Abstract
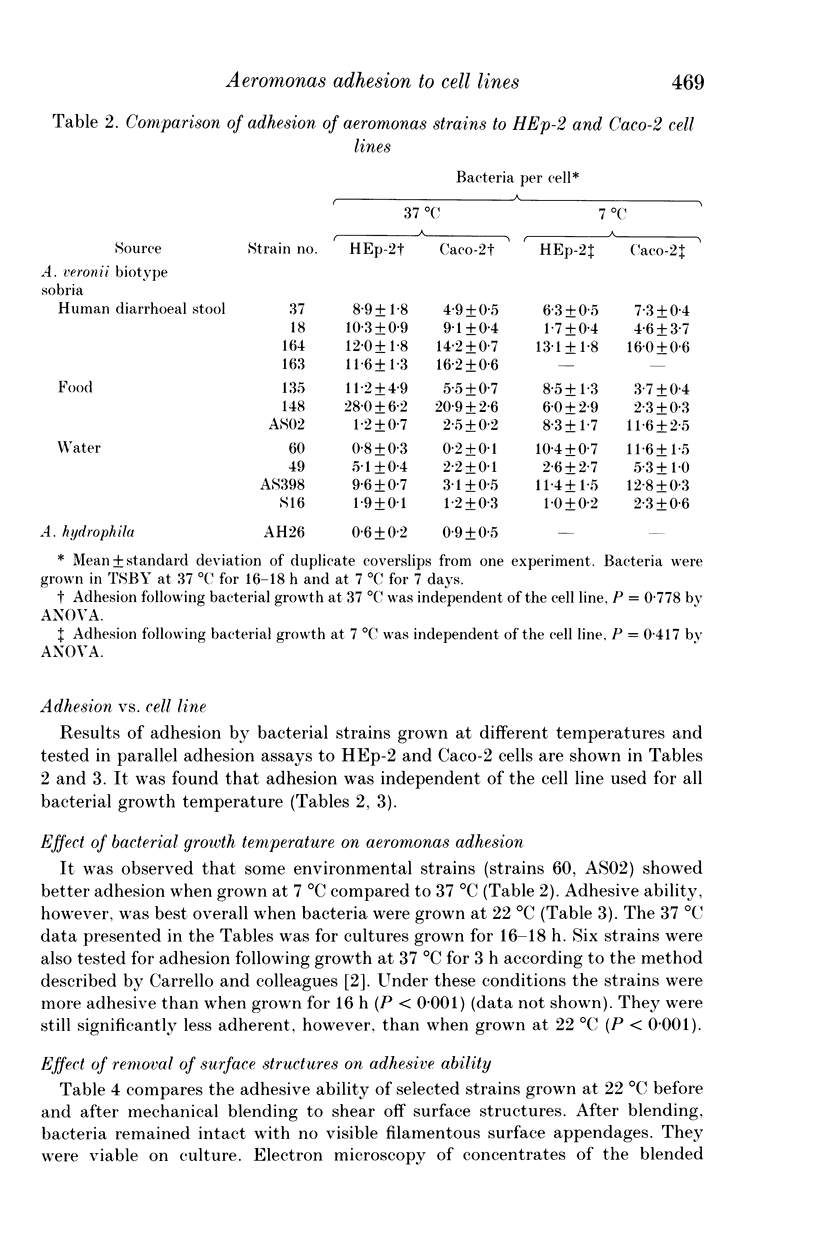
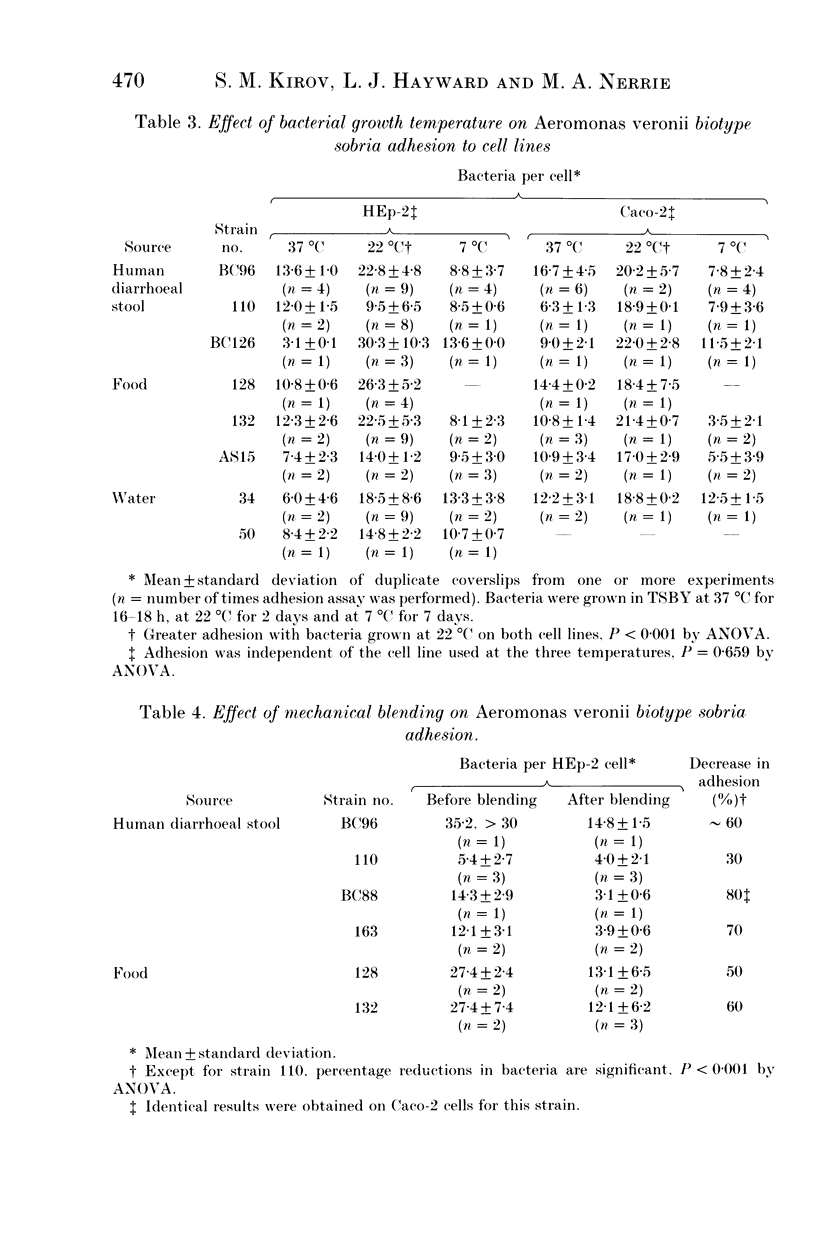
Adhesion to HEp-2 cells has been shown to correlate with enteropathogenicity for Aeromonas species. Such adhesion is thought to reflect the ability of strains to adhere to human intestinal enterocytes, although HEp-2 cells are not of intestinal origin. In this study strains of Aeromonas veronii biotype sobria isolated from various sources were investigated in parallel assays for their ability to adhere to HEp-2 cells and to an intestinal cell line (Caco-2). Quantitative assays showed identical adhesion values were obtained with both cell lines. Adhesion was best when bacteria were grown at 22 degrees C compared with 37 degrees C and 7 degrees C. Some environmental isolates showed greater adhesion when grown at 7 degrees C than when grown at 37 degrees C. Filamentous structures on these strains are also optimally expressed under the above conditions (reported elsewhere). Mechanical shearing or trypsin treatment to remove surface structures from several adhesive strains grown at 22 degrees C decreased adhesion to cell lines by 50-80% providing further indirect evidence that filamentous adhesins may play a role in cell adhesion for this Aeromonas species.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carrello A., Silburn K. A., Budden J. R., Chang B. J. Adhesion of clinical and environmental Aeromonas isolates to HEp-2 cells. J Med Microbiol. 1988 May;26(1):19–27. doi: 10.1099/00222615-26-1-19. [DOI] [PubMed] [Google Scholar]
- Daily O. P., Joseph S. W., Coolbaugh J. C., Walker R. I., Merrell B. R., Rollins D. M., Seidler R. J., Colwell R. R., Lissner C. R. Association of Aeromonas sobria with human infection. J Clin Microbiol. 1981 Apr;13(4):769–777. doi: 10.1128/jcm.13.4.769-777.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francki K. T., Chang B. J. Variable expression of O-antigen and the role of lipopolysaccharide as an adhesin in Aeromonas sobria. FEMS Microbiol Lett. 1994 Sep 15;122(1-2):97–101. doi: 10.1111/j.1574-6968.1994.tb07150.x. [DOI] [PubMed] [Google Scholar]
- Grey P. A., Kirov S. M. Adherence to HEp-2 cells and enteropathogenic potential of Aeromonas spp. Epidemiol Infect. 1993 Apr;110(2):279–287. doi: 10.1017/s0950268800068217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A. S., Mietzner T. A., Smith A. J., Schoolnik G. K. The pili of Aeromonas hydrophila: identification of an environmentally regulated "mini pilin". J Exp Med. 1990 Sep 1;172(3):795–806. doi: 10.1084/jem.172.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokama A., Iwanaga M. Purification and characterization of Aeromonas sobria Ae24 pili: a possible new colonization factor. Microb Pathog. 1992 Oct;13(4):325–334. doi: 10.1016/0882-4010(92)90042-m. [DOI] [PubMed] [Google Scholar]
- Hokama A., Iwanaga M. Purification and characterization of Aeromonas sobria pili, a possible colonization factor. Infect Immun. 1991 Oct;59(10):3478–3483. doi: 10.1128/iai.59.10.3478-3483.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga M., Hokama A. Characterization of Aeromonas sobria TAP13 pili: a possible new colonization factor. J Gen Microbiol. 1992 Sep;138(9):1913–1919. doi: 10.1099/00221287-138-9-1913. [DOI] [PubMed] [Google Scholar]
- Janda J. M., Oshiro L. S., Abbott S. L., Duffey P. S. Virulence markers of mesophilic aeromonads: association of the autoagglutination phenomenon with mouse pathogenicity and the presence of a peripheral cell-associated layer. Infect Immun. 1987 Dec;55(12):3070–3077. doi: 10.1128/iai.55.12.3070-3077.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda J. M., Reitano M., Bottone E. J. Biotyping of Aeromonas isolates as a correlate to delineating a species-associated disease spectrum. J Clin Microbiol. 1984 Jan;19(1):44–47. doi: 10.1128/jcm.19.1.44-47.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamperman L., Kirov S. M. Pili and the interaction of Aeromonas species with human peripheral blood polymorphonuclear cells. FEMS Immunol Med Microbiol. 1993 Aug;7(2):187–195. doi: 10.1111/j.1574-695X.1993.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Kirov S. M., Jacobs I., Hayward L. J., Hapin R. H. Electron microscopic examination of factors influencing the expression of filamentous surface structures on clinical and environmental isolates of Aeromonas veronii Biotype sobria. Microbiol Immunol. 1995;39(5):329–338. doi: 10.1111/j.1348-0421.1995.tb02209.x. [DOI] [PubMed] [Google Scholar]
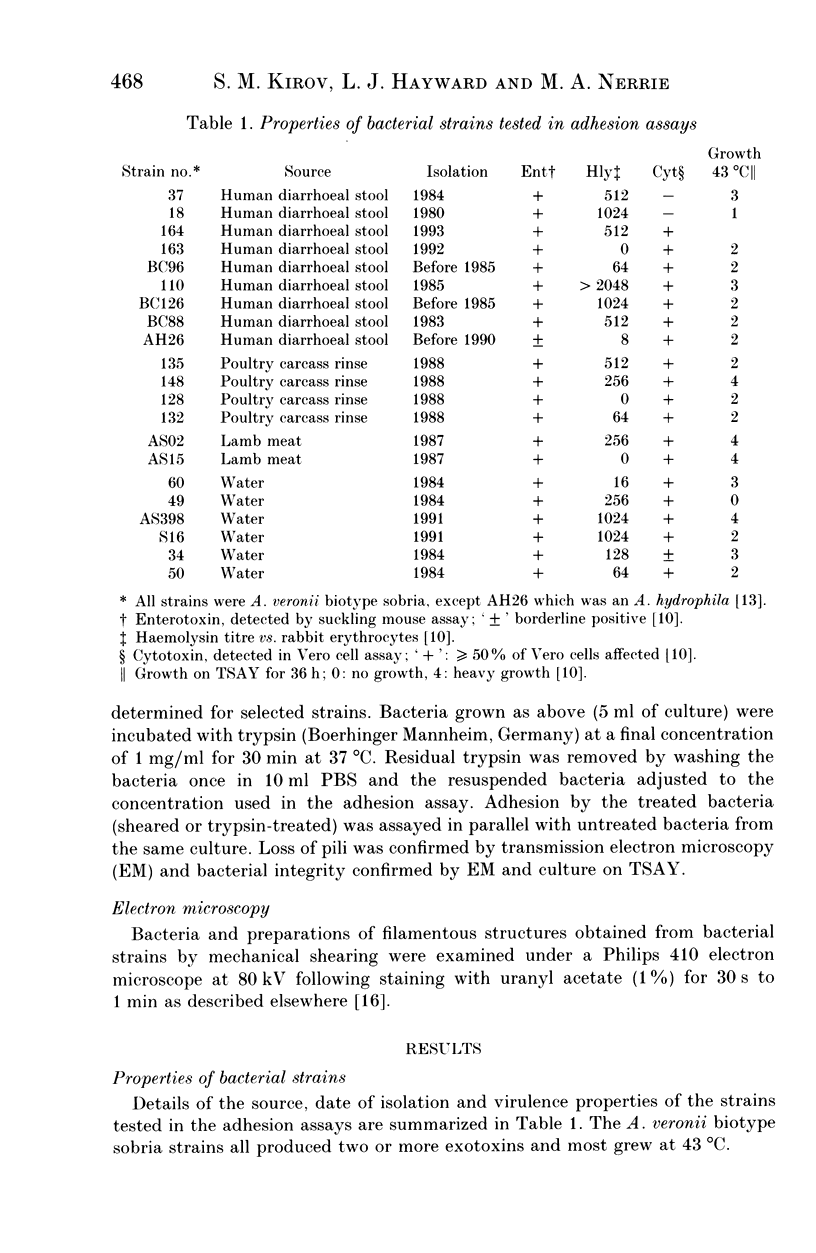
- Kirov S. M., Rees B., Wellock R. C., Goldsmid J. M., Van Galen A. D. Virulence characteristics of Aeromonas spp. in relation to source and biotype. J Clin Microbiol. 1986 Nov;24(5):827–834. doi: 10.1128/jcm.24.5.827-834.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruvada R., Das P., Ghosh A. N., Pal S. C., Nair G. B. Electrophoretic mobility and immunoblot analysis of the outer membrane proteins of Aeromonas hydrophila, A. sobria and A. caviae. Microbios. 1992;71(287):105–113. [PubMed] [Google Scholar]
- Mateos D., Anguita J., Naharro G., Paniagua C. Influence of growth temperature on the production of extracellular virulence factors and pathogenicity of environmental and human strains of Aeromonas hydrophila. J Appl Bacteriol. 1993 Feb;74(2):111–118. doi: 10.1111/j.1365-2672.1993.tb03003.x. [DOI] [PubMed] [Google Scholar]
- Merino S., Camprubí S., Tomás J. M. Effect of growth temperature on outer membrane components and virulence of Aeromonas hydrophila strains of serotype O:34. Infect Immun. 1992 Oct;60(10):4343–4349. doi: 10.1128/iai.60.10.4343-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandapalan N., Chang B. J. Production and characterization of monoclonal antibodies to Aeromonas sobria surface antigens. FEMS Microbiol Immunol. 1989 Dec;1(8-9):515–524. doi: 10.1111/j.1574-6968.1989.tb02444.x. [DOI] [PubMed] [Google Scholar]
- Neves M. S., Nunes M. P., Milhomem A. M. Aeromonas species exhibit aggregative adherence to HEp-2 cells. J Clin Microbiol. 1994 Apr;32(4):1130–1131. doi: 10.1128/jcm.32.4.1130-1131.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa Y., Hase A., Ogawasara J., Scotland S. M., Smith H. R., Kimura T. Adhesion to and invasion of human colon carcinoma Caco-2 cells by Aeromonas strains. J Med Microbiol. 1994 Jan;40(1):55–61. doi: 10.1099/00222615-40-1-55. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y., Kimura T., Kishi T. Mannose-resistant adhesion of motile Aeromonas to INT407 cells and the differences among isolates from humans, food and water. Epidemiol Infect. 1991 Aug;107(1):171–179. doi: 10.1017/s0950268800048792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn D. M., Atkinson H. M., Bretag A. H., Tester M., Trust T. J., Wong C. Y., Flower R. L. Carbohydrate-reactive, pore-forming outer membrane proteins of Aeromonas hydrophila. Infect Immun. 1994 Sep;62(9):4054–4058. doi: 10.1128/iai.62.9.4054-4058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn D. M., Wong C. Y., Atkinson H. M., Flower R. L. Isolation of carbohydrate-reactive outer membrane proteins of Aeromonas hydrophila. Infect Immun. 1993 Feb;61(2):371–377. doi: 10.1128/iai.61.2.371-377.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. G., Blake D. C., Jr Cell association and invasion of Caco-2 cells by Campylobacter jejuni. Infect Immun. 1994 Sep;62(9):3773–3779. doi: 10.1128/iai.62.9.3773-3779.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


