Abstract
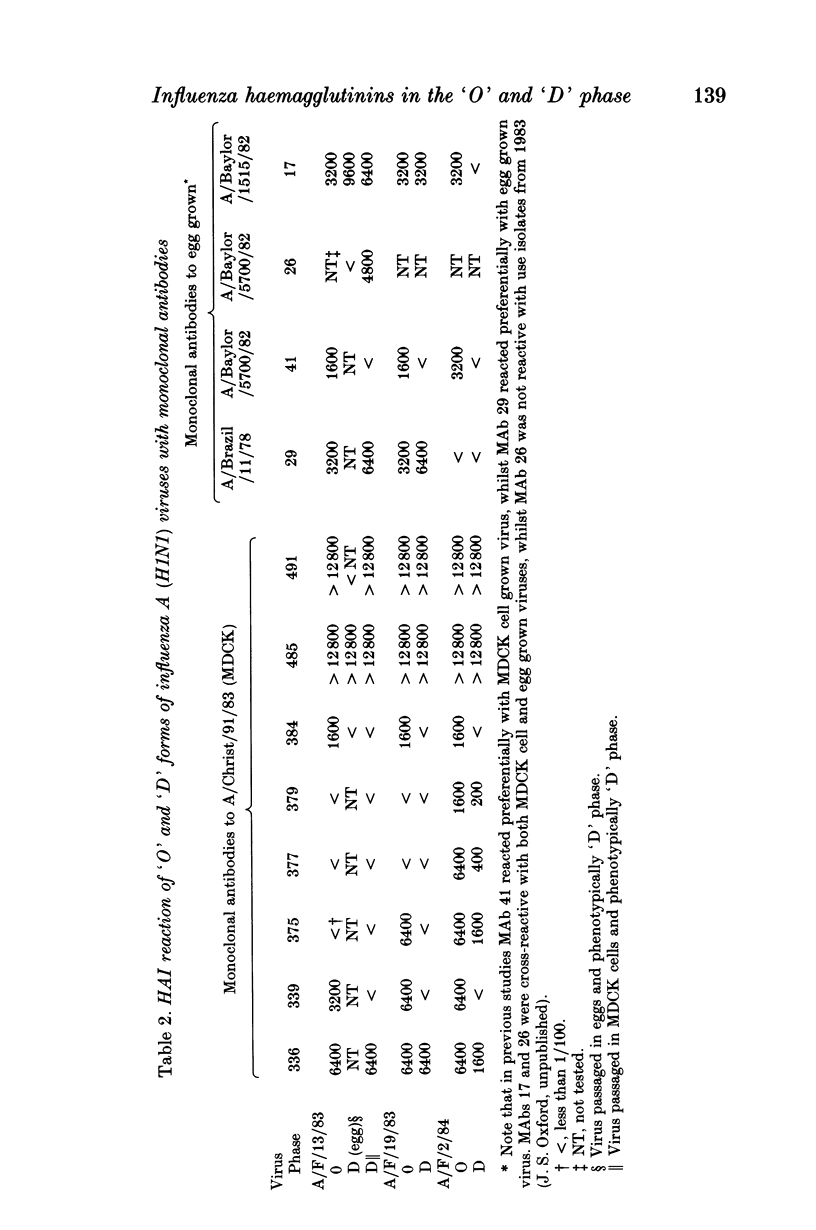
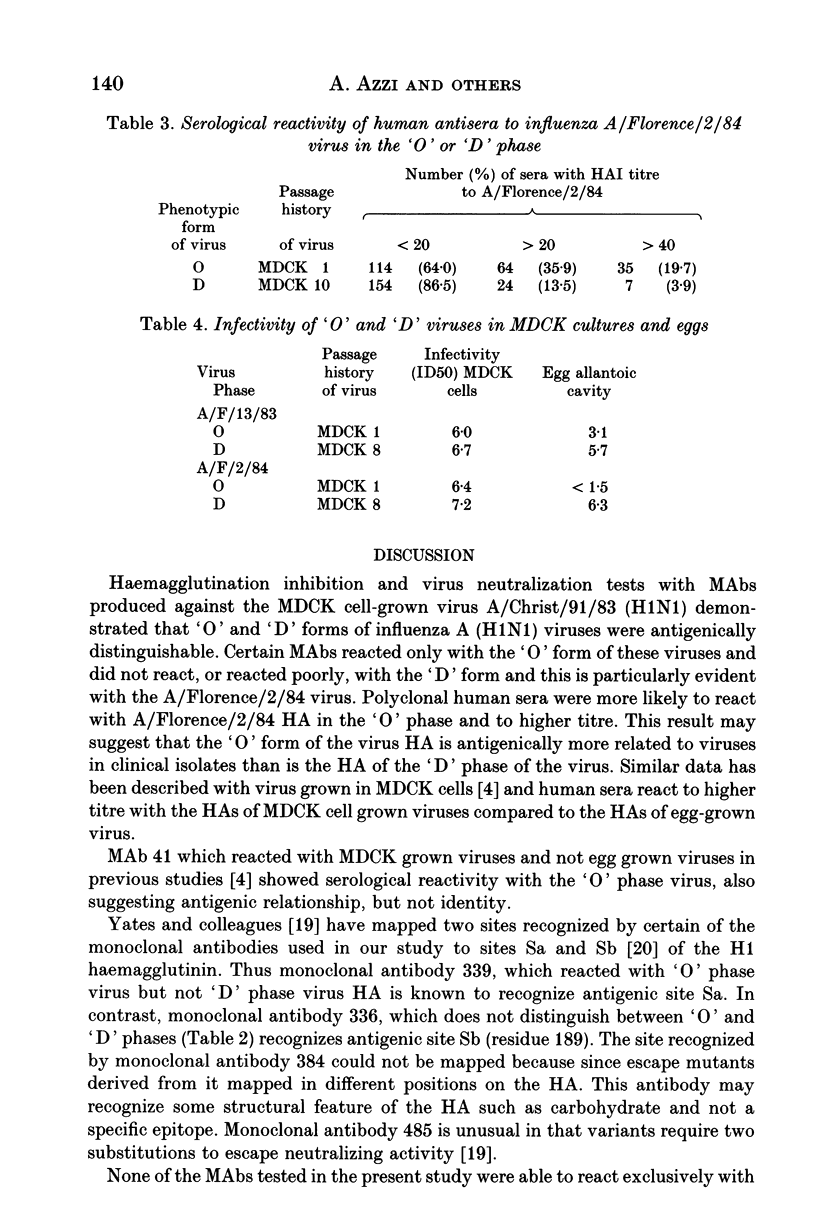
Influenza A (H1N1) viruses when initially isolated in mammalian cell cultures (MDCK cells) had different agglutination reactions with chicken and guinea-pig erythrocytes compared to the same viruses after passage. On first isolation the virus HA resembled the 'O' phase viruses described originally by Burnet and Bull and agglutinated mammalian but not avian erythrocytes. After passage, the virus HA resembled a classical 'D' phase virus and agglutinated both avian and mammalian erythrocytes. Monoclonal and polyclonal antisera detected antigenic differences between the HAs of the viruses in the 'O' and 'D' phases. The 'O' phase virus HA reacted preferentially with antibodies in post infection human antisera. Viruses in the 'O' phase replicated poorly in the allantoic cavity of embryonated hens' eggs whilst 'D' phase virus replicated in both MDCK cells and in embryonated hens' eggs. At least three distinguishable subpopulations of influenza A (H1N1) viruses may co-exist in clinical throat swab material, including viruses possessing HAs in the 'O' and 'D' phases and other 'D' phase viruses cultivable in embryonated hens' eggs but antigenically distinguishable from the corresponding 'D' phase virus in MDCK cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aytay S., Schulze I. T. Single amino acid substitutions in the hemagglutinin can alter the host range and receptor binding properties of H1 strains of influenza A virus. J Virol. 1991 Jun;65(6):3022–3028. doi: 10.1128/jvi.65.6.3022-3028.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton A. J., Brownlee G. G., Yewdell J. W., Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell. 1982 Dec;31(2 Pt 1):417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- Katz J. M., Naeve C. W., Webster R. G. Host cell-mediated variation in H3N2 influenza viruses. Virology. 1987 Feb;156(2):386–395. doi: 10.1016/0042-6822(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Katz J. M., Wang M., Webster R. G. Direct sequencing of the HA gene of influenza (H3N2) virus in original clinical samples reveals sequence identity with mammalian cell-grown virus. J Virol. 1990 Apr;64(4):1808–1811. doi: 10.1128/jvi.64.4.1808-1811.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J. M., Webster R. G. Antigenic and structural characterization of multiple subpopulations of H3N2 influenza virus from an individual. Virology. 1988 Aug;165(2):446–456. doi: 10.1016/0042-6822(88)90588-0. [DOI] [PubMed] [Google Scholar]
- Krystal M., Elliott R. M., Benz E. W., Jr, Young J. F., Palese P. Evolution of influenza A and B viruses: conservation of structural features in the hemagglutinin genes. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4800–4804. doi: 10.1073/pnas.79.15.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Nobusawa E., Nakajima K. Amino acid substitution at position 226 of the hemagglutinin molecule of influenza (H1N1) virus affects receptor binding activity but not fusion activity. Virology. 1988 Nov;167(1):8–14. doi: 10.1016/0042-6822(88)90048-7. [DOI] [PubMed] [Google Scholar]
- Oxford J. S., Corcoran T., Knott R., Bates J., Bartolomei O., Major D., Newman R. W., Yates P., Robertson J., Webster R. G. Serological studies with influenza A(H1N1) viruses cultivated in eggs or in a canine kidney cell line (MDCK). Bull World Health Organ. 1987;65(2):181–187. [PMC free article] [PubMed] [Google Scholar]
- Oxford J. S., Corcoran T., Schild G. C. Naturally occurring temperature-sensitive influenza A viruses of the H1N1 and H3N2 subtypes. J Gen Virol. 1980 Jun;48(Pt 2):383–389. doi: 10.1099/0022-1317-48-2-383. [DOI] [PubMed] [Google Scholar]
- Oxford J. S., Schild G. C., Corcoran T., Newman R., Major D., Robertson J., Bootman J., Higgins P., al-Nakib W., Tyrrell D. A. A host-cell-selected variant of influenza B virus with a single nucleotide substitution in HA affecting a potential glycosylation site was attenuated in virulence for volunteers. Arch Virol. 1990;110(1-2):37–46. doi: 10.1007/BF01310701. [DOI] [PubMed] [Google Scholar]
- Patterson S., Oxford J. S. Analysis of antigenic determinants on internal and external proteins of influenza virus and identification of antigenic subpopulations of virions in recent field isolates using monoclonal antibodies and immunogold labelling. Arch Virol. 1986;88(3-4):189–202. doi: 10.1007/BF01310874. [DOI] [PubMed] [Google Scholar]
- Rajakumar A., Swierkosz E. M., Schulze I. T. Sequence of an influenza virus hemagglutinin determined directly from a clinical sample. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4154–4158. doi: 10.1073/pnas.87.11.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S., Naeve C. W., Webster R. G., Bootman J. S., Newman R., Schild G. C. Alterations in the hemagglutinin associated with adaptation of influenza B virus to growth in eggs. Virology. 1985 May;143(1):166–174. doi: 10.1016/0042-6822(85)90105-9. [DOI] [PubMed] [Google Scholar]
- Rogers G. N., Paulson J. C., Daniels R. S., Skehel J. J., Wilson I. A., Wiley D. C. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983 Jul 7;304(5921):76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- Schild G. C., Oxford J. S., de Jong J. C., Webster R. G. Evidence for host-cell selection of influenza virus antigenic variants. Nature. 1983 Jun 23;303(5919):706–709. doi: 10.1038/303706a0. [DOI] [PubMed] [Google Scholar]
- Wang M. L., Katz J. M., Webster R. G. Extensive heterogeneity in the hemagglutinin of egg-grown influenza viruses from different patients. Virology. 1989 Jul;171(1):275–279. doi: 10.1016/0042-6822(89)90538-2. [DOI] [PubMed] [Google Scholar]
- Yates P. J., Bootman J. S., Robertson J. S. The antigenic structure of a human influenza A (H1N1) virus isolate grown exclusively in MDCK cells. J Gen Virol. 1990 Aug;71(Pt 8):1683–1688. doi: 10.1099/0022-1317-71-8-1683. [DOI] [PubMed] [Google Scholar]


