Abstract
Feedback controls of estrogen in LHRH-1 neurons play a pivotal role in reproductive function. However, the mechanism of estrogen action in LHRH-1 neurons is still unclear. In the present study, the effect of estrogens on intracellular calcium ([Ca2+]i) oscillations in primate LHRH-1 neurons was examined. Application of 17β-estradiol (E2, 1 nm) for 10 min increased the frequency of [Ca2+]i oscillations within a few minutes. E2 also increased the frequency of [Ca2+]i synchronization among LHRH-1 neurons. Similar E2 effects on the frequency of [Ca2+]i oscillations were observed under the presence of tetrodotoxin, indicating that estrogen appears to cause direct action on LHRH-1 neurons. Moreover, application of a nuclear membrane-impermeable estrogen dendrimer conjugate, not control dendrimer, resulted in a robust increase in the frequencies of [Ca2+]i oscillations and synchronizations, indicating that effects estrogens on [Ca2+]i oscillations and their synchronizations do not require their entry into the cell nucleus. Exposure of cells to E2 in the presence of the estrogen receptor antagonist ICI 182,780 did not change the E2-induced increase in the frequency of [Ca2+]i oscillations or the E2-induced increase in the synchronization frequency. Collectively, estrogens induce rapid, direct stimulatory actions through receptors located in the cell membrane/cytoplasm of primate LHRH-1 neurons, and this action of estrogens is mediated by an ICI 182,780-insensitive mechanism yet to be identified.
FEEDBACK CONTROLS of estrogen in LHRH-1 neurons play a pivotal role in reproductive function. However, the mechanism of estrogen action in LHRH-1 neurons is still elusive. In fact, it has been believed for many years that feedback effects of estrogen on LHRH-1 release were mediated by interneurons, because in earlier studies, estrogen receptors (ER) were not found in LHRH-1 neurons (1,2,3), whereas colocalization of ER in other types of neurons, such as norepinephrine and neuropeptide Y neurons, was readily observed (see Ref. 4). Recent advances in technology and the discovery of ERβ, however, have opened up a new avenue for the direct action of estrogen through ER in LHRH-1 neurons. It has been shown that immortalized LHRH-1 neurons (GT1-7 cells) express both ERα and ERβ (5,6), and estrogen exerts stimulatory and inhibitory action, depending on its concentrations, in GT-1 cells (6). A subpopulation of LHRH-1 neurons express the ERβ transcript (7,8,9) and ERβ protein (10,11), and estrogen induces an increase in the frequency and synchronization of intracellular calcium ([Ca2+]i) in mouse LHRH-1 neurons, which appears to be mediated by ERβ (12).
Previously, we have shown that a primary cell culture system of LHRH-1 neurons is a useful approach for cellular and molecular studies (13). LHRH-1 neurons derived from embryonic olfactory placode of rhesus monkeys exhibited pulsatile LHRH-1 release and [Ca2+]i oscillations, which synchronize at a frequency similar to LHRH-1 release (14,15). We further reported that estrogen induced a rapid increase in firing activity in LHRH-1 neurons (16). However, the mechanism of the rapid action of estrogen in primate LHRH-1 neurons is still unclear. Therefore, in the present study, we examined whether estrogens induce rapid changes in [Ca2+]i oscillations in LHRH-1 neurons and, if it does, whether estrogen dendrimer conjugate (EDC), which is impermeable to the nuclear membrane (17), also induces results similar to 17β-estradiol (E2). Because both estrogen and EDC resulted in rapid changes in [Ca2+]i oscillations, we further examined whether estrogens’ effects were blocked by the nuclear ER blocker ICI 182,780 (ICI). Results suggest that estrogens’ effects observed in this study appear to be mediated by an ICI-insensitive mechanism yet to be identified.
Materials and Methods
Animals and tissue culture
Rhesus monkey embryos (Macaca mulatta) from time-mated pregnancies were delivered by cesarean section under isoflurane anesthesia. A total of 20 fetuses at embryonic d 34–38 were used in this study. All experimental procedures were carried out in accordance with the standards outlined in Principles for Use of Animals and Guide for the Care and Use of Laboratory Animals. The protocol used in the studies was approved by the Animal Care and Use Committee of the University of Wisconsin-Madison.
Culture methods for LHRH-1 neurons derived from olfactory placode region have been described in detail elsewhere (13,14). Briefly, the olfactory placode region and ventral migratory pathway of LHRH-1 neurons (terminal nerve region) were dissected out and divided into small (<1 mm3) pieces, and two to three pieces were placed on each glass coverslip producing 24–32 coverslips per embryo. Cultures were grown in growth medium (medium 199; Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 0.6% glucose, and 50 mg/ml gentamycin (Sigma) under 1.5% CO2/98.5% air at 37 C for at least 2 wk before experiments. Medium was replaced every 1–3 d. All experiments were conducted at 2–5 wk of culture. In the study, most experiments were conducted in a phenol red-free M199 medium, to which cells were exposed starting 24 h before the experiment. Because of concerns regarding unknown effects of serum, in some cultures, we also repeated the experiments with cultures grown in a modified growth medium (based on DMEM/F-12; Sigma), which does not contain either serum or phenol red.
Measurement of [Ca2+]i
[Ca2+]i levels were assessed by the method described previously (15,18) with some modifications. Culture medium (2 ml) was mixed with 18 μm fura-2 AM (Tef Lab, Austin, TX) and 6 μl of a mixture of pluronic F-127 (BASF Corp., Parsippany, NY) and dimethylsulfoxide (1:2) by vortexing for 15 sec. Cells on a coverslip were incubated in fura-2 for 20–40 min at 37 C under 1.5% CO2. The coverslip was then placed in a Dvorak-Stotler recording chamber. Fluorescence imaging of the dye-loaded cells was achieved with an inverted microscope. A culture was viewed through a ×20 objective lens, and a 750- × 750-μm recording field that contained the appearance of LHRH-1 neurons (see below) was selected for data capture. Cultures were continuously perifused at a speed of 50 μl/min with either M199 medium or serum-free medium at pH 7.4, under 95% O2 and 5% CO2 at room temperature (22–25 C).
[Ca2+]i was monitored as a function of the ratio of the 510-nm fura-2 emission excited by illuminations at 340 and 380 nm with a Lambda DG-4 light source and filter exchanger (Sutter Instruments, Novato, CA). Fura-2 fluorescence was recorded at 10-sec intervals with a CCD camera (Photometrics, Tucson, AZ) and Metafluor imaging software (Molecular Devices Corp., Downingtown, PA). The ratio of the fluorescence intensities (ΔF/F0) from the 340- and 380-nm excitation wavelength-evoked images were used to calculate the free [Ca2+]i levels as described previously (15) and saved as a text file with Excel for later analysis.
LHRH-1 neurons for [Ca2+]i imaging in cultures were easily identifiable based on their morphology as well as their migratory pattern: They appeared in ovoid shape with a large (≥10 μm) soma and large somatic processes, which form neuronal bundles. However, our cultures contain numerous epithelial cells, fibroblasts, and other types of unidentified cells and occasionally a small number of non-LHRH neurons (13,18). To confirm recorded cells were LHRH-1 neurons, after the completion of each [Ca2+]i imaging experiment, we took a photomicrograph of the viewing area and recorded a reference grid number of the coverslip for later identification of LHRH-1 neurons with immunostaining (see below), as described previously (15,18).
Experimental design
Experiment 1.
To examine whether estrogens cause rapid action, cultured LHRH-1 neurons were exposed to 1 nm E2 (Schering Co., Bloomfield, NJ) or vehicle for 10 min, after at least 20 min of control recording.
Experiment 2.
The effects of E2 on LHRH-1 neurons might be transsynaptic, rather than direct. Although most of our cultures contain only LHRH-1 neurons and nonneuronal cells, occasionally non-LHRH neurons were found in cultures (13,18). Thus, to address the issue of direct effects of E2 on LHRH-1 neurons, cells were exposed to 1 μm tetrodotoxin (TTX) (Sigma) for 14 min, during which 1 nm E2 challenge was applied for 10 min (TTX was applied 2 min before through 2 min after E2). In a few cases, cells were exposed to TTX for 62 min; i.e. TTX was applied 2 min before through 50 min after E2.
Experiment 3.
Rapid action of estrogens may occur via a nongenomic pathway. To examine this possibility, we have used a nuclear membrane-impermeable estrogen, which has recently been developed by Drs. John and Benita Katzenellenbogen (University of Illinois, Urbana, IL) and described as an estrogen (17α-ethynylestradiol) conjugated with 6-G PAMAM dendrimer (EDC) (17). Similar to experiment 1, cells were challenged with 1 μm or 1 nm EDC for 10 min. For control, 1 μm or 1 nm dendrimer control (DC, 6-G PAMAM) was similarly examined.
Experiment 4.
To address the question of whether ERα and ERβ are involved in the rapid action of estrogens, cells were exposed to the ER blocker, 0.1 μm ICI (Tocris, Bristol, UK) for at least 20 min before, through the 10-min E2 application, and until the end of the experiment. For the control, ICI without E2 was examined. After at least 20 min of control recording, the ICI was applied for 60 min or longer (until the end of the experiment).
Immunocytochemistry
To identify whether the recorded cells were LHRH-1 neurons, cultures were stained using standard immunocytochemical procedures with an antisera cocktail, GF-6 and LR-1 [gifts from Dr. N. M. Sherwood, University of British Victoria, Victoria, Canada (1:9000 dilution) and Dr. R. A. Benoit, University of Montreal, Montreal, Canada (1:15,000)] for 40–42 h, Vectastain ABC peroxidase system (Vector Laboratories, Burlingame, CA), and 3.3′-diaminobenzidine as the chromogen. LHRH-1-immunopositive neurons were identified by matching the photomicrograph and digitized fluorescence images (18). Neurons that were not LHRH-1 immunopositive were excluded from analysis. Other cells, such as fibroblasts and epithelial cells as well as cells yet to be identified, were defined as nonneural cells and excluded from analysis.
Data analysis
In the present study, we analyzed cultures with LHRH-1 neurons, from which the [Ca2+]i peaks were unambiguously detected by pulsar algorithm (see below). Approximately one third of cultures with LHRH-1 neurons did not have any clear [Ca2+]i oscillatory pattern, and pulsar algorithm could not be reliably applied for the data analysis. We did not analyze the data from non-LHRH cells, because of the volume of the data. For statistical analysis, we combined the results from the two culture medium conditions (M199 without phenol red and DMEM/F-12-based serum-free medium), because they were not different.
To detect peaks in [Ca2+]i oscillations, we used Pulsar algorithm (19); cutoff criteria were G(1) = 3.8, G(2) = 2.26, G(3) = 1.56, G(4) = 1.13, G(5) = 0.83, and the coefficient of variation for our calcium imaging was described by the formula Y = 1.95X − 0.589. With these parameters, we were able to reliably detect [Ca2+]i peaks. After the detection of [Ca2+]i peaks, the number of [Ca2+]i peaks, the amplitude of [Ca2+]i peaks, and the interpeak interval (IPI) during 20-min blocks (−20 to 0, 0–20, 20–40, and 40–60 min after the treatment) were calculated in individual LHRH-1 neurons from the Pulsar results. Subsequently, mean values for these parameters of all LHRH-1 neurons (13–65 cells per culture) in each culture were calculated. Finally, for statistical comparison, overall mean (± sem) for each treatment group was calculated from the mean value of each culture. For the purpose of graphic presentation the frequency (numbers per 20-min period) of [Ca2+]i peaks was normalized by designating the mean value at −20 to 0 min as 100%, although statistical analysis for the frequency was conducted with raw data.
The effects of treatments (E2, E2 plus TTX, EDC, and E2 plus ICI) on the proportion of cells that increased, decreased, or had no change in [Ca2+]i oscillations during each 20-min block after the initiation of treatment were examined in each LHRH-1 neuron by comparing them to the frequency of [Ca2+]i oscillations during the control period (−20 to 0 min before the treatment). Frequency of spontaneous [Ca2+]i oscillations fluctuated with time; during any given 20-min period in a culture, approximately one third of cells exhibit a higher frequency than the previous 20 min, another third exhibit a lower frequency than the previous 20 min, and the remaining third exhibit no change (often silent cells). In fact, the results showed that approximately 30% of cells with vehicle treatment were stimulated during the consecutive 20-min periods (see Results). After the calculation of the proportion (percent) for each culture, overall mean (± sem) for each treatment consisting of six cultures was derived.
Synchronization of [Ca2+]i peaks among multiple numbers of cells in a culture was defined as follows. First, based on the [Ca2+]i peaks determined by Pulsar algorithm, the rate of [Ca2+]i peaks occurring during 50-sec periods were plotted. Second, the average rate of [Ca2+]i peaks during the entire recording period was calculated. Subsequently, we considered a synchronization to have occurred if the mean rate during the two successive 50-sec periods was greater than the mean + 3 sd, based on our previous observations (15,18). We found that with this criterion, an average of 60% recorded cells contributed to a synchronization. Synchronization frequency was expressed as a total number of synchronizations occurring during a 60-min period after the initiation of treatment (i.e. 10 min during and 50 min after).
The effects of treatments (E2, E2 plus TTX, EDC, and E2 plus ICI) on the frequency, amplitude, IPI, and proportion of the cell number with increased [Ca2+]i oscillations during the 20-min block were compared with those in respective controls (vehicle, TTX alone, ICI alone, and DC) using two-way ANOVA repeated measures, followed by Dunnett multiple comparison test. The effects of treatments on the synchronization frequency during the 60 min after the initiation of a treatment were compared with that in respective controls in the same 60-min period using t test (unpaired, two-tailed). All groups consisted of six cultures per group. Data are presented as means ± sem. Statistical significance was established at P < 0.05.
Results
Effects of E2 on [Ca2+]i oscillations and synchronizations
Individual LHRH-1 neurons exhibited spontaneous [Ca2+]i oscillations with frequencies similar to those described previously (18). The average IPI and amplitude of LHRH-1 neurons in six cultures without treatment was 5.7 ± 1.4 min and 49.5 ± 7.0 nm, respectively (n = 275).
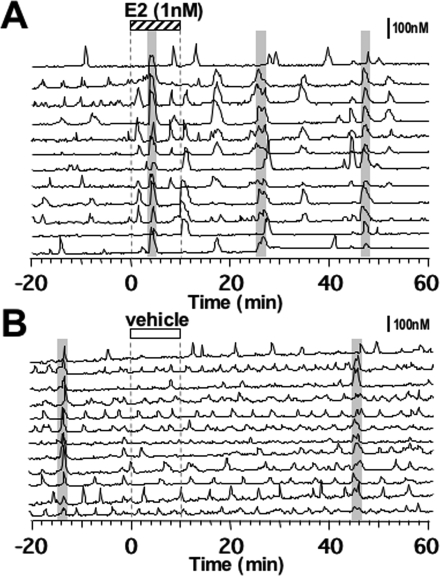
Application of E2 (1 nm; 10 min) induced an increase in the number of [Ca2+]i peaks (Fig. 1A). E2 also resulted in [Ca2+]i oscillations in cells that were not exhibiting spontaneous [Ca2+]i peaks before E2 exposure. In contrast, vehicle infusion did not cause any significant effects (Fig. 1B).
Figure 1.
Effects of E2 (A) or vehicle (B) on [Ca2+]i oscillations and synchronization of primate LHRH-1 neurons. Twelve of 41 LHRH-1 neurons from a culture treated with E2 and 12 of 50 LHRH-1 neurons from a culture treated with vehicle are shown. After 20 min of control recording, cells were exposed to E2 (1 nm, stippled bar on the top) or vehicle for 10 min (white bar). Changes in [Ca2+]i levels are shown as ratio values of fura-2 dye (nanomolar). A shaded bar over the traces indicates synchronization of [Ca2+]i peaks among LHRH-1 neurons based on the criteria described in Materials and Methods. Note that E2 induced an increase in the frequency of [Ca2+]i peaks in LHRH-1 neurons as well as frequent synchronization among LHRH-1 neurons.
Statistical analysis indicated that the mean number of [Ca2+]i peaks (5.9 ± 1.0 pulses/20 min) during the first 20 min after the initiation of E2 application was significantly (P < 0.05) higher than that during the control 20-min period (3.3 ± 0.7 pulses/20 min; n = 296 from six cultures; Fig. 2A). This increase returned to control levels in the next 40 min. Although there was a tendency of an increase in the amplitude of [Ca2+]i peaks after the E2 exposure, overall significance was not attained (before, 23.7 ± 3.5 nm vs. 20 min after E2, 36.4 ± 6.9 nm, P > 0.05). Vehicle application did not cause any change in the mean frequency (Fig. 2A) or amplitude of [Ca2+]i oscillations (frequency before, 4.0 ± 0.6 pulses/20 min vs. 20 min after, 3.6 ± 0.5 pulses/20 min; amplitude before, 49.5 ± 7.2 nm vs. 20 min after vehicle, 43.8 ± 6.9 nm; n = 275 from six cultures). Moreover, the E2-induced increase in the mean frequency of [Ca2+]i peaks during the initial 20 min after E2 (Fig. 2A) was parallel with a significantly higher percentage (P < 0.001) of LHRH-1 neurons (70.6 ± 6.8%; n = 296) exhibiting stimulated activity (Fig. 2B) when compared with that (31.7 ± 3.4%; n = 275) in vehicle controls.
Figure 2.
Changes in the frequency of [Ca2+]i oscillations (A) and the percentage (B) of stimulated LHRH-1 neurons induced by E2 or vehicle treatment. For changes in the frequency of [Ca2+]i oscillations, normalized data are shown. Note that E2 stimulated the frequency of [Ca2+]i oscillations and the percentage of excited cells. The n for E2 and vehicle are 296 and 275, respectively. *, P < 0.05 vs.−20 to 0; #, P < 0.05; ###, P < 0.001 vs. vehicle at the corresponding time.
E2 also resulted in more frequent synchronization of [Ca2+]i peaks among LHRH-1 neurons (Fig. 1A), whereas vehicle treatment did not induce any change in the synchronization pattern (Fig. 1B). In fact, the number of synchronizations in the 60-min period after the initiation of E2 treatment was significantly higher (P < 0.01) than that in the same period after vehicle (Table 1). Interestingly, not all LHRH-1 neurons that exhibited the E2-induced increase in [Ca2+]i oscillations participated in the synchronization (Fig. 1A).
Table 1.
Effects of E2 on the synchronization of calcium oscillations in primate LHRH neurons
| Treatments | No. of synchronizations in the 60-min period after initiation of treatment |
|---|---|
| E2 (1 nm) | 2.67 ± 0.33b |
| Vehicle | 1.00 ± 0.26 |
| TTX (1 μm) + E2 (1 nm) | 1.83 ± 0.48 |
| TTX (1 μm) | 0.67 ± 0.33 |
| EDC (1 nm) | 2.00 ± 0.51a |
| DC (1 nm) | 0.67 ± 0.21 |
| EDC (1 μm) | 3.17 ± 0.48b |
| DC (1 μm) | 1.00 ± 0.26 |
| ICI (100 nm) + E2 (1 nm) | 2.17 ± 0.54a |
| ICI (100 nm) | 0.83 ± 0.17 |
n = 6 cultures in each treatment group.
P < 0.05 vs. respective control.
P < 0.01 vs. respective control.
Effects of E2 in the presence of TTX
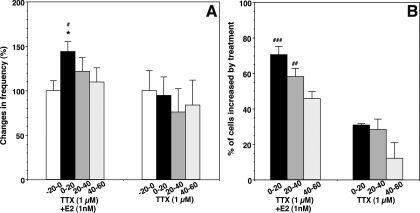
E2 application in the presence of TTX (1 μm) also caused a significant increase (P < 0.05) in the frequency of [Ca2+]i peaks (before, 3.2 ± 0.4 pulses/20 min vs. 0–20 min after E2, 4.6 ± 0.3 pulses/20 min; n = 149; Figs. 3A and 4A). Similarly, in the presence of TTX, E2 induced a significantly higher percentage (P < 0.001) of stimulated cells (70.1%) than TTX alone (30.9%; Fig. 4B). E2 in the presence of TTX did not modify the amplitude of [Ca2+]i oscillations (data not shown). However, TTX caused a slight delay in the timing of the first synchronization after E2 (Fig. 3A). As a consequence, despite the trend of increase in the synchronization frequency during the 60-min period, the E2-induced increase in synchronization frequency did not attain significant levels (P = 0.076) when compared with the TTX control (Table 1). Treatment with TTX (1 μm) alone did not cause any significant changes in [Ca2+]i oscillations (before, 3.7 ± 0.9 pulses/20 min vs. 0–20 min after vehicle, 3.5 ± 0.7 pulses/20 min; n = 194; Figs. 3B and 4, A and B). TTX alone also had a slight tendency to suppress the synchronization frequency (Table 1). The results from longer exposures to TTX [32 min (n = 6) and 62 min (n = 3)] indicated that E2 also induced an increase in pulse frequency of in [Ca2+]i peaks and that synchronization occurred after E2 with a similar timing to the shorter TTX exposure, which was still under the presence of TTX (data not shown).
Figure 3.
A representative example of TTX plus E2 (A) or TTX (B) treatment on [Ca2+]i oscillations. A, After 20 min of control recording, cells were exposed to E2 (1 nm, stippled bar on the top) for 10 min. Cells were also exposed to TTX (1 μm) 2 min before E2 through 2 min after E2. Shaded bars over the traces indicate synchronization of [Ca2+]i peaks among LHRH-1 neurons. B, Note that the E2-induced increase in the frequency of [Ca2+]i oscillations and synchronizations was observed in the presence of TTX, whereas TTX alone (white bar on the top) did not induce any significant effects. Nine of 26 LHRH-1 neurons from a culture treated with TTX plus E2 and nine of 50 LHRH-1 neurons from a culture treated with TTX alone are shown.
Figure 4.
Changes in the frequency of [Ca2+]i oscillations (A) and the percentage (B) of stimulated LHRH-1 neurons induced by E2 in the presence of TTX. For changes in the frequency of [Ca2+]i oscillations, normalized data are shown. Note that TTX did not block the E2-induced increase in the frequency of [Ca2+]i oscillations or the percentage of excited cells. The n for TTX plus E2 and TTX are 149 and 194, respectively. *, P < 0.05 vs.−20 to 0; #, P < 0.05; ##, P < 0.01; ###, P < 0.001 vs. TTX alone at the corresponding time.
Response to a nuclear membrane-impermeable analog of estrogen
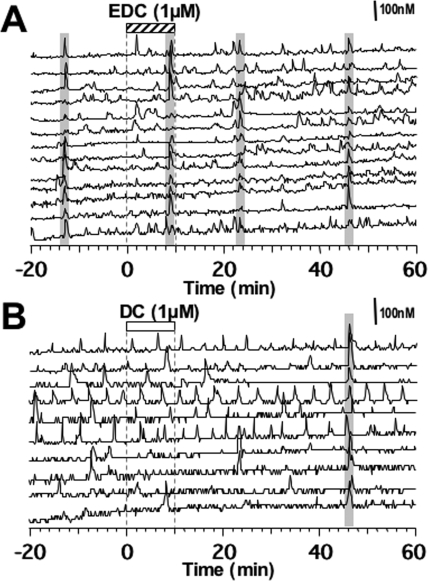
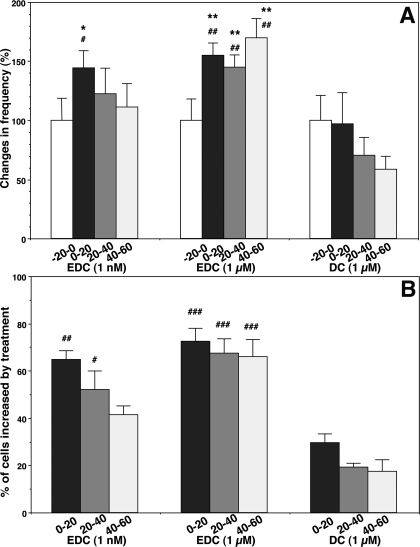
To investigate whether the rapid action of E2 requires its entrance into the cell nucleus, we examined the effects of EDC, a nuclear membrane-impermeable estrogen molecule. As shown in Fig. 5, a 10-min exposure to EDC at 1 μm stimulated [Ca2+]i oscillations. Statistical analysis indicated that EDC significantly (P < 0.01) increased the number of [Ca2+]i peaks (EDC frequency before, 2.0 ± 0.4 pulses/20 min vs. 0–20 min after EDC, 3.5 ± 0.3 pulses/20 min; n = 149; Fig. 6A) as well as the percentage (72.5%) of stimulated cells (Fig. 6B), which was significantly (P < 0.001) higher than that (29.7%) in DC. EDC at 1 nm also significantly increased the number of [Ca2+]i peaks (EDC frequency before, 2.7 ± 0.5 pulses/20 min vs. 0–20 min after EDC, 3.9 ± 0.5 pulses/20 min; n = 195; P < 0.05; Fig. 6A) as well as the percentage (64.9%) of stimulated cells (Fig. 6B). Although EDC at a higher dose induced an increase in [Ca2+]i oscillations for a longer period than E2, this was not observed at a lower dose (Fig. 6, A and B). The peak amplitude was not significantly altered by EDC at either dose (data not shown).
Figure 5.
Effects of EDC (A) or DC (B) on [Ca2+]i oscillations and synchronizations of primate LHRH-1 neurons. After a 20-min control recording, cells were exposed to EDC (1 μm, stippled bar on the top) or DC (white bar) for 10 min. DC did not induce any changes in [Ca2+]i oscillations. Note that EDC induced an increase in the frequency of [Ca2+]i peaks in LHRH-1 neurons as well as frequent synchronization among LHRH-1 neurons (shaded bars over the traces). Thirteen of 27 and 10 of 35 LHRH-1 neurons from cultures treated with EDC and DC, respectively, are shown.
Figure 6.
Changes in the frequency of [Ca2+]i oscillations (A) and the percentage (B) of stimulated LHRH-1 neurons induced by EDC (1 nm or 1 μm) or DC (1 μm) treatment. Note that EDC at both doses stimulated the frequency of [Ca2+]i oscillations and the percentage of excited cells. EDC at 1 μm resulted in a more prolonged effect than that of EDC at 1 nm. The n for EDC at 1 nm, EDC at 1 μm, and DC are 195, 149, and 182, respectively. *, P < 0.05; **, P < 0.01 vs.−20 to 0; #, P < 0.05; ##, P < 0.01; ###, P < 0.001 vs. DC at the corresponding time.
Similarly, EDC at both 1 μm and 1 nm resulted in a significant increase (P < 0.01 and P < 0.05, respectively) in the frequency of synchronization, when compared with DC treatment (Table 1). In contrast to the effect of EDC, DC (1 μm) treatment did not cause any significant changes in [Ca2+]i oscillations (frequency before DC, 3.0 ± 0.7 pulses/20 min vs. 0–20 min after DC, 2.6 ± 0.3 pulses/20 min; n = 182; Figs. 5B and 6, A and B) or the synchronization of [Ca2+]i oscillations (Table 1).
Effects of ICI on the E2-induced changes in [Ca2+]i oscillations and synchronization
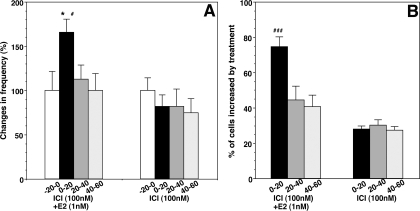
To determine the possible involvement of the nuclear receptors ERα and ERβ, we examined the effects of ICI. Cells were treated with ICI (100 nm) starting 20 min before E2, throughout E2 application, and continuously until the end of experiments. As shown in Fig. 7A, exposure to ICI did not block the E2-induced increase in the frequency of [Ca2+]i peaks (ICI plus E2 before, 3.2 ± 0.7 pulses/20 min vs. 0–20 min after, 5.3 ± 0.8 pulses/20 min; n = 180; Fig. 8A) or the percentage (74.6%) of stimulated cells, which was significantly higher (P < 0.001) than that (30.0%) with ICI alone (Fig. 8B). Similarly, ICI did not block the E2-induced increase in synchronization (P < 0.01, Table 1). ICI itself did not cause any significant effects on the frequency of [Ca2+]i oscillations (ICI alone before, 2.8 ± 0.4 pulses/20 min vs. 0–20 min after, 2.3 ± 0.5 pulses/20 min; n = 186) or on the synchronization pattern (Figs. 7B and 8, A and B; Table 1).
Figure 7.
Effects of ICI (100 nm) on the E2-induced changes in [Ca2+]i oscillations. A, Cells were treated with ICI for the entire period of the experiment as indicated by the white bar on the top, and E2 was applied for 10 min starting 20 min after the initiation of ICI treatment (stippled bar on the top). E2 induced increases in the frequency of [Ca2+]i oscillations and synchronizations in the presence of ICI. B, ICI alone did not cause any significant changes in [Ca2+]i oscillations and synchronization. Thirteen of 30 and eight of 32 LHRH-1 neurons from cultures treated with ICI plus E2 and ICI alone, respectively, are shown.
Figure 8.
Changes in the frequency of [Ca2+]i oscillations (A) and the percentage (B) of stimulated LHRH-1 neurons induced by E2 in the presence of ICI. Note that ICI did not block the E2-induced increase in the frequency of [Ca2+]i oscillations or the percentage of excited cells. The n for ICI plus E2 and ICI are 180 and 186, respectively. *, P < 0.05 vs.−20 to 0; #, P < 0.05; ###, P < 0.001 vs. ICI alone at the corresponding time.
Discussion
In the present study, the effects of estrogens on activity of primate LHRH-1 neurons were investigated in vitro. E2 induced an increase in the frequency of [Ca2+]i oscillations and the number of synchronizations among LHRH-1 neurons with a short latency. E2 did not alter the amplitude of [Ca2+]i peaks. Although TTX slightly interfered with the frequency of synchronizations, the E2-induced increase in [Ca2+]i oscillations in the presence of TTX was similar to that without TTX, suggesting that E2 appears to cause changes in LHRH-1 neurons directly. Moreover, EDC, a nuclear membrane-impermeable estrogen, increased the number of [Ca2+]i peaks in LHRH-1 neurons as well as the frequency of synchronization of [Ca2+]i oscillations. These results indicate that estrogens cause a rapid stimulatory action in primate LHRH-1 neurons, and this estrogen action occurs with an extranuclear mechanism through the plasma membrane and/or cytoplasm.
Rapid action of estrogens on neuronal activity has been reported for some time. In fact, for nearly a half-century, numerous studies with electrophysiological recording have shown that the ovarian steroids, estrogen and progesterone, rapidly modify activity of hypothalamic neurons (20,21). More recently, direct rapid action of estrogens on LHRH-1 neurons has been documented. Lagrange and colleagues (22) showed that estrogens hyperpolarized guinea pig LHRH-1 neurons by opening K+ channels in a slice preparation, corresponding to the negative feedback effects of estrogens in vivo. Navarro and colleagues (6) reported that E2 at picomolar doses rapidly induced inhibitory effects, whereas E2 at nanomolar doses induced stimulatory effects on cAMP production in GT1-7 cells. Temple and colleagues (12) further described that E2 at nanomolar doses resulted in a stimulatory change in the pattern of [Ca2+]i oscillations in mouse LHRH-1 neurons in the presence of TTX. In our laboratory as well, Abe and Terasawa (16) demonstrated that firing activity of primate LHRH-1 neurons was accelerated by E2 at 1 nm. The present findings that E2 stimulates the frequency of [Ca2+]i oscillations in primate LHRH-1 neurons with a short latency in the presence of TTX are consistent with the previous findings.
Unlike classical action of estrogens through ERα and ERβ modulating gene transcription in the cell nucleus, rapid action of estrogens occurs through receptors located in the cell membrane/cytoplasm. To distinguish extranuclear action of estrogens from nuclear (or genomic) action of estrogens, membrane-impermeable estrogen compounds, such as BSA-conjugated estradiol (E2-BSA) have been widely used. However, use of E2-BSA is problematic. First, there is a possibility that free estrogen can leak from the conjugate, because the linkage between estrogen and BSA is not chemically stable (23), and even after filtration of free E2, E2-BSA could be degraded in cells (17), and second, the effects of E2-BSA on [Ca2+]i oscillations in mouse LHRH-1 neurons differed depending on the site selected to link the estrogen carbon position with BSA (24). Accordingly, we have used EDC, estrogen conjugated to a large, abiotic, nondegradable poly(amido)-amine dendrimer macromolecule, which has been shown to cause extranuclear effects of E2 in cancer cells (17). Our results show that EDC either at 1 nm or 1 μm induced stimulatory effects on [Ca2+]i oscillations in primate LHRH-1 neurons. Interestingly, EDC at 1 μm concentration resulted in a prolonged effect as compared with E2 or EDC at 1 nm. These results indicate that stimulatory effects of E2 occur at the cell membrane and/or cytoplasm.
ERs in the neuronal membrane could be classical ERα and/or ERβ or novel receptors yet to be identified. Abraham et al. (25) show that E2, not E2-BSA, stimulates the phosphorylation of cAMP response element-binding protein (CREB) in mouse LHRH-1 neurons within 15 min, and this rapid estrogen effect requires intracellular ERβ, because estrogens did not cause the effect in ERβ knockout mice. Similarly, the estrogen-induced frequency of [Ca2+]i oscillations and the number of activated cells in mouse LHRH-1 neurons appears to be mediated by ERβ, because ICI blocks this effect of estrogens, and mouse LHRH-1 neurons contain only ERβ (10). Boulware et al. (26) also reported in cultured hippocampal neurons with glutamate receptors that E2 stimulation of membrane ERα and ERβ triggered metabotropic glutamate receptor signaling, leading to a decrease in L-type calcium channel-mediated and the MAPK-dependent CREB phosphorylation.
Several researchers have proposed novel membrane ERs. First, Toran-Allerand et al. (27) report the presence of high-affinity ERs, named ER-X, in developing neocortical neurons. ER-X are associated with caveolar-like microdomains and coupled to the activation of the MAPK signaling cascade. They are insensitive to ICI (28) and not observed in ERα and ERβ knockout mice (29). Second, Kelly, Ronnekleiv, and their colleagues (30) propose novel G protein-coupled ERs in hypothalamic β-endorphin, dopamine, and GABAergic neurons with coupling of μ-opioid and/or GABAB receptors. This G protein-coupled membrane receptor is sensitive to ICI, yet it differs from ERα and ERβ, because STX, a newly synthesized nonsteroidal compound, which mimics the membrane E2 action in guinea pigs and normal mice, was also effective in ERα,β knockout mice (31). Third, Filardo and colleagues suggest that the orphan receptor, G protein–coupled receptor 30 (GPR30), is linked to estrogen-mediated signaling in certain types of cells. The seven transmembrane G protein-coupled ER, GPR30, is required for membrane action of E2 in human breast cancer cells (32,33), membrane GPR30 possesses estrogen-binding sites, independent of ERα and ERβ (34), and GPR30 is expressed in reproductive tissues such as placenta syncytiotrophoblasts (34). Stimulation of GPR30 by E2 activates adenylyl cyclase and release of epidermal growth factor-related ligand, resulting in second messenger cascades. Interestingly, ICI and tamoxifen also bind GPR30 and induce agonistic action with estrogens (33).
In the present study, we found that in the presence of ICI, E2 was still fully effective for all parameters analyzed. This ineffectiveness of ICI is not due to chemical degradation, because a collateral study in our laboratory indicated that ICI (the same batch at the same dose) blocked the E2-induced pCREB induction in GT1-7 cells (Noel, S. D., and E. Terasawa, unpublished). These data are interpreted to mean that the rapid action of estrogens in primate LHRH-1 neurons is mediated by neither ERα nor ERβ, because 100 nm ICI should be able to compete with 1 nm E2. It is, rather, similar to Torand-Allerand’s ER-X (27) but differs from a G protein-coupled receptor described by Kelly, Ronnekleiv, and colleagues (31). We have hypothesized that the rapid action of estrogen on an increase in [Ca2+]i oscillations in primate LHRH-1 neurons is mediated by GPR30, described by Filardo and colleagues (33) in cancer cells. Our preliminary data support this hypothesis. GPR30 was expressed in a subset of primate LHRH-1 neurons (35), E2 action did not occur in LHRH-1 neurons pretreated with an activated form of pertussis toxin but did occur with an inactivated form of pertussis toxin (35), and E2 failed to induce an increase in [Ca2+]i peaks and synchronization in LHRH-1 neurons treated with siRNA for GPR30 (36). Nonetheless, a question arises as to why ICI alone did not stimulate [Ca2+]i oscillations. We believe that the dose of ICI at 100 nm was too low. In cancer cells, the agonistic action of ICI occurred at 1 μm (33,37), but not 100 nm (Filardo, E., personal communication). Additional experiments will clarify the hypothesis.
The results of this study using monkey LHRH-1 neurons are quite similar to those found in mouse LHRH-1 neurons (12,24). In both studies, estrogen increased the frequency of [Ca2+]i oscillations and their synchronizations. Moreover, membrane-impermeable estrogens induced effects similar to those with E2, although in the mouse study (12), E2-BSA was used, and in this study, EDC was used. However, there are also some differences. In monkey LHRH-1 neurons, ICI did not block the estrogen-induced increase in both frequency of [Ca2+]i oscillations and synchronizations, whereas in mouse LHRH-1 neurons, it did block the frequency of [Ca2+]i oscillations as well as synchronizations (24). Moreover, in monkey LHRH-1 neurons, TTX suppressed synchronization of [Ca2+]i oscillations. We speculate that differences are due to the properties of the LHRH-1 neurons in the two species; i.e. monkey LHRH-1 neurons often exhibit synchronization of [Ca2+]i oscillations as a Ca2+ wave (15,18) across LHRH-1 neurons and nonneuronal cells, which form a network (18), whereas synchronization in mouse LHRH-1 neurons is not propagated as a Ca2+ wave (38). Synchronization propagated as a Ca2+ wave has also been shown in GT1-1 cells (39). Nonetheless, a slight suppression of the E2-induced synchronization by TTX in monkey LHRH-1 neurons may be due to the failure of propagating depolarization signals for synchronizations. We previously have shown that synchronization of [Ca2+]i oscillations between LHRH-1 neurons and nonneuronal cells is, in part, mediated by ATP through P2X receptors (40). Thus, P2X receptor activation by ATP could lead to open voltage-dependent calcium channels and subsequent depolarization.
Because it has been reported that in the presence of TTX corticosterone induced miniature excitatory postsynaptic glutamatergic currents in the hippocampal neurons of mineralocorticoid and glucocorticoid receptor knockout mice (41), one may argue that the negative data with TTX in the present study are due to the generation of miniature excitatory postsynaptic currents by E2 under TTX, even though input from other neurons were blocked. Although this possibility needs to be examined, it might be important to point out that our cultures rarely contain non-LHRH neuronal cells (13). Rather, E2 could change the activity of nonneuronal cells, through which activity of LHRH-1 may be modified by nonsynaptic transmission. Additional studies are needed to clarify these possibilities.
The rapid action of E2 could be mediated by more than one mechanism. Although in mouse LHRH-1 neurons ERβ appears to be important for E2 action (12), blockade of E2 action by ICI does not entirely exclude the possible involvement of STX-sensitive membrane receptors (21). Mouse and guinea pig hypothalamic neurons, which were hyperpolarized by GABAB receptor activation, were stimulated by E2 through STX-sensitive membrane receptors (31). Because over 90% of mouse LHRH-1 neurons in 3-wk cultures were stimulated by GABAB receptor activation (38), E2 action in mouse LHRH-1 neurons may also be mediated through STX-sensitive membrane receptors. Similarly, the rapid E2 action in monkey LHRH-1 neurons may be mediated by more than one mechanism. In monkey LHRH-1 neurons, the E2-induced increase in [Ca2+]i peaks in the present study was not blocked by ICI, whereas the E2-induced increase in firing activity was blocked by ICI (16). The difference in the results from two approaches is unclear at this time, because the direct comparison between the E2-induced increases in [Ca2+]i oscillations and firing activity has not been made. The investigation of how firing activity correlates with each [Ca2+]i oscillation in monkey LHRH-1 neurons remains. Nonetheless, it is plausible that a subset of LHRH-1 neurons is ICI insensitive, whereas another set of LHRH-1 neurons is ICI sensitive. Therefore, we hypothesize that the rapid E2 action in primate LHRH-1 neurons is mediated by GPR30 as well as by extranuclear ERα and/or ERβ.
An elevation of estrogens during the late follicular phase results in preovulatory LHRH-1/LH surges in primates (42). For many years, it has been believed from the ample evidence in in vivo studies that stimulatory effects of estrogens on the LHRH-1 surge are mediated by interneurons, such as norepinephrine and neuropeptide Y neurons (4,43,44). More recently, a role of metastin/kisspeptin neurons in mediating negative and positive feedback effects of E2 on LHRH-1 neurons has been demonstrated (45,46). In the present in vitro study, however, we have found that estrogens can induce rapid, direct stimulatory effects through membrane receptors in LHRH-1 neurons and partly mediated by unidentified membrane receptors. Therefore, the results from the present study shed new light on the complex mechanism of estrogen action in the LHRH-1 neurosecretory system.
Acknowledgments
We are indebted to Drs. John and Benita Katzenellenbogen (University of Illinois, Urbana, IL) for the gift of estrogen dendrimer conjugate and control dendrimer. We also thank Rafael Connemara, Amanda Marsh, and Samuel Frost for their technical assistance.
Footnotes
First Published Online December 13, 2007
Abbreviations: CREB, cAMP response element-binding protein; DC, dendrimer control; E2, 17β-estradiol; EDC, estrogen dendrimer conjugate; ER, estrogen receptor; GPR30, G protein-coupled receptor 30; ICI, ICI 182,780; IPI, interpeak interval; TTX, tetrodotoxin.
This work was supported by National Institutes of Health Grants R01HD15433 and R01HD11355, and was possible to perform by National Institutes of Health support (P51RR000167, RR15459, and RR020141) to the Wisconsin National Primate Research Center.
Present address for H.A.: Department of Biological Sciences, Graduate School of Science, University of Tokyo, Tokyo 113-0033, Japan.
Disclosure Statement: The authors have nothing to disclose.
References
- Shivers BD, Harlan RE, Morrell JI, Pfaff DW 1983 Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature 304:345–347 [DOI] [PubMed] [Google Scholar]
- Lehman MN, Karsch FJ 1993 Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and β-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology 133:887–895 [DOI] [PubMed] [Google Scholar]
- Herbison AE, Horvath TL, Naftolin F, Leranth C 1995 Distribution of estrogen receptor-immunoreactive cells in monkey hypothalamus: relationship to neurones containing luteinizing hormone-releasing hormone and tyrosine hydroxylase. Neuroendocrinology 61:1–10 [DOI] [PubMed] [Google Scholar]
- Herbison AE 1998 Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 19:302–330 [DOI] [PubMed] [Google Scholar]
- Roy D, Angelini NL, Belsham DD 1999 Estrogen directly respresses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-α (ERα)- and ERβ-expressing GT1–7 GnRH neurons. Endocrinology 140:5045–5053 [DOI] [PubMed] [Google Scholar]
- Navarro CE, Saeed SA, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ 2003 Regulation of cyclic adenosine 3′,5′-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotrophin-releasing hormone neurons. Mol Endocrinol 17:1792–1804 [DOI] [PubMed] [Google Scholar]
- Herbison AE, Pape JR 2001 New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol 22:292–308 [DOI] [PubMed] [Google Scholar]
- Sharifi N, Reuss AE, Wray S 2002 Prenatal LHRH neurons in nasal explant cultures express estrogen receptor beta transcript. Endocrinology 143:2503–2507 [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL 2000 Detection of estrogen receptor-β messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 141:3506–3509 [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z 2001 Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 142:3261–3264 [DOI] [PubMed] [Google Scholar]
- Kallo I, Butler JA, Barkovics-Kallo M, Goubillon ML, Coen CW 2001 Oestrogen receptor β-immunoreactivity in gonadotropin releasing hormone-expressing neurones: regulation by oestrogen. J Neuroendocrinol 13:741–748 [DOI] [PubMed] [Google Scholar]
- Temple JL, Laing E, Sunder A, Wray S 2004 Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci 24:6326–6333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Quanbeck CD, Schulz CA, Burich AJ, Luchansky LL, Claude P 1993 A primary cell culture system of luteinizing hormone releasing hormone neurons derived from embryonic olfactory placode in the rhesus monkey. Endocrinology 133:2379–2390 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Keen KL, Mogi K, Claude P 1999 Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology 140:1432–1441 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Schanhofer WK, Keen KL, Luchansky L 1999 Intracellular Ca2+ oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci 19:5898–5909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Terasawa E 2005 Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology 146:4312–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS 2006 Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol 20:491–502 [DOI] [PubMed] [Google Scholar]
- Richter TA, Keen KL, Terasawa E 2002 Synchronization of Ca2+ oscillations among primate LHRH neurons and nonneuronal cells in vitro. J Neurophysiol 88:1559–1567 [DOI] [PubMed] [Google Scholar]
- Merriam GR, Wachter KW 1982 Algorithms for the study of episodic hormone secretion. Am J Physiol 243:E310–E318 [DOI] [PubMed] [Google Scholar]
- Kawakami M, Sawyer CH 1959 Neuroendocrine correlates of changes in brain activity thresholds by sex steroids and pituitary hormones. Endocrinology 65:652–668 [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK, Kelly MJ 2007 Membrane-initiated signaling of estrogen in the brain. Semin Reprod Med 25:165–177 [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Ronnekleiv OK, Kelly MJ 1995 Estradiol-17β and μ-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology 136:2341–2344 [DOI] [PubMed] [Google Scholar]
- Stevis PE, Deecher DC, Suhadolnik L, Mallis LM, Frail DE 1999 Differential effects of estradiol and estradiol-BSA conjugates. Endocrinology 140:5455–5458 [DOI] [PubMed] [Google Scholar]
- Temple JL, Wray S 2005 Bovine serum albumin-estrogen compounds differentially alter gonadotropin-releasing hormone-1 neuronal activity. Endocrinology 146:558–563 [DOI] [PubMed] [Google Scholar]
- Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE 2003 Estrogen receptor β mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci 23:5771–5777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG 2005 Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci 25:5066–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES Jr., Nethrapalli IS, Tinnikov AA 2002 ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci 22:8391–8401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Setalo Jr G, Guan X, Warren M, Toran-Allerand CD 1999 Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci 19:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Setalo Jr G, Guan X, Frail DE, Toran-Allerand CD 2000 Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-α knock-out mice. J Neurosci 20:1694–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ 2003 Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci 23:9529–9540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Ronnekleiv OK, Kelly MJ 2006 A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci 26:5649–5655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton Jr AR 2000 Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14:1649–1660 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton Jr AR, Bland KI 2002 Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol 16:70–84 [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J 2005 Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146:624–632 [DOI] [PubMed] [Google Scholar]
- Frost SI, Keen KL, Noel SD, Filardo EJ, Terasawa E, Rapid and membrane mediated estrogen effects and a possible role of G protein-coupled receptor GPR30 in primate LHRH neurons. Program of the of the 89th Annual Meeting of The Endocrine Society, Boston, MA, 2006 (Abstract P3-265) [Google Scholar]
- Noel SD, Keen KL, Abe H, Terasawa E, Mechanism of rapid action of estrogen in primate LHRH neurons: a possible role of G-protein coupled receptor 30. Proc 37th Annual Meeting of the Society for Neuroscience, San Diego, CA, 2007 (Abstract 556.8) [Google Scholar]
- Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P 2007 Activation of the novel estrogen receptor, GPR30, at the plasma membrane. Endocrinology 148:3236–3245 [DOI] [PubMed] [Google Scholar]
- Moore Jr JP, Shang E, Wray S 2002 In situ GABAergic modulation of synchronous gonadotropin releasing hormone-1 neuronal activity. J Neurosci 22:8932–8941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AC, Kodali SK, Tyndale RF 1996 Intercellular calcium waves in neurons. Mol Cell Neurosci 7:337–353 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Keen KL, Grendell RL, Golos TG 2007 Possible role of 5′-adenosine triphosphate in synchronization of Ca2+ oscillations in primate luteinizing hormone-releasing hormone neurons. Mol Endocrinol 19:2736–2747 [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M 2005 Mineral corticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA 102:19204–19207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss J, Knobil E 1994 The menstrual cycle and its neuroendocrine control. In: Knobil E, Neill JD, eds. The physiology of reproduction. 2nd ed. New York: Raven Press; 711–749 [Google Scholar]
- Spies HG, Pau KY, Yang SP 1997 Coital and estrogen signals: a contrast in the preovulatory neuroendocrine networks of rabbits and rhesus monkeys. Biol Reprod 56:310–319 [DOI] [PubMed] [Google Scholar]
- Terasawa E 2001 Luteinizing hormone-releasing hormone (LHRH) neurons: mechanism of pulsatile LHRH release. Vitam Horm 63:91–129 [DOI] [PubMed] [Google Scholar]
- Smith JT, Clifton DK, Steiner RA 2006 Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction 131:623–630 [DOI] [PubMed] [Google Scholar]
- Maeda K, Adachi S, Inoue K, Ohkura S, Tsukamura H 2007 Metastin/kisspeptin and control of estrous cycle in rats. Rev Endocr Metab Disord 8:21–29 [DOI] [PubMed] [Google Scholar]