Abstract
Chronic exposure of humans to high concentrations of arsenic in drinking water is associated with skin lesions, peripheral vascular disease, hypertension, blackfoot disease and a high risk of cancer. Arsenic induces single strand breaks, DNA-protein crosslinks and apurinic sites in DNA, which are prerequisites for induction of cancer. Amelioration of such damages with natural compounds could be an effective strategy to combat arsenic toxicity. Curcumin is the active ingredient of turmeric, a common household spice, which is a rich source of polyphenols and this compound has been extensively studied as a chemopreventive agent against many types of cancer. The present study investigates whether curcumin could counteract the DNA damage caused by arsenic as assessed by single cell gel electrophoresis (SCGE) using peripheral blood lymphocytes, from healthy donors. It was observed that DNA damage induced by arsenic could be efficiently reduced by curcumin and the effect was more pronounced when lymphocytes were pre-incubated with curcumin prior to arsenic insult. Arsenic caused DNA damage by generation of reactive oxygen species (ROS) and enhancement of lipid peroxidation levels. Curcumin counteracted the damage by quenching ROS, decreasing the level of lipid peroxidation and increasing the level of phase II detoxification enzymes like catalase, superoxide dismutase and glutathione peroxidase. Curcumin also enhanced the DNA repair activity against arsenic induced damage. The expression of polymerase, a repair enzyme, was found to be highly elevated when arsenite induced damaged cells were allowed to repair in presence of curcumin. Results indicate that curcumin has significant role in confronting the deleterious effect caused by arsenic, which could be an economic mode of arsenic mitigation among rural population in West Bengal, India.
Keywords: arsenite, DNA damage, comet assay, curcumin, ROS
Introduction
Arsenic has been considered as an environmental and occupational toxin [1]. Since decades, chronic arsenic toxicity is a widespread global problem affecting millions of people all over the world [2]. Among the affected countries, high levels of arsenic in India [3] and Bangladesh [4] constitute a serious public health concern. In India, the Gangetic plain of West Bengal has been engulfed by a disastrous environmental calamity of arsenic contamination of the ground water [5]. In areas of high arsenic exposure in West Bengal, India, arsenic has been found in drinking water at a concentration of 200–600 µg/l whereas WHO recommended permissible limit is 50 µg/l only [6]. Being ubiquitous element detected in all environmental media, arsenic commonly occurs in pentavalent (As V) and trivalent (As III) forms. As III is found to be relatively more toxic compared to As V [7] and this trivalent inorganic arsenic seems to be the ultimate responsible form mediating most of the toxic effects of the semimetal [8]. Upon ingestion these inorganic forms through drinking water undergo methylation in the biological system and get converted to their organic forms, which are also potent genotoxic compounds.
Chronic exposure to arsenic causes a unique peripheral vascular disorder called ‘black foot disease’. Acute arsenic poisoning causes peripheral neuropathy and also various types of cancer in humans [9, 10]. Other arsenic related clinical manifestations are keratosis, hyperkeratosis, melanosis, depigmentation, gastrointestinal disorder and neurological disorder. Although arsenic compounds are generally perceived as poor mutagens in bacterial and animal cells, they exhibit aneugenic potential [11] and clastogenic properties in many cell types in vivo and in vitro [12, 13]. As III was reported to induce DNA damage in human lymphocytes [14].
Arsenite-induced proposed mechanisms of toxicity are mainly interference with DNA repair process and the induction of oxidative stress [6, 15]. It has been reported that generation of oxidative DNA damage leads to DNA strand breaks [16–18], oxidative DNA base modifications [18, 19] and DNA-protein crosslinks [16, 18, 20]. As III has been known to exert its toxicity by generating reactive oxygen species (ROS) and thereby oxidative stress [1]. ROS are associated not only with initiation, but also with promotion and progression in the multistage carcinogenesis model [21]. Nowadays, plant derived natural compounds and their active principles have received great attention as potential antioxidant agent [22] and have focused much attention as promising agents for reducing the risk of oxidative stress-induced disease [23]. These products are known to exert their protective effects by scavenging free radicals, modulating carcinogen detoxification and antioxidant defense system [24]. Curcumin, an important constituent of turmeric (Curcuma longa L.), a well known medicinal herb, has been widely used for centuries as an indigenous medicine [25]. It has been shown to exhibit a variety of biological activities including antioxidative activity [26]. This phenolic compound has been reported to scavenge superoxide anions [27], nitric oxide radical [28], inhibits lipid peroxidation [26, 29] and lipoxygenase, cycloxygenase activity [30]. Moreover, curcumin was also reported to elevate the activities of some of the Phase 2 detoxification enzymes such as glutathione transferase [31] and NAD(P)H: quinine reductase [32]. Curcumin possessing strong antioxidant activity is thought to be a probable candidate to counteract the oxidative stress-induced damage induced by arsenic. The present study has been designed to investigate the modulatory role of curcumin against As III-induced DNA damage in normal lymphocytes isolated from healthy donors. It is also aimed at investigating the mechanisms lying behind the protection afforded by curcumin against this arsenic-induced genotoxicity.
Materials and Methods
Chemicals
RPMI-1640, fetal bovine serum (FBS), phytohaemagglutinin (PHA), gentamycin, penicillin, streptomycin, agarose (normal as well as low melting point), and Tris were purchased from GIBCO-BRL India Pvt. Ltd. (New Delhi, India). Dithiothreitol (DTT), Histopaque 1077, dichlorofluorescein diacetate (DCFH-DA), 2-thiobarbituric acid (TBA), glutathione reductase (GR), 1,1,3,3-tetramethoxy propane (TMP), ethidium bromide, Triton-X 100, hydroxyurea, Curcumin [1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] were obtained from Sigma-Aldrich (St Louis, MO). [Methyl-3H]thymidine (17.2 Ci/mmol) was purchased from BRIT (Mumbai, India). Nitrocellulose membrane was purchased from Hybond ECL, Amersham Biosciences, U.K. and Poly (ADP ribose) polymerase (PARP) monoclonal antibody (IgG 1, C-2-10) was purchased from BD Biosciences, DNA polymerase β (n-19): sc 5925 goat polyclonal antibody was purchased from Santa Cruz Biotechnology, California, Goat antimouse IgG, rabbit antigoat IgG, both alkaline phosphatase conjugated secondary antibodies and 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (BCIP/NBT) substrate were purchased from Bangalore Genei, India. Analytical grade of As III was procured from S.D. Fine-Chem. Ltd (Mumbai, India). β-mercaptoethanol was purchased from Loba Chemie, Mumbai, India. Trichloroacetic acid (TCA) was procured from Spectrochem India Pvt Ltd, Mumbai, India. Ethylene glycol-bis(β-aminoethylether-)-N,N,N',N'-tetraacetic acid (EGTA), 3-[(3-cholamidopropyl)dimethylaminonio]-1-propanesulphonate (CHAPS), glycerol, N-2 hydroxyethyl piperazine N-2 ethane sulphonic acid (HEPES buffer), Folin Ciocaltaeu, H2O2, NADP, reduced glutathione (GSH), sodium dodecyl sulphate (SDS), ethylenediaminetetraacetic acid disodium salt (Na2EDTA), pyrogallol, formaldehyde, acetone and other reagents of analytical grade were procured locally.
Lymphocyte isolation and maintenance
Freshly collected blood from normal (male individuals, between the age group 25–35 years, who were non-smokers and not under any medication) donors was carefully layered on top of Histopaque and centrifuged at 1000 rpm for 20 min. The buffy coat interface, which represented the lymphocytes, was aspirated and again centrifuged at 1500 rpm for 15 min. The supernatant was discarded; pellets were disrupted and washed with normal saline. Finally the lymphocytes were seeded in RPMI-1640 supplemented with 10% FBS and 20 µg/ml PHA. Cells were grown at 37°C in a humidified atmosphere of 5% CO2/95% air.
Comet assay
DNA damage (single strand breaks) was measured by alkaline single cell gel electrophoresis (SCGE), also known as comet assay. The procedure of Singh et al. [33] was followed with minor modifications. Briefly, lymphocyte cells (1 × 104) were suspended in 0.6% (w/v) low melting agarose and layered over a frosted microscopic slide previously coated with a layer of 0.75% normal melting agarose. The slides were then immersed in a lysing solution of pH 10.0 and left overnight at 4°C. Slides were then transferred into a horizontal electrophoresis chamber containing alkaline solution (300 mM NaOH, I mM Na2EDTA; pH 13.0) and presoaked in this solution for 20 min for unwinding of DNA. Electrophoresis was then carried out for 20 min (300 mA, 20 V). Slides were then washed thrice with neutralizing buffer (Tris 0.4 M, pH 7.5), stained with ethidium bromide (final concentration 50 µg/ml), examined under a Nikon fluorescence microscope and subjected to image analysis using comet assay software programme (CASP). DNA damage was quantitated by tail moment measurement. It was calculated by multiplying the total intensity of the comet tail by the tail length, measured from the centre of the comet head.
Determination of intracellular ROS production
To measure intracellular ROS production according to Balasubramanyam et al. [34], cells after treatment were loaded with 10 µM DCFH-DA for 45 min. ROS levels were measured using spectrofluorimeter (Waters, USA 474 Scanning Fluorescence Detector, with an excitation set at 485 nm and emission at 530 nm) as a change in fluorescence because of the conversion of non-fluorescent DCFH-DA to the highly fluorescent compound 2',7'-dichlorofluorescein (DCF) in the cells. As III (1000 µM)-treated cells were incubated with different concentrations of curcumin (10, 25 and 50 µM respectively) for 1 h and resuspended in HEPES buffered saline (HBS, pH 7.4 containing 140 mM NaCl, 5 mM KCl, 10 mM HEPES, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose) loaded with the dye prior to each experiment. The non-fluorescent dye passively diffused into the cells where the acetates were cleaved by intracellular esterases. The resulting diol was retained by the cell membrane.
Estimation of lipid peroxidation
Lipid peroxidation was analyzed by the method of Ohkawa et al. [35]. The reaction mixture in a final volume of 3.0 ml contained the cell lysate, 100 µl of 10% SDS, 600 µl of 20% glacial acetic acid, 600 µl of 0.8% TBA, and water. The mixture was placed in a boiling water bath for 1 h and immediately shifted to crushed ice bath for 10 min. The mixture was centrifuged at 2500 × g for 10 min. The amount of thiobarbituric acid reactive substances (TBARS) formed was assayed by measuring the optical density of the supernatant at 535 nm against a blank devoid of the cell lysate. The activity was expressed as nmoles of TBARS/mg of protein using 1,1,3,3,-tetramethoxypropane (TMP) as standard.
Estimation of antioxidant enzymes
Catalase—Catalase was assayed by the method of Aebi [36]. The cell supernatant was treated with ethanol (10 µl/ml) and was kept on ice for 30 min. Triton X-100 (1%) was added subsequently and kept on ice for 30 min. Supernatant was added to assay mixture which contained 0.5 M sodium phosphate buffer (pH 7.0) and 10 mM H2O2. The decrease in absorbance was measured at 240 nm. The activity was calculated using extinction coefficient 0.04 mmole−1cm−1. One unit of catalase activity is defined as the amount of enzyme required to decompose 1 mole of H2O2/min.
Superoxide dismutase (SOD)—SOD was assayed by the method of Marklund and Marklund [37] with slight modifications. The assay is based on the ability of enzyme to inhibit auto-oxidation of pyrogallol. The cytosolic supernatant treated with triton X-100 (1%) was kept at 4°C for 30 min and was added to the assay mixture, which contained 0.05 M sodium phosphate buffer (pH 8.0), 0.1 mM EDTA and 0.27 mM pyrogallol. Solution of pyrogallol was made fresh in 10 mM HCl. The absorbance was measured for 5 min at 420 nm. One unit of SOD activity is defined as the amount of SOD required to cause unit change in absorbance per minute.
Glutathione peroxidese (GPx)—The activity of GPx was measured by the procedure described by Paglia and Valentine [38]. The procedure is an indirect measurement of GPx activity. GSSG (glutathione disulphide i.e. oxidized GSH) produced as a result of action of GPx was immediately reduced in the presence of excess GR thereby maintaining a constant level of GSH in the reaction system. The assay made use of oxidation of NADPH by GR, which could be measured at 340 nm. The final concentration in 3 ml reaction volume contained 50 mM sodium phosphate buffer (pH 7) containing EDTA (0.1 M buffer with 1 mM EDTA), 0.24 U/ml yeast GR, 0.3 mM GSH, 0.2 mM NADPH, 1.5 mM H2O2 and cytosolic sample. Reaction was started by addition of NADPH and the decrease in absorbance was monitored at 340 nm for 5 min. The GPx activity was expressed as nmoles of NADPH consumed /min/mg of protein.
DNA repair synthesis
Unscheduled DNA synthesis was examined in As-treated cells using [3H]thymidine incorporation in presence of different curcumin concentrations used. Normal human lymphocytes (2 × 105) were seeded in 35 mm plates and, after 24 h, hydroxyurea (final concentration 10 µM) was added followed by treatment with As III for 1 h. The cells were then washed in fresh medium containing hydroxyurea and curcumin of different concentrations was added along with [methyl 3H]thymidine (final concentration 2 µCi/ml). Cells harvested after 2 and 4 h were washed with phosphate buffered saline (PBS), suspended in PBS and aliquot was spotted on Whatman 3 mm filter paper disc [39]. The discs were washed successively in 10% trichloroacetic acid (TCA) followed by 5% TCA, ethanol (2x) and air-dried. Amount of [3H]thymidine incorporation was determined by liquid scintillation counting in an LKB 1217 Rackbeta liquid scintillation spectrometer.
Immunocytochemistry study
To determine the expression different DNA repair enzymes, immunocytochemistry was performed. Briefly, after AsIII treatment followed by curcumin post-treatment for different time periods cells were harvested and seeded on coverslips which were then air dried and fixed in formaldehyde solution. After permeabilization with ice cold acetone followed by blocking with bovine serum albumin (BSA), coverslips were incubated with primary antibodies (anti-PARP monoclonal) and (anti-DNA polymerase β (n-19) goat polyclonal) for overnight and then washed extensively with PBS followed by incubation with FITC conjugated secondary antibody at 37°C for 90 min in a dark humid chamber. Finally, after mounting with glycerol these coverslips were viewed under fluorescent microscope.
Preparation of cell lysate
Cells after treatment were harvested and washed in wash buffer (HEPES-KOH 10 mM, MgCl2 1 mM, KCl 10 mM, DTT 1 mM). The pellet was lysed with lysis buffer (Tris-HCl 10 mM, pH 7.5, MgCl2 1 mM, EGTA 1 mM, β-mercaptoethanol 0.65%, CHAPS 0.5% and glycerol 10%) and the lysate was centrifuged at 10,000 × g for 20 min at 4°C and the supernatant was collected for protein estimation. The protein concentration of the cell lysate was estimated following Lowry’s method [40] and the extract was utilized for further studies.
Western blotting
The cell lysate containing 30 µg of protein was analyzed on a 12.5% SDS-PAGE under reducing conditions. The gel was electro-blotted on to a nitrocellulose membrane. Membrane was incubated with primary antibodies for repair enzymes (PARP/DNA polymerase β) 1:1000 dilution in TBS overnight at 4°C with constant shaking. The blots were washed 4 times with TBST followed by treatment with alkaline phosphatase conjugated anti-mouse immunoglobulin G (1:2000 dilutions in TBS) at room temperature. The membrane was then washed 4 times and treated with BCIP/NBT to visualize the proteins.
Experiments
For comet assay, lymphocytes were seeded in 55 mm culture petri-dishes at a cell density of 5 × 105 cells per plate. Exponentially growing cells were subjected to different modes of treatment. For dosimetry analysis the lymphocytes were treated with different concentrations of As III (100, 250, 500 and 1000 µM respectively). The other treatment modes were carried out with the highest damage causing concentration of As III (1000 µM). During simultaneous treatment lymphocytes were treated with As III (1000 µM) and different doses of curcumin (10, 25 and 50 µM) for 1 h to assess the extent of DNA damage. Pre treatment analysis was followed where lymphocytes were incubated with very low doses of curcumin (5, 10 and 15 µM) for 24 h and washed with fresh medium. Subsequently cells were treated with As III (1000 µM) treatment for 1 h. In third set of experiments, As III (1000 µM) treated cells were post-incubated with or without different concentrations of curcumin for different time intervals. For assessing modulatory role of curcumin in inhibiting lipid peroxidation, lymphocytes were simultaneously treated with arsenic and curcumin of different doses. Investigation for antioxidant potential of curcumin against As-induced genotoxicity was carried out by the simultaneous mode of treatment. Assessment of additional repair inducing capability of curcumin using [3H]thymidine was observed by following post-treatment of As-damaged lymphocytes with different curcumin concentrations.
Results
The present experiments were carried out on human lymphocytes with the most potent form of As, As III. Treatment of lymphocytes with different concentrations of As III for 1 h showed a gradual increase in tail moment, giving a perfect dose-response relationship (Fig. 1). The concentrations of curcumin used in this study showed no genotoxic as well as cytotoxic effect after 1 h incubation with lymphocytes from healthy donor (data not shown). The extent of DNA damage induced by 1000 µM As III (that induces highest amount of DNA damage) was considerably reduced when the cells were simultaneously treated with different concentrations of curcumin (10, 25 and 50 µM). The results are shown in Fig. 2 (a). When cells were preincubated with very low concentrations of curcumin (5, 10 and 15 µM) for 24 h and then subsequently treated with As III (1000 µM), the comet tail moment was reduced remarkably in proportion to the pretreatment dose (Fig. 2 (b)). Based on these two results inhibition concentration of curcumin required to reduce the genotoxicity by 50% i.e. IC50 value was calculated in both sets of experiments. On the basis of IC50 values it was observed that during simultaneous treatment the value was 38 µM, whereas during pre-treatment this could be achieved even at a much lower value (11 µM), indicating better efficacy of curcumin in protecting genotoxicity.
Fig. 1.
Dose dependent increase in comet tail moment with As III treatment in normal human lymphocytes reflecting DNA damage as measured by SCGE.
Fig. 2.
Reduction in As III (1000 µM)-induced comet tail moment in normal human lymphocytes with curcumin by (a) simultaneous treatment and by (b) low dose pre treatment.
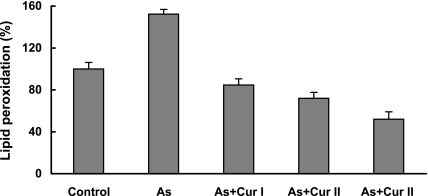
When the cells were analyzed for intracellular ROS production with the fluorescent dye DCFH-DA using a spectrofluorimeter, it was found that 1000 µM As III enhanced the level of ROS generation over the background level found in control cells. This increased ROS generation was effectively quenched by curcumin at different concentrations (10, 25 and 50 µM respectively). As depicted in Fig. 3 (a), arsenic exposure increases generation of ROS production three times higher than that of the control value which in presence of curcumin was effectively reduced and with 50 µM curcumin it was almost at the background level. When these arsenic treated cells were viewed under a fluorescence microscope, very intense fluorescence compared to the background was observed indicating generation of ROS; presence of curcumin appreciably reduced the fluorescence intensity indicating quenching action of curcumin. Photographs showing generation of ROS by arsenite and its quenching by curcumin are shown in Fig. 3 (b). Curcumin was also proved effective in inhibiting the lipid peroxidation caused by As III (Fig. 4). As represented in the figure the levels of TBARS were significantly increased from 100% in control lymphocytes (without any treatment) to 153% in cells treated with As 1000 µM. Administration of curcumin during this arsenite treatment significantly decreased the levels of TBARS by 84.6%, 72% and 52% in presence of 10, 25 and 50 µM respectively.
Fig. 3.
Quenching of ROS production by curcumin, Cur I (10 µM), Cur II (25 µM) and Cur III (50 µM) during treatment with As III (1000 µM) in normal human lymphocytes as measured by (a) fluorescence intensity; (b) fluorescence microscopic pictures showing arsenite induced ROS production and its modulation by curcumin. (i) indicates background ROS level, (ii) ROS generation after 1000 µM As treatment, (iii), (iv) and (v) indicate modulation of ROS by 10, 25 and 50 µM of curcumin.
Fig. 4.
Inhibition of lipid peroxidation by curcumin during treatment with As III (1000 µM) in normal human lymphocytes. Cur I (10 µM), Cur II (25 µM) and Cur III (50 µM).
Analysis of catalase, SOD and GPx as represented in Fig. 5 (a), (b), (c) in cells treated with curcumin showed that their activity was higher than that found in control cells. The control levels of catalase as shown in Fig. 5 (a) were greatly reduced by 1000 µM. arsenite. However, when these cells were treated simultaneously with curcumin the level of catalase was much higher than that of the control or basal level value. This might be due to elevated levels of catalase as induced by curcumin. Similar trends were observed in case of SOD as well as GPx as depicted in Fig. 5 (b) and 5 (c). Moreover it was observed that the elevated levels of antioxidant enzyme activity induced by curcumin were retained up to 4–5 h after which they gradually dropped to their original value during the next 24 h (data not shown).
Fig. 5.
Induction of antioxidant defense enzymes Catalase (a), SOD (b) and GPx (c) by curcumin(10, 25 and 50 µM respectively) during simultaneous treatment with As III (1000 µM) in normal human lymphocytes.
During post-treatment study, As-treated cells post-incubated with curcumin for different time periods, displayed the ability to repair DNA damage in a dose dependent manner. It was observed that after arsenic exposure there was very little repair after 2 h and even after 4 h. During this repair time when curcumin was administered the process was accelerated remarkably. These results are represented in Fig. 6 (a). Using hydroxyurea (which suppresses the replicative DNA synthesis but not the repair synthesis), unscheduled DNA synthesis as measured by incorporation of [3H]thymidine was examined in treated cells. Results (Fig. 6b) revealed that curcumin induced repair synthesis of DNA at a faster rate, as evident by the increase in the [3H]thymidine incorporation in presence of curcumin. To understand the role of curcumin in the DNA repair process expression of two important DNA repair enzymes were examined. When immunocytochemistry study was performed using PARP antibody it was observed that PARP expression increased in presence of curcumin mainly after 4 h of post-incubation (data not shown). Another repair enzyme, DNA polymerase β, was also examined but FITC stained slides hardly showed any influence of curcumin in inducing the expression of this enzyme (results not shown). Western blot analysis using antibodies for PARP and DNA polymerase β also reflected similar trend as represented in Fig. 7. The bands clearly indicate that expression of PARP was much more pronounced when arsenite induced damaged cells were allowed to repair in presence of curcumin. However, curcumin showed insignificant influence on the expression of DNA polymerase β.
Fig. 6.
(a) Enhancement of repair activity in As III (1000 µM)-damaged human lymphocytes when post-incubated with curcumin (10, 25 and 50 µM). (b) DNA repair synthesis of As III (1000 µM)-damaged lymphocytes by curcumin was assessed by measuring incorporation of [3H]thymidine in isolated DNA in presence of hydroxyurea.
Fig. 7.

Expression of repair enzymes PARP and DNA polymerase β as measured by Western blot following treatment with curcumin in As III (1000 µM)-treated human lymphocytes. Lane 1: represents control, Lane 2: As III (1000 µM) treatment, Lane 3: As III (1000 µM) followed by curcumin 25 µM and Lane 4 represents As III (1000 µM) followed by curcumin 50 µM.
Discussion
Arsenite, a potent gene and chromosomal mutagen and oxyradical-generating element is predominantly responsible for inducing multilocus deletion [41]. Despite extensive research on the toxicity of arsenic, many questions remain unanswered, making risk assessment difficult [42]. Although multiple hypotheses have been proposed to explain arsenic induced carcinogenesis, the exact mechanisms remain elusive [43]. Most stronger theories of arsenic causing carcinogenicity are production of chromosomal abnormalities, promotion of carcinogenesis and oxidative stress [44]. In the last few years, increasing evidence of the correlation between the generation of ROS, DNA damage, tumor promotion and arsenic exposure was observed [45]. However the exact mechanisms or pathways involved in free radicals induced damage remained to be elucidated. Liu and his coworkers [46] are of the view that primary target in As-induced genotoxic response is mitochondrial damage which in turn causes the release of superoxide anions that can react with nitric oxide (NO) to produce highly reactive peroxynitrites. Recent evidences support the fact that As exerts its toxicity through the generation of ROS, which include hydrogen peroxide (H2O2), super oxide anion (O2·−), hydroxyl radical (OH·), and peroxyl radical (ROO·) [19]. The present study here revealed that As III induced DNA damage in human lymphocytes in a dose dependent manner as monitored by comet assay. The comet assay or alkaline single cell gel electrophoresis is a technique used for quantification of DNA strand breaks, cross links and alkali labile sites induced by any agents. DNA migration in an electric field is considered to be proportional to strand breakage, which gives an estimation of genotoxicity [47]. The comet tail moment as measured here not only provided an indication of the extent of DNA damage but was also an effective indicator of the reduction of damage as obtained by treatment with curcumin, confirming the antagonistic effect of curcumin towards DNA damage. The natural compounds particularly polyphenols seem to possess many of the desirable qualities for chemopreventive properties and curcumin is such a natural agent. Significant research interest has been generated in recent years around curcumin, the major constituent of turmeric, because of its antioxidant, anti-tumor, anti-inflammatory and chemopreventive effects.
Lymphocytes preincubated with curcumin as observed here were protected from damage to DNA when subsequently exposed to As III. This definitely attributes a true chemopreventive role of the compound. In cultured cells, it was reported previously that As III increased the formation of fluorescent dichlorofluorescein by oxidation of non- fluorescent form dichlorofluorescein dihydroacetate (DCF-DAc) [48, 49]. In our findings also As III was reported to generate ROS level as measured by fluorescent technique, the value being much higher than the background one. Curcumin could efficiently counteract this As-induced damage by quenching the generation of ROS. As a consequence of ROS generation, lipid peroxidation level in treated cells gets increased which was significantly reduced by treatment with curcumin. Having polyphenolic structure and β-diketone functional group, curcumin is reported to be a stronger antioxidant inhibitor of lipid peroxidation than other flavonoids having single phenolic hydroxyl group [50]. Due to such functional groups curcumin is able to scavenge or neutralize free radicals by interacting with oxidative cascade, quenches oxygen and by chelating some metal ions inhibits peroxidation of membrane lipids thereby maintaining membrane integrity and their function [51]. From the present results it is evident that arsenic induces oxidative DNA damage in human lymphocytes. However, the exact mechanism by which this inorganic form causes damage is not very clear. The present study showed that arsenic inhibits the activities of some antioxidant enzymes like catalase, SOD and also glutathione peroxidase. Arsenic was reported to inhibit the activities of catalase and GPx leading to accumulation of H2O2, [49, 52]. Other recent studies suggest that arsenite activates NADH oxidase to produce O2·−, which causes oxidative DNA damage [53]. This H2O2 and O2·− are considered the main ROS involved in arsenic induced DNA damage [19]. Chronic As toxicity in experimental animals caused significant reduction of hepatic antioxidant enzymes like glucose-6-phosphate dehydrogenase, catalase, GPx, GR, glutathione-S-transferase (GST), and plasma membrane Na+/K+ ATPase activity [54]. Similar observations of decrease in catalase and SOD activities were also found in CH V79 mammalian cells with As III treatment [55]. These GPx and catalase, which act as preventive antioxidants and SOD, a chain breaking antioxidant, play an important role in protection against the deleterious effects of lipid peroxidation [56]. The results presented here showed that depleted levels of these antioxidant enzymes were brought back to their normal form after administration of curcumin indicating a phase II enzyme inducing activity of the phenolic. An interesting finding was that these elevated enzyme activities remained elevated up to 5 h (with 1 h exposure of curcumin) after which they gradually dropped back to their original level. Another aspect of As-mediated carcinogenesis might be inhibition of repair phenomena. Ability to cause DNA damage without inducing direct mutations has led to the concept that arsenic promotes DNA damage by inhibiting DNA repair [57]. As III has been found to induce gene expression of a number of stress response proteins such as ubiquitin that result in altering the DNA repair mechanism, which thereby causes DNA damage [58]. Snow [59] was of the view that arsenic inhibits the enzymes involved in repair thus leading to the alteration of DNA replication and repair mechanism. Li and Rossman [60] stated that inhibition of DNA repair is due to inhibitory effect of As III on DNA ligase II and to a lesser extent on DNA ligase I, which is a possible mechanism for co-mutagenesis. The present findings also explain here that As III greatly affects DNA repair efficacy of normal lymphocytes. But the significant observation of the present work is that the curcumin not only prevented DNA damage, but also helped in recovery of DNA damage by accelerating DNA repair efficiency in the damaged cells. Reduction in comet tail moment even after 2 h of post-incubation with curcumin indicate a faster DNA repair rate which was further supported by the observation on unscheduled DNA synthesis. Lymphocytes with As III insult exhibited greater expression of repair enzyme PARP compared to cells without such treatment during the recovery process. Previous reports suggest that As III might generate nitric oxide to damage DNA, which then stimulate poly (ADP-ribosylation) [61]. Moreover the expression of PARP was much more pronounced when these As-damaged cells were treated with curcumin. This is in accordance with the findings of other researchers who suggest that chemopreventive effects of [(−)-epigallocatechin-3-gallate] (EGCG), natural phenolic constituent of green tea, may partly be attributed to an induction of PAR formation [62]. However, the expression of another DNA repair enzyme, DNA polymerase β, seemed unaltered even after curcumin treatment. This finding was in support of the fact that over-expression of PARP 1 antagonizes expression of DNA polymerase β [63].
In conclusion it can be summarized that curcumin could be a potential antioxidant against the oxidative stress generated by As III. The additional repair inducing property of curcumin was an interesting finding in the reduction of As induced DNA damage. These observations indicate a promising role of curcumin in combating the disastrous health effects of arsenic in India, particularly in the state of West Bengal. Before establishing the usage of curcumin in arsenic affected areas in vivo studies need to be conducted, which are in progress. In addition, field trials on the effect of curcumin consumption in As-exposed humans are required which might pave a safer future for the population suffering from the severe health hazards of As.
Acknowledgments
The authors are indebted to Director CNCI, for providing the infrastructural facility and fellowship to SM.
Abbreviations
- As
arsenic
- As III
trivalent sodium arsenite
- As V
pentavalent sodium arsenate
- BCIP/NBT
5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium
- CASP
comet assay software programme
- CHAPS
(3-[(3-cholamidoprppyl) dimethylaminonio]-1-propanesulphonate
- DCFHDA, 2',7'
dichlorofluorescein diacetate
- DMSO
dimethyl sulphoxide
- DTT
dithiotheritol
- Na2EDTA
ethylenediaminetetraacetic acid disodium salt
- EGTA
ethylene glycol-bis(β-aminoethylether-)-N,N,N',N'-tetraacetic acid
- FACS
fluorescence activated cell sorter
- FBS
fetal bovine serum
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GSH
glutathione
- GSSG
glutathione disulphide
- GST
glutthione S trnsferase
- H2O2
hydrogen peroxide
- HEPES
N-2hydroxyethyl piperazine N-2 ethane sulphonic acid
- PHA
phytohaemagglutinin
- PARP
poly(ADP ribose)polymerase
- ROO·
peroxyl radical
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SCGE
single cell gel electrophoresis
- SDS
sodium dodecyl sulphate
- SDS-PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- SOD
superoxide dismutase
- O2·−
super oxide anion
- TBA
thiobarbituric acid
- TBARS
thiobarbituric acid reactive substances
- TCA
trichloroacetic acid
- TMP
1,1,3,3-tetramethoxypropane
References
- 1.Shi H., Hudson L.G., Ding W., Wang S., Cooper K.L., Liu S., Chen Y., Shi X., Liu K.J. Arsenic causes DNA damage in keratinocytes via generation of hydroxyl radicals. Chem. Res. Toxicol. 2004;17:871–878. doi: 10.1021/tx049939e. [DOI] [PubMed] [Google Scholar]
- 2.Flora S.J.S. Arsenic-induced oxidative stress and its reversibility following combined administration of N-acetyl cysteine and meso 2,3-dimercaptosuccinic acid in rats. Clin. Exp. Pharmacol. Physiol. 1999;26:865–869. doi: 10.1046/j.1440-1681.1999.03157.x. [DOI] [PubMed] [Google Scholar]
- 3.Bagla P., Kaisar J. India’s spreading health crisis draws global arsenic experts. Science. 1996;274:174–175. doi: 10.1126/science.274.5285.174. [DOI] [PubMed] [Google Scholar]
- 4.Nickson R., McArthur J., Burgess W., Ahmed K.M., Ravenscroft P., Rahman M. Arsenic poisoning of Bangladesh groundwater. Nature. 1998;395:338. doi: 10.1038/26387. [DOI] [PubMed] [Google Scholar]
- 5.Sinha D., Roy M., Siddiqi M., Bhattacharya R.K. Arsenic induced micronuclei formation in mammalian cells and its counteraction by tea. J. Env. Pathol. Toxicol. Oncol. 2005;24:43–54. doi: 10.1615/jenvpathtoxoncol.v24.i1.50. [DOI] [PubMed] [Google Scholar]
- 6.Basu A., Mahata J., Basu A., Gupta S., Giri A.K. Genetic toxicology of a paradoxical human carcinogen, arsenic: a review. Mutat. Res. 2001;488:171–194. doi: 10.1016/s1383-5742(01)00056-4. [DOI] [PubMed] [Google Scholar]
- 7.Aposhian H.V. Biochemical toxicology of arsenic. Rev. Biochem. Toxicol. 1989;10:265–299. [Google Scholar]
- 8.Tinwell H., Stephens S.C., Ashby J. Arsenite as the probable active species in human carcinogenicity of arsenic: mouse micronucleus assay on Na and K arsenite, orpiment and Fowler’s solution. Environ. Health Perspect. 1991;95:205–210. doi: 10.1289/ehp.9195205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin T.H., Huang Y.L., Wang M.Y. Arsenic species in drinking water, hair, fingernails, and urine of patients with blackfoot disease. J. Toxicol. Environ. Health. 1998;53:85–93. doi: 10.1080/009841098159376. [DOI] [PubMed] [Google Scholar]
- 10.Abernathy C.O., Liu Y.P., Longfellow D., Aposhian H.V., Fowler B., Goyer R., Menzer R., Rossman T., Thompson C., Walkes M. Arsenic: health effects, mechanisms of actions and research issues. Environ. Health Perspect. 1999;107:593–597. doi: 10.1289/ehp.99107593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashiwada E., Kuroda K., Endo G. Aneuploidy induced by dimethyl arsinic acid in mouse bone marrow cells. Mutat. Res. 1998;413:33–38. doi: 10.1016/s1383-5718(98)00011-4. [DOI] [PubMed] [Google Scholar]
- 12.Jha A.N., Noditi M., Nilsson R., Natarajan A.T. Genotoxic effects of sodium arsenite on human cells. Mutat. Res. 1992;284:215–221. doi: 10.1016/0027-5107(92)90005-m. [DOI] [PubMed] [Google Scholar]
- 13.Hartmann A., Speit G. Comparative investigations of the genotoxic effects of metals in the single cell gel (SCG) assay and their sister chromatid exchange test. Environ. Mol. Mutagen. 1994;23:299–305. doi: 10.1002/em.2850230407. [DOI] [PubMed] [Google Scholar]
- 14.Schaumloffel N., Gebel T. Heterogeneity of the DNA damage provoked by antimony and arsenic. Mutagenesis. 1998;13:281–286. doi: 10.1093/mutage/13.3.281. [DOI] [PubMed] [Google Scholar]
- 15.Gebel T.W. Genotoxicity of arsenical compounds. Int. J. Hyg. Environ. Health. 2001;203:249–262. doi: 10.1078/S1438-4639(04)70036-X. [DOI] [PubMed] [Google Scholar]
- 16.Gebel T.W., Birkenkamp P., Luthin S., Dunkelberg H. Arsenic (III), but not antimony (III), induces DNA-protein crosslinks. Anticancer Res. 1998;18:4253–4257. [PubMed] [Google Scholar]
- 17.Li D., Morimoto K., Takeshita T., Lu Y. Arsenic induces DNA damage via reactive oxygen species in mammalian cells. Environ. Health Prev. Med. 2001;6:27–32. doi: 10.1007/BF02897306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T.S., Hsu T.Y., Chung C.H., Wang A.S., Bau D.T., Jan K.Y. Arsenite induces oxidative DNA adducts and DNA-protein cross-links in mammalian cells. Free Radic. Biol. Med. 2001;31:321–330. doi: 10.1016/s0891-5849(01)00581-0. [DOI] [PubMed] [Google Scholar]
- 19.Li D., Morimoto K., Takeshita T., Lu Y. Formamidopyrimidine-DNA glycosylase enhances arsenic-induced DNA strand breaks in PHA-stimulated and unstimulated human lymphocytes. Environ. Health Perspect. 2001;109:523–526. doi: 10.1289/ehp.01109523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramirez P., Del Razo L.M., Gutierrez-Ruiz M.C., Gonsebatt M.E. Arsenite induces DNA-protein crosslinks and cytokeratin expression in the WRL-68 human hepatic cell line. Carcinogenesis. 2000;21:701–706. doi: 10.1093/carcin/21.4.701. [DOI] [PubMed] [Google Scholar]
- 21.Nishigori C., Hattori Y., Toyokuni S. Role of reactive oxygen species in skin carcinogenesis. Antioxid. Redox Signal. 2004;6:561–570. doi: 10.1089/152308604773934314. [DOI] [PubMed] [Google Scholar]
- 22.Lee B.M., Park K.K. Beneficial and adverse effects of chemopreventive agents. Mutat. Res. 2003:265–270. 523–524. doi: 10.1016/s0027-5107(02)00342-1. [DOI] [PubMed] [Google Scholar]
- 23.Mahakunakorn P., Tohda M., Murakami Y., Matsumoto K., Watanabe H., Vajaragupta O. Cytoprotective and cytotoxic effects of curcumin: Dual action on H2O2-induced oxidative cell damage in NG108-15 cells. Biol. Pharma. Bull. 2003;26:725–728. doi: 10.1248/bpb.26.725. [DOI] [PubMed] [Google Scholar]
- 24.Kalpana C., Menon V.P. Modulatory effects of curcumin on lipid peroxidation and antioxidant status during nicotine induced toxicity. Pol. J. Pharmacol. 2004;56:581–586. [PubMed] [Google Scholar]
- 25.Ammon H.P.T., Wahl M.A. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 26.Wei Q.Y., Chen W.F., Zhou B., Yang L., Liu Z.L. Inhibition of lipid peroxidation and protein oxidation in rat liver mitochondria by curcumin and its analogues. Biochem. Biophys. Acta. 2006;1760:70–77. doi: 10.1016/j.bbagen.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Kunchandy E., Rao M.N.A. Effect of curcumin on hydroxyl radical generation through Fenton reaction. Int. J. Pharm. 1989;57:173–176. [Google Scholar]
- 28.Sreejayan N., Rao M.N.A. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 29.Sreejayan N., Rao M.N.A. Curcuminoids as potent inhibitors of lipid peroxidation. J. Pharma. Pharmacol. 1994;46:1013–1016. doi: 10.1111/j.2042-7158.1994.tb03258.x. [DOI] [PubMed] [Google Scholar]
- 30.Huang M.T., Lystz T., Ferraro T., Abidi T.F., Laskin J.D., Conney J.H. Inhibitory effect of curcumin in vivo lipoxygenase and cycloxygenase activities in mouse epidermis. Cancer Res. 1991;51:813–819. [PubMed] [Google Scholar]
- 31.Susan M., Rao M.N.A. Induction of glutathione S-transferase activity by curcumin in mice. Arneim.-Forsch. 1992;42:962–964. [PubMed] [Google Scholar]
- 32.Arbisor J.L., Klauber N., Rohan R., van Leeuwen R., Huang M.-T., Fisher C., Flynn E., Byers H.R. Curcumin is an in vivo inhibitor of angiogenesis. Mol. Med. 1998;4:376–383. [PMC free article] [PubMed] [Google Scholar]
- 33.Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell. Res. 1988;75:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 34.Balasubramanyam M., Koteswari A.A., Sampath Kumar R., Monickaraj S.F., Maheswari J.U., Mohan V. Curcumin induced inhibition of reactive oxygen species generation: Novel therapeutic implications. J. Biosc. 2003;28:715–721. doi: 10.1007/BF02708432. [DOI] [PubMed] [Google Scholar]
- 35.Ohkawa H., Ohisi N., Yagi Y. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 36.Aebi H. Catalase in vitro. Methods in Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 37.Marklund S., Marklund G. Involvement of superoxide anion radical in autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 38.Paglia D.E., Valentine W.M. Studies on the qualitative and quantitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 39.Bollum F.J. Filter paper disk techniques for assaying radioactive macromolecules. Methods in Enzymol. 1968;12B:169–173. [Google Scholar]
- 40.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 41.Kessel M., Liu S.X., Xu A., Santella R., Hei T.K. Arsenic induces oxidative DNA damage in mammalian cells. Mol. Cell. Biochem. 2002;234235:301–308. [PubMed] [Google Scholar]
- 42.Shen J., Wanibuchi H., Salim E.L., Wei M., Kinoshita A., Yoshida K., Endo G., Fukushima S. Liver tumorigenicity of trimethylarsine oxide in male Fischer 344 rats-association with oxidative DNA damage and enhanced cell proliferation. Carcinogenesis. 2003;21:1827–1835. doi: 10.1093/carcin/bgg143. [DOI] [PubMed] [Google Scholar]
- 43.Kitchin K.T. Recent advances in arsenic carcinogenesis: modes of action, animal model system and methylated arsenic metabolites. Toxicol. Appl. Pharmacol. 2001;172:249–261. doi: 10.1006/taap.2001.9157. [DOI] [PubMed] [Google Scholar]
- 44.Kitchin K.T., Ahmad S. Oxidative stress as a possible mode of action for arsenic carcinogenesis. Toxicol. Lett. 2003;137:3–13. doi: 10.1016/s0378-4274(02)00376-4. [DOI] [PubMed] [Google Scholar]
- 45.Shi H., Shi X., Liu K.J. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol. Cell. Bio. Chem. 2004;255:67–78. doi: 10.1023/b:mcbi.0000007262.26044.e8. [DOI] [PubMed] [Google Scholar]
- 46.Liu S.X., Athar M., Lippai I., Waldren C., Hei T.K. Induction of oxyradicals by arsenic: implication of mechanism of genotoxicity. PNAS. 2001;98:1643–1648. doi: 10.1073/pnas.031482998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duez P., Dehon G., Kumps A., Dubois J. Statistics of the comet assay: a key to discriminate between genotoxic effects. Mutagenesis. 2003;18:159–166. doi: 10.1093/mutage/18.2.159. [DOI] [PubMed] [Google Scholar]
- 48.Barchowsky A., Dudek E.J., Treadwell M.D., Wetterhahn K.E. Arsenic induces oxidative stress and NF-kB activation in cultured aortic endothelial cells. Free Radic. Biol. Med. 1996;21:783–790. doi: 10.1016/0891-5849(96)00174-8. [DOI] [PubMed] [Google Scholar]
- 49.Lee T.-C., Ho I.-C. Modulation of cellular antioxidant defense activities by sodium arsenite in human fibroblasts. Arch. Toxicol. 1995;69:498–504. doi: 10.1007/s002040050204. [DOI] [PubMed] [Google Scholar]
- 50.Phan T.T., See P., See S.T., Chan S.Y. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J. Trauma. 2001;51:927–931. doi: 10.1097/00005373-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 51.Pulla Reddy A., Lokesh B.R. Effect of dietary turmeric (Curcuma longa) on iron-induced lipid peroxidation in the rat liver. Food Chem. Toxicol. 1994;32:279–283. doi: 10.1016/0278-6915(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 52.Jing Y., Dai J., Chalmers-Redman R.M., Tastton W.G., Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–2111. [PubMed] [Google Scholar]
- 53.Liu F., Jan K.Y. DNA damage in arsenite and cadmium treated bovine aortic endothelial cells. Free Radic. Biol. Med. 2000;28:55–63. doi: 10.1016/s0891-5849(99)00196-3. [DOI] [PubMed] [Google Scholar]
- 54.Santra A., Maiti A., Das S., Lahiri S., Chakraborty S.K., Mazumdar D.N. Hepatic damage caused by chronic arsenic toxicity in experimental animals. Toxicol. Clin. Toxicol. 2000;38:395–405. doi: 10.1081/clt-100100949. [DOI] [PubMed] [Google Scholar]
- 55.Sinha D., Bhattacharya R.K., Siddiqi M., Roy M. Amelioration of sodium arsenite induced clastogenicity by tea extracts in Chinese hamster V79 cells. J. Env. Pathol. Toxicol. Oncol. 2005;24:129–139. doi: 10.1615/jenvpathtoxoncol.v24.i2.60. [DOI] [PubMed] [Google Scholar]
- 56.Ray G., Hussain S.A. Oxidants, antioxidants and carcinogenesis. Indian J. Exp. Biol. 2002;42:1213–1232. [PubMed] [Google Scholar]
- 57.Saleha Banu B., Danadevi K., Jamil K., Ahuja Y.R., Rao K.V., Ishaq M. In vivo genotoxic effect of arsenic trioxide in mice using comet assay. Toxicology. 2001;162:171–177. doi: 10.1016/s0300-483x(01)00359-6. [DOI] [PubMed] [Google Scholar]
- 58.Parag H.A., Raboy B., Kulka R.G. Effects of heat shock on protein degradation in mammalian cells involvement of the ubiquitin system. EMBO J. 1987;6:55–61. doi: 10.1002/j.1460-2075.1987.tb04718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snow E.T. Metal carcinogenesis: mechanistic implications. Pharmacol. Ther. 1992;53:31–65. doi: 10.1016/0163-7258(92)90043-y. [DOI] [PubMed] [Google Scholar]
- 60.Li J.H., Rossman T.G. Inhibition of DNA ligase activity by arsenite: a possible mechanism of its comutagenesis. Mol. Toxicol. 1989;2:1–9. [PubMed] [Google Scholar]
- 61.Lynn S., Shiung J.N., Gurr J.R., Jan K.Y. Arsenite stimulates poly (ADP ribosylation) by generation of nitric oxide. Free Radic. Biol. Med. 1998;24:442–449. doi: 10.1016/s0891-5849(97)00279-7. [DOI] [PubMed] [Google Scholar]
- 62.Bertam B., Bollow U., Rajaee-Behbahani N., Burkle A., Schmezer P. Induction of poly (ADP ribosyl)ation and DNA damage in human peripheral lymphocytes after treatment (−)-epigallocatechin gallate. Mutat. Res. 2003;534:77–84. doi: 10.1016/s1383-5718(02)00245-0. [DOI] [PubMed] [Google Scholar]
- 63.Sukhanova M.V., Khodyreva S.N., Lebedeva N.A., Prasad R., Wilson S.H., Lavrik O.I. Human base excision repair enzymes apurinic/apyrimidinic endonuclease1 (APE 1), DNA polymerase β and poly (ADP ribose) polymerase 1: interplay between strand displacement DNA synthesis and proof reading exonuclease activity. Nucleic Acids Res. 2005;33:1222–1229. doi: 10.1093/nar/gki266. [DOI] [PMC free article] [PubMed] [Google Scholar]