Abstract
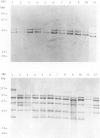
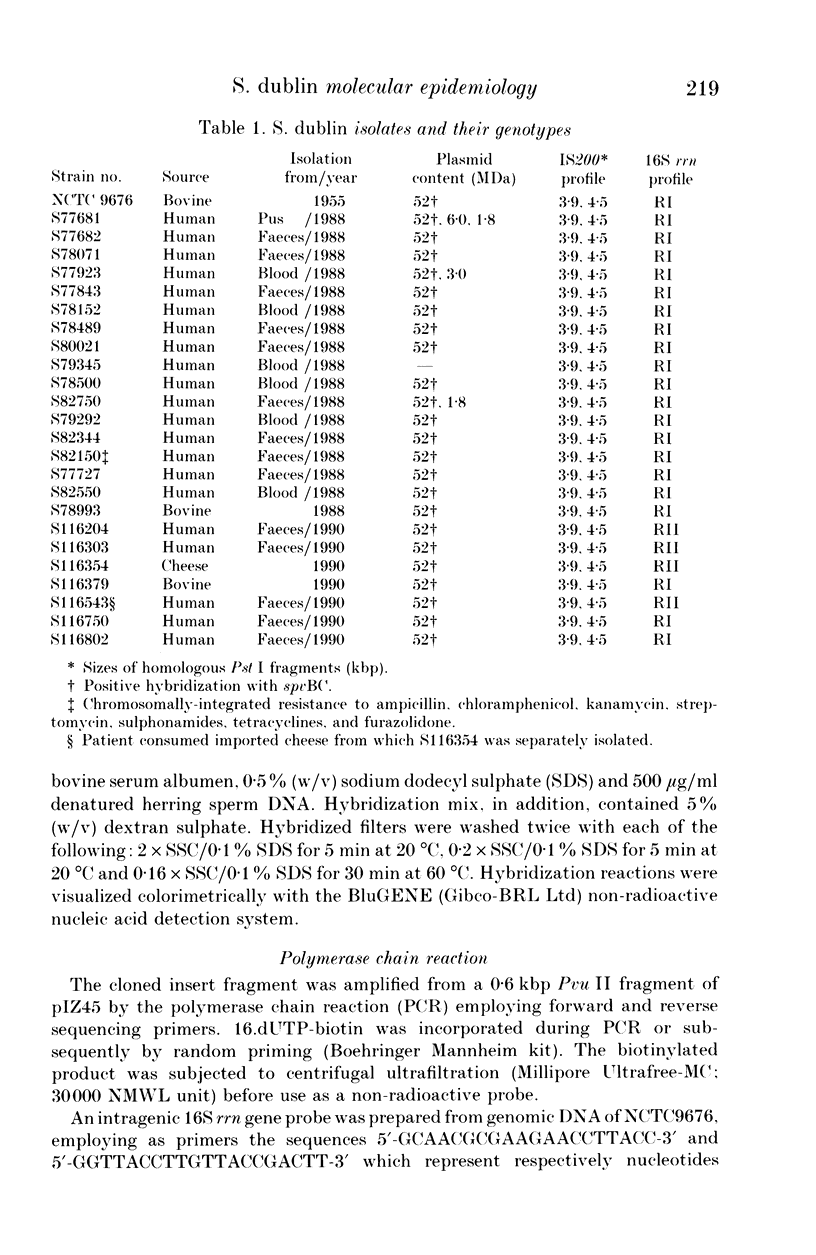
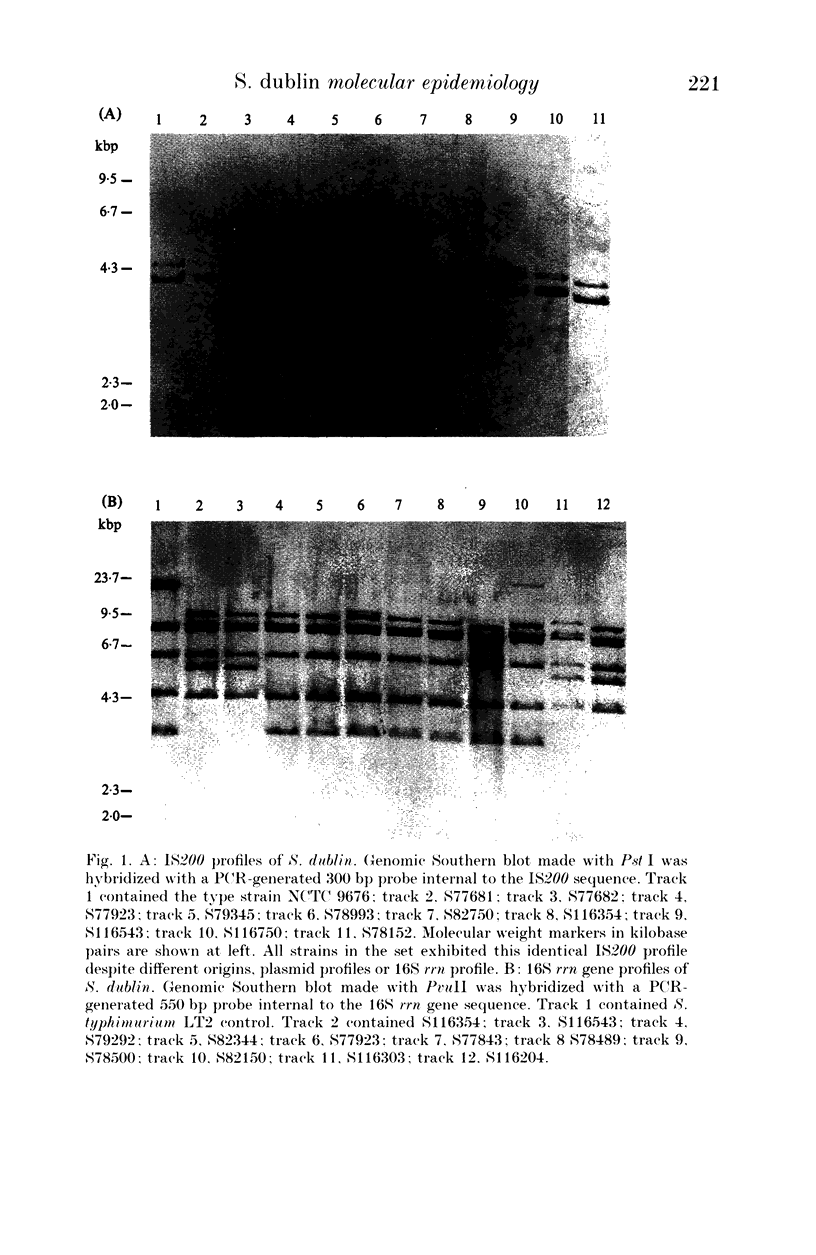
An analysis of genotype was made for representative strains of Salmonella dublin. The collection consisted primarily of strains isolated from humans in England and Wales, and were of both intestinal and extra-intestinal origin. Three genetic elements were characterized by DNA hybridization. They were the spvBC genes, extrachromosomal virulence determinants, the salmonella-specific insertion sequence IS200, and the 16S ribosomal RNA genes, a phylogenetic marker. Two clones of S. dublin (SdRI and SdRII) which shared an identical IS200 profile, were identified on the basis of restriction fragment length polymorphism at the 16S rRNA locus. With one exception, all strains harboured a 52 MDa plasmid which contained a conserved 3.7 kbp Hind III fragment homologous to the spvBC mouse-virulence genes of S. typhimurium. However, a single plasmid-free strain of SdRI, isolated from a patient with septicaemia exhibited no spc homology. In SdRI there was no observable genotype distinction between strains causing gastroenteritis or bacteraemia. In contrast, none of the strains of SdRII were from cases of bacteraemia, and all human isolates of this clone were from cases of gastroenteritis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beninger P. R., Chikami G., Tanabe K., Roudier C., Fierer J., Guiney D. G. Physical and genetic mapping of the Salmonella dublin virulence plasmid pSDL2. Relationship to plasmids from other Salmonella strains. J Clin Invest. 1988 May;81(5):1341–1347. doi: 10.1172/JCI113461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibert I., Carroll K., Hillyard D. R., Barbé J., Casadesus J. IS200 is not a member of the IS600 family of insertion sequences. Nucleic Acids Res. 1991 Mar 25;19(6):1343–1343. doi: 10.1093/nar/19.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Woods D. R. Effect of oxygen on liquid holding recovery of Bacteroides fragilis. J Bacteriol. 1981 Jan;145(1):1–7. doi: 10.1128/jb.145.1.1-7.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S., Roth J. R. IS200: a Salmonella-specific insertion sequence. Cell. 1983 Oct;34(3):951–960. doi: 10.1016/0092-8674(83)90552-4. [DOI] [PubMed] [Google Scholar]
- Lax A. J., Pullinger G. D., Baird G. D., Williamson C. M. The virulence plasmid of Salmonella dublin: detailed restriction map and analysis by transposon mutagenesis. J Gen Microbiol. 1990 Jun;136(6):1117–1123. doi: 10.1099/00221287-136-6-1117. [DOI] [PubMed] [Google Scholar]
- O'Reilly C., Black G. W., Laffey R., McConnell D. J. Molecular analysis of an IS200 insertion in the gpt gene of Salmonella typhimurium LT2. J Bacteriol. 1990 Nov;172(11):6599–6601. doi: 10.1128/jb.172.11.6599-6601.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J. E., Baggesen D. L., Nielsen B. B., Larsen H. E. The prevalence of plasmids in Danish bovine and human isolates of Salmonella dublin. APMIS. 1990 Aug;98(8):735–740. doi: 10.1111/j.1699-0463.1990.tb04994.x. [DOI] [PubMed] [Google Scholar]
- Roudier C., Krause M., Fierer J., Guiney D. G. Correlation between the presence of sequences homologous to the vir region of Salmonella dublin plasmid pSDL2 and the virulence of twenty-two Salmonella serotypes in mice. Infect Immun. 1990 May;58(5):1180–1185. doi: 10.1128/iai.58.5.1180-1185.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer S. A., Dykhuizen D. E., DuBose R. F., Green L., Mutangadura-Mhlanga T., Wolczyk D. F., Hartl D. L. Distribution and abundance of insertion sequences among natural isolates of Escherichia coli. Genetics. 1987 Jan;115(1):51–63. doi: 10.1093/genetics/115.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Smith N. H., Li J., Beltran P., Ferris K. E., Kopecko D. J., Rubin F. A. Molecular evolutionary genetics of the cattle-adapted serovar Salmonella dublin. J Bacteriol. 1992 Jun;174(11):3587–3592. doi: 10.1128/jb.174.11.3587-3592.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Goldsworthy M., Threlfall E. J. Molecular phylogenetic typing of pandemic isolates of Salmonella enteritidis. FEMS Microbiol Lett. 1992 Jan 1;69(2):153–160. doi: 10.1016/0378-1097(92)90620-4. [DOI] [PubMed] [Google Scholar]
- Stull T. L., LiPuma J. J., Edlind T. D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988 Feb;157(2):280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- Threlfall E. J., Frost J. A. The identification, typing and fingerprinting of Salmonella: laboratory aspects and epidemiological applications. J Appl Bacteriol. 1990 Jan;68(1):5–16. doi: 10.1111/j.1365-2672.1990.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Valone S. E., Chikami G. K. Characterization of three proteins expressed from the virulence region of plasmid pSDL2 in Salmonella dublin. Infect Immun. 1991 Oct;59(10):3511–3517. doi: 10.1128/iai.59.10.3511-3517.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]