Abstract
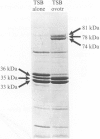
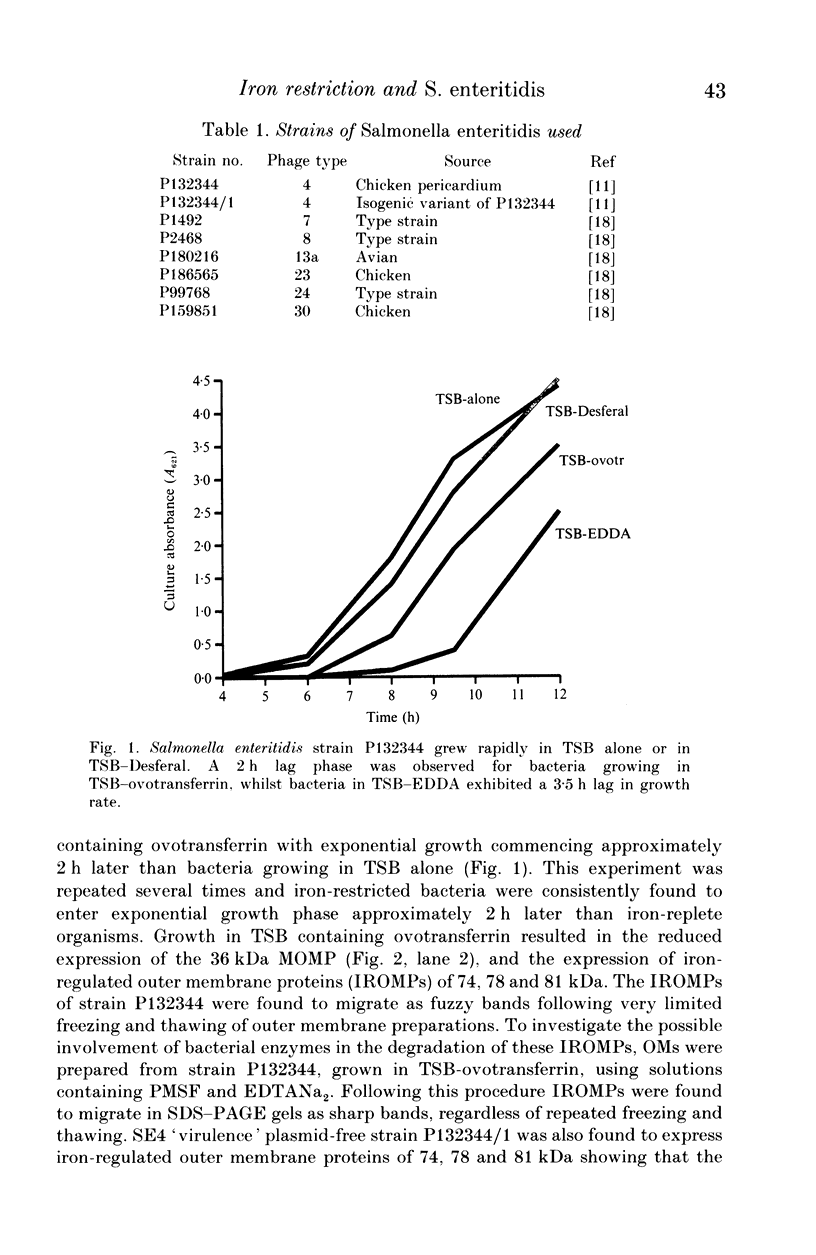
Strains of Salmonella enteritidis were examined for their ability to remove ferric-ions from the iron chelating agents ovotransferrin, Desferal and EDDA. Growth of S. enteritidis phage type (PT) 4 (SE4) in trypticase soy broth containing ovotransferrin resulted in the expression of iron regulated outer membrane proteins (OMPs) of 74, 78 and 81 kDa, and unexpectedly the repression of expression of OMP C. The 38 MDa 'mouse virulence' plasmid was not required for the expression of the iron-regulated OMPs (IROMPs). SE4 was able to obtain iron bound to the iron chelator Desferal and EDDA without expressing a high-affinity iron uptake system. Strains of S. enteritidis belonging to PTs 7, 8, 13a, 23, 24 and 30 were also able to remove ferric ions from Desferal and EDDA without expressing a high-affinity iron uptake system. We conclude that strains of SE4 possess a high-affinity iron sequestering mechanism that can readily remove iron from ovotransferrin. It is likely that iron limitation, and not iron restriction, is responsible for the bacteriostatic properties of fresh egg whites.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chart H., Buck M., Stevenson P., Griffiths E. Iron regulated outer membrane proteins of Escherichia coli: variations in expression due to the chelator used to restrict the availability of iron. J Gen Microbiol. 1986 May;132(5):1373–1378. doi: 10.1099/00221287-132-5-1373. [DOI] [PubMed] [Google Scholar]
- Chart H., Griffiths E. Antigenic and molecular homology of the ferric enterobactin receptor protein of Escherichia coli. J Gen Microbiol. 1985 Jun;131(6):1503–1509. doi: 10.1099/00221287-131-6-1503. [DOI] [PubMed] [Google Scholar]
- Chart H., Stevenson P., Griffiths E. Iron-regulated outer-membrane proteins of Escherichia coli strains associated with enteric or extraintestinal diseases of man and animals. J Gen Microbiol. 1988 Jun;134(6):1549–1559. doi: 10.1099/00221287-134-6-1549. [DOI] [PubMed] [Google Scholar]
- Chart H., Trust T. J. Acquisition of iron by Aeromonas salmonicida. J Bacteriol. 1983 Nov;156(2):758–764. doi: 10.1128/jb.156.2.758-764.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay C. E., Board R. G. Growth of Salmonella enteritidis in artificially contaminated hens' shell eggs. Epidemiol Infect. 1991 Apr;106(2):271–281. doi: 10.1017/s095026880004841x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle E. F., Palmer S. R., Ribeiro C. D., Jones H. I., Howard A. J., Ward L., Rowe B. Salmonella enteritidis phage type 4 infection: association with hen's eggs. Lancet. 1988 Dec 3;2(8623):1295–1297. doi: 10.1016/s0140-6736(88)92902-9. [DOI] [PubMed] [Google Scholar]
- Faundez G., Aron L., Cabello F. C. Chromosomal DNA, iron-transport systems, outer membrane proteins, and enterotoxin (heat labile) production in Salmonella typhi strains. J Clin Microbiol. 1990 May;28(5):894–897. doi: 10.1128/jcm.28.5.894-897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths E., Humphreys J. Alterations in tRNAs containing 2-methylthio-N6-(delta2-isopentenyl)-adenosine during growth of enteropathogenic Escherichia coli in the presence of iron-binding proteins. Eur J Biochem. 1978 Jan 16;82(2):503–513. doi: 10.1111/j.1432-1033.1978.tb12044.x. [DOI] [PubMed] [Google Scholar]
- Humphrey T. J., Baskerville A., Mawer S., Rowe B., Hopper S. Salmonella enteritidis phage type 4 from the contents of intact eggs: a study involving naturally infected hens. Epidemiol Infect. 1989 Dec;103(3):415–423. doi: 10.1017/s0950268800030818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey T. J., Whitehead A., Gawler A. H., Henley A., Rowe B. Numbers of Salmonella enteritidis in the contents of naturally contaminated hens' eggs. Epidemiol Infect. 1991 Jun;106(3):489–496. doi: 10.1017/s0950268800067546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J. L., Board R. G. Persistence of contamination of hens' egg albumen in vitro with Salmonella serotypes. Epidemiol Infect. 1992 Jun;108(3):389–396. doi: 10.1017/s095026880004989x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M. Iron and virulence in Shigella. Mol Microbiol. 1989 Sep;3(9):1301–1306. doi: 10.1111/j.1365-2958.1989.tb00281.x. [DOI] [PubMed] [Google Scholar]
- Payne S. M. Iron and virulence in the family Enterobacteriaceae. Crit Rev Microbiol. 1988;16(2):81–111. doi: 10.3109/10408418809104468. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade A. L., Caroline L. RAW HEN EGG WHITE AND THE ROLE OF IRON IN GROWTH INHIBITION OF SHIGELLA DYSENTERIAE, STAPHYLOCOCCUS AUREUS, ESCHERICHIA COLI AND SACCHAROMYCES CEREVISIAE. Science. 1944 Jul 7;100(2584):14–15. doi: 10.1126/science.100.2584.14. [DOI] [PubMed] [Google Scholar]
- Threlfall E. J., Rowe B., Ward L. R. Subdivision of Salmonella enteritidis phage types by plasmid profile typing. Epidemiol Infect. 1989 Jun;102(3):459–465. doi: 10.1017/s095026880003017x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A. C., Simpson L. M., Oliver J. D. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect Immun. 1981 Nov;34(2):503–507. doi: 10.1128/iai.34.2.503-507.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]