Abstract
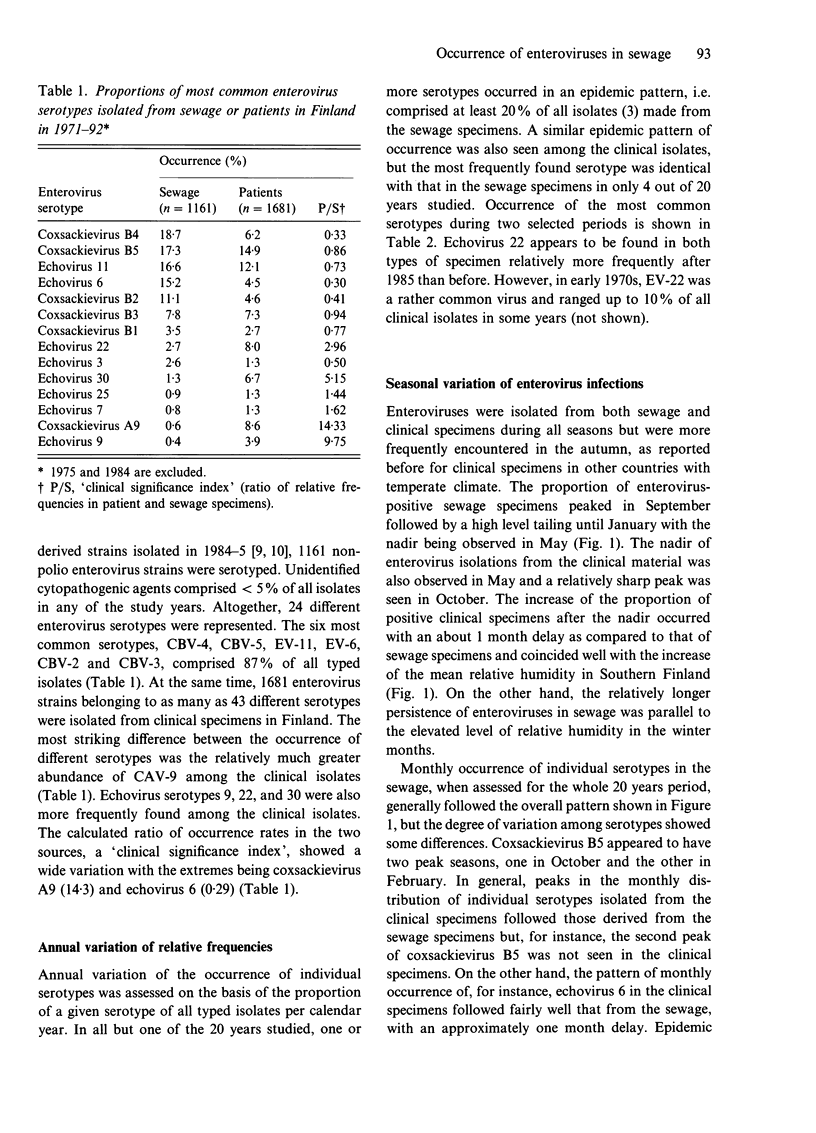
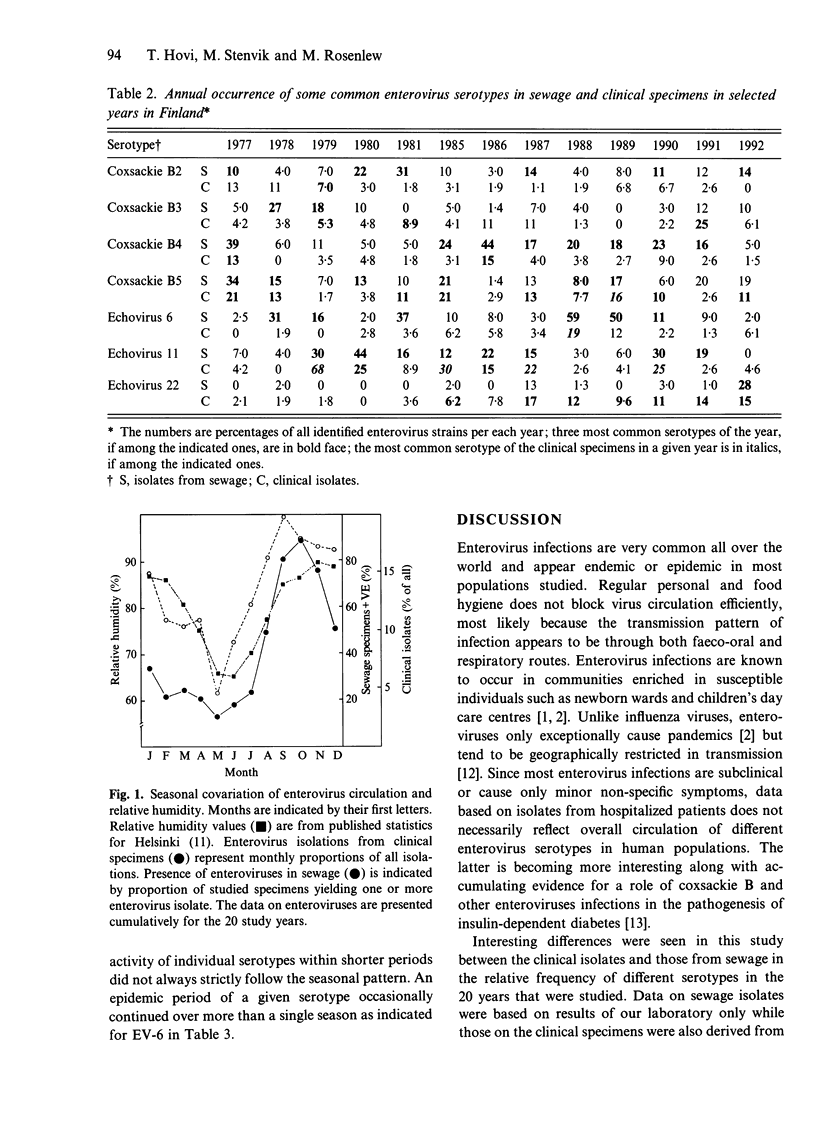
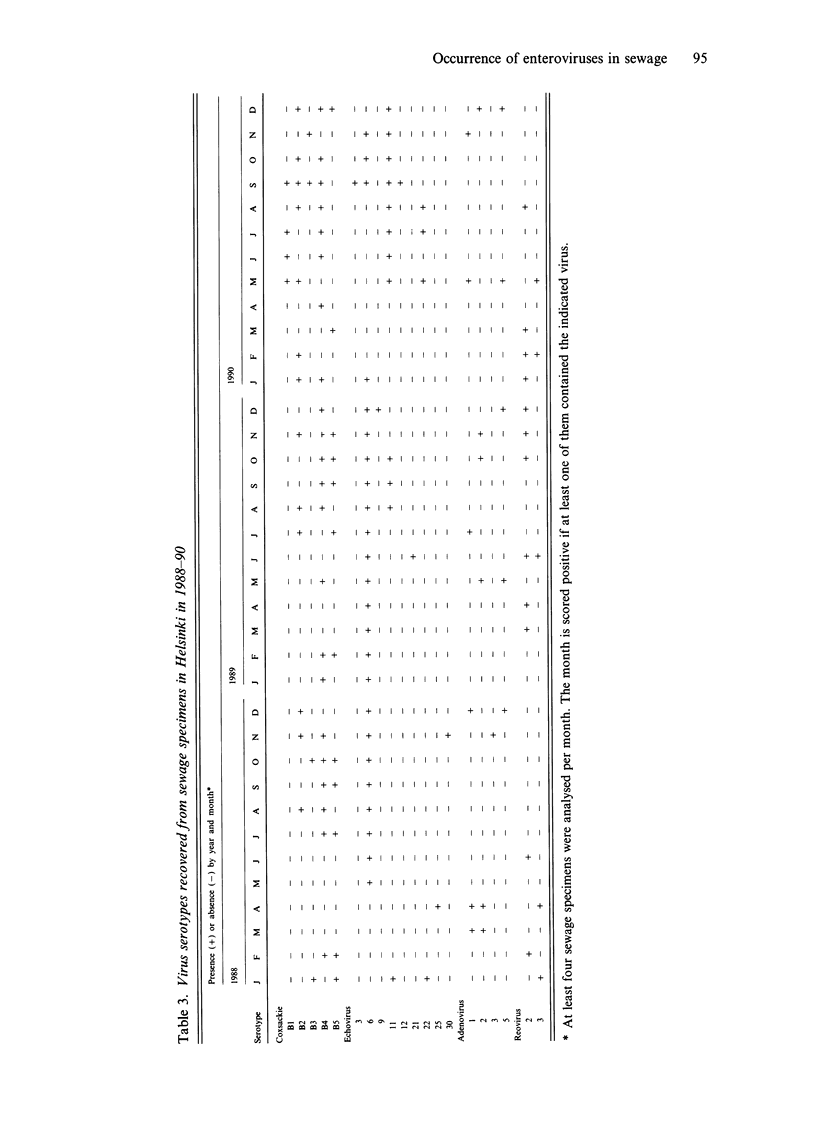
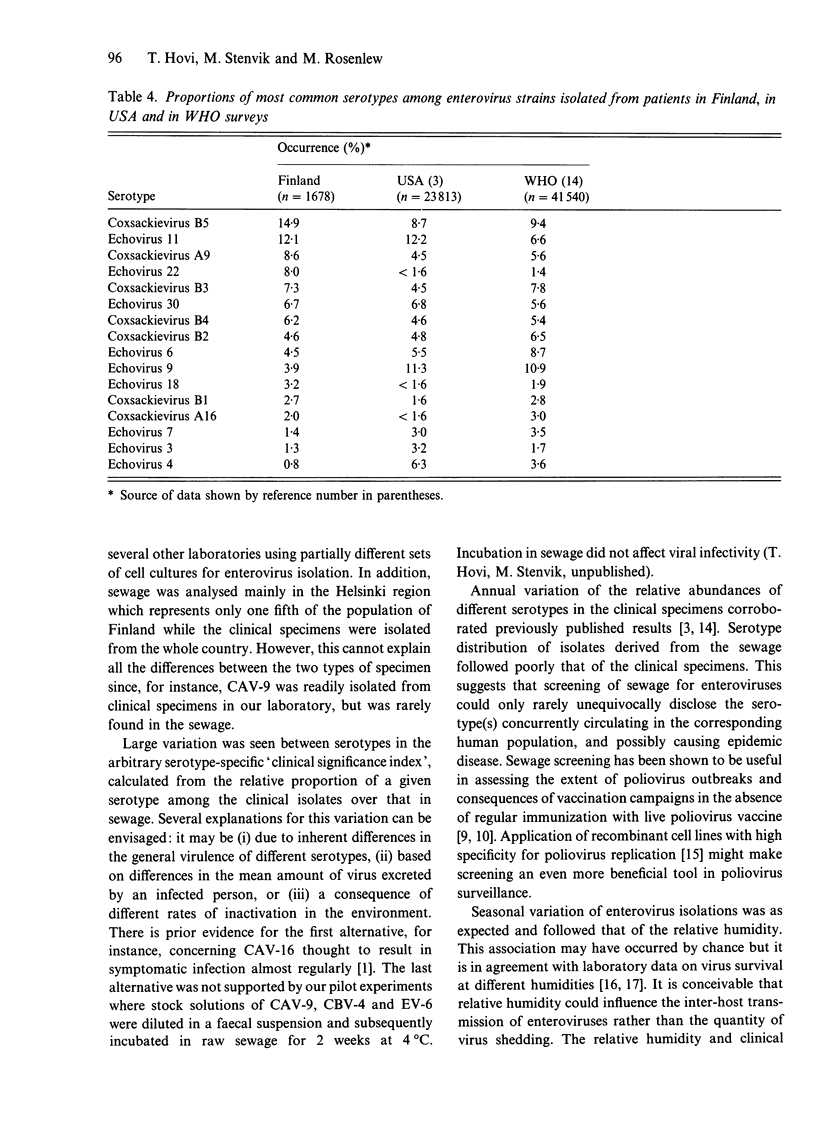
One thousand one hundred and sixty-one non-polio enterovirus strains, isolated during regular screening of Finnish sewage specimens, were analysed for serotype distribution seasonally through 20 years, and the findings were compared with similar data based on 1681 clinical isolates. Coxsackievirus B4 (CBV-4), CBV-5, echovirus 11 (EV-11), EV-6, CBV-2 and CBV-3 were the most common serotypes in sewage, whilst CBV-5, EV-11, coxsackievirus A9, EV-22, CBV-3 and EV-30 were the most common clinical isolates. Reasons for the differences are not known but several explanations are possible. Seasonal variation of enterovirus occurrence in both sources showed an expected peak in the autumn with a trough in the spring. The occurrence of enteroviruses was closely correlated with monthly recordings of mean relative humidity. A further observation concerning the clinical specimens in Finland was the relative excess of some serotypes, such as echovirus 22 and coxsackievirus A9, and paucity of others, for instance, echoviruses 4 and 9, when compared to published data from other countries. This is consistent with the idea of geographically restricted circulation of enteroviruses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Grist N. R., Bell E. J., Assaad F. Enteroviruses in human disease. Prog Med Virol. 1978;24:114–157. [PubMed] [Google Scholar]
- HEMMES J. H., WINKLER K. C., KOOL S. M. Virus survival as a seasonal factor in influenza and polimyelitis. Nature. 1960 Oct 29;188:430–431. doi: 10.1038/188430a0. [DOI] [PubMed] [Google Scholar]
- Hovi T., Cantell K., Huovilainen A., Kinnunen E., Kuronen T., Lapinleimu K., Pöyry T., Roivainen M., Salama N., Stenvik M. Outbreak of paralytic poliomyelitis in Finland: widespread circulation of antigenically altered poliovirus type 3 in a vaccinated population. Lancet. 1986 Jun 21;1(8495):1427–1432. doi: 10.1016/s0140-6736(86)91566-7. [DOI] [PubMed] [Google Scholar]
- Hovi T., Stenvik M. Selective isolation of poliovirus in recombinant murine cell line expressing the human poliovirus receptor gene. J Clin Microbiol. 1994 May;32(5):1366–1368. doi: 10.1128/jcm.32.5.1366-1368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyöty H., Hiltunen M., Knip M., Laakkonen M., Vähäsalo P., Karjalainen J., Koskela P., Roivainen M., Leinikki P., Hovi T. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes. 1995 Jun;44(6):652–657. doi: 10.2337/diab.44.6.652. [DOI] [PubMed] [Google Scholar]
- Ijaz M. K., Sattar S. A., Johnson-Lussenburg C. M., Springthorpe V. S. Comparison of the airborne survival of calf rotavirus and poliovirus type 1 (Sabin) aerosolized as a mixture. Appl Environ Microbiol. 1985 Feb;49(2):289–293. doi: 10.1128/aem.49.2.289-293.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogon A., Spigland I., Frothingham T. E., Elveback L., Williams C., Hall C. E., Fox J. P. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VII. Observations on viral excretion, seroimmunity, intrafamilial spread and illness association in coxsackie and echovirus infections. Am J Epidemiol. 1969 Jan;89(1):51–61. doi: 10.1093/oxfordjournals.aje.a120915. [DOI] [PubMed] [Google Scholar]
- Lapinleimu K., Stenvik M. Experiences with polio vaccination and herd immunity in Finland. Dev Biol Stand. 1981;47:241–246. [PubMed] [Google Scholar]
- Pöyry T., Stenvik M., Hovi T. Viruses in sewage waters during and after a poliomyelitis outbreak and subsequent nationwide oral poliovirus vaccination campaign in Finland. Appl Environ Microbiol. 1988 Feb;54(2):371–374. doi: 10.1128/aem.54.2.371-374.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. C., Metcalf T. G., Melnick J. L. Human viruses in sediments, sludges, and soils. Bull World Health Organ. 1986;64(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- Rico-Hesse R., Pallansch M. A., Nottay B. K., Kew O. M. Geographic distribution of wild poliovirus type 1 genotypes. Virology. 1987 Oct;160(2):311–322. doi: 10.1016/0042-6822(87)90001-8. [DOI] [PubMed] [Google Scholar]
- Strikas R. A., Anderson L. J., Parker R. A. Temporal and geographic patterns of isolates of nonpolio enterovirus in the United States, 1970-1983. J Infect Dis. 1986 Feb;153(2):346–351. doi: 10.1093/infdis/153.2.346. [DOI] [PubMed] [Google Scholar]


