747–1753. doi: 10.1016/j.clinph.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clinical Neurophysiology. 2006a;117:348–368. doi: 10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: II. Adequacy of low-density estimates. Clinical Neurophysiology. 2006b;117:369–380. doi: 10.1016/j.clinph.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Bruder GE. Dissociation of brain ERP topographies for tonal and phonetic oddball tasks. Psychophysiology. 1998;35:576–590. doi: 10.1017/s0048577298970214. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Gates NA, Bruder GE. Reference-independent ERP generator patterns of auditory and visual word recognition memory. Psychophysiology. 2007;44 doi: 10.1111/j.1469-8986.2007.00562.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL, Clayworth CC. Contributions of temporal-parietal junction to the human auditory P3. Brain Research. 1989;502:109–116. doi: 10.1016/0006-8993(89)90466-6. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Gunter T, Friederici AD, Schroger E. Brain indices of music processing: “nonmusicians” are musical. Journal of Cognitive Neuroscience. 2000;12:520–541. doi: 10.1162/089892900562183. [DOI] [PubMed] [Google Scholar]
- Krumbholz K, Schonwiesner M, Rubsamen R, Zilles K, Fink GR, von Cramon DY. Hierarchical processing of sound location and motion in the human brainstem and planum temporale. European Journal of Neuroscience. 2005;21:230–238. doi: 10.1111/j.1460-9568.2004.03836.x. [DOI] [PubMed] [Google Scholar]
- Kujala A, Alhoc K, Service E, Ilmoniemi RJ, Connolly JF. Activation in the anterior left auditory cortex associated with phonological analysis of speech input: localization of the phonological mismatch negativity response with MEG. Cognitive Brain Research. 2004;21:106–113. doi: 10.1016/j.cogbrainres.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Laguitton V, Demany L, Semal C, Liegeois-Chauvel C. Pitch perception: a difference between right- and left-handed listeners. Neuropsychologia. 1998;36:201–7. doi: 10.1016/s0028-3932(97)00122-x. [DOI] [PubMed] [Google Scholar]
- Mulert C, Juckel G, Augustin H, Hegerl U. Comparison between the analysis of the loudness dependency of the auditory N1/P2 component with LORETA and dipole source analysis in the prediction of treatment response to the selective serotonin reuptake inhibitor citalopram in major depression. Clinical Neurophysiology. 2002;113:1566–1572. doi: 10.1016/s1388-2457(02)00252-3. [DOI] [PubMed] [Google Scholar]
- Mulert C, Juckel G, Brunnmeier M, Karch S, Leicht G, Mergl R, Möller HJ, Hegerl U, Pogarell O. Prediction of treatment response in major depression: Integration of concepts. Journal of Affective Disorders. 2007;98:215–225. doi: 10.1016/j.jad.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Neelon MF, Williams J, Garell PC. The effects of auditory attention measured from human electrocorticograms. Clinical Neurophysiology. 2006;117:504–521. doi: 10.1016/j.clinph.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric Fields of the Brain: The Neurophysics of EEG. 2nd ed. Oxford University Press; New York: 2006. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Opitz B, Mecklinger A, Friederici AD, von Cramen DY. The functional anatomy of novelty processing: integrating ERP and fMRI results. Cerebral Cortex. 1999a;9:379–391. doi: 10.1093/cercor/9.4.379. [DOI] [PubMed] [Google Scholar]
- Opitz B, Mecklinger A, von Cramen DY, Kruggel F. Combining electrophysiological and hemodynamic measures of the auditory oddball. Psychophysiology. 1999b;36:142–147. doi: 10.1017/s0048577299980848. [DOI] [PubMed] [Google Scholar]
- Overby LA, III, Harris AE, Leck MR. Perceptual asymmetry in schizophrenia and affective disorder: Implications from a right hemisphere task. Neuropsychologia. 1989;27:861–870. doi: 10.1016/0028-3932(89)90009-2. [DOI] [PubMed] [Google Scholar]
- Paige SR, Fitzpatrick DF, Kline JP, Balogh SE, Hendricks SE. Event-related potential amplitude/intensity slopes predict response to antidepressants. Neuropsychobiology. 1994;30:197–201. doi: 10.1159/000119161. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, Castillo E, Breier JI, Davis RN, Simos PG, Diehl RL. Differential brain activation patterns during perception of voice and tone onset time series: A MEG study. NeuroImage. 2003;18:448–459. doi: 10.1016/s1053-8119(02)00020-4. [DOI] [PubMed] [Google Scholar]
- Patel AD, Gibson E, Ratner J, Besson M, Holcomb PJ. Processing syntactic relations in language and music: an event-related potential study. Journal of Cognitive Neuroscience. 1998;10:717–733. doi: 10.1162/089892998563121. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. Corrigenda EEG 02274, Electroencephalography and Clinical Neurophysiology, 1990, 76, 565. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr., Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox N, Nuwer MR. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30:547–558. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Post RM, DeLisi LE, Holcomb HH, Uhde TW, Cohen R, Buchsbaum MS. Glucose utilization in the temporal cortex of affectively ill patients: Positron emission tomography. Biological Psychiatry. 1987;22:545–553. doi: 10.1016/0006-3223(87)90182-x. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Eichele T, Hugdahl K. The effect of voice-onset-time on dichotic listening with consonant-vowel syllables. Neuropsychologia. 2006;44:191–6. doi: 10.1016/j.neuropsychologia.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Rosburg T, Trautner P, Dietl T, Korzyukov OA, Boutros NN, Schaller C, Elger CE, Kurthen M. Subdural recordings of the mismatch negativity (MMN) in patients with focal epilepsy. Brain. 2005;128:819–828. doi: 10.1093/brain/awh442. [DOI] [PubMed] [Google Scholar]
- Roth WT, Duncan CC, Pfefferbaum A, Timsit-Berthier M. Applications of cognitive ERPs in psychiatric patients. In: McCallum WC, Zapppoli R, Denoth F, editors. Cerebral Psychophysiology: Studies in Event-Related Potentials. EEG Suppl 38. Elsevier; Amsterdam: 1986. pp. 419–438. [PubMed] [Google Scholar]
- Saint-Amour D, De Sanctis P, Molholm S, Ritter W, Foxe JJ. Seeing voices: high-density electrical mapping and source-analysis of the multisensory mismatch negativity evoked during the McGurk illusion. Neuropsychologia. 2007;45:587–97. doi: 10.1016/j.neuropsychologia.2006.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ. The complex tone test: implications for the assessment of auditory laterality effects. Neuropsychologia. 1981;19:103–112. doi: 10.1016/0028-3932(81)90050-6. [DOI] [PubMed] [Google Scholar]
- Simson R, Vaughan HG, Ritter W. The scalp topography of potentials associated with missing visual or auditory stimuli. Electroencephalography and Clinical Neurophysiology. 1976;40:33–42. doi: 10.1016/0013-4694(76)90177-2. [DOI] [PubMed] [Google Scholar]
- Smith ME, Halgren E, Sokolik M, Baudena P, Musolino A, Liegeois-Chauvel C, Chauvel P. The intracranial topography of the P3 event-related potential elicited during auditory oddball. Electroencephalography and Clinical Neurophysiology. 1990;76:235–248. doi: 10.1016/0013-4694(90)90018-f. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38:343–358. [PubMed] [Google Scholar]
- Spencer KM, Wijesinghe1 RS, Dien J, Donchin E. Prefrontal activity in oddball paradigms as measured by scalp current density. Psychophysiology. 1999;36:S111. [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalography and Clinical Neurophysiology. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Steinschneider M, Schroeder CE, Arezzo JC, Vaughan HG., Jr. Physiologic correlates of the voice onset time boundary in primate auditory cortex (A1) of the awake monkey: temporal response patterns. Brain and Language. 1995;48:326–340. doi: 10.1006/brln.1995.1015. [DOI] [PubMed] [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Sutton S, Ruchkin DS. The late positive complex: advances and new problems. Annals of the New York Academy of Sciences. 1984;425:1–23. doi: 10.1111/j.1749-6632.1984.tb23520.x. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. A convenient method for detecting electrolyte bridges in multichannel EEG and ERP recordings. Clinical Neurophysiology. 2001;112:545–550. doi: 10.1016/s1388-2457(00)00553-8. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. Reference-free quantification of EEG spectra: combining current source density (CSD) and frequency principal components analysis (fPCA) Clinical Neurophysiology. 2005;116:2826–2846. doi: 10.1016/j.clinph.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Bruder GE, Towey J, Leite P, Sidtis JJ. Correspondence between ERP and behavioral asymmetries in a dichotic complex tone test. Psychophysiology. 1993a;30:62–70. doi: 10.1111/j.1469-8986.1993.tb03205.x. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Schroeder CE, Arezzo JC, Vaughan HG., Jr. Interpretation of high-resolution current source density profiles: a simulation of sublaminar contributions to the visual evoked potential. Experimental Brain Research. 1993b;94:183–192. doi: 10.1007/BF00230286. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, Fong R, Leite P, Towey JP, Bruder GE. Response- and stimulus-related ERP asymmetries in a tonal oddball task: a Laplacian analysis. Brain Topography. 1998;10:201–10. doi: 10.1023/a:1022261226370. [DOI] [PubMed] [Google Scholar]
- Trébuchon-Da Fonseca A, Giraud K, Badier JM, Chauvel P, Liégeois-Chauvel C. Hemispheric lateralization of voice onset time (VOT) comparison between depth and scalp EEG recordings. NeuroImage. 2005;27:1–14. doi: 10.1016/j.neuroimage.2004.12.064. [DOI] [PubMed] [Google Scholar]
- Vaughan HG, Jr., Ritter W. The sources of auditory evoked responses recorded from the human scalp. Electroencephalography and Clinical Neurophysiology. 1970;28:360–367. doi: 10.1016/0013-4694(70)90228-2. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E, Gjedde A. Lateralization of phonetic and pitch discrimination in speech processing. Science. 1992;256:846–849. doi: 10.1126/science.1589767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The impact of target ear and response hand on P3 factor score topographies, based on a subset of fifteen participants (7 male) with eight or more correct, artifact-free trials in each of the eight target averages. Averaged topographies for tonal and phonetic tasks (left two columns) are shown for each ear, as well as for differences between ears (bottom row). For the tonal task, the posterior source for left ear targets was larger and extended more prominently into the mid-parietal sites in the right hemisphere when compared to right ear targets (red and orange areas of difference map). Conversely, the source amplitude for phonetic targets was smaller for left ear stimuli, with a difference topography showing least source activity over the left hemisphere (blue and green areas of difference map). A repeated measures ANOVA produced a marginal Task × Ear effect (F[1,11] = 4.52, p = .057), evidenced by simple effects at Tonal (larger source for left than right ear; F[1,11] = 4.01, p = .07), but not Phonetic stimuli (F < 1.0).
Fig. S2. All eight target ear-by-response hand P3 target topographies for 8 controls (4 male) and 7 patients (3 male) having eight or more correct, artifact-free trials in each average. The general reduction of the posterior source topography in patients is evident, with notable exception of the parietal midline for right ear responses in the phonetic task for both hands (row 2, cols 4 and 8). However, a statistically reliable Group main effect was not observed in the corresponding posterior ANOVA. Instead, there was a Group × Ear × Hemisphere effect (F[1,11] = 5.7, p <.05; greater over left hemisphere for left targets in controls, but the opposite asymmetry for patients), a robust topographic interaction with Ear (Group × Ear × Site: F[5,55] = 4.21, p<.01, epsilon = .68; smaller sources for right than left targets at lateral sites for patients, but not controls), as well as a higher order Group × Task × Ear × Hand interaction (F[5,55] = 4.21, p < .05). In view of the reduced power available for between-subject effects, and the lack of a gender factor in the ANOVA model, it is would be difficult to justify interpretations of these differences.
Fig. S3. Grand mean surface Laplacian waveforms (source down) for tonal dichotic oddball targets (left panel) and nontargets (right panel) in healthy controls (solid black) and depressed patients (dashed gray). Compared to controls, patients showed P3 source reductions for both targets and nontargets that were greatest over the left hemisphere at lateral sites where the N1 source was found (circled for nontargets).
Fig. S4. Grand mean surface Laplacian waveforms (source down) for phonetic dichotic oddball targets (left panel) and nontargets (right panel) in healthy controls (solid black) and depressed patients (dashed gray). Compared to controls, patients showed P3 source reductions for both targets and nontargets that were greatest over the left hemisphere at lateral sites where the N1 source was found (circled for nontargets).