Abstract
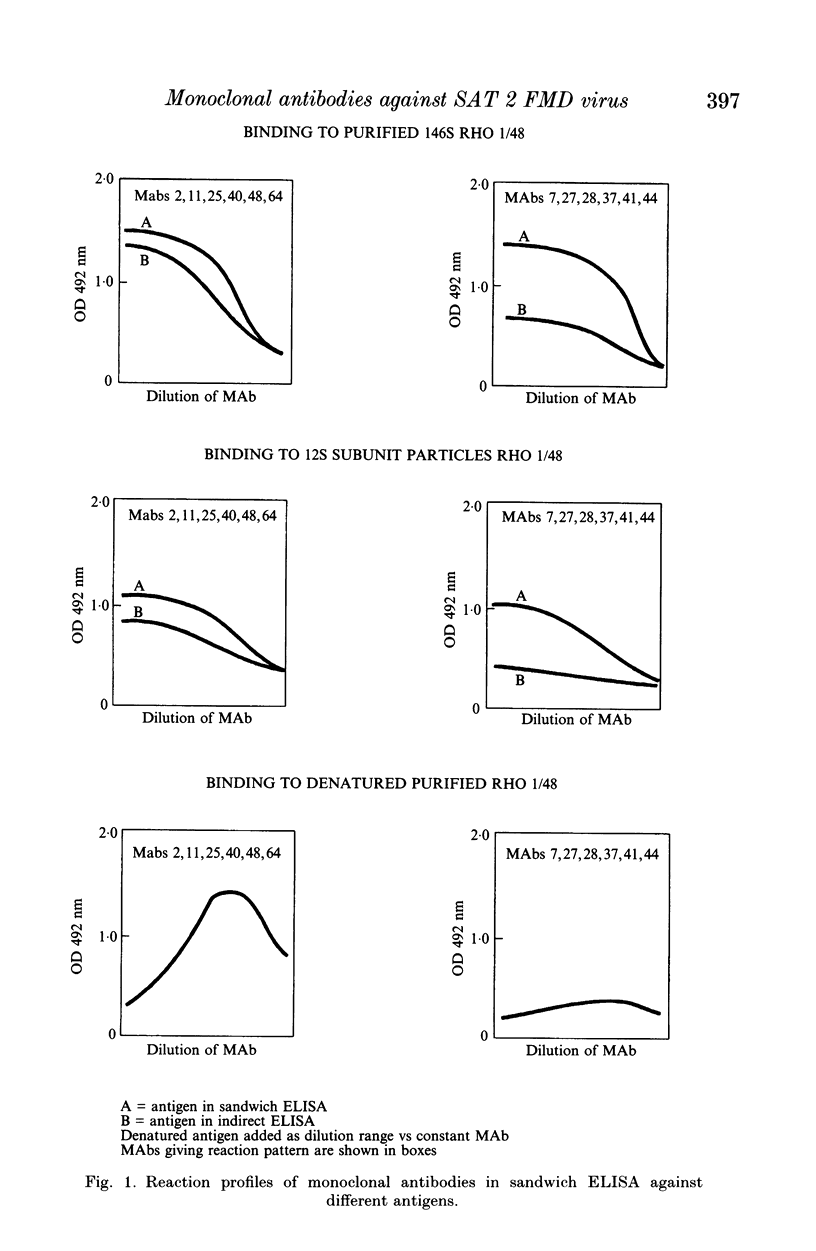
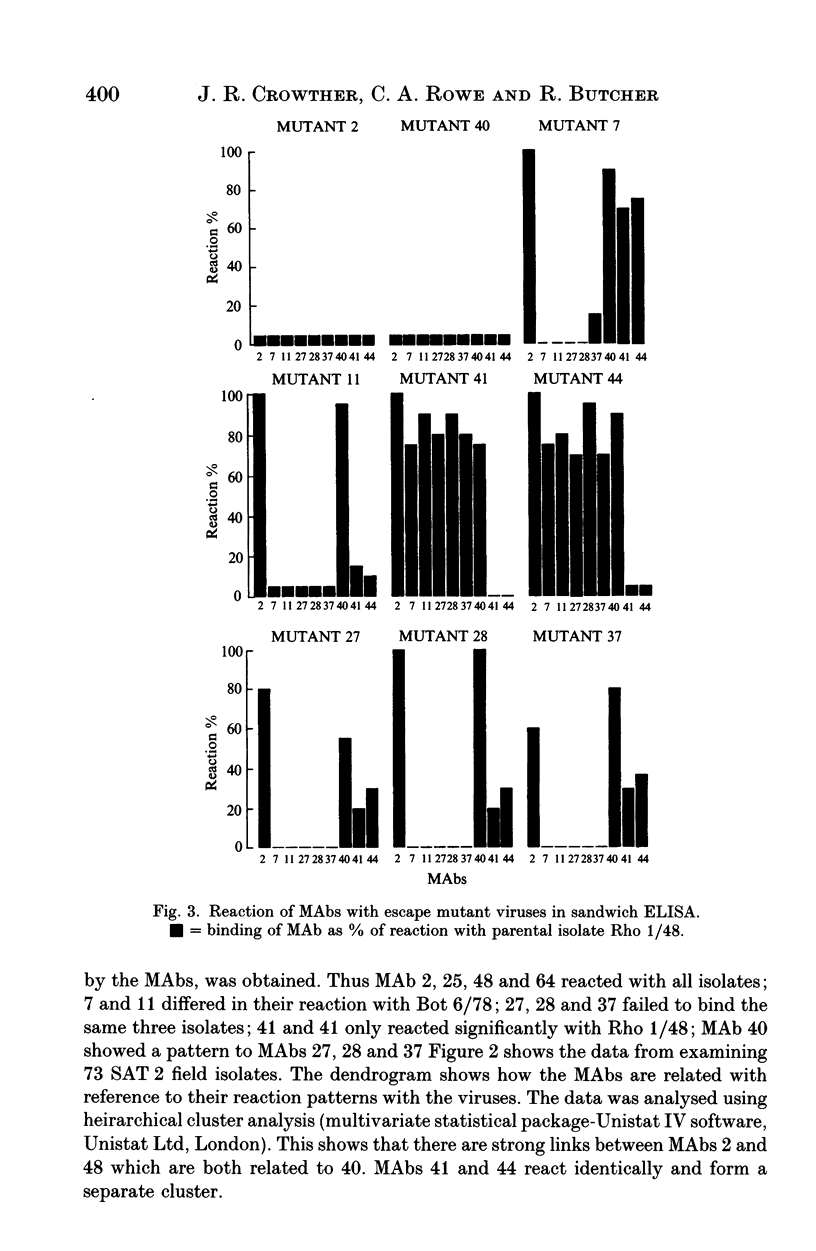
This paper is the first to describe characterization of monoclonal antibodies (MAbs) against a South African Territories 2 (SAT 2) foot-and-mouth disease virus (isolate Rho 1/48). Twelve MAbs which neutralized homologous virus were characterized in indirect and sandwich ELISA using purified Rho 1/48 virus particles, subunits, trypsin-treated, and chemically denatured virus. All the MAbs inhibited haemagglutination by parental virus. Binding of the MAbs to 73 SAT 2 field isolates was measured in a sandwich ELISA and defined four distinct antigenic regions. Preliminary characterization of escape mutants selected with some of the MAbs using virus neutralization tests, ELISA, and amino acid sequencing is included. MAbs 2, 25, 40, 48 and 64, reacted with a linear epitope on the VP1 loop region. An amino acid change at position 149 (valine to glutamic acid) was detected in mutants selected by MAb 2 and 40 and this eliminated binding and neutralization by all the other MAb. This epitope was conformation-dependent and was conserved in all 73 isolates of SAT 2 examined. Escape mutants isolated with MAb 41 and 44, had changes at positions 156 (glycine to aspartic acid), or 158 (serine to leucine) respectively. These MAbs bound with Rho 1/48 only out of 73 field strain viruses studies and the reactions of MAbs from the other groups was unaltered. MAb 27, 28 and 37 reacted with a conformation-dependent epitope on VP1 which was not conserved in field isolates. All mutants selected by these MAbs had a single amino acid substitution at position 149 (valine to alanine). The same change was always found in field isolates which did not bind MAbs from this group. MAb 11 reacted with a linear epitope associated with amino acids 147 or 148 on VP1 and showed similar binding characteristics to a conformation dependent MAb 7, no amino acid residue changes were found within VP1 for monoclonal antibody 7 mutants.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN F., CARTWRIGHT B. PURIFICATION OF RADIOACTIVE FOOT-AND-MOUTH DISEASE VIRUS. Nature. 1963 Sep 21;199:1168–1170. doi: 10.1038/1991168a0. [DOI] [PubMed] [Google Scholar]
- Barnett P. V., Ouldridge E. J., Rowlands D. J., Brown F., Parry N. R. Neutralizing epitopes of type O foot-and-mouth disease virus. I. Identification and characterization of three functionally independent, conformational sites. J Gen Virol. 1989 Jun;70(Pt 6):1483–1491. doi: 10.1099/0022-1317-70-6-1483. [DOI] [PubMed] [Google Scholar]
- Booth J. C., Pay T. W. Haemagglutination by type SAT 2 foot-and-mouth disease viruses. J Gen Virol. 1973 Jun;19(3):397–404. doi: 10.1099/0022-1317-19-3-397. [DOI] [PubMed] [Google Scholar]
- Butchaiah G., Rao B. U. Hybridoma cell lines secreting monoclonal antibodies to foot-and-mouth disease virus type Asia-1. Acta Virol. 1989 Mar;33(2):121–130. [PubMed] [Google Scholar]
- Butcher R. N., Obi T. U., McCullough K. C. Rapid isolation of monoclonal hybridoma cultures by a 'fusion-cloning' method: the requirement for aminopterin. Biologicals. 1991 Jul;19(3):171–175. doi: 10.1016/1045-1056(91)90031-e. [DOI] [PubMed] [Google Scholar]
- Haresnape J. M., McCahon D. Four independent antigenic determinants on the capsid polypeptides of aphthovirus. J Gen Virol. 1983 Nov;64(Pt 11):2345–2355. doi: 10.1099/0022-1317-64-11-2345. [DOI] [PubMed] [Google Scholar]
- Kitson J. D., McCahon D., Belsham G. J. Sequence analysis of monoclonal antibody resistant mutants of type O foot and mouth disease virus: evidence for the involvement of the three surface exposed capsid proteins in four antigenic sites. Virology. 1990 Nov;179(1):26–34. doi: 10.1016/0042-6822(90)90269-w. [DOI] [PubMed] [Google Scholar]
- McCahon D., Crowther J. R., Belsham G. J., Kitson J. D., Duchesne M., Have P., Meloen R. H., Morgan D. O., De Simone F. Evidence for at least four antigenic sites on type O foot-and-mouth disease virus involved in neutralization; identification by single and multiple site monoclonal antibody-resistant mutants. J Gen Virol. 1989 Mar;70(Pt 3):639–645. doi: 10.1099/0022-1317-70-3-639. [DOI] [PubMed] [Google Scholar]
- McCullough K. C., Crowther J. R., Butcher R. N. Alteration in antibody reactivity with foot-and-mouth disease virus (FMDV) 146S antigen before and after binding to a solid phase or complexing with specific antibody. J Immunol Methods. 1985 Sep 3;82(1):91–100. doi: 10.1016/0022-1759(85)90228-5. [DOI] [PubMed] [Google Scholar]
- McCullough K. C., Crowther J. R., Carpenter W. C., Brocchi E., Capucci L., De Simone F., Xie Q., McCahon D. Epitopes on foot-and-mouth disease virus particles. I. Topology. Virology. 1987 Apr;157(2):516–525. doi: 10.1016/0042-6822(87)90294-7. [DOI] [PubMed] [Google Scholar]
- Meloen R. H., Barteling S. J. Epitope mapping of the outer structural protein VP1 of three different serotypes of foot-and-mouth disease virus. Virology. 1986 Feb;149(1):55–63. doi: 10.1016/0042-6822(86)90086-3. [DOI] [PubMed] [Google Scholar]
- Ouldridge E. J., Barnett P. V., Parry N. R., Syred A., Head M., Rweyemamu M. M. Demonstration of neutralizing and non-neutralizing epitopes on the trypsin-sensitive site of foot-and-mouth disease virus. J Gen Virol. 1984 Jan;65(Pt 1):203–207. doi: 10.1099/0022-1317-65-1-203. [DOI] [PubMed] [Google Scholar]
- Parry N., Fox G., Rowlands D., Brown F., Fry E., Acharya R., Logan D., Stuart D. Structural and serological evidence for a novel mechanism of antigenic variation in foot-and-mouth disease virus. Nature. 1990 Oct 11;347(6293):569–572. doi: 10.1038/347569a0. [DOI] [PubMed] [Google Scholar]
- Robertson B. H., Morgan D. O., Moore D. M. Location of neutralizing epitopes defined by monoclonal antibodies generated against the outer capsid polypeptide, VP1, of foot-and-mouth disease virus A12. Virus Res. 1984 Sep;1(6):489–500. doi: 10.1016/0168-1702(84)90006-6. [DOI] [PubMed] [Google Scholar]
- Rowlands D. J., Clarke B. E., Carroll A. R., Brown F., Nicholson B. H., Bittle J. L., Houghten R. A., Lerner R. A. Chemical basis of antigenic variation in foot-and-mouth disease virus. Nature. 1983 Dec 15;306(5944):694–697. doi: 10.1038/306694a0. [DOI] [PubMed] [Google Scholar]
- Saiz J. C., Gonzalez M. J., Morgan D. O., Card J. L., Sobrino F., Moore D. M. Antigenic comparison of different foot-and-mouth disease virus types using monoclonal antibodies defining multiple neutralizing epitopes on FMDV A5 subtypes. Virus Res. 1989 May;13(1):45–60. doi: 10.1016/0168-1702(89)90086-5. [DOI] [PubMed] [Google Scholar]
- Samuel A. R., Knowles N. J., Samuel G. D., Crowther J. R. Evaluation of a trapping ELISA for the differentiation of foot-and-mouth disease virus strains using monoclonal antibodies. Biologicals. 1991 Oct;19(4):299–310. doi: 10.1016/s1045-1056(05)80019-3. [DOI] [PubMed] [Google Scholar]
- Thomas A. A., Woortmeijer R. J., Puijk W., Barteling S. J. Antigenic sites on foot-and-mouth disease virus type A10. J Virol. 1988 Aug;62(8):2782–2789. doi: 10.1128/jvi.62.8.2782-2789.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q. C., McCahon D., Crowther J. R., Belsham G. J., McCullough K. C. Neutralization of foot-and-mouth disease virus can be mediated through any of at least three separate antigenic sites. J Gen Virol. 1987 Jun;68(Pt 6):1637–1647. doi: 10.1099/0022-1317-68-6-1637. [DOI] [PubMed] [Google Scholar]


