Abstract
The genetic and biochemical basis of ampicillin resistance amongst the aerobic Gram-negative commensal faecal flora of healthy volunteers in South Africa has been determined. Amongst 608 ampicillin resistant strains isolated from 320 of the participants, 158 were able to transfer their ampicillin resistant determinants into Escherichia coli K-12 J62-2. Iso-electric focusing of the beta-lactamases, extracted from the transconjugants, demonstrated that ampicillin resistance resulted from the presence of the TEM-1, TEM-2 and SHV-1 beta-lactamases in 94.3%, 2.5% and 3.2% of isolates respectively. Endonuclease restriction digests of the plasmids isolated from the transconjugants showed that the beta-lactamase genes were present on a wide variety of plasmid types; 101 distinct plasmid endonuclease restriction patterns were identified. Transferable ampicillin resistance was associated with resistance to other antibiotics at the following frequencies: trimethoprim (48.7%), streptomycin (35.4%), tetracycline (27.2%), spectinomycin (9.5%), chloramphenicol (3.2%) and gentamicin (1.3%). One antibiotic resistance pattern, ampicillin and trimethoprim, predominated (28%). In total, 77.9% of the plasmids conferred resistance to other antibiotics raising the possibility that use of any of these agents, not simply ampicillin, may contribute to the maintenance of resistance genes.
Full text
PDF







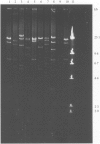
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amyes S. G., Gould I. M. Trimethoprim resistance plasmids. Ann Microbiol (Paris) 1984 Sep-Oct;135B(2):177–186. [PubMed] [Google Scholar]
- Amyes S. G., Tait S., Thomson C. J., Payne D. J., Nandivada L. S., Jesudason M. V., Mukundan U. D., Young H. K. The incidence of antibiotic resistance in aerobic faecal flora in south India. J Antimicrob Chemother. 1992 Apr;29(4):415–425. doi: 10.1093/jac/29.4.415. [DOI] [PubMed] [Google Scholar]
- Amyes S. G. The success of plasmid-encoded resistance genes in clinical bacteria. An examination of plasmid-mediated ampicillin and trimethoprim resistance genes and their resistance mechanisms. J Med Microbiol. 1989 Feb;28(2):73–83. doi: 10.1099/00222615-28-2-73. [DOI] [PubMed] [Google Scholar]
- Bauernfeind A., Rosenthal E., Eberlein E., Holley M., Schweighart S. Spread of Klebsiella pneumoniae producing SHV-5 beta-lactamase among hospitalized patients. Infection. 1993 Jan-Feb;21(1):18–22. doi: 10.1007/BF01739303. [DOI] [PubMed] [Google Scholar]
- Bonten M., Stobberingh E., Philips J., Houben A. Antibiotic resistance of Escherichia coli in fecal samples of healthy people in two different areas in an industrialized country. Infection. 1992 Sep-Oct;20(5):258–262. doi: 10.1007/BF01710790. [DOI] [PubMed] [Google Scholar]
- Cullmann W. The threat of resistance to the new oral cephalosporins. Chemotherapy. 1992;38 (Suppl 2):10–17. doi: 10.1159/000239092. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. E. Antibiotic resistance in developing countries. J Infect Dis. 1985 Dec;152(6):1103–1106. doi: 10.1093/infdis/152.6.1103. [DOI] [PubMed] [Google Scholar]
- Lester S. C., del Pilar Pla M., Wang F., Perez Schael I., Jiang H., O'Brien T. F. The carriage of Escherichia coli resistant to antimicrobial agents by healthy children in Boston, in Caracas, Venezuela, and in Qin Pu, China. N Engl J Med. 1990 Aug 2;323(5):285–289. doi: 10.1056/NEJM199008023230501. [DOI] [PubMed] [Google Scholar]
- Levy S. B., Marshall B., Schluederberg S., Rowse D., Davis J. High frequency of antimicrobial resistance in human fecal flora. Antimicrob Agents Chemother. 1988 Dec;32(12):1801–1806. doi: 10.1128/aac.32.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamun K. Z., Shears P., Hart C. A. The prevalence and genetics of resistance to commonly used antimicrobial agents in faecal Enterobacteriaceae from children in Bangladesh. Epidemiol Infect. 1993 Jun;110(3):447–458. doi: 10.1017/s0950268800050871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Medeiros A. A. Beta-lactamases. Br Med Bull. 1984 Jan;40(1):18–27. doi: 10.1093/oxfordjournals.bmb.a071942. [DOI] [PubMed] [Google Scholar]
- Reves R. R., Murray B. E., Pickering L. K., Prado D., Maddock M., Bartlett A. V., 3rd Children with trimethoprim- and ampicillin-resistant fecal Escherichia coli in day care centers. J Infect Dis. 1987 Nov;156(5):758–762. doi: 10.1093/infdis/156.5.758. [DOI] [PubMed] [Google Scholar]
- Roy C., Teruel D., Reig R., Hermida M., Teixell M. beta-Lactamases and susceptibility phenotypes to beta-lactam antibiotics in Escherichia coli strains. J Antimicrob Chemother. 1992 May;29(5):593–594. doi: 10.1093/jac/29.5.593. [DOI] [PubMed] [Google Scholar]
- Shanahan P. M., Wylie B. A., Adrian P. V., Koornhof H. J., Thomson C. J., Amyes S. G. The prevalence of antimicrobial resistance in human faecal flora in South Africa. Epidemiol Infect. 1993 Oct;111(2):221–228. doi: 10.1017/s0950268800056922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears P., Suliman G., Hart C. A. Occurrence of multiple antibiotic resistance and R plasmids in Enterobacteriaceae isolated from children in the Sudan. Epidemiol Infect. 1988 Feb;100(1):73–81. doi: 10.1017/s0950268800065572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears P., Suliman G., Hart C. A. Restriction endonuclease characterization of resistant plasmids in Enterobacteriaceae isolated from children in the Sudan. Epidemiol Infect. 1989 Dec;103(3):487–496. doi: 10.1017/s0950268800030892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot D. L., Goldstein F. W., Soussy C. J., Courtieu A. L., Husson M. O., Lemozy J., Meyran M., Morel C., Perez R., Quentin-Noury C. Resistance to cefotaxime and seven other beta-lactams in members of the family Enterobacteriaceae: a 3-year survey in France. Antimicrob Agents Chemother. 1992 Aug;36(8):1677–1681. doi: 10.1128/aac.36.8.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984 Oct;20(4):608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson C. J., Shanahan P. M., Amyes S. G. TEM-1 plasmids in the community. Lancet. 1994 Apr 9;343(8902):921–921. doi: 10.1016/s0140-6736(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Thomson C. J., Tait S., Jesudason M. V., Amyes S. G. Dissemination of the TEM-I beta-lactamase gene into disparate plasmids in faecal strains of Escherichia coli isolated in Vellore, south India. J Infect. 1993 Jul;27(1):47–50. doi: 10.1016/0163-4453(93)93698-4. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- Wu P. J., Shannon K., Phillips I. Effect of hyperproduction of TEM-1 beta-lactamase on in vitro susceptibility of Escherichia coli to beta-lactam antibiotics. Antimicrob Agents Chemother. 1994 Mar;38(3):494–498. doi: 10.1128/aac.38.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H. K., Nandivada L. S., Amyes S. G. Antibiotic resistance in the tropics. 1. The genetics of bacterial ampicillin resistance in tropical areas. Trans R Soc Trop Med Hyg. 1989 Jan-Feb;83(1):38–41. doi: 10.1016/0035-9203(89)90699-8. [DOI] [PubMed] [Google Scholar]