Abstract
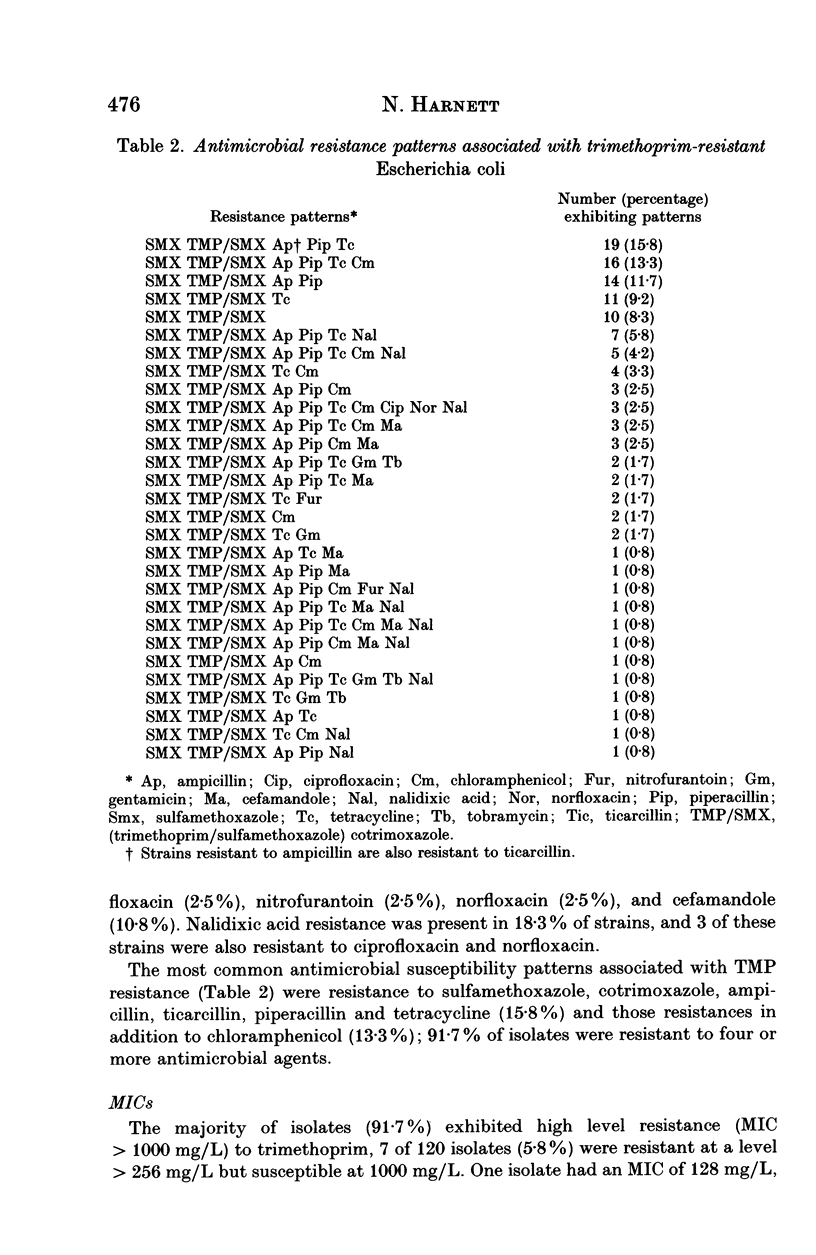
Of 1171 isolates of Escherichia coli isolated from urine samples at the Public Health Laboratory, Toronto, Ontario, Canada, between May 1990 and December 1991, 120 (10.3%) were resistant to trimethoprim (TMP), cotrimoxazole (TMP/SMX), sulfamethoxazole (SMX) and other antimicrobial agents; 110 of the 120 isolates (91.7%) were resistant to four or more agents. The majority of resistant isolates (91.7%) exhibited high-level resistance (MIC > 1000 mg/L) to TMP. The MIC of TMP/SMX for all 120 isolates was > 2.0/38.0 mg/L and for SMX > 1024 mg/L. High-level resistances were also present among the beta-lactam antimicrobials with MICs ranging from 16- > 256 mg/L. Forty-three of 120 TMP-resistant (35.8%) isolates conjugally transferred TMP-resistance to E. coli K-12. Co-transfer of several other resistances was observed. SMX cotransferred from 86% of the 43 donors and beta-lactams together with SMX cotransferred from 70%. Nalidixic acid resistance was present among 22 (18.3%) of the 120 resistant isolates, however, nalidixic acid resistance was not transferred to E. coli K-12.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavalieri S. J., Snyder I. S. Effect of Escherichia coli alpha-hemolysin on human peripheral leukocyte viability in vitro. Infect Immun. 1982 May;36(2):455–461. doi: 10.1128/iai.36.2.455-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen M. E., Wyke A. W., Kuroda R., Fisher L. M. Cloning and characterization of a DNA gyrase A gene from Escherichia coli that confers clinical resistance to 4-quinolones. Antimicrob Agents Chemother. 1989 Jun;33(6):886–894. doi: 10.1128/aac.33.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett N. Antimicrobial susceptibilities of Shigella species isolated in Ontario in 1990. Can Dis Wkly Rep. 1991 Dec 14;17(50):275–277. [PubMed] [Google Scholar]
- Harnett N. High level resistance to trimethoprim, cotrimoxazole and other antimicrobial agents among clinical isolates of Shigella species in Ontario, Canada--an update. Epidemiol Infect. 1992 Dec;109(3):463–472. doi: 10.1017/s0950268800050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett N., McLeod S., AuYong Y., Krishnan C. Increasing incidence of resistance among shigellae to trimethoprim. Lancet. 1991 Mar 9;337(8741):622–622. doi: 10.1016/0140-6736(91)91694-p. [DOI] [PubMed] [Google Scholar]
- Heikkilä E., Renkonen O. V., Sunila R., Uurasmaa P., Huovinen P. The emergence and mechanisms of trimethoprim resistance in Escherichia coli isolated from outpatients in Finland. J Antimicrob Chemother. 1990 Feb;25(2):275–283. doi: 10.1093/jac/25.2.275. [DOI] [PubMed] [Google Scholar]
- Heikkilä E., Siitonen A., Jahkola M., Fling M., Sundström L., Huovinen P. Increase of trimethoprim resistance among Shigella species, 1975-1988: analysis of resistance mechanisms. J Infect Dis. 1990 Jun;161(6):1242–1248. doi: 10.1093/infdis/161.6.1242. [DOI] [PubMed] [Google Scholar]
- Heikkilä E., Sundström L., Huovinen P. Trimethoprim resistance in Escherichia coli isolates from a geriatric unit. Antimicrob Agents Chemother. 1990 Oct;34(10):2013–2015. doi: 10.1128/aac.34.10.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe H., Shah P., Krcmery V., Antal M., Mitsuhashi S. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection. 1983 Nov-Dec;11(6):315–317. doi: 10.1007/BF01641355. [DOI] [PubMed] [Google Scholar]
- Lamikanra A., Ndep R. B. Trimethoprim resistance in urinary tract pathogens in two Nigerian hospitals. J Antimicrob Chemother. 1989 Jan;23(1):151–154. doi: 10.1093/jac/23.1.151. [DOI] [PubMed] [Google Scholar]
- Mayer K. H., Fling M. E., Hopkins J. D., O'Brien T. F. Trimethoprim resistance in multiple genera of Enterobacteriaceae at a U.S. hospital: spread of the type II dihydrofolate reductase gene by a single plasmid. J Infect Dis. 1985 May;151(5):783–789. doi: 10.1093/infdis/151.5.783. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Alvarado T., Kim K. H., Vorachit M., Jayanetra P., Levine M. M., Prenzel I., Fling M., Elwell L., McCracken G. H. Increasing resistance to trimethoprim-sulfamethoxazole among isolates of Escherichia coli in developing countries. J Infect Dis. 1985 Dec;152(6):1107–1113. doi: 10.1093/infdis/152.6.1107. [DOI] [PubMed] [Google Scholar]
- Nandivada L. S., Amyes S. G. Plasmid-mediated beta-lactam resistance in pathogenic gram-negative bacteria isolated in south India. J Antimicrob Chemother. 1990 Aug;26(2):279–290. doi: 10.1093/jac/26.2.279. [DOI] [PubMed] [Google Scholar]
- Ronald A. R., Turck M., Petersdorf R. G. A critical evaluation of nalidixic acid in urinary-tract infections. N Engl J Med. 1966 Nov 17;275(20):1081–1089. doi: 10.1056/NEJM196611172752001. [DOI] [PubMed] [Google Scholar]
- Tauxe R. V., Puhr N. D., Wells J. G., Hargrett-Bean N., Blake P. A. Antimicrobial resistance of Shigella isolates in the USA: the importance of international travelers. J Infect Dis. 1990 Nov;162(5):1107–1111. doi: 10.1093/infdis/162.5.1107. [DOI] [PubMed] [Google Scholar]
- Trimethoprim--resistant coliforms. Lancet. 1977 Oct 29;2(8044):926–926. [PubMed] [Google Scholar]
- Tsakris A., Johnson A. P., George R. C., Mehtar S., Vatopoulos A. C. Distribution and transferability of plasmids encoding trimethoprim resistance in urinary pathogens from Greece. J Med Microbiol. 1991 Mar;34(3):153–157. doi: 10.1099/00222615-34-3-153. [DOI] [PubMed] [Google Scholar]
- Waalwijk C., MacLaren D. M., de Graaff J. In vivo function of hemolysin in the nephropathogenicity of Escherichia coli. Infect Immun. 1983 Oct;42(1):245–249. doi: 10.1128/iai.42.1.245-249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., van den Bosch J. F., MacLaren D. M., de Graaff J. Hemolysin plasmid coding for the virulence of a nephropathogenic Escherichia coli strain. Infect Immun. 1982 Jan;35(1):32–37. doi: 10.1128/iai.35.1.32-37.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- Young H. K., Jesudason M. V., Koshi G., Amyes S. G. Trimethoprim resistance amongst urinary pathogens in south India. J Antimicrob Chemother. 1986 May;17(5):615–621. doi: 10.1093/jac/17.5.615. [DOI] [PubMed] [Google Scholar]
- Young H. K., Jesudason M. V., Koshi G., Amyes S. G. Unusual expression of new low-level-trimethoprim-resistance plasmids. J Clin Microbiol. 1986 Jul;24(1):61–64. doi: 10.1128/jcm.24.1.61-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


