Abstract
The nuclear PXR (pregnane X receptor) was originally characterized as a key transcription factor that activated hepatic genes encoding drug-metabolizing enzymes. We have now demonstrated that PXR also represses glucagon-activated transcription of the G6Pase (glucose-6-phosphatase) gene by directly binding to CREB [CRE (cAMP-response element)-binding protein]. Adenoviral-mediated expression of human PXR (hPXR) and its activation by rifampicin strongly repressed cAMP-dependent induction of the endogenous G6Pase gene in Huh7 cells. Using the −259 bp G6Pase promoter construct in cell-based transcription assays, repression by hPXR of PKA (cAMP-dependent protein kinase)-mediated promoter activation was delineated to CRE sites. GST (glutathione transferase) pull-down and immunoprecipitation assays were employed to show that PXR binds directly to CREB, while gel-shift assays were used to demonstrate that this binding prevents CREB interaction with the CRE. These results are consistent with the hypothesis that PXR represses the transcription of the G6Pase gene by inhibiting the DNA-binding ability of CREB. In support of this hypothesis, treatment with the mouse PXR activator PCN (pregnenolone 16α-carbonitrile) repressed cAMP-dependent induction of the G6Pase gene in primary hepatocytes prepared from wild-type, but not from PXR-knockout, mice, and also in the liver of fasting wild-type, but not PXR-knockout, mice. Moreover, ChIP (chromatin immunoprecipitation) assays were performed to show a decreased CREB binding to the G6Pase promoter in fasting wild-type mice after PCN treatment. Thus drug activation of PXR can repress the transcriptional activity of CREB, down-regulating gluconeogenesis.
Keywords: cAMP-response element-binding protein (CREB), glucagon, gluconeogenesis, glucose-6-phosphatase (G6Pase), insulin, pregnane X receptor (PXR)
Abbreviations: AF2, activation function 2; CAR, constitutive active/androstane receptor; ChIP, chromatin immunoprecipitation; CRE, cAMP-response element; CREB, CRE-binding protein; CYP, cytochrome P450; DBD, DNA-binding domain; DR1, direct repeat 1; FBS, foetal bovine serum; FoxO, Forkhead box O; G6Pase, glucose-6-phosphatase; GST, glutathione transferase; HNF4, hepatic nuclear factor 4; IRS, insulin-response sequence; KO, knockout; MEM, minimal essential medium; MOI, multiplicity of infection; PCN, pregnenolone 16α-carbonitrile; PEPCK1, phosphoenolpyrvate carboxykinase 1; PGC1α, peroxisome-proliferator-activated receptor γ co-activator 1α; PKA, cAMP-dependent protein kinase; PXR, pregnane X receptor; RXR, retinoid X receptor
INTRODUCTION
In response to fasting and/or starvation, the liver increases production of glucose by increasing both gluconeogensis and glycogenolysis, in which glucagon up-regulates the transcription of the hepatic genes that encode rate-limiting enzymes, such as G6Pase (glucose-6-phosphatase) and PEPCK1 (phosphoenolpyrvate carboxykinase 1) [1–5]. Glucagon stimulates PKA (cAMP-dependent protein kinase) that phosphorylates the CREB [CRE (cAMP-response element)-binding protein] [1,6,7]. Phosphorylated CREB binds to CRE and activates the transcription of CRE-bearing genes such as those for G6Pase and PEPCK1 [2,3,8,9]. The PGC1A (peroxisome-proliferator-activated receptor γ co-activator 1α) gene is a glucagon-activated gene [4,7]. PGC1α acts as a glucagon-response factor up-regulating these genes by co-activating their HNF4 (hepatocyte nuclear factor 4)-mediated transcription [4,10,11]. Treatment with drugs such as phenobarbital and PCN (pregnenolone 16α-carbonitrile), which activate the nuclear receptors CAR (constitutive active/androstane receptor) and/or PXR (pregnane X receptor), results in the down-regulation of these enzymes [12–15]. Since G6Pase is the critical enzyme that controls the serum level of glucose by catalysing the dephosphorylation of glucose 6-phosphate generated from both gluconeogensis and glycogenolysis, in the present study, we investigated the molecular mechanism by which PXR represses the glucagon-mediated activation of the G6Pase gene.
Mouse PXR was characterized as a hepatocyte-enriched transcription factor that is activated by CYP3A (where CYP is cytochrome P450) inducers, such as PCN [16]. Conserving its activation by drugs, the corresponding human PXR (hPXR) was subsequently shown to be activated by a large number of human CYP3A inducers, such as rifampicin [17]. Along with the other drug-activated nuclear receptor CAR, PXR provides a major defence mechanism against drug-induced adverse events by increasing the hepatic capability to metabolize and excrete these drugs [13,18]. Upon activation and transcription of a similar set of hepatic genes, these receptors are also involved in the defence against bile acid and bilirubin toxicity [19–22]. In regenerating mouse liver, activation of CAR decreases the levels of reverse T3 (a T3 antagonist) by activating the deiodinase 1 gene, resulting in the increased T3 activity and in the induction of hepatic T3-target genes such as the tyrosine transaminase gene [23]. In addition, PXR and CAR regulate energy metabolism by decreasing gluconeogenesis, β-oxidation and ketogenesis, and by increasing lipogenesis [12,14,15,24]. We have shown previously that, in this receptor-dependent repression, PXR and CAR bind directly to FoxO1 (Forkhead box O1) and repress its activation of IRS (insulin-response sequence)-mediated transcription, thus repressing the expression of IRS-bearing genes in gluconeogenesis [14]. Although the receptors utilize the same insulin-response signal to repress gluconeogenesis, the receptor-mediated mechanism differs from the mechanism by which insulin regulates the activity of FoxO1, in which insulin indirectly phosphorylates FoxO1 and excludes it from the nucleus [25,26].
Fasting increases hepatic glucose production, in part, by augmenting glucagon-dependent activation of CREB-mediated transcription of gluconeogenic genes such as those for G6Pase and PEPCK1. A transgenic mouse bearing dominant-negative CREB exhibited attenuated levels of blood glucose, and both the PEPCK1 and G6Pase genes were repressed in the liver [7]. Therefore, in the present study, we investigated whether PXR represses CREB-mediated transcription by focusing on its direct binding to CREB. First, we have demonstrated that drug activation of PXR results in the repression of the cAMP-mediated induction of the endogenous G6Pase gene as well as in that of the CREB-mediated activation of the G6Pase promoter in human hepatocarcinoma cells. Subsequently, we used PXR-KO (knockout) and wild-type mice to confirm that PXR represses the interaction of CREB with the G6Pase promoter in the fasting liver. Experimental considerations will be presented to propose the regulatory mechanism by which PXR represses glucagon-dependent transcription by directly binding to CREB and inhibiting its interaction with CRE in the fasting liver.
EXPERIMENTAL
Materials
PCN, rifampicin and dibutyryl cAMP were purchased from Sigma–Aldrich, restriction endonucleases and DNA-modifying enzymes were from New England Biolabs, [32P]dATP and [35S]methionine were from GE Healthcare Biosciences.
Animals and primary hepatocytes
Wild-type and PXR-KO male mice (kindly provided by Dr Jeff Staudinger, Department of Pharmacology and Toxicology, University of Kansas, Lawrence, KS, U.S.A.) were maintained on a 12 h light/12 h dark cycle. Mice (6–8 weeks old) were randomly divided into two groups, of which one group was intraperitoneally injected with PCN (40 mg/kg of body weight) once every 2 days three times and the other group with DMSO. After the last injection, mice were fasted for 16 h before being killed. For ChIP (chromatin immunoprecipitation) assays, mice that had been fasted for 16 h were intraperitoneally administered with PCN (40 mg/kg of body weight) and were killed 4 h after administration to prepare liver nuclei. Mouse primary hepatocytes were prepared as described previously [27], cultured in William E medium supplemented with 2 mM L-glutamate and penicillin (100 units/ml) and streptomycin (100 μg/ml), and treated with 10 μM PCN and 0.75 mM dibutyryl cAMP for 16 h.
Quantitative real-time PCR
Total RNA was extracted using TRIzol® reagent (Invitrogen), from which cDNA was synthesized using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). Real-time PCR was performed with an ABI prism 7700 sequence detection system (Applied Biosystems). Assays-on-Demand probes (Applied Biosystems) were used for PCR, and included Hs00430021_m1 for the human CYP3A4 gene, Hs00609178_m1 for the human G6Pase gene, Hs00159918_m1 for the human PEPCK1 gene, Mm00731567_m1 for the mouse Cyp3a11 gene, Mm00839363_m1 for the mouse G6Pase gene and Mm00440636_m1 for the mouse Pepck1 gene. The TaqMan® human β-actin and rodent GAPDH (glyceraldehyde-3-phosphate dehydrogenase) control regents (Applied Biosystems) were used as internal controls.
Recombinant vectors
In all vectors, prefixes m and h in front of inserts denote mouse and human respectively. To construct pAdEasyTrackCMV/hPXR, cDNA was subcloned into pAdtrackCMV (A.T.C.C., Manassas, VA, U.S.A.) to generate pAdtrackCMVhPXR, which was subsequently recombinated with pAdEasy1 vector (A.T.C.C.) by co-transforming them into BJ5183 cells. pAdEasyTrackCMV/hPXR and pAdeasy1/GFP/β-gal (A.T.C.C.) were transfected into HEK-293A (human embryonic kidney 293A) cells (Invitrogen). Amplified recombinant adenoviruses were purified as a supernatant of cell lysates by freeze–thawing three times and were stored at −80 °C. To construct pGEX/hCREB, pGEX/hCREBDBD (residues 281–341) and pGEX/hPXR plasmids, their full-length and partial cDNAs were amplified from pCR3/hPXR and pCR3/hCREB with PCR primers containing proper restriction enzyme sites, digested and inserted into pGEX4T-1 vectors (GE Healthcare Biosciences). A double-stranded oligonucleotide encoding the FLAG epitope sequence was inserted into the BamHI-digested site of pCR3/hPXR to yield pCR3/FLAGhPXR. To construct the AF2 (activation function 2)-domain-deleted mutant of hPXR, the cDNA was amplified from pCR3/hPXR with proper PCR primers and cloned into pcDNA3.1/V5-His-TOPO to yield pcDNA3/hPXRΔAF2. A series of CREB-deletion mutants, pCR3/hCREB100, pCR3/hCREB160 and pCR3/hCREB280, were generated by inserting a stop codon into pCR3/hCREB using site-directed mutagenesis. To construct pcDNA3/hCREBV5, the full-length hCREB cDNA was amplified from pCR3/hCREB with appropriate PCR primers and cloned into pcDNA3.1/V5-His-TOPO. A −259 bp G6Pase promoter–luciferase plasmid was constructed by ampliying the −259/+60 region of G6Pase promoter from mouse genomic DNAs using LA Taq™ DNA polymerase (TaKaRa) and the primers 5′-GGATCCAGCACTGTCAAGCAGTGTGCCC-3′ and 5′-CATCCTTCCTCCCTTGGATCCTCAGGAAGC-3′ (BamHI recognition sequences are underlined). Amplified DNA was first cloned into pCR2.1-TOPO (Invitrogen), from which the DNA insert was cut out by digestion with BamHI and was inserted at the BglII site of pGL3-basic vector (Promega) to yield a −259 bp G6Pase promoter (pGL3/mG6Pase259). To construct mutated promoters, pGL3/mG6Pase259 was used as the template for site-directed mutagenesis using the QuikChange® mutagenesis kit (Stratagene). The following mutagenic oligo-nucleotides were used: to mutate the IRS site, 5′-CAATGGCGATCAGGCTCTTTTTGTGTGCCTCTTTTGCTCTTTTACGTAAATC-3′ and 5′-GATTTACGTAAAAGAGCAAAAGAGGCACACAAAAAGAGCCTGATCGCCATTG-3′; to mutate both IRS and CRE1 sites, 5′-CAATGGCGATCAGGCTCTTTTTGTGTGCCTCTTTTGCTCTTTTAGGTCCATC-3′ and 5′-GATGGACCTAAAAGAGCAAAAGAGGCACACAAAAAGAGCCTGATCGCCATTG-3′; to mutate the HNF4 site, 5′-AGACAAAAGTGGTTTTTTGCTTCACACTAGACGGGCTGGATTGACCTACAGAC-3′ and 5′-GTCTGTAGGTCAATCCAGCCCGTCTAGTGTGAAGCAAAAAACCACTTTTGTCT-3′; and to make CRE1 and CRE2 double mutations, 5′-GAACCTGTTTTGCTATTTTAGGTCCATCACCCTGAACATG-3′, 5′-CATGTTCAGGGTGATGGACCTAAAATAGCAAAACAGGCAC-3′, 5′-CACCCTGAACATGTTTCTAGAAACCTACTGATGATGC-3′ and 5′-GCATCATCAGTAGGTTTCTAGAAACATGTTCAGGGTG-3′. pcDNA3/mFoxO1, pCR3/hPXR and pCMX/hRXR have been described previously [14,28]. pCR3/hCREB, pCR3/hPKA and pCR3/hHNF4 were kindly provided by Dr Joyce Goldstein (National Institute of Environmental Health Sciences), and pCR3/hPGC1α was kindly provided by Dr Anastasia Kralli (Scripps Research Institute, La Jolla, CA, U.S.A.).
Transfections and infections
Human hepatocarcinoma HepG2 and Huh7 cells were maintained in MEM (minimal essential medium) supplemented with 10% FBS (fetal bovine serum), 2 mM L-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin in an atmosphere of 5% CO2 at 37 °C. HepG2 cells plated on 24-well plates at 60–70% confluence were transfected with 30 ng of G6Pase promoter–firefly luciferase, 1.5 ng of pRL-CMV for Renilla luciferase control (Promega) and 30 ng of a given gene expression plasmid (7.5 ng pCR3/hPKA), using FuGENE6 (Roche) according to the manufacturer's instruction. The amount of DNA transfected was adjusted to 99 ng by adding pcDNA3-V5-His (Invitrogen) as empty vector control. At 24 h after transfection, HepG2 cells were washed with PBS and treated with drugs in MEM without FBS for an additional 24 h. Luciferase reporter assays were performed as described previously [14]. For adenoviral infection, Huh7 cells (4.0×105 cells/well) were cultured in medium containing adeno-hPXR or adeno-β-gal at 15 MOI (multiplicity of infection) for 24 h. After being washed with PBS, the cells were treated with drugs for 14 h, from which RNAs were extracted using TRIzol® reagent or cell extracts were prepared for subsequent Western blot analysis with an anti-PXR antibody [28].
GST (glutathione transferase) pull-down assays
GST fusion proteins GST–hCREB and GST–hCREB DBD (DNA-binding domain) were expressed in Escherichia coli strain BL21 cells and were purified with glutathione–Sepharose. A series of cDNAs for hPXR and hCREB in pCR3 or pcDNA3.1-V5-His-TOPO vector were in vitro-translated in the presence of [35S]methionine using the TNT-coupled reticulocyte lysate system (Promega) according to the manufacturer's instructions. GST pull-down assays were carried out as described previously [29]. Western blots were performed to examine the phosphorylation of CREB using anti-CREB (Santa Cruz Biotechnology) and anti-phospho-CREB (Cell Signaling Technology) antibodies.
Immunoprecipitation assays
Huh7 cells were transfected with pCR3/FLAGhPXR and pcDNA3/hCREBV5 for 24 h and were subsequently treated with 0.1% DMSO or 10 μM rifampicin for 2 h before additional treatment with 100 μM cAMP for 30 min. Then, cells were lysed in ice-cold immunoprecipitation buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris/HCl, pH 7.5, 1 mM EDTA, 1 mM EGTA, 0.2 mM sodium orthovanadate, 0.2 mM PMSF, 0.5% Nonidet P40 and 0.1% DMSO or 20 μM rifampicin) containing phosphatase inhibitor cocktail 1 (Sigma–Aldrich) for 30 min at 4 °C, and were centrifuged at 16000 g for 15 min to prepare whole-cell extracts. For co-immunoprecipitation assays, anti-FLAG M2–agarose (Sigma–Aldrich) was incubated with cell extracts for 2 h at 4 °C, washed with immunoprecipitation buffer and were subjected to Western blot analysis with horseradish-peroxidase-conjugated anti-FLAG M2 antibody (Sigma–Aldrich) or horseradish-peroxidase-conjugated anti-V5 antibody (Invitrogen).
Gel-shift assays
hPXR, hRXR (retinoid X receptor) and hCREB proteins were produced by in vitro translation as described above. For probes, two sets of double-stranded oligonucleotides containing wild-type CRE1 (5′-GATCTTGCTATTTTACGTAAATCACCC-3′) and of mutant CRE1 (5′-GATCTTGCTATTTTAAAGCTTTCACCC-3′) were synthesized and labelled by using [32P]dATP and DNA polymerase Klenow fragment (New England Biolabs). Gel-shift assays were performed as described previously [30]. For competition or antibody supershift assays, unlabelled oligonu-cleotide, normal rabbit IgG (Santa Cruz Biotechnology) or anti-CREB antibody was added 15 min before adding radioactive oligonucleotide to start the binding reaction.
ChIP assays
ChIP assays were performed using a ChIP assay kits (Upstate) according to the manufacturer's instruction with some modifications. Mouse liver nuclei were prepared as described previously [31]. For cross-linking, nuclear pellets were suspended and incubated in PBS containing 3 mM MgCl2 and 1% (v/v) formaldehyde at room temperature (25 °C) for 30 min with rotation. The reaction was stopped by adding glycine to 125 mM and by subsequent incubation for 5 min. After washing with ice-cold PBS containing 3 mM MgCl2, the cross-linked nuclei were suspended and incubated in SDS lysis buffer for 10 min on ice. The lysate was then sonicated to shear DNA to lengths between 200 and 1000 bp. After centrifugation at 1000 g for 1 min to remove insoluble material, 200 μl of the supernatant was diluted 10-fold with ChIP dilution buffer. The chromatin sample was pre-cleared by incubating with 500 ng of sheared salmon sperm DNA, 50 μl of pre-immune serum and 75 μl of Protein A–agarose/salmon sperm DNA for 2 h at 4 °C with rotation. The pre-cleared chromatin solution was incubated with either 5 μg of anti-CREB antibody (Cell Signaling Technology or Santa Cruz Biotechnology) or normal rabbit IgG (Santa Cruz Biotechnology) at 4 °C overnight. Immunocomplexes were collected by centrifugation at 1000 g for 1 min after incubation with 60 μl of Protein A–agarose/salmon sperm DNA and 300 ng of sheared salmon sperm DNA. Immunoprecipitated DNA was purified by following the manufacturer's instruction combined with the QIAquick PCR purification kit (Qiagen). The purified DNA was used as a template for semi-quantitative PCR with following primers: 5′-GCCTCTAGCACTGTCAAGCAGTGTGCC-3′ and 5′-GGTCAATCCAGCCCTGATCTTTGGACTC-3′.
RESULTS
Repressing gluconogenic genes in Huh7 cells
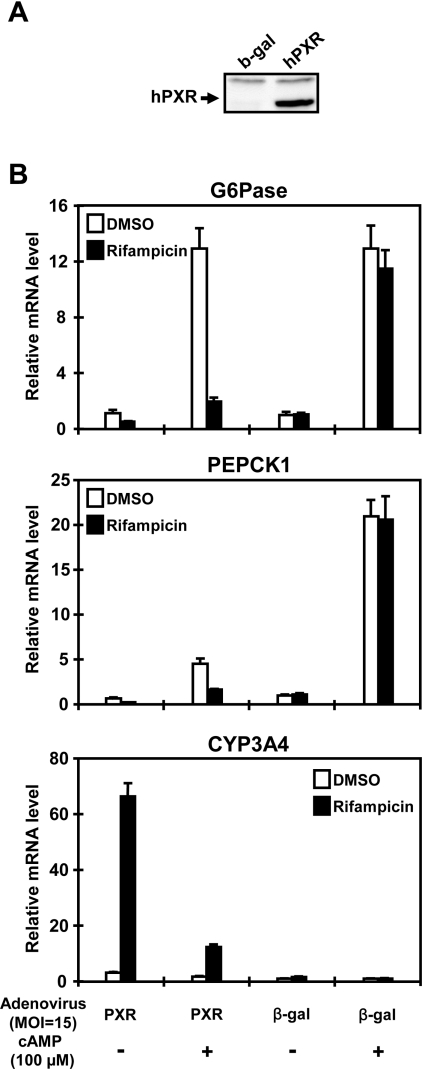
First, adenovirus-based expression of hPXR was established with human hepatocarcinoma Huh7 cells, in which Western blot analysis confirmed ectopic expression of the hPXR protein (Figure 1A). The levels of G6Pase and PEPCK1 mRNAs were increased over 13- and 20-fold respectively after cAMP treatment in Huh7 cells (Figure 1B). In strongly depending on the presence of ligand rifampicin, hPXR effectively repressed the cAMP-induced increase in these mRNAs (Figure 1B). This repression by hPXR was specific, since infection of adeno-β-gal did not affect the levels of G6Pase and PXPCK1 mRNAs. hPXR also attenuated the basal levels of these mRNAs (in the absence of cAMP) (Figure 1B). Over 20-fold induction of CYP3A4 mRNA by hPXR in the presence of rifampicin provided a positive control for ectopic hPXR being active in the infected Huh7 cells (Figure 1B). Thus these results indicated that hPXR could repress cAMP-regulated expression of G6Pase as well as PEPCK1 genes in Huh7 cells. An unexpected and intriguing finding was that cAMP effectively attenuated a rifampicin-dependent induction of CYP3A4 (Figure 1B).
Figure 1. Repression of cAMP-dependent gene expression in Huh7 cells.
Human hepatocarcinoma Huh7 cells were infected with adeno-β-gal or adeno-hPXR at an MOI of 15. After 16 h of infection, the cells were washed with PBS and were incubated in serum-free medium in the presence or absence of rifampicin (10 μM) and cAMP (100 μM) for an additional 14 h. (A) Western blot analysis using cell lysates to determine the expression of hPXR. (B) Quantitative real-time PCR was performed as described in the Experimental section. Relative levels of mRNAs were expressed by taking the levels in DMSO-treated Huh7 cells as 1. Results are means±S.D. for three experiments.
Repressing CREB–CRE activity of the G6Pase promoter
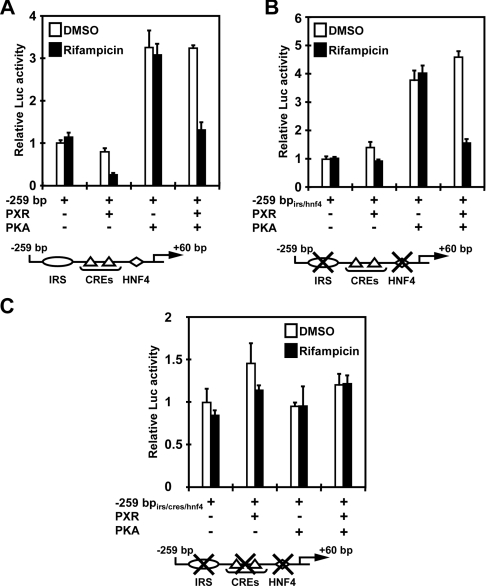
The promoters of the G6Pase genes have been well characterized, in which the CREB–CRE activity resides near the transcription start sites [3,8,9]. For the present study, we cloned a −259 bp promoter of the G6Pase gene that contains two CREs and also the IRS and HNF4 sites. In cell-based reporter assays, we co-expressed PKA to activate the CREB–CRE pathway because cAMP strongly affected the internal standard used. The −259 bp G6Pase construct exhibited basal promoter activity, as well as activation by PKA. hPXR effectively repressed both basal and PKA-activated activities of this promoter (Figure 2A). Under the experimental conditions used, activation by PKA was mediated only by CRE sites, since mutations of these sites abrogated PKA activation of the promoter (results not shown). To delineate the hPXR-dependent repression to CREs, both the IRS and HNF4 sites were mutated within the context of the −259 bp G6Pase promoter. This mutated promoter was activated by PKA 4-fold and hPXR repressed the activated promoter to the level of basal activity (Figure 2B). However, when two CRE sites were also mutated in addition to the IRS and HNF4 sites, the promoter was neither activated by PKA nor repressed by hPXR (Figure 2C). Thus these results indicate that, upon activation by rifampicin, hPXR could effectively repress CREB–CRE activity of the G6Pase promoter, consistent with the hypothesis that hPXR represses cAMP-mediated induction of the G6Pase gene through its interaction with CREB.
Figure 2. Repression of PKA–CRE-mediated activity.
The −259 bp G6Pase promoter–luciferase reporter plasmid and its mutant constructs were co-transfected with pCR3/hPXR and/or pCR3/hPKA, and the reporter activity was measured as described in the Experimental section. (A) The −259 bp wild-type promoter, (B) the −259 bpirs/hnf4 promoter bearing the double mutations of IRS and HNF4 sites, (C) the −259 bpirs/cres/hnf4 promoter having the triple mutations at the IRS, two CRE and HNF4 sites. pRL-CMV was always co-transfected for the control. Total amount of DNA was adjusted by adding pcDNA3-V5-His as an empty vector control. Relative fold activities were calculated by taking the activity of the cells that were transfected by the reporter plasmid only in the presence of DMSO as 1. Results are means±S.D. for three experiments.
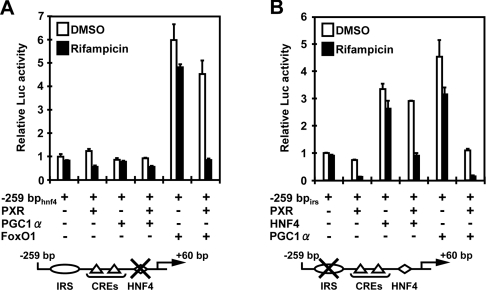
PXR is known to repress the activation of IRS by FoxO1 and the co-activation by PGC1α of HNF4-mediated transcription [14,15,32]. First, the DR1 (direct repeat 1) (HNF4) site in the context of the −259 bp G6Pase promoter (−259 bphnf4) was mutated to examine the role of PXR in regulation of the FoxO1-mediated activation of the G6Pase promoter. Co-expression of FoxO1 effectively up-regulated the mutated −259 bphnf4 promoter, and hPXR strongly repressed this FoxO1-dependent activation in a rifampicin-dependent fashion (Figure 3A). Although it has been reported that PGC1α co-activates FoxO1-mediated activation of the IRS [33], we found that neither was co-expression of PGC1α able to up-regulate the −259 bphnf4 promoter nor did hPXR affect the activity of this promoter when co-expressed with PGC1α (Figure 3A). However, our results were consistent with the other report showing that PGC1α does not co-regulate FoxO1 [34]. Next, the IRS site in the context of the −259 bp G6Pase promoter (−259 bpirs) was mutated to examine the role of PXR in the regulation of PGC1α- and HNF4-mediated activations of the G6Pase promoter. Co-expression of either HNF4 or PGC1α effectively up-regulated the mutated −259 bpirs promoter, and hPXR strongly repressed both the HNF4-activated and the PGC1α-activated promoter activities in a rifampicin-dependent manner (Figure 3B). Because of the presence of the endogenous HNF4 and PGC1α in HepG2 cells, it could not definitely be determined whether PXR attenuated the interaction of HNF4 with the DR1 site, the co-activation by PGC1α of HNF4 or both at the present time. Nevertheless, these observations clearly showed that PXR is capable of repressing both the IRS- and DR1-regulated transcription of the G6Pase promoter.
Figure 3. Repression of the G6Pase promoter through insulin-response signal and HNF4 sites.
The −259 bphnf4 and −259 bpirs G6Pase promoters were co-transfected with pCR3/hPXR and/or pcDNA3/mFoxO1, pCR3/hHNF4 or pCR3/hPGC1α, and the reporter activity was measured as described in the Experimental section. (A) The −259 bphnf4 promoter bearing the mutation of the HNF4 site, and (B) the −259 bpirs promoter having the mutation of the IRS site. pRL-CMV was always co-transfected as the control. Relative fold activities were calculated by taking the activity of the cells that were transfected by the reporter plasmid only in the presence of DMSO as 1. Results are means±S.D. for three experiments.
Direct binding to CREB
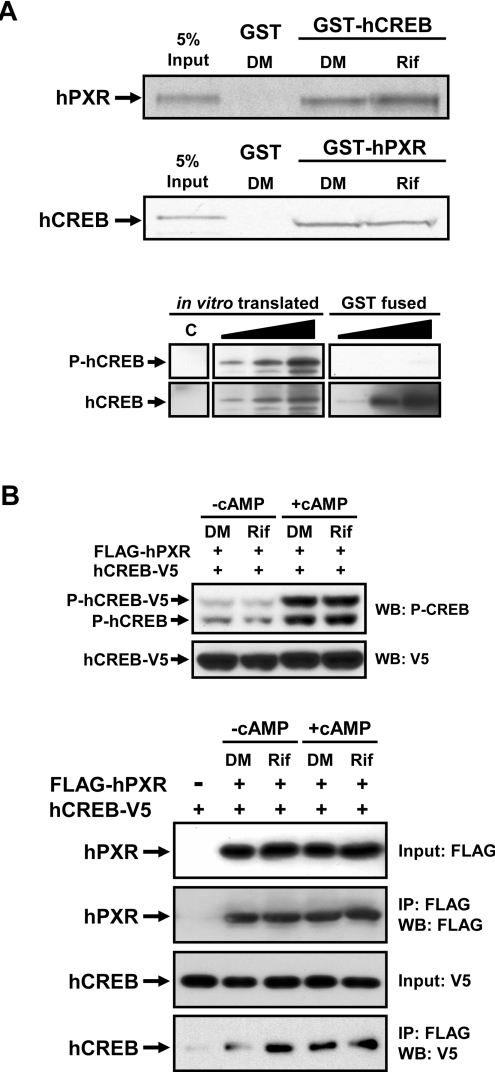
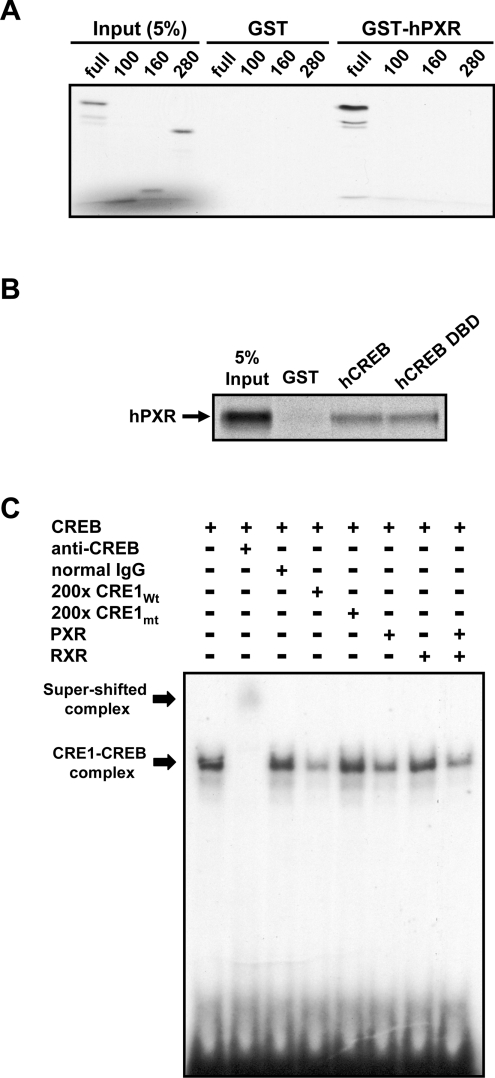
To decipher the molecular mechanism of how hPXR represses CREB–CRE activity, we employed GST pull-down, co-immunoprecipitation and gel-shift assays to examine the direct interaction of hPXR with CREB. In GST pull-down assays, an in vitro-translated hPXR bound to GST–CREB, and this binding became greater when rifampicin was present (Figure 4A). Although this rifampicin-dependency was weak, it was reproducible and suggested ligand-dependent binding of hPXR to CREB. When an in vitro-translated CREB was used, it was also found to bind GST–hPXR, but binding did not increase in the presence of rifampicin (Figure 4A). To look for a reason for the ligand-independent binding in this combination of GST–hPXR and in vivo-translated CREB, we examined the phosphorylation status of CREB used for these assays (Figure 4A): the in vitro-translated CREB was phosphorylated, whereas the bacterially expressed CREB was not. When the ligand-dependency was observed, CREB was not phosphorylated (Figure 4A). To provide additional evidence for the interaction of PXR with CREB, we performed immunoprecipitation assays. For this, FLAG–hPXR and CREB–V5 were co-expressed in Huh7 cells. After drug treatments, cell extracts were prepared for subsequent immunoprecipitation assays: anti-FLAG M2 antibody co-precipitated CREB with hPXR (Figure 4B). In the absence of cAMP, rifampicin treatment resulted in an increase in CREB co-precipitated with FLAG–hPXR, suggesting that hPXR formed a complex with CREB in a ligand-dependent manner (Figure 4B). The degree of this increase calculated from three independent assays was 330±125% (P<0.0166). From cell extracts treated with cAMP, CREB was similarly co-precipitated with hPXR in the presence (247±58%; P<0.0058) and absence (349±137%; P<0.0173) of rifampicin (Figure 4B). Under these assay conditions, both endogenous and overexpressed CREB were strongly phosphorylated when cells were treated with cAMP (Figure 4B). Thus complex formation of PXR with CREB exhibited a strong ligand-dependency only in the absence of cAMP treatment. Presuming that cAMP treatment increased the phosphorylated form of CREB, complex formation was ligand-dependent only when CREB was not phosphorylated, as observed with our GST pull-down assays.
Figure 4. Formation of a PXR complex with CREB.
(A) GST pull-down assays were performed using an in vitro-translated 35S-labelled hPXR and CREB with GST–CREB and GST–hPXR fusion proteins respectively in the presence of 0.1% DMSO (DM) or 50 μM rifampicin (Rif). GST was used as negative control as described in the Experimental section. (B) Immunoprecipitation assays: Huh7 cells were transfected with pCR3/FLAGhPXR and pcDNA3/hCREBV5 for 24 h. Subsequently, these cells were treated with rifampicin (Rif; 10 μM) for 2 h, followed by the treatment with cAMP (100 μM) or DMSO (DM) for additional 30 min. Cell extracts were prepared and subjected to Western blotting (WB) of CREB phosphorylation as well as immunoprecipitation (IP) using anti-FLAG M2–agarose as described in the Experimental section. The anti-FLAG precipitants for Western blotting were detected by anti-FLAG or anti-V5 antibodies. The assays were repeated three times.
Using a series of deletion mutants of in vitro-translated CREBs, GST pull-down assays were performed to determine the region that binds to PXR (Figure 5A). GST–hPXR bound only to the full-length CREB and was not able to bind to three different fragments lacking the DBD. Furthermore, GST–CREB DBD interacted with hPXR at an equal strength to its interaction with GST–CREB. These results clearly indicated that PXR bound directly to the DBD of CREB. In gel-shift assays, a 32P-labelled oligonucleotide probe including the CRE1 site of the G6Pase promoter formed a specific band with in vitro-translated CREB on a polyacrylamide gel, based on the supershift by anti-CREB antibody, but not with normal IgG and by the competition with unlabelled wild-type, but not by mutated, probes (Figure 5C). Addition of hPXR diminished the formation of the specific CREB–CRE complex, suggesting that hPXR binds directly to the DBD of CREB and prevents its interaction with the CREs of the G6Pase promoter (Figure 5B). In addition, we performed luciferase reporter assays using the hPXR lacking its AF2 domain and showed that the hPXRΔAF2 was unable to repress the PKA-dependent activation of the −259 bpirs/hnf4 promoter. Subsequently, the repression by hPXR was found to depend on the presence of the AF2 domain (results not shown), thus strengthening the notion that the repression is, in fact, ligand-dependent.
Figure 5. Inhibiting CREB–CRE interaction via binding to the CREB DBD.
GST pull-down assays were performed using a bacterially expressed GST–hPXR (A) or GST–CREB DBD (B) with the in vitro-translated 35S-labelled hPXR, CREB and deleted fragments of CREB (full-length and amino acid residues 1–100, 1–160 and 1–280). (B) Gel-shift assays were performed using the in vitro-translated CREB, hPXR and hRXR and the radiolabelled oligonucleotides of wild-type CRE1 (CRE1wt) and mutant CRE1 (CRE1mt) as probes, as described in the Experimental section. Both supershift by anti-CREB antibody and competition with non-labelled oligonucleotides of CRE1wt and CRE1mt were carried out to confirm the specificity of the complex formation. The assays were repeated three times.
Repressing CREB interaction with G6Pase promoter in mouse liver
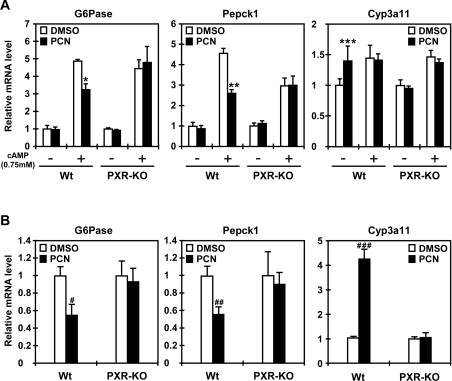
First, the ability of PXR to repress the G6Pase gene was examined using mouse primary hepatocytes prepared from wild-type and PXR-KO mice. Treatment with the mouse PXR activator PCN resulted in the 30–40% decrease in the cAMP-induced expression of both G6Pase and PEPCK1 genes in the wild-type hepatocytes only (Figure 6A). PCN also repressed the expression of G6Pase and PEPCK1 genes in the wild-type mice only, showing that PXR is responsible for the repression of the genes (Figure 6B). As expected, the Cyp3a11 gene was induced by PCN in both primary hepatocytes and livers only when mPXR was present, thus serving as a positive control for the activation of mPXR by PCN in these experimental systems (Figure 6).
Figure 6. Repression of the G6Pase and PEPCK1 genes in vivo.
(A) Mouse primary hepatocytes were prepared from wild-type (Wt) and PXR-KO mice as described in the Experimental section, and were treated with 0.1% DMSO or 10 μM PCN in the presence or absence of 0.75 mM cAMP for 16 h. Total RNA was extracted and subjected to quantitative real-time PCR analysis with the sets of specific probes. Relative levels were expressed by taking the level in DMSO-treated hepatocytes without cAMP treatment as 1. The assays were repeated three times. (B) Mice were treated with PCN or DMSO as described in the Experimental section. Liver RNAs were prepared from four mice for each of the wild-type (Wt) and PXR-KO groups and were individually subjected to quantitative real-time PCR. Relative mRNA levels were expressed by taking those with DMSO as 1. Results are means±S.D. of replicates (n=4). *P<0.0007, **P<0.0002 and ***P<0.03 for cAMP plus DMSO-treated wild-type hepatocytes compared with cAMP plus PCN-treated wild-type hepatocytes. #P<0.01, ##P<0.007 and ###P<0.00005 for the DMSO-injected wild-type group compared with the PCN-injected wild-type group.
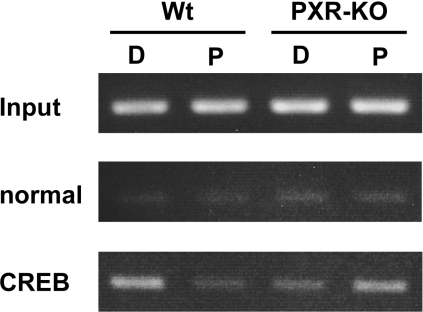
Lastly, we performed ChIP assays to examine whether mPXR altered the interaction of CREB with the G6Pase promoter in mouse liver, correlating with the repression of the G6Pase gene. Treatment with PCN decreased the binding of CREB to the G6Pase promoter in the wild-type mice only: the degree of this decrease calculated from three independent assays was 52±18% (P<0.0167) (Figure 7). A small increase in CREB binding to the promoter after PCN treatment was consistently observed in the PXR-KO mice, although this increase was not statistically significant (179.2±52.2%; P<0.0762) (Figure 7). Since PCN did not induce the G6Pase gene in the absence of mPXR (Figure 6), this mPXR-independent increase in CREB binding remained enigmatic, but did not appear to be involved in the regulation of G6Pase gene. Thus these results suggest that the inhibition of CREB binding to the CRE by mPXR is part of the molecular mechanism regulating the repression of the G6Pase gene by PCN treatment in mouse liver.
Figure 7. Dissociation of CREB from the G6Pase promoter in vivo.
ChIP assays were performed as described in the Experimental section. From the liver nuclear extracts, DNA fragments were immunoprecipitated with either rabbit normal IgG or an anti-CREB antibody, semi-quantified by PCR for the G6Pase promoter, separated on an agarose gel and visualized by ethidium bromide staining. D and P denote DMSO and PCN respectively; Wt, wild-type. The assays were repeated three times.
DISCUSSION
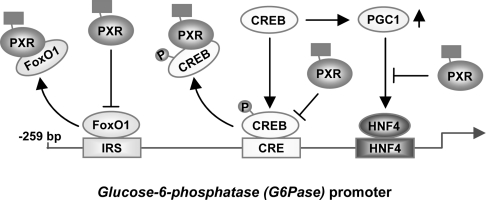
Hepatic production of glucose fluctuates as required by physiological conditions to maintain its levels in blood [5]. Glucagon up-regulates the production, whereas insulin down-regulates it, in which the G6Pase gene is the major target regulated by these pancreatic hormones [1,4]. In the present study, we have shown that drug activation of the nuclear receptor PXR represses glucagon-regulated transcription of the G6Pase gene. This repression is now found to be mediated by the direct interaction of PXR with the glucagon-response transcription factor CREB, thus preventing transcriptional activation of the CRE-bearing G6Pase gene by CREB. In the liver of fasting mice, treatment with PCN resulted in a PXR-dependent decrease of CREB binding to the G6Pase promoter. Since PXR also binds directly to the insulin-response transcription factor FoxO1 repressing the IRS activation of the G6Pase promoter, through its cross-talk with CREB and FoxO1, drugs activating PXR act like insulin and concurrently antagonize glucagon to attenuate glucose production by repressing gluconeogenic genes such as G6Pase and PEPCK1 (Figure 8).
Figure 8. Schematic representation of cross-talk.
Arrows indicate activation and co-activation, while stop bars indicate repression and co-repression.
Treatment with the mouse PXR activator PCN decreased blood glucose levels in fasting wild-type, but not PXR-KO, mice [24], thus suggesting that PXR is capable of down-regulating the expression of gluconeogenic genes. In support of this suggestion, our previous [14] and present studies have shown that PXR, in fact, represses the FoxO1-mediated IRS activation as well as the FoxO1-mediated activation of the G6Pase promoter. Moreover, the G6Pase and PEPCK1 genes were found to be down-regulated in transgenic mice bearing a constitutively activated mutant of hPXR [35]. We have now confirmed that, upon activation by rifampicin, hPXR represses cAMP-induced expression of endogenous G6Pase and PEPCK1 genes in Huh7 cells. In cell-based transfection assays, hPXR effectively repressed the CRE-mediated transcription of the G6Pase gene by PKA in a clear ligand-dependent manner. PCN treatment repressed cAMP-induced expression of G6Pase in mouse primary hepatocytes as well as in fasting mouse liver, but only in those of wild-type mice, not those of PXR-KO mice. ChIP assays in the liver of both wild-type and PXR-KO mice were employed to demonstrate that repression of the G6Pase gene by PXR was regulated via CREB in liver in vivo. Since PXR binds directly to the DBD of CREB to prevent CREB interactions with CRE in our gel-shift assays, a direct binding of PXR to CREB appears to be the cause of the repression of the G6Pase gene. However, the possibility remains that direct binding by itself may not be sufficient to explain the molecular mechanism of PXR-dependent repression of CREB-mediated transcription. For instance, the thyroid receptor is known to inhibit phosphorylation of CREB to repress its transcriptional activity [36]. Although PXR did not appear to inhibit CREB phosphorylation under our experimental conditions (results not shown), some type of phosphorylation-dependent regulation may be required for PXR to repress CREB activity in addition to direct binding.
During fasting under our experimental conditions, the levels of blood glucagon were constant [24], confirming that CREB remains to be a critical factor in up-regulating gluconeogenic genes. In addition to the direct binding to the CRE site, CREB indirectly activates gluconeogenic genes: for example, adenovirus-based overexpression of PGC1α in a heterozygote Creb+/− mouse recovered the blood glucose back to levels like those of the wild-type mice, the mechanism for which increase in PGC1α via CREB-mediated activation of the Pgc1a gene has been suggested [7]. We did not observe PXR regulating the expression of PGC1α, since the nuclear receptor neither repressed nor enhanced cAMP induction of the PGC1A gene in Huh7 cells or the Pgc1a expression in fasting mouse liver (results not shown). PXR has been reported to repress the HNF4-mediated transcription of the PEPCK1 gene by dissociating PGC1α from HNF4 in HepG2 cells [15]. Consistent with this report, we also found that PXR represses the PGC1α-mediated co-activation of HNF4 activity on the G6Pase promoter. Thus PXR may also repress the G6Pase gene via the HNF4 site in addition to the CRE sites of its promoter.
Our and others' studies have now shown that PXR enable to attenuate the expression of the G6Pase gene via CREB, FoxO1 and PGC1α. However, there is no way to know to what degree each of these three factors contributes to the PXR-dependent repression in the liver in vivo. Transgenic mouse bearing constitutively active FoxO1 exhibited an increase in levels of blood glucose, with the PEPCK1 and G6Pase genes being up-regulated [37]. It is intriguing that Pgc1a−/− mice had an approx. 20% decrease in blood glucose during fasting compared with wild-type mice, while the Pepck1 and G6Pase genes were expressed at the similar levels in both Pgc1a−/− and wild-type mice [38]. Since Creb−/− mice are perinatally lethal [39], information with respect to the corresponding gene expression and blood glucose in adult mice lacking the CREB gene is not available at the present time.
It has long been known that treatment with drugs, which now turn out to be activators of the nuclear receptors CAR and PXR, represses hepatic gluconeogenic enzymes and genes [13–15,40,41]. Moreover, there is a clinical study reporting that chronic phenobarbital treatment reduces serum glucose levels and improves insulin responsiveness [40]. In insulin-resistant Type 2 diabetes, high blood glucose is often complicated with hyperglycaemia. We have shown that PXR directly binds to FoxA2 and represses transcription of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene and that PCN treatment decreased blood 3-hydroxybutylate levels [24]. Because of its ability in repressing FoxO1-, FoxA2-, CREB- and PGC1α-mediated transcription, PXR may be a therapeutic target for Type 2 diabetes and its medical complications. Apparently, PXR may also become a factor negatively affecting disease; activation of PXR led mice to increase hepatic triacylglycerols, another complication associated with Type 2 diabetes [35]. Moreover, we observed both hyperglycaemic and high hepatic triacylglycerol conditions in non-treated PXR-KO mice [24]. Thus PXR plays complex and contradicting roles in regulating glucose and lipid metabolism, affecting the state of liver diseases. Although these entangled functions of PXR must first be elucidated for us to consider PXR as a viable target for drug development, the cross-talk of drug-activating nuclear receptors with insulin- and glucagon-response transcription factors provides an excellent concept in helping us to understand the drug-induced regulation of hepatic energy metabolism.
Acknowledgments
S. K. is a JSPS (Japan Society for the Promotion of Science) Research Fellow in the Biomedical and Behavioral Research Program at NIH (National Institutes of Health). This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
References
- 1.Hanson R. W., Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu. Rev. Biochem. 1997;66:581–611. doi: 10.1146/annurev.biochem.66.1.581. [DOI] [PubMed] [Google Scholar]
- 2.Quinn P. G., Wong T. W., Magnuson M. A., Shabb J. B., Granner D. K. Identification of basal and cyclic AMP regulatory elements in the promoter of the phosphoenolpyruvate carboxykinase gene. Mol. Cell. Biol. 1988;8:3467–3475. doi: 10.1128/mcb.8.8.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmoll D., Wasner C., Hinds C. J., Allan B. B., Walther R., Burchell A. Identification of a cAMP response element within the glucose-6-phosphatase hydrolytic subunit gene promoter which is involved in the transcriptional regulation by cAMP and glucocorticoids in H4IIE hepatoma cells. Biochem. J. 1999;338:457–463. [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon J. C., Puigserver P., Chen G., Donovan J., Wu Z., Rhee J., Adelmant G., Stafford J., Kahn C. R., Granner D. K., et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 5.Van Schaftingen E., Gerin I. The glucose-6-phosphatase system. Biochem. J. 2002;362:513–532. doi: 10.1042/0264-6021:3620513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez G. A., Montminy M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 7.Herzig S., Long F., Jhala U. S., Hedrick S., Quinn R., Bauer A., Rudolph D., Schutz G., Yoon C., Puigserver P., et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 8.Thiel G., Al Sarraj J., Stefano L. cAMP response element binding protein (CREB) activates transcription via two distinct genetic elements of the human glucose-6-phosphatase gene. BMC Mol. Biol. 2005;6:2. doi: 10.1186/1471-2199-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin B., Morris D. W., Chou J. Y. The role of HNF1α, HNF3γ, and cyclic AMP in glucose-6-phosphatase gene activation. Biochemistry. 1997;36:14096–14106. doi: 10.1021/bi9703249. [DOI] [PubMed] [Google Scholar]
- 10.Boustead J. N., Stadelmaier B. T., Eeds A. M., Wiebe P. O., Svitek C. A., Oeser J. K., O'Brien R. M. Hepatocyte nuclear factor-4α mediates the stimulatory effect of peroxisome proliferator-activated receptor γ co-activator-1α (PGC-1α) on glucose-6-phosphatase catalytic subunit gene transcription in H4IIE cells. Biochem. J. 2003;369:17–22. doi: 10.1042/BJ20021382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee J., Inoue Y., Yoon J. C., Puigserver P., Fan M., Gonzalez F. J., Spiegelman B. M. Regulation of hepatic fasting response by PPARγ coactivator-1α (PGC-1): requirement for hepatocyte nuclear factor 4α in gluconeogenesis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4012–4017. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miao J., Fang S., Bae Y., Kemper J. K. Functional inhibitory cross-talk between constitutive androstane receptor and hepatic nuclear factor-4 in hepatic lipid/glucose metabolism is mediated by competition for binding to the DR1 motif and to the common coactivators, GRIP-1 and PGC-1α. J. Biol. Chem. 2006;281:14537–14546. doi: 10.1074/jbc.M510713200. [DOI] [PubMed] [Google Scholar]
- 13.Ueda A., Hamadeh H. K., Webb H. K., Yamamoto Y., Sueyoshi T., Afshari C. A., Lehmann J. M., Negishi M. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol. Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Kodama S., Koike C., Negishi M., Yamamoto Y. Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol. Cell. Biol. 2004;24:7931–7940. doi: 10.1128/MCB.24.18.7931-7940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhalla S., Ozalp C., Fang S., Xiang L., Kemper J. K. Ligand-activated pregnane X receptor interferes with HNF-4 signaling by targeting a common coactivator PGC-1α: functional implications in hepatic cholesterol and glucose metabolism. J. Biol. Chem. 2004;279:45139–45147. doi: 10.1074/jbc.M405423200. [DOI] [PubMed] [Google Scholar]
- 16.Kliewer S. A., Moore J. T., Wade L., Staudinger J. L., Watson M. A., Jones S. A., McKee D. D., Oliver B. B., Willson T. M., Zetterstrom R. H., et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann J. M., McKee D. D., Watson M. A., Willson T. M., Moore J. T., Kliewer S. A. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J. Clin. Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maglich J. M., Stoltz C. M., Goodwin B., Hawkins-Brown D., Moore J. T., Kliewer S. A. Nuclear pregnane X receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol. Pharmacol. 2002;62:638–646. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- 19.Staudinger J. L., Goodwin B., Jones S. A., Hawkins-Brown D., MacKenzie K. I., LaTour A., Liu Y., Klaassen C. D., Brown K. K., Reinhard J., et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo G. L., Lambert G., Negishi M., Ward J. M., Brewer H. B., Jr, Kliewer S. A., Gonzalez F. J., Sinal C. J. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J. Biol. Chem. 2003;278:45062–45071. doi: 10.1074/jbc.M307145200. [DOI] [PubMed] [Google Scholar]
- 21.Huang W., Zhang J., Chua S. S., Qatanani M., Han Y., Granata R., Moore D. D. Induction of bilirubin clearance by the constitutive androstane receptor (CAR) Proc. Natl. Acad. Sci. U.S.A. 2003;100:4156–4161. doi: 10.1073/pnas.0630614100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugatani J., Kojima H., Ueda A., Kakizaki S., Yoshinari K., Gong Q. H., Owens I. S., Negishi M., Sueyoshi T. The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase UGT1A1 gene and regulation by the nuclear receptor CAR. Hepatology. 2001;33:1232–1238. doi: 10.1053/jhep.2001.24172. [DOI] [PubMed] [Google Scholar]
- 23.Tien E. S., Matsui K., Moore R., Negishi M. The nuclear receptor constitutively active/androstane receptor regulates type 1 deiodinase and thyroid hormone activity in the regenerating mouse liver. J. Pharmacol. Exp. Ther. 2007;320:307–313. doi: 10.1124/jpet.106.112706. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura K., Moore R., Negishi M., Sueyoshi T. Nuclear pregnane X receptor cross-talk with FOXA2 to mediate the drug-induced regulation of lipid metabolism in fasting mouse liver. J. Biol. Chem. 2007;282:9768–9776. doi: 10.1074/jbc.M610072200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo S., Rena G., Cichy S., He X., Cohen P., Unterman T. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J. Biol. Chem. 1999;274:17184–17192. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X., Gan L., Pan H., Guo S., He X., Olson S. T., Mesecar A., Adam S., Unterman T. G. Phosphorylation of serine 256 suppresses transactivation by FKHR (FOXO1) by multiple mechanisms: direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. J. Biol. Chem. 2002;277:45276–45284. doi: 10.1074/jbc.M208063200. [DOI] [PubMed] [Google Scholar]
- 27.Honkakoski P., Moore R., Gynther J., Negishi M. Characterization of phenobarbital-inducible mouse Cyp2b10 gene transcription in primary hepatocytes. J. Biol. Chem. 1996;271:9746–9753. doi: 10.1074/jbc.271.16.9746. [DOI] [PubMed] [Google Scholar]
- 28.Squires E. J., Sueyoshi T., Negishi M. Cytoplasmic localization of pregnane X receptor and ligand-dependent nuclear translocation in mouse liver. J. Biol. Chem. 2004;279:49307–49314. doi: 10.1074/jbc.M407281200. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi K., Sueyoshi T., Inoue K., Moore R., Negishi M. Cytoplasmic accumulation of the nuclear receptor CAR by a tetratricopeptide repeat protein in HepG2 cells. Mol. Pharmacol. 2003;64:1069–1075. doi: 10.1124/mol.64.5.1069. [DOI] [PubMed] [Google Scholar]
- 30.Honkakoski P., Zelko I., Sueyoshi T., Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol. Cell. Biol. 1998;18:5652–5658. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986;47:767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- 32.Li T., Chiang J. Y. Mechanism of rifampicin and pregnane X receptor inhibition of human cholesterol 7 α-hydroxylase gene transcription. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G74–G84. doi: 10.1152/ajpgi.00258.2004. [DOI] [PubMed] [Google Scholar]
- 33.Puigserver P., Rhee J., Donovan J., Walkey C. J., Yoon J. C., Oriente F., Kitamura Y., Altomonte J., Dong H., Accili D., Spiegelman B. M. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 34.Schilling M. M., Oeser J. K., Boustead J. N., Flemming B. P., O'Brien R. M. Gluconeogenesis: re-evaluating the FOXO1–PGC-1α connection. Nature. 2006;443:E10–11. doi: 10.1038/nature05288. [DOI] [PubMed] [Google Scholar]
- 35.Zhou J., Zhai Y., Mu Y., Gong H., Uppal H., Toma D., Ren S., Evans R. M., Xie W. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J. Biol. Chem. 2006;281:15013–15020. doi: 10.1074/jbc.M511116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendez-Pertuz M., Sanchez-Pacheco A., Aranda A. The thyroid hormone receptor antagonizes CREB-mediated transcription. EMBO J. 2003;22:3102–3112. doi: 10.1093/emboj/cdg295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W., Patil S., Chauhan B., Guo S., Powell D. R., Le J., Klotsas A., Matika R., Xiao X., Franks R., et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J. Biol. Chem. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
- 38.Lin J., Wu P. H., Tarr P. T., Lindenberg K. S., St-Pierre J., Zhang C. Y., Mootha V. K., Jager S., Vianna C. R., Reznick R. M., et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1α null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Rudolph D., Tafuri A., Gass P., Hammerling G. J., Arnold B., Schutz G. Impaired fetal T cell development and perinatal lethality in mice lacking the cAMP response element binding protein. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4481–4486. doi: 10.1073/pnas.95.8.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lahtela J. T., Arranto A. J., Sotaniemi E. A. Enzyme inducers improve insulin sensitivity in non-insulin-dependent diabetic subjects. Diabetes. 1985;34:911–916. doi: 10.2337/diab.34.9.911. [DOI] [PubMed] [Google Scholar]
- 41.Argaud D., Halimi S., Catelloni F., Leverve X. M. Inhibition of gluconeogenesis in isolated rat hepatocytes after chronic treatment with phenobarbital. Biochem. J. 1991;280:663–669. doi: 10.1042/bj2800663. [DOI] [PMC free article] [PubMed] [Google Scholar]