Abstract
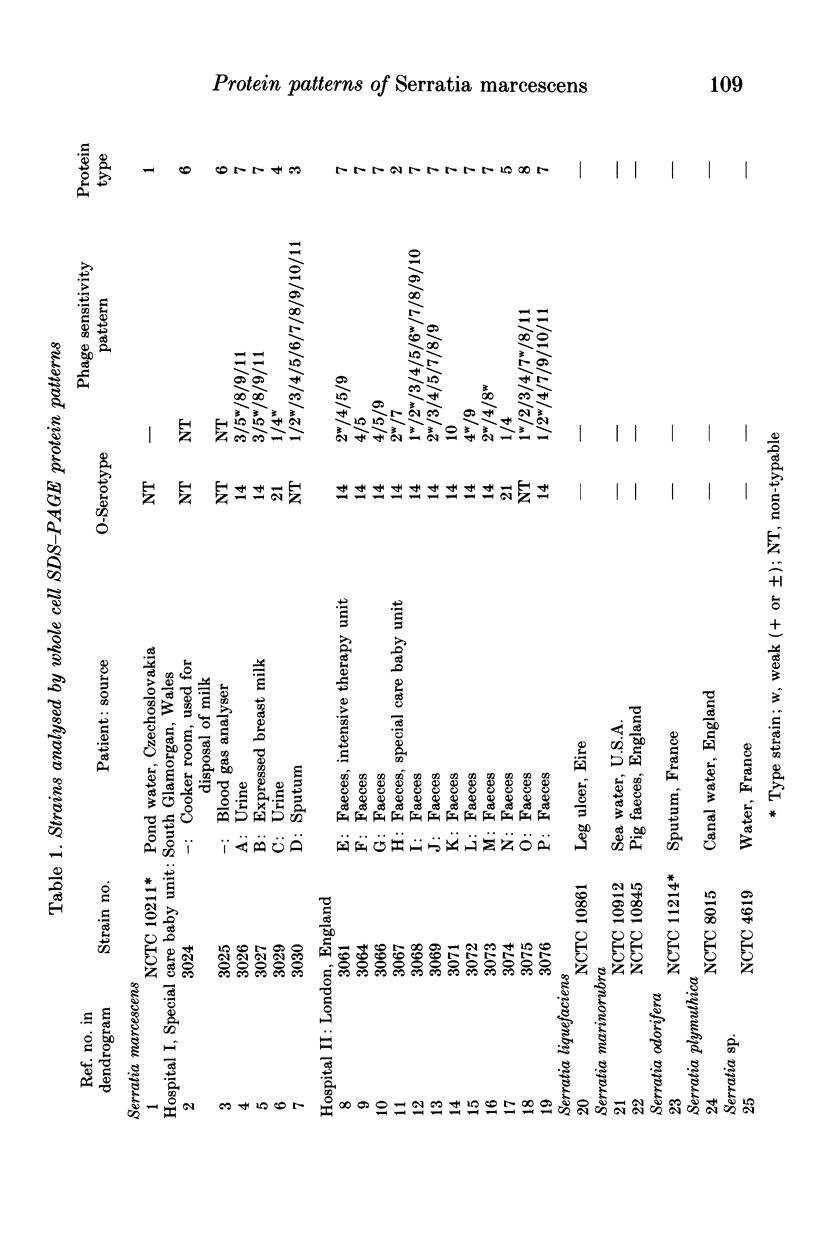
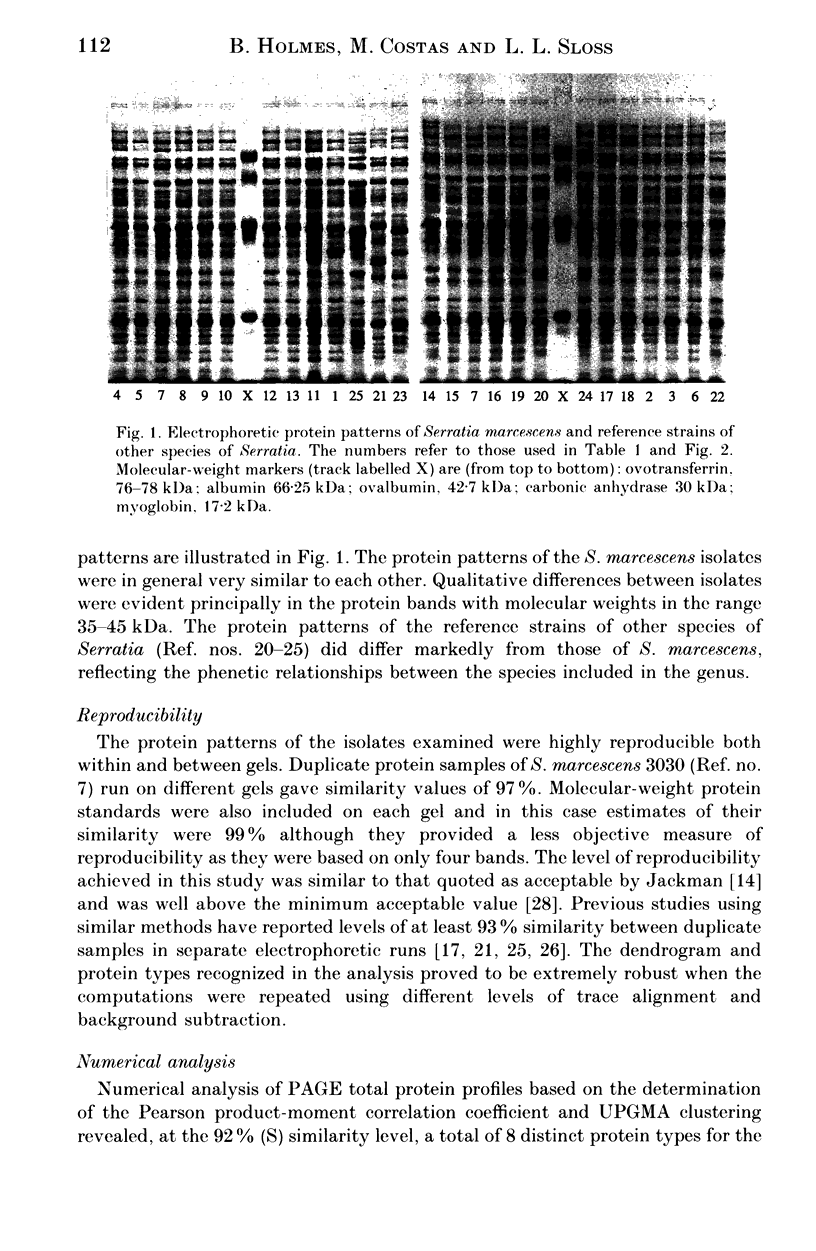
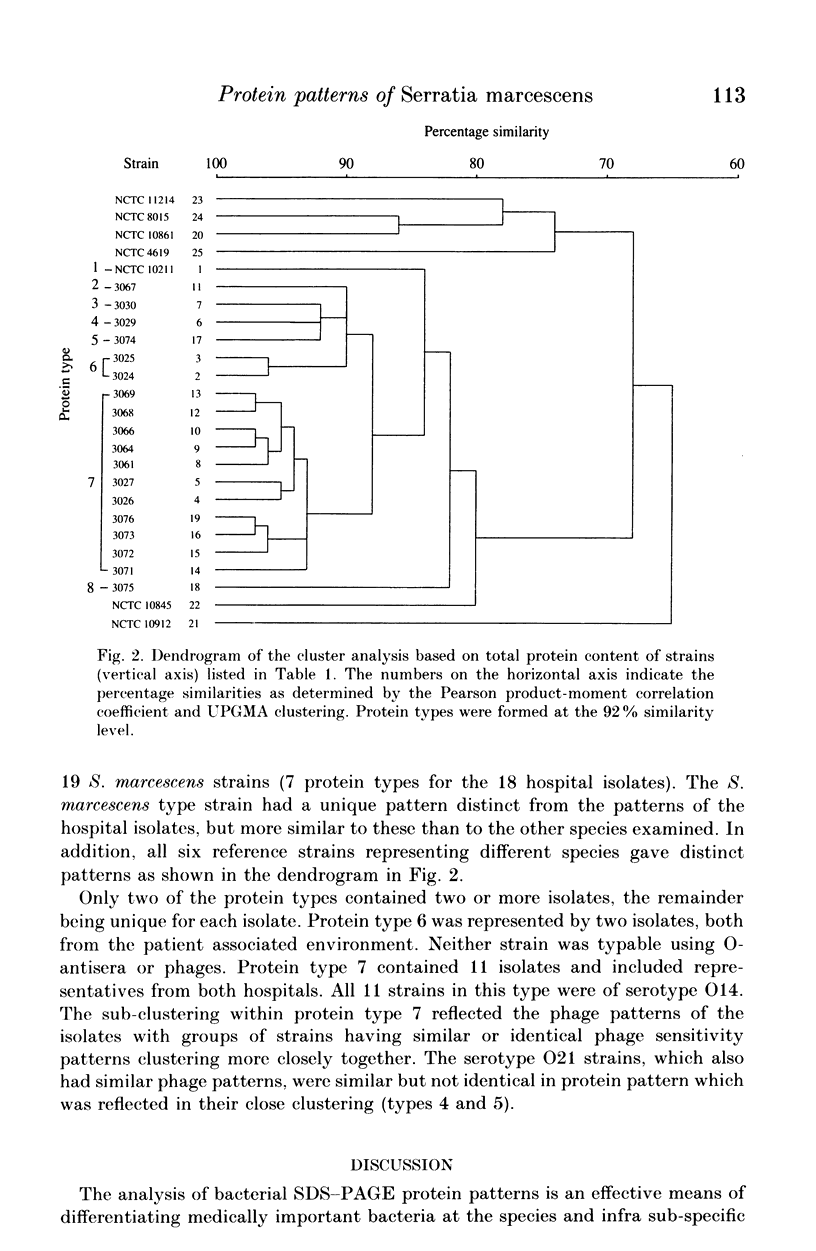
Twenty-five cultures comprising 18 clinical isolates of Serratia marcescens from two hospitals, the type strain of S. marcescens, two reference strains of S. marinorubra, the type or a reference strain of three other Serratia species and a reference strain of undetermined species, were characterized by one-dimensional sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) of whole-cell proteins. The protein patterns were highly reproducible and were used as the basis of a numerical analysis which divided the clinical isolates into eight protein types. Comparison with O-serotyping indicated that the level of discrimination by SDS-PAGE was similar. As with O-serotyping, a secondary scheme, such as phage typing, is necessary to differentiate strains of the same protein type. We conclude that high-resolution SDS-PAGE of proteins provides an effective adjunct to other methods for typing isolates of S. marcescens.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M., Rahman M., Taylor M., Noble W. C. A study of the value of electrophoretic and other techniques for typing Acinetobacter calcoaceticus. J Hosp Infect. 1988 Nov;12(4):273–287. doi: 10.1016/0195-6701(88)90069-2. [DOI] [PubMed] [Google Scholar]
- Arzese A., Botta G. A., Gesu G. P., Schito G. Evaluation of a computer-assisted method of analysing SDS-PAGE protein profiles in tracing a hospital outbreak of Serratia marcescens. J Infect. 1988 Jul;17(1):35–42. doi: 10.1016/s0163-4453(88)92284-0. [DOI] [PubMed] [Google Scholar]
- Costas M., Cookson B. D., Talsania H. G., Owen R. J. Numerical analysis of electrophoretic protein patterns of methicillin-resistant strains of Staphylococcus aureus. J Clin Microbiol. 1989 Nov;27(11):2574–2581. doi: 10.1128/jcm.27.11.2574-2581.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costas M., Holmes B., Sloss L. L. Numerical analysis of electrophoretic protein patterns of Providencia rustigianii strains from human diarrhoea and other sources. J Appl Bacteriol. 1987 Oct;63(4):319–328. doi: 10.1111/j.1365-2672.1987.tb02709.x. [DOI] [PubMed] [Google Scholar]
- Costas M., Holmes B., Wood A. C., On S. L. Numerical analysis of electrophoretic protein patterns of Providencia rettgeri strains from human faeces, urine and other specimens. J Appl Bacteriol. 1989 Oct;67(4):441–452. doi: 10.1111/j.1365-2672.1989.tb02515.x. [DOI] [PubMed] [Google Scholar]
- Costas M., Sloss L. L., Owen R. J., Gaston M. A. Evaluation of numerical analysis of SDS-PAGE of protein patterns for typing Enterobacter cloacae. Epidemiol Infect. 1989 Oct;103(2):265–274. doi: 10.1017/s0950268800030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston M. A., Pitt T. L. O-antigen specificities of the serotype strains of Serratia marcescens. J Clin Microbiol. 1989 Dec;27(12):2697–2701. doi: 10.1128/jcm.27.12.2697-2701.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont P. A., Grimont F. Biotyping of Serratia marcescens and its use in epidemiological studies. J Clin Microbiol. 1978 Jul;8(1):73–83. doi: 10.1128/jcm.8.1.73-83.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Costas M., Sloss L. L. Numerical analysis of electrophoretic protein patterns of Providencia alcalifaciens strains from human faeces and veterinary specimens. J Appl Bacteriol. 1988 Jan;64(1):27–35. doi: 10.1111/j.1365-2672.1988.tb02426.x. [DOI] [PubMed] [Google Scholar]
- Le Minor S., Pigache F. Etude antigénique de souches de Serratia marcescens isolées en France. I--Antigènes H: individualisation de six nouveaux facteurs H. Ann Microbiol (Paris) 1977 Aug-Sep;128(2):207–214. [PubMed] [Google Scholar]
- Le Minor S., Sauvageot-Pigache F. Nouveaux facteurs antigéniques H (H21-H25) et O (O21) de Serratia marcescens: subdivision des facteurs O5, O10, O16. Ann Microbiol (Paris) 1981 May-Jun;132(3):239–252. [PubMed] [Google Scholar]
- Mulligan M. E., Peterson L. R., Kwok R. Y., Clabots C. R., Gerding D. N. Immunoblots and plasmid fingerprints compared with serotyping and polyacrylamide gel electrophoresis for typing Clostridium difficile. J Clin Microbiol. 1988 Jan;26(1):41–46. doi: 10.1128/jcm.26.1.41-46.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J., Costas M., Morgan D. D., On S. L., Hill L. R., Pearson A. D., Morgan D. R. Strain variation in Campylobacter pylori detected by numerical analysis of one-dimensional electrophoretic protein patterns. Antonie Van Leeuwenhoek. 1989 Mar;55(3):253–267. doi: 10.1007/BF00393854. [DOI] [PubMed] [Google Scholar]
- Pitt T. L., Erdman Y. J., Bucher C. The epidemiological type identification of Serratia marcescens from outbreaks of infection in hospitals. J Hyg (Lond) 1980 Apr;84(2):269–283. doi: 10.1017/s0022172400026772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifuentes-Osornio J., Gröschel D. H. Modification of Grimont biotyping system for epidemiologic studies with nosocomial Serratia marcescens isolates. J Clin Microbiol. 1987 Mar;25(3):567–568. doi: 10.1128/jcm.25.3.567-568.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneath P. H., Johnson R. The influence on numerical taxonomic similarities of errors in microbiological tests. J Gen Microbiol. 1972 Sep;72(2):377–392. doi: 10.1099/00221287-72-2-377. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Crook S. J., Tabaqchali S. New method for typing Staphylococcus aureus resistant to methicillin based on sulphur-35 methionine labelled proteins: its application in an outbreak. Br Med J (Clin Res Ed) 1986 Sep 6;293(6547):581–583. doi: 10.1136/bmj.293.6547.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabaqchali S., O'Farrell S., Holland D., Silman R. Method for the typing of Clostridium difficile based on polyacrylamide gel electrophoresis of [35S]methionine-labeled proteins. J Clin Microbiol. 1986 Jan;23(1):197–198. doi: 10.1128/jcm.23.1.197-198.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W. H. Serotyping of Serratia marcescens: identification of a new O-antigen (O24). Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Jul;259(4):485–488. doi: 10.1016/s0176-6724(85)80080-8. [DOI] [PubMed] [Google Scholar]
- Wilfert J. N., Barrett F. F., Ewing W. H., Finland M., Kass E. H. Serratia marcescens: biochemical, serological, and epidemiological characteristics and antibiotic susceptibility of strains isolated at Boston City Hospital. Appl Microbiol. 1970 Feb;19(2):345–352. doi: 10.1128/am.19.2.345-352.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



