Abstract
Genotypic and phenotypic analysis of 42 strains of Streptococcus uberis isolated from mammary secretions of 17 cows collected at different periods of the lactation cycle and from episodes of clinical mastitis were performed. Seventeen restriction endonuclease fingerprint (REF) patterns and 12 bacteriocin-like inhibitory substance (BLIS) fingerprints were observed. REF identified and differentiated closely related strains of S. uberis isolated from mammary secretions collected from the same cow at different periods of the lactation cycle and from episodes of clinical mastitis. BLIS fingerprinting of S. uberis complemented REF results. REF and BLIS fingerprinting provided evidence concerning persistence of infection in the same quarter or different quarters of the mammary gland over different periods of the lactation cycle, and occurrence of infection with similar and dissimilar strains of S. uberis. Biochemical profiles could not identify closely related strains nor did they complement REF results. Antibiotic resistance patterns alone were of little value in differentiating closely related strains, but were identical with isolates having same REF pattern. None of the S. uberis strains was found to carry plasmids. REF and BLIS fingerprinting can be utilized effectively to differentiate closely related and unrelated strains of S. uberis isolated from bovine mammary secretions.
Full text
PDF



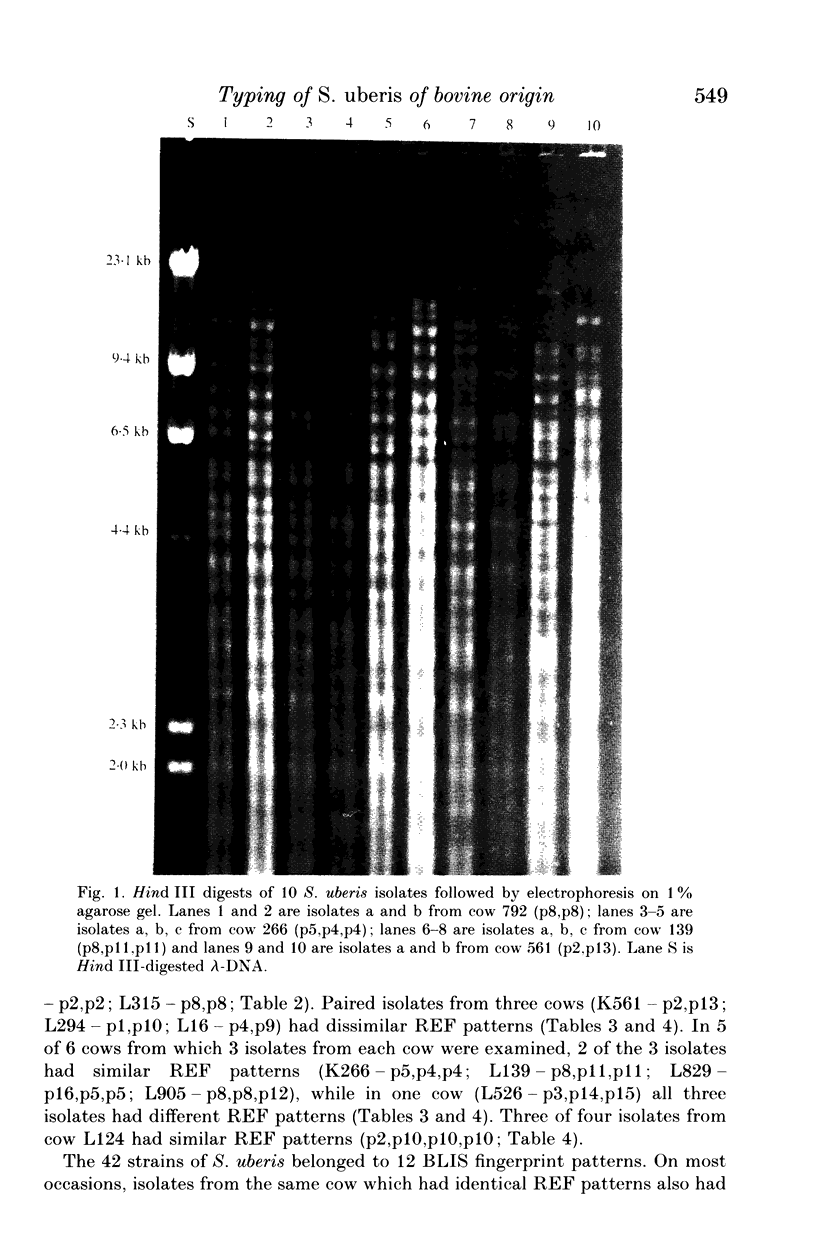
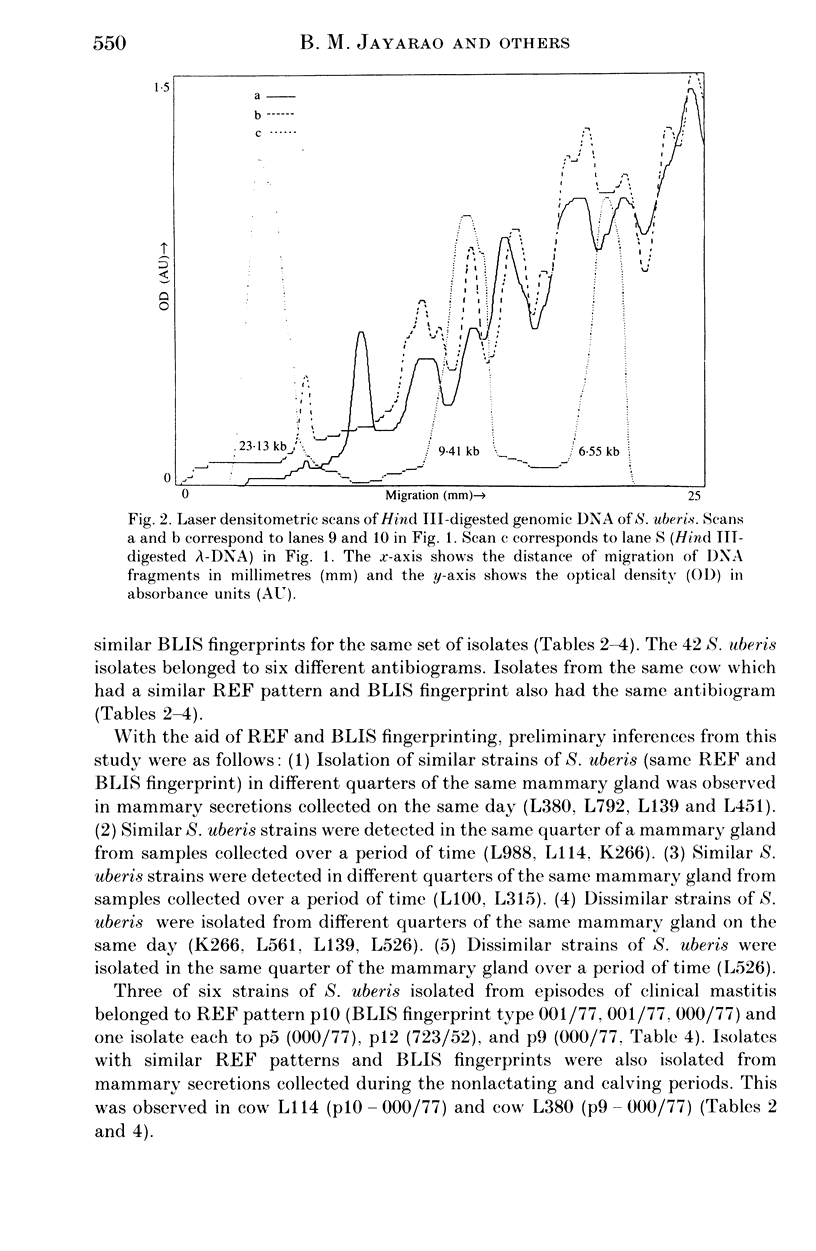
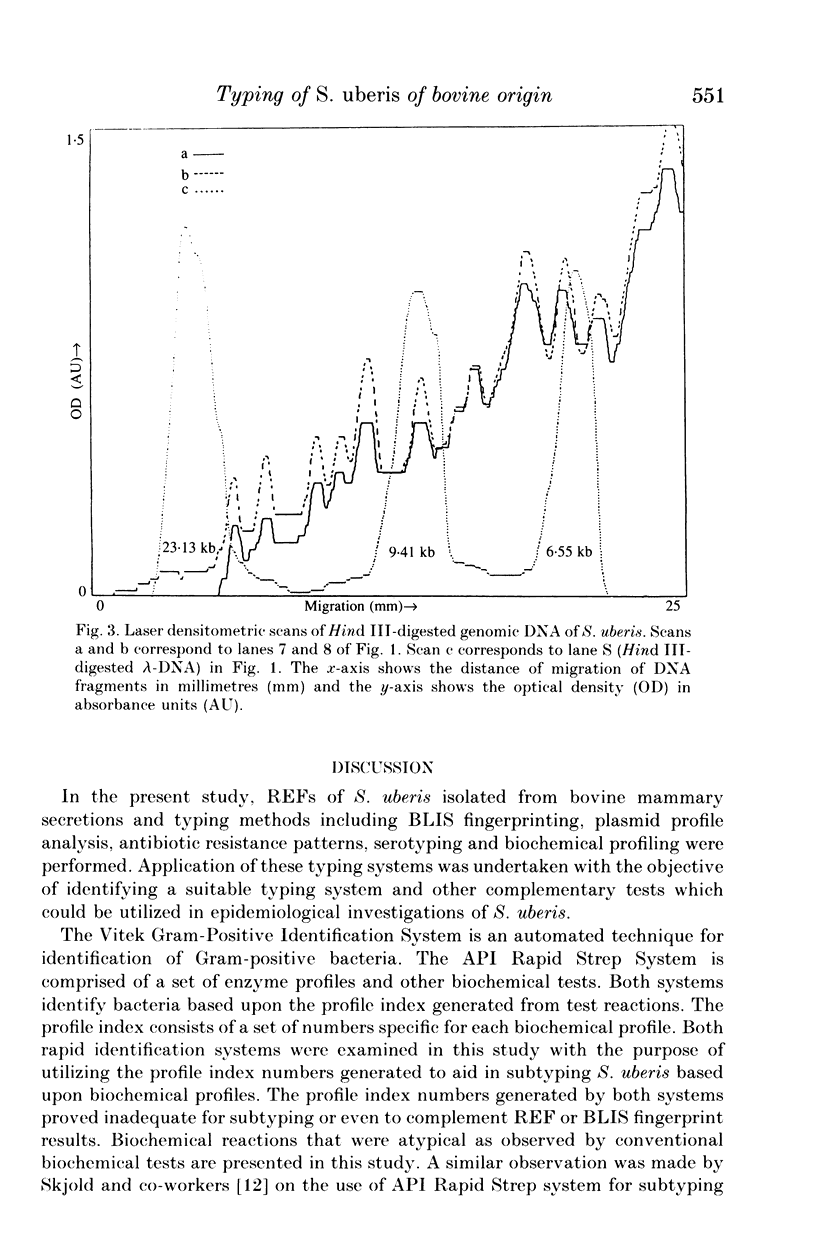




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Buddle B. M., Tagg J. R., Ralston M. J. Use of an inhibitor typing scheme to study the epidemiology of Streptococcus uberis mastitis. N Z Vet J. 1988 Sep;36(3):115–119. doi: 10.1080/00480169.1988.35504. [DOI] [PubMed] [Google Scholar]
- Caufield P. W., Walker T. M. Genetic diversity within Streptococcus mutans evident from chromosomal DNA restriction fragment polymorphisms. J Clin Microbiol. 1989 Feb;27(2):274–278. doi: 10.1128/jcm.27.2.274-278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary P. P., Kaplan E. L., Livdahl C., Skjold S. DNA fingerprints of Streptococcus pyogenes are M type specific. J Infect Dis. 1988 Dec;158(6):1317–1323. doi: 10.1093/infdis/158.6.1317. [DOI] [PubMed] [Google Scholar]
- Denning D. W., Baker C. J., Troup N. J., Tompkins L. S. Restriction endonuclease analysis of human and bovine group B streptococci for epidemiologic study. J Clin Microbiol. 1989 Jun;27(6):1352–1356. doi: 10.1128/jcm.27.6.1352-1356.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. W., Brady C. A. A note on the isolation and propagation of lytic phages from Streptococcus uberis and their potential for strain typing. J Appl Bacteriol. 1989 Oct;67(4):425–431. doi: 10.1111/j.1365-2672.1989.tb02513.x. [DOI] [PubMed] [Google Scholar]
- Hill A. W., Leigh J. A. DNA fingerprinting of Streptococcus uberis: a useful tool for epidemiology of bovine mastitis. Epidemiol Infect. 1989 Aug;103(1):165–171. doi: 10.1017/s0950268800030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayarao B. M., Oliver S. P., Matthews K. R., King S. H. Comparative evaluation of Vitek gram-positive identification system and API Rapid Strep system for identification of Streptococcus species of bovine origin. Vet Microbiol. 1991 Feb 1;26(3):301–308. doi: 10.1016/0378-1135(91)90023-9. [DOI] [PubMed] [Google Scholar]
- Leblanc D. J., Lee L. N. Rapid screening procedure for detection of plasmids in streptococci. J Bacteriol. 1979 Dec;140(3):1112–1115. doi: 10.1128/jb.140.3.1112-1115.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N. J., Kaplan E. L., Gerber M. A., Menegus M. A., Randolph M., Bell K., Cleary P. P. Comparison of epidemic and endemic group G streptococci by restriction enzyme analysis. J Clin Microbiol. 1990 Sep;28(9):1881–1886. doi: 10.1128/jcm.28.9.1881-1886.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogollon J. D., Pijoan C., Murtaugh M. P., Kaplan E. L., Collins J. E., Cleary P. P. Characterization of prototype and clinically defined strains of Streptococcus suis by genomic fingerprinting. J Clin Microbiol. 1990 Nov;28(11):2462–2466. doi: 10.1128/jcm.28.11.2462-2466.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Heath J. D., Sharma B. R., Weinstock G. M. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990 Sep;28(9):2059–2063. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S. P. Frequency of isolation of environmental mastitis-causing pathogens and incidence of new intramammary infection during the nonlactating period. Am J Vet Res. 1988 Nov;49(11):1789–1793. [PubMed] [Google Scholar]
- Oliver S. P., Mitchell B. A. Prevalence of mastitis pathogens in herds participating in a mastitis control program. J Dairy Sci. 1984 Oct;67(10):2436–2440. doi: 10.3168/jds.S0022-0302(84)81592-1. [DOI] [PubMed] [Google Scholar]
- Oliver S. P., Sordillo L. M. Udder health in the periparturient period. J Dairy Sci. 1988 Sep;71(9):2584–2606. doi: 10.3168/jds.S0022-0302(88)79847-1. [DOI] [PubMed] [Google Scholar]
- Roguinsky M. Réactions de Streptococcus uberis avec les sérums G et P. Ann Inst Pasteur (Paris) 1969 Oct;117(4):529–532. [PubMed] [Google Scholar]
- Schofield C. R., Tagg J. R. Bacteriocin-like activity of group B and group C streptococci of human and of animal origin. J Hyg (Lond) 1983 Feb;90(1):7–18. doi: 10.1017/s0022172400063774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjold S. A., Quie P. G., Fries L. A., Barnham M., Cleary P. P. DNA fingerprinting of Streptococcus zooepidemicus (Lancefield group C) as an aid to epidemiological study. J Infect Dis. 1987 Jun;155(6):1145–1150. doi: 10.1093/infdis/155.6.1145. [DOI] [PubMed] [Google Scholar]
- Smith K. L., Todhunter D. A., Schoenberger P. S. Environmental pathogens and intramammary infection during the dry period. J Dairy Sci. 1985 Feb;68(2):402–417. doi: 10.3168/jds.s0022-0302(85)80838-9. [DOI] [PubMed] [Google Scholar]
- Tagg J. R., Bannister L. V. "Fingerprinting" beta-haemolytic streptococci by their production of and sensitivity to bacteriocine-like inhibitors. J Med Microbiol. 1979 Nov;12(4):397–411. doi: 10.1099/00222615-12-4-397. [DOI] [PubMed] [Google Scholar]
- Tagg J. R., Vugler L. G. An inhibitor typing scheme for Streptococcus uberis. J Dairy Res. 1986 Aug;53(3):451–456. doi: 10.1017/s0022029900025061. [DOI] [PubMed] [Google Scholar]
- Tompkins L. S., Troup N. J., Woods T., Bibb W., McKinney R. M. Molecular epidemiology of Legionella species by restriction endonuclease and alloenzyme analysis. J Clin Microbiol. 1987 Oct;25(10):1875–1880. doi: 10.1128/jcm.25.10.1875-1880.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. M., Collins M. D. Molecular taxonomic studies on Streptococcus uberis types I and II. Description of Streptococcus parauberis sp. nov. J Appl Bacteriol. 1990 May;68(5):485–490. doi: 10.1111/j.1365-2672.1990.tb02900.x. [DOI] [PubMed] [Google Scholar]