Abstract
LH and FSH play crucial roles in mammalian reproduction by mediating steroidogenesis and gametogenesis. Gonadal steroid hormones influence gonadotropin production via feedback to the hypothalamus and pituitary. We previously demonstrated that progesterone and testosterone can stimulate expression of the FSH β-subunit gene in immortalized gonadotrope-derived LβT2 cells. Herein, we investigate how these gonadal steroids modulate activin signaling in the gonadotrope. Cotreatment of LβT2 cells or mouse primary pituitary cells with steroids and activin results in a synergistic induction of FSHβ gene expression. This synergy decreases when DNA-binding mutations are introduced into the steroid receptors or when mutations that reduce steroid hormone responsiveness are introduced into the FSHβ promoter, indicating that synergy requires direct DNA binding of the steroid receptors. Furthermore, classical activin signaling via Smad proteins is necessary for this synergy. In addition, these steroid receptors physically interact with Smads and are sufficient for the synergism to occur on the FSHβ promoter. Disruption of Smad binding to the promoter with a Smad protein lacking the DNA-binding domain or an FSHβ promoter containing mutated activin-response elements prevents the synergistic enhancement of FSHβ transcription. Collectively, our data demonstrate that the molecular mechanism for gonadal steroid hormone action on the FSHβ promoter involves cross-talk between the steroid and activin signaling pathways. They also reveal that this synergism requires binding of both the steroid receptors and Smad proteins to their cognate DNA-binding elements and likely involves a direct protein-protein interaction between the two types of transcription factors.
MAMMALIAN REPRODUCTION DEPENDS upon a complex orchestration of hormonal regulation in the hypothalamic-pituitary-gonadal axis. The gonadotrope cell in the anterior pituitary acts as a central integrator of steroid and peptide hormone signaling in this axis. Signaling events in the gonadotrope culminate in the synthesis and secretion of two hormones: LH and FSH. These hormones are secreted into the bloodstream where they bind to receptors on the female ovary and male testes and play essential roles in ovulation, steroidogenesis, and gametogenesis (1,2,3).
LH and FSH are heterodimeric glycoproteins composed of an α-subunit shared by both hormones as well as a unique β-subunit that confers the biological specificity of LH and FSH (4). Synthesis of the LH and FSH β-subunits appears to be the rate-limiting step for production of FSH and LH (5,6). Understanding the mechanisms by which steroid and peptide hormones regulate the transcription of LHβ and FSHβ is critical for comprehending the process of LH and FSH production. Although much is known about the responsiveness of the gonadotropin genes to individual hormones, it is still unclear how interactions among these various hormones contribute to the regulation of LHβ and FSHβ gene expression.
Both steroid and peptide hormones have been shown to modulate LHβ and FSHβ gene expression. For example, the hypothalamic neuropeptide GnRH mediates the synthesis of both gonadotropin β-subunits in addition to its well-characterized role in secretion (7). The induction of FSHβ and LHβ gene expression by activin has also been well characterized (8,9,10,11,12,13). Moreover, steroid hormones have been shown to modulate the transcription of LHβ and FSHβ subunit genes at the level of the pituitary (reviewed by Burger et al. in Ref. 14). Recently, we demonstrated that two gonadal steroid hormones, progesterone and testosterone, can differentially modulate LHβ and FSHβ gene expression in immortalized pituitary gonadotrope cells and, therefore, potentially play a role in differential regulation of LH and FSH (15). Because activin is a potent activator of FSHβ and its levels remain relatively uniform during the reproductive cycle, we focused on whether gonadal steroid hormones modulate activin signaling in the pituitary gonadotrope. Our previous study supports the hypothesis that progesterone and testosterone act to increase FSHβ gene expression directly at the level of the gonadotrope through the classical steroid receptor signaling pathway. However, other studies suggest that gonadal steroid hormones may also regulate FSHβ indirectly through modulation of components of the activin signaling pathway. Activin signaling may be modulated by gonadal steroid hormones via altering the bioavailability of activin or the intracellular signaling pathways. Follistatin is a glycoprotein expressed in both gonadotropes and pituitary folliculostellate cells that binds and inactivates activin (16). Testosterone has been shown to suppress follistatin mRNA and primary transcripts in rat primary cell cultures (17,18). Smad mRNA and Smad phosphorylation have also been reported to change after testosterone treatment in rat primary cells (19). Furthermore, cotreatment of rat primary cells with testosterone and activin enhances FSHβ gene expression levels beyond those observed with activin alone (20), and its action can be blunted by follistatin (21,22).
Development of the LβT2 cells provided the opportunity to analyze the mechanisms of steroid and peptide hormonal regulation of the gonadotropin genes in the context of a pure population of gonadotrope cells (23). Because the gonadotrope cell is exposed to multiple hormonal signals at the same time, there is a high potential for cooperation or synergy among these hormones. The immortalized LβT2 cell line is an excellent model system in which to study gonadotropin gene expression because it expresses many markers of a mature gonadotrope including FSHβ, LHβ, GnRH receptor, activin, activin receptor, follistatin, and inhibin (24,25,26,27). This cell model became even more useful when it became apparent that folliculostellate cells producing follistatin rapidly overgrow primary pituitary cell cultures (28), thus complicating the interpretation of experiments performed in vitro.
In this study, we investigated the potential for cross-talk between gonadal steroid and activin signaling in gonadotrope cells. Specifically, we used LβT2 and mouse primary pituitary cells to determine whether progesterone, as well as testosterone, can synergize with activin to mediate expression of the FSHβ gene. We used transient transfection experiments with a murine FSHβ-luciferase reporter gene to characterize the responsiveness of the proximal promoter to the combined action of gonadal steroids and activin. In addition, we determined whether the synergy between steroids and activin occurred through classical Smad-dependent signaling. Glutathione-S-transferase (GST) interaction assays were used to determine whether progesterone receptor (PR) and androgen receptor (AR) could partner with Smad proteins. And finally, we investigated whether DNA binding of the steroid receptors and/or Smad proteins to the FSHβ promoter was required for the synergy.
Materials and Methods
Reagents
Promegestone (R5020) and methyltrienolone (R1881) were purchased from NEN Life Science Products Life Sciences (Boston, MA). Progesterone and dihydrotestosterone (DHT) were purchased from Sigma Chemical Co. (St. Louis, MO), whereas activin and follistatin were obtained from R&D Systems (Minneapolis, MN). The inhibitors SB-202190 and SB-431541 were obtained from Sigma.
Plasmids
Construction of the −1000FSHβluc plasmid was described previously (15,29). The GRAS-tk-luc plasmid was also previously described (22). It is a luciferase reporter gene linked to four repeats of the GRAS element (−340 to −315) from the mouse GnRH receptor gene (30) upstream of a minimal −81 herpes simplex virus thymidine kinase promoter. The pGL3 MMTV plasmid was provided by Jeff Miner of Ligand Pharmaceuticals. The steroid receptor expression plasmids pSG5-rAR and pSG5-rARC562G were provided by Jorma Palvimo (31). Benita Katzenellenbogen donated the pCMV5-rPRB plasmid, and Dean Edwards provided the pcDNA-I-mPRB and pcDNA-I-mPRA plasmids. Rik Derynck kindly provided the pRK5-Smad3 and PRK5-Smad7 plasmids.
Mutagenesis
Generation of the mutated −381 hormone response element (HRE) in FSHβ and the PRC577A mutant unable to bind DNA were described previously (15). We used the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) to generate mutations in three Smad-binding elements (SBE) in the proximal FSHβ promoter. The first round of mutagenesis was performed using the −1000FSHβluc plasmid and the appropriate oligonucleotides according to the manufacturer’s protocol: −267SBEmut (5′-AGATCAGAAAGAATAGTAGCTACTCTAGAGTCACA TTTAATTTAC-3′). Then a second round used the plasmid with the mutated −267 SBE and the appropriate oligos: 153&120SBEmut (5′-TGGCATTTCTACTGCTTTGGCGAGGCTTGATCTCCCTGTCCGTAGAAACAATG-3′) to generate the 3xSBE mutation (the mutated residues are underlined). We confirmed the sequences of all promoter fragments through dideoxynucleotide sequencing performed by the DNA Sequencing Shared Resource, UCSD Cancer Center.
Cell culture and transient transfection
Cell culture and transient transfection experiments were performed with the LβT2 cell line (24). LβT2 cells were maintained in 10-cm-diameter dishes in DMEM (Cellgro; Mediatech., Herndon, VA) supplemented with 10% fetal bovine serum (Omega Scientific, Inc., Tarzana, CA) at 37 C with 5% CO2. One day before transfection, 3 × 105 cells were plated per well into 12-well plates. Transient transfection was performed using Fugene 6 reagent (Roche Molecular Biochemicals, Indianapolis, IN) following the manufacturer’s instructions. Each well was transfected with 0.4 μg reporter plasmid. Additionally, the cells were transfected with 0.2 μg steroid receptor. As an internal control for transfection efficiency, we cotransfected the LβT2 cells with a reporter plasmid containing β-galactosidase driven by the Rous sarcoma virus promoter (RSV-β-gal). The cells were switched to serum-free DMEM 6 h after transfection to eliminate the influence of activin, growth factors, and steroids in the serum. The following day, the cells were treated with ethanol (vehicle control) or hormone for 24 h.
Luciferase and β-galactosidase activity assays
The cells were washed once with 1× PBS and then lysed with 0.1 m K-phosphate buffer (pH 7.8) containing 0.2% Triton X-100. After lysing the cells, we assayed the luciferase activity using a buffer containing 100 mm Tris-HCl (pH 7.8), 15 mm MgSO4, 10 mm ATP, and 65 μm luciferin. β-Galactosidase activity was measured using the Galacto-light assay (Tropix, Bedford, MA) according to the manufacturer’s protocol. Both luciferase and β-galactosidase activity were measured using an EG&G Berthold Microplate Luminometer (PerkinElmer Corp., Norwalk, CT).
Mouse primary pituitary cell culture
Eight-week-old, male C57 Black 6 mice, purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN), were killed, and the pituitaries were collected in ice-cold Dulbecco’s A PBS. After rinsing in PBS, the pituitaries were placed in Hanks’ balanced salt solution with 25 mm HEPES (pH 7.2) containing 0.25% collagenase and 0.25% trypsin. After cell dispersion for 30 min at 37 C in a shaker water bath, the same volume of DMEM with 10% fetal bovine serum was added to stop the reaction, and DNase was added to a final concentration of 20 μg/ml and incubated for another 15 min at 37 C. After removal of tissue debris, the cells were pelleted by centrifugation and plated at 106 cells/2-cm2 well density. The media was changed to serum-free DMEM with 0.1% BSA 16 h before treatment. Cells were treated with the relevant hormones for 24 h, after which the cells were lysed to obtain total RNA. These studies were approved by the UCSD Institutional Animal Care and Use Committee.
Quantitative real-time PCR
RNA was obtained with Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Contaminating DNA was removed with DNA-free reagent (Ambion, Austin, TX), and 2 μg RNA was reverse transcribed using Superscript III First-Strand Synthesis System (Invitrogen). Quantitative real-time PCR was performed using an iCycler from Bio-Rad (Hercules, CA), using QuantiTect SYBR Green PCR Kit (QIAGEN, Valencia, CA) and the following primers: FSH forward, GCCGTTTCTGCATAAGC; FSH reverse, CAATCTTACGGTCTCGTATACC; GAPDH forward, TGCACCACCAACTGCTTAG; GAPDH reverse, GGATGCAGGGATGATGTTC. The PCR was performed under the following conditions: 95 C for 15 min followed by 40 cycles at 95 C for 15 sec, 54 C for 30 sec, and 72 C for 30 sec. For FSHβ measurements, the equivalent of 50 ng starting RNA (as quantified before reverse transcription) was used in each reaction, whereas for GAPDH, 10 ng was sufficient. Each sample was assayed in triplicate. A standard curve with dilutions of 10 pg/well, 1 pg/well, 100 fg/well, and 10 fg/well of a plasmid containing FSHβ or GAPDH cDNA was generated in each run with the samples. In each experiment, the amount of FSHβ was calculated by comparing the threshold cycle obtained for each sample with the standard curve generated in the same run. After each run, a melting-curve analysis was performed to confirm that a single amplicon was generated in each reaction.
GST interaction assay
The GST-Smad2, -Smad3, and -Smad4 plasmids were kindly provided by Rik Derynck. The GFP expression plasmid was obtained from Douglass Forbes (UCSD). 35S-labeled proteins were produced using the TnT Coupled Reticulolysate System (Promega Corp., Madison, WI). Bacteria transformed with the GST plasmids were grown to an OD of 0.6 and then induced with isopropyl-β-d-thiogalactoside overnight at 30 C. The bacterial pellets were sonicated in 0.1% Triton X-100 and 5 mm EDTA in 1× PBS and centrifuged, and the supernatant was bound to glutathione Sepharose 4B resin (Amersham Pharmacia Biotech, Piscataway, NJ). The beads were washed four times in PBS and then in HND buffer [10 mg/ml BSA, 20 mm HEPES (pH 7.8), 50 mm NaCl, 5 mm dithiothreitol, and 0.1% Nonidet P-40]. For the interaction assay, 40 μl 35S-labeled in vitro-transcribed and -translated AR, PRB, or PRA was added to the beads with 400 μl HND buffer, after which 5 μl GFP was added to the beads. The beads were incubated overnight at 4 C and then washed twice with HND buffer and twice with 0.1% Nonidet P-40 in PBS. Thirty microliters of 2× Laemmli load buffer were added, and the samples were boiled and then electrophoresed on a 10% SDS-polyacrylamide gel. One tenth of the 35S-labeled in vitro-transcribed and -translated product was loaded onto the gel as input. The gel was dried, and the proteins were visualized by autoradiography.
Statistical analyses
Transient transfections were performed in triplicate, and each experiment was repeated independently at least three times. We normalized the data for transfection efficiency by expressing luciferase activity relative to β-galactosidase activity and relative to the empty pGL3 plasmid to control for hormone effects on the vector DNA. The data were analyzed by one-way ANOVA, followed by post hoc comparisons with the Tukey-Kramer honestly significant difference test or two-way ANOVA (32) using the statistical package JMP 5.0 (SAS, Cary, NC). In the figures, an asterisk indicates significant differences from the vehicle-treated control, whereas a dagger indicates a synergistic interaction as defined by a two-way ANOVA (P < 0.05).
Results
Synergy between gonadal steroid hormones and activin enhances FSHβ gene expression in pituitary gonadotropes
The immortalized LβT2 gonadotrope cell line was used to test whether the gonadal steroid hormones, progesterone and testosterone, cooperate with activin to alter FSHβ gene expression directly in the pituitary gonadotrope. Although LβT2 cells have been shown to express AR and PR (15,27,33), the levels appear to be low because the hormone response with the endogenous receptors is less than 2-fold. Thus, to amplify the hormone response and allow us to compare the effects of different receptor isoforms and receptor mutants, we transfected exogenous steroid receptors into the cells. We demonstrated previously that a significant hormone response could be detected on the FSHβ promoter with even a small amount of exogenous receptor added to the cells and that the responsiveness continued to increase with additional receptor (15). These results indicate that the responsiveness is not likely due to artificially high levels of exogenous receptors because the receptor is limiting in the cells.
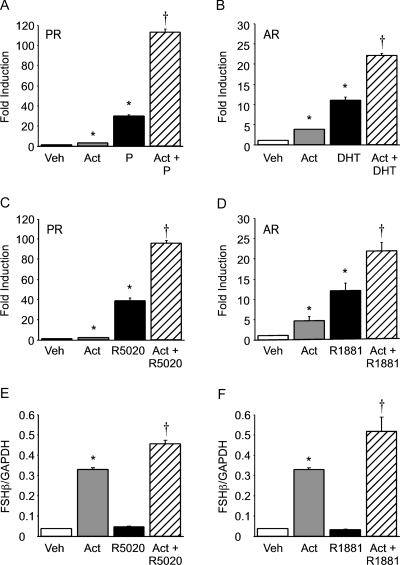
Activin or progesterone treatment significantly induced the −1000FSHβluc reporter gene (3- and 30-fold, respectively) in cells transfected with the rat PR (Fig. 1A). The induction of FSHβ gene expression was also greatly enhanced (110-fold) after the cells were treated with both activin and progesterone (Fig. 1A). This positive interaction was determined to be synergistic using a two-way ANOVA [see Slinker (32) for details on using a two-way ANOVA to determine whether two factors are additive or synergistic].
Figure 1.
Progestins and androgens synergize with activin to induce FSHβ gene expression in immortalized gonadotrope and mouse primary pituitary cells. A–D, The −1000FSHβluc reporter plasmid was transiently transfected into LβT2 cells along with 200 ng rat PR (A and C) or AR (B and D). After overnight starvation in serum-free media, the cells were treated for 24 h with vehicle (Veh), 10 ng/μl activin (Act), 100 nm progesterone (P) (A), DHT (B), R5020 (C), R1881 (D), or both activin and the relevant steroid hormone, as indicated. Luciferase activity was normalized to β-galactosidase activity and set relative to the empty reporter vector. Results represent the mean ± sem of at least three independent experiments performed in triplicate and are presented as fold induction of hormone treatment relative to the vehicle control. E and F, Primary pituitary cells were dispersed from 8-wk-old male mice and plated in culture. The cells were treated for 24 h with vehicle (Veh), 10 ng/μl activin (Act), 100 nm R5020 (E), R1881 (F), or both activin and the relevant steroid hormone, as indicated. Total RNA was purified and reverse transcribed, and the level of FSHβ expression was assayed by real-time PCR. In each sample, the amount of FSHβ, calculated from the standard curve, was compared with the amount of GAPDH. The results represent the mean ± sem of FSHβ levels relative to GAPDH performed in triplicate. *, Significant differences from the vehicle-treated control; †, synergistic interaction as defined by a two-way ANOVA (P < 0.05).
We recently demonstrated that androgens synergize with activin on the ovine FSHβ proximal promoter and that this synergy was dependent upon an androgen response element (−245ARE) and an activin response element (−138SBE) (22). Thus, we were interested in determining whether a similar effect occurred on the murine FSHβ promoter, because not all of the androgen- and activin-responsive regions are conserved between the murine and ovine promoters and because the LβT2 cells are of murine origin. Activin or DHT significantly induced FSHβ gene expression 4- and 11-fold, respectively (Fig. 1B). Synergy was observed after cotreatment of the cells with activin and DHT (22-fold induction), indicating that both the ovine and murine FSHβ promoters respond similarly in this regard and that AR is necessary for this cooperative effect.
A similar synergism on the FSHβ promoter was also observed using synthetic analogs of progesterone and testosterone in concert with activin. Activin or the synthetic progestin R5020 significantly induced FSHβ gene expression 2- and 39-fold, respectively (Fig. 1C). Synergy was also observed after cotreatment of the cells with activin and R5020 (96-fold induction). Activin or the synthetic androgen R1881 induced FSHβ gene expression 5- and 12-fold, respectively (Fig. 1D). Synergy was observed after cotreatment of the cells with activin and R1881 (21-fold induction).
We then determined whether the synergy between gonadal steroid hormones and activin could also be observed in cultured pituitary cells. Treatment of mouse primary pituitary cells with activin resulted in a robust induction of FSHβ mRNA levels by approximately 8-fold (Fig. 1, E and F). Although treatment of the cells with either R5020 or R1881 alone did not result in a significant induction over vehicle control, cotreatment of the primary cells with activin and R5020 synergistically stimulated FSHβ mRNA levels (12-fold). Similar to the findings of Leal et al. (20) with rat pituitary cells, we also observed a synergistic induction of FSHβ by 14-fold in mouse primary pituitary cells after cotreatment with activin and R1881.
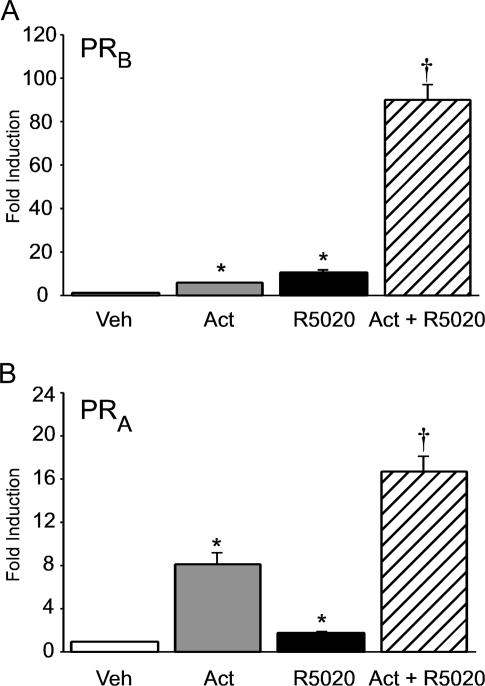
Maximal synergy between progestins and activin requires the full-length PRB isoform
Activin or the synthetic progestin R5020 significantly induced the −1000FSHβluc reporter gene (6- and 13-fold, respectively) in cells transfected with the full-length mouse PRB isoform (Fig. 2A). The induction of FSHβ gene expression was also greatly enhanced (94-fold) after the cells were treated with both activin and R5020 (Fig. 2A). We then tested whether the alternative isoform of PR, PRA, which lacks the amino-terminal 164 amino acids of PRB, could also interact with activin to modulate FSHβ expression. Activin still induced FSHβ gene expression 7-fold when the cells were transfected with mouse PRA (Fig. 2B). In addition, progestins significantly induced the −1000FSHβluc reporter 2-fold, although the hormonal response was 85% less than with PRB. The combination of activin with R5020 also synergistically induced FSHβ by 16-fold. However, the hormonal response was reduced by 83% compared with the synergistic response with PRB. These results indicate that maximal progesterone responsiveness on the FSHβ promoter occurs in the context of the full-length PRB isoform and in concert with activin.
Figure 2.
Full-length PRB is required for maximal synergy between progesterone and activin. The −1000FSHβluc reporter plasmid was transiently transfected into LβT2 cells along with 200 ng mouse PRB (A) or PRA (B). After overnight starvation in serum-free media, the cells were treated for 24 h with vehicle (Veh), 10 ng/μl activin (Act), 100 nm R5020, or activin and R5020, as indicated. Results represent the mean ± sem of at least three independent experiments performed in triplicate and are presented as fold induction of hormone treatment relative to the vehicle control. *, Significant differences from the vehicle-treated control; †, synergistic interaction as defined by a two-way ANOVA (P < 0.05).
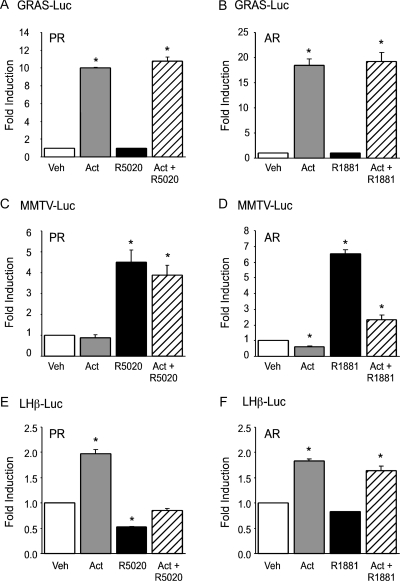
Synergy between progestins or androgens and activin is specific to FSHβ
Because the interaction between gonadal steroid hormones and activin has been proposed to be due to steroid modulation of activin signaling inhibitors such as follistatin and inhibin, we tested whether promoters known to exhibit steroid hormone and/or activin responsiveness other than FSHβ exhibited a synergistic induction upon cotreatment with activin and the relevant steroid hormone. Initially, we determined whether synergy occurred on a luciferase reporter gene linked to an activin-responsive element derived from the GnRH receptor promoter (the GRAS element) (30). As indicated in Fig. 3, the GRAS-tk-luc reporter gene was induced by activin, whereas there was no response to progestins or androgens on the GRAS promoter. In contrast to the synergy obtained on the FSHβ promoter (Fig. 1), there was no increase in expression from the GRAS element after cotreatment with activin and gonadal steroid hormones compared with activin treatment alone (Fig. 3, A and B). We then tested whether synergy between steroid hormones and activin could be observed on a steroid hormone-responsive mouse mammary tumor virus promoter linked to a luciferase reporter gene (MMTV-luc). Treatment of the LβT2 cells with activin did not lead to an induction of the MMTV promoter, whereas treatment with R5020 or R1881 resulted in a 4.5- and 6.5-fold induction, respectively (Fig. 3, C and D). There was no detectable synergistic induction on the MMTV promoter after treatment with both activin and R5020; cotreatment with activin and R1881 actually resulted in a decrease in transcription compared with R1881 alone. Finally, we determined whether synergy could be observed on a luciferase reporter linked to 1.8 kb of the rat LHβ promoter. Unlike the previous two promoters, the LHβ promoter has been shown to be responsive to both activin and steroid hormones (13,15,34). Similar to our previous report, activin induced expression of the LHβ promoter approximately 2-fold (Fig. 3, E and F). No synergy was observed on the LHβ promoter after cotreatment with activin and either R5020 or R1881. Instead, progestins decreased LHβ gene expression both alone and in combination with activin. Similar to the findings of Curtin et al. (34), we also observed a decrease of LHβ expression with androgen treatment, although it was less striking than the repressive effect we observed with progesterone. These experiments indicate that the synergy seen on the FSHβ promoter after cotreatment of activin and steroid hormones is not due to a global change in steroid hormone or activin signaling in the gonadotrope.
Figure 3.
Synergistic activation by progestins or androgens and activin is specific to the FSHβ promoter. The GRAS-tk-luc reporter gene was transiently transfected into LβT2 cells along with 200 ng PR (A) or AR (B). The MMTV-luc reporter gene was transiently transfected into the cells with PR (C) or AR (D). The LHβ-luc reporter gene was transiently transfected into the cells with PR (E) or AR (F). After overnight starvation in serum-free media, the cells were treated with vehicle (Veh), 10 ng/μl activin (Act), 100 nm R5020 (A, C, and E), 100 nm R1881 (B, D, and F), or both activin and the relevant steroid hormone for 24 h, as indicated. *, Significant differences from the vehicle-treated control.
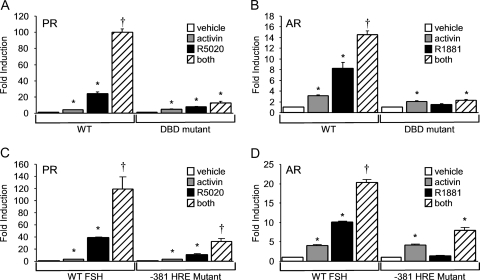
Cross-talk between gonadal steroid hormones and activin requires steroid receptor binding to HREs in the FSHβ promoter
To determine whether the synergy between progestins or androgens and activin occurs directly at the level of the FSHβ promoter, we first tested whether DNA-binding-deficient steroid receptors would still elicit a synergistic hormonal response. The PRC577A mutant is analogous to a C587A mutation in the human PRB that prevents DNA binding (35), and the AR C562G mutation has also been reported to inhibit DNA binding (31). Figure 4 shows that whereas activin induced FSHβ gene expression in the presence of both wild-type and mutant steroid receptors, the response to R5020 (Fig. 4A) and R1881 (Fig. 4B) was greatly diminished in the presence of the mutant receptors. Additionally, use of the PR C577A mutant did not result in synergy after cotreatment of the cells with activin and R5020, in contrast to the synergy observed with wild-type PR (Fig. 4A). Similar results were obtained using the ARC562G mutant receptor (Fig. 4B), indicating that the DNA-binding domain (DBD) regions of PR and AR are critical for the synergistic response between activin and the gonadal steroid hormones.
Figure 4.
DNA binding of PR or AR to the proximal FSHβ promoter is required for the synergistic activation by steroids and activin. A, The −1000FSHβluc reporter plasmid was transiently cotransfected with wild-type (WT) or mutant PR (PRC577A). B, The −1000FSHβluc reporter was transiently cotransfected into LβT2 cells with the wild-type or mutant AR (ARC562G). C and D, The wild-type −1000FSHβluc reporter or the −381 mutant was transiently cotransfected into LβT2 cells with PR or AR, respectively. After overnight starvation in serum-free media, cells were treated with vehicle, 10 ng/μl activin, 100 nm steroid, or both hormones for 24 h. *, Significant differences from the vehicle-treated control; †, synergistic interaction as defined by a two-way ANOVA (P < 0.05).
To further test whether the synergy between activin and progestins or androgens requires binding of PR or AR, respectively, to the FSHβ promoter, we transfected LβT2 cells with a reporter plasmid containing a mutated HRE at −381 of the FSHβ promoter (−381 HRE). The −381 HRE was previously characterized as an element critical for steroid hormone responsiveness on the murine FSHβ promoter (15). Activin induced FSHβ gene expression 3- and 4-fold in the presence of exogenous PR and AR, respectively (Fig. 4, C and D), but the steroid hormone response was significantly reduced on the −381 HRE mutant compared with the wild-type FSHβ promoter. The synergy between progestins and activin was also substantially decreased by 73% (Fig. 4C). The remaining synergy is probably due to PR binding to other HREs present in the FSHβ promoter (15). In contrast, there was no significant interaction between androgens and activin on the −381 HRE mutant promoter (Fig. 4D).
Smad-dependent signaling is critical for synergy between steroid hormones and activin
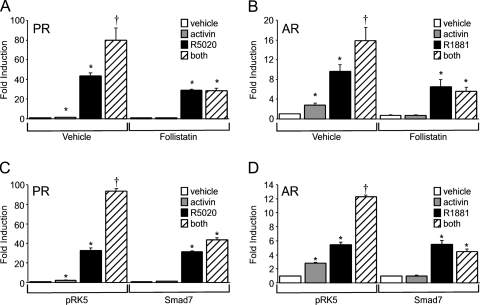
The next set of experiments explored whether the classical activin signaling pathway was required for synergy. First, we tested whether the synergy between activin and progestins or androgens would be abrogated by reducing the bioavailability of activin with an inhibitor such as follistatin. Treatment of the LβT2 cells with follistatin resulted in diminished basal gene expression as reported previously (24), probably due to follistatin inhibition of the actions of autocrine activin secreted by the LβT2 cells themselves (Fig. 5, A and B). Induction of FSHβ gene expression by either R5020 (Fig. 5A) or R1881 (Fig. 5B) was also reduced after treatment of the cells with follistatin. Furthermore, treatment with follistatin completely blocked FSHβ gene expression induced by activin and reduced the synergy between activin and the gonadal steroid hormones to the level of induction seen with steroid hormone alone (Fig. 5).
Figure 5.
The activin signaling pathway is critical for cross-talk between activin and progestins or androgens. The −1000FSHβluc reporter gene was transiently transfected into LβT2 cells along with PR (A) or AR (B). After overnight starvation in serum-free media, cells were treated with vehicle, 10 ng/μl activin, 100 ng/μl follistatin, 100 nm steroid, or combinations of hormones, as indicated, for 24 h. The −1000FSHβluc reporter gene was transiently transfected into LβT2 cells along with PR (C) or AR (D) and Smad7 or empty vector control (pRK5) as indicated. After overnight starvation in serum-free media, cells were treated with vehicle, 10 ng/μl activin, 100 nm R5020, or R1881, or both activin and the relevant steroid hormone for 24 h. *, Significant differences from the vehicle-treated control; †, synergistic interaction as defined by a two-way ANOVA (P < 0.05).
Second, we tested whether the synergy between activin and gonadal steroid hormones was due to activation of the Smad signaling pathway using an inhibitory Smad protein. Smad7 has been shown to act as an inhibitor of the activin signaling pathway by binding to the activin receptors and preventing the phosphorylation of Smad2 and Smad3 (36,37). Transient transfection of the LβT2 cells with the pRK5 empty vector did not affect synergy between activin and progestins or androgens (Fig. 5, C and D). However, transfection of LβT2 cells with 200 ng Smad7 abolished the activin induction and the synergy between activin and the gonadal steroid hormones without affecting the activation by steroid hormones alone.
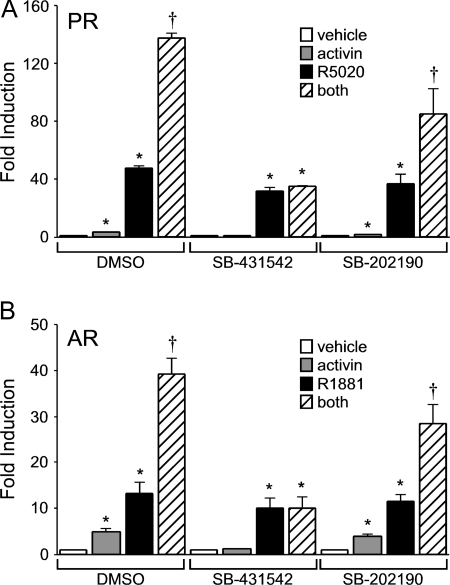
Finally, because Smad7 appears to have other cellular functions in addition to blocking the activation of Smad2/3 (38,39,40), additional experiments were needed to confirm whether the synergy required activation of the Smad-dependent signaling pathway. Specifically, we determined whether pharmacological inhibitors of these signaling pathways could block the synergy. The inhibitor SB-431541 has been shown to specifically block the phosphorylation of Smad2 and Smad3 (41), whereas the SB-202190 compound prevents Smad-independent activation of p38 MAPK (42,43). Treatment of the LβT2 cells with dimethylsulfoxide (the vehicle control for the inhibitors) had no effect on the synergy between activin and progestins or androgens (Fig. 6). On the other hand, treatment with 2 μm SB-431541 blocked both the activin induction and the synergy between activin and the steroid hormones. In contrast, the p38 MAPK inhibitor SB-202190 significantly reduced FSHβ gene expression after activin treatment compared with the vehicle control (P < 0.05) but did not abolish synergy between activin and progestins or androgens. These results indicate that Smad-dependent signaling is required for synergy between gonadal steroid hormones and activin on the FSHβ promoter.
Figure 6.
Smad-dependent signaling is required for the synergy between gonadal steroid hormones and activin. The −1000FSHβluc reporter gene was transiently transfected into LβT2 cells along with PR (A) or AR (B). After overnight starvation in serum-free media, cells were treated for 24 h with vehicle, 10 ng/μl activin, 100 nm of either R5020 or R1881, or both activin and steroid hormones in the presence of dimethylsulfoxide or 2 μm of the Alk-4/5 or p38 MAPK inhibitors SB-431542 or SB-202190, as indicated. *, Significant differences from the vehicle-treated control; †, synergistic interaction as defined by a two-way ANOVA (P < 0.05).
Gonadal steroid hormone receptors and Smad proteins physically interact
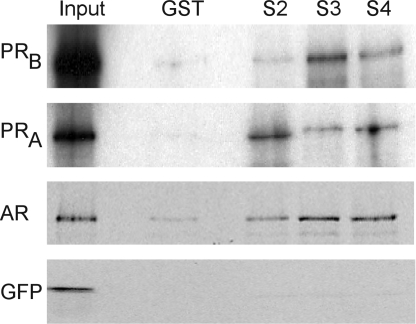
After demonstrating that steroid receptors and Smad-dependent signaling appear to be essential for the synergy between gonadal steroid hormones and activin, we then tested whether PR and AR can interact with Smad proteins because Smads can alter gene transcription by tethering to other transcription factors as well as by direct DNA binding (44,45,46). AR has been shown previously to interact with Smad3 and Smad4 in vitro and in prostate cancer cells (47,48,49). We tested whether PR and AR interact with Smad proteins by incubating these steroid receptors with GST-Smad fusion proteins in pull-down experiments. Bacterially expressed GST-Smad2, -Smad3, and -Smad4 fusion proteins interacted with in vitro-transcribed and -translated PR (Fig. 7). Both the full-length PRB and PRA bound to Smad2, Smad3, and Smad4. As a positive control, we also showed that AR interacts with Smad3 and Smad4, as previously reported, as well as with Smad2. In contrast, there was minimal interaction between any of the GST-fusion Smad proteins and the negative control, green fluorescent protein (GFP), or with GST alone incubated with the transcribed and translated proteins (Fig. 7).
Figure 7.
PR and AR bind specifically to Smad proteins. GST interaction assays were performed using bacterially expressed GST-fusion proteins (indicated above each lane) and 35S-labeled in vitro-transcribed and -translated AR, PRB, PRA, and GFP (indicated on the left of the panels). GFP was used as a negative control. The GST-fusion proteins included GST alone, GST-Smad2 (S2), GST-Smad3 (S3), and GST-Smad4 (S4). One tenth of the protein used in the interaction assay was loaded in the lane marked Input. The experiment was repeated several times with the same results, and a representative experiment is shown.
Overexpression of Smad proteins and gonadal steroid receptors is sufficient for the synergy
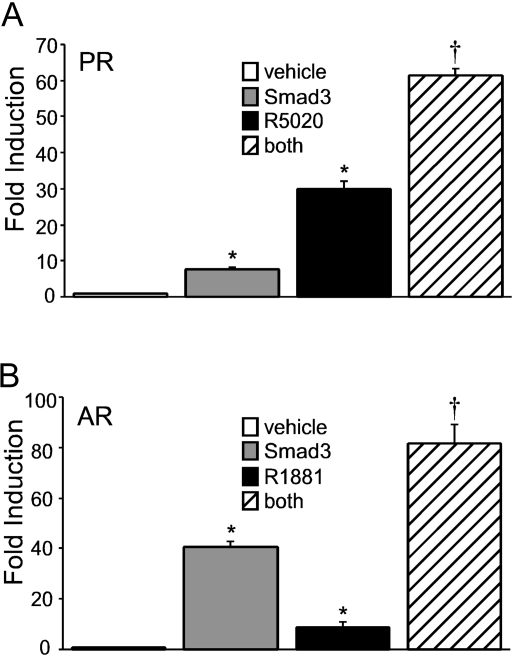
Because the synergy between progestins or androgens and activin is dependent on the Smad signaling pathway and Smad2/3/4 can interact with PR and AR, we then asked whether overexpression of Smad proteins and ligand-bound steroid receptors is sufficient for the synergy to occur. We chose to focus on Smad3 because Smad2 is difficult to overexpress in LβT2 cells (50). Transient transfection of the LβT2 cells with an expression plasmid containing Smad3 resulted in a significant increase in FSHβ gene expression compared with the empty vector (Fig. 8). LβT2 cells containing Smad3 were also treated with the vehicle control or either 100 nm R5020 (Fig. 8A) or 100 nm R1881 (Fig. 8B). Treatment of cells with Smad3 and steroid hormones resulted in a synergistic induction compared with the steroid hormone alone, indicating that overexpression of Smad3 was sufficient to elicit cooperation between the steroid receptors and the Smad-dependent signaling pathway.
Figure 8.
Overexpression of Smads and steroid receptors is sufficient for synergistic induction of the proximal FSHβ promoter. The −1000FSHβluc reporter gene was transiently cotransfected into LβT2 cells with 200 ng PR (A) or AR (B). Cells were also transfected with the pRK5 plasmid as the empty vector control or the Smad3 expression vector. After overnight starvation in serum-free media, the cells were treated with the vehicle control and 100 nm R5020 or R1881 for 24 h, as indicated. *, Significant differences from the vehicle-treated control; †, synergistic interaction as defined by a wo-way ANOVA (P < 0.05).
Cooperation between gonadal steroid hormones and activin requires binding of Smads to SBEs in the FSHβ promoter
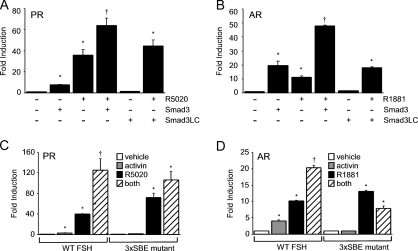
Because Smad proteins physically interact with PR and AR (Fig. 7) and they are sufficient to elicit a synergistic response with steroid receptors (Fig. 8), we asked whether Smad proteins bind to their cognate response elements or whether they act through tethering to the steroid receptors. To address this issue, we first transfected the LβT2 cells with a Smad3 mutant (Smad3LC) lacking the amino-terminal DBD (MH1) but containing the linker region and the carboxyl-terminal domain (MH2) necessary for interaction with Smad4 (51). The MH1 domain of Smad3 was previously shown to be critical for activin-mediated stimulation of the rat FSHβ promoter (52). Both progestins and androgens significantly induced FSHβ gene expression (Fig. 9). In contrast to the induction observed in the presence of the wild-type Smad3, there was no response to the mutant Smad3LC (Fig. 9, A and B). The Smad3LC also did not elicit a synergistic response after treatment with R5020 (Fig. 9A) or R1881 (Fig. 9B), indicating that the Smad DBD is critical for the synergistic interaction between gonadal steroid hormones and activin.
Figure 9.
Disruption of Smad binding to the FSHβ promoter blocks cross-talk between activin and progestins or androgens. A and B, The −1000FSHβluc reporter was transiently cotransfected into LβT2 cells with PR or AR, as indicated. Cells were also transfected with the full-length Smad3 (S3) expression vector or a truncation containing the linker and carboxyl-terminal domain of Smad3 (S3LC). After overnight starvation in serum-free media, the cells were treated with vehicle (−) and 100 nm R5020 or R1881 (+), as indicated. C and D, The wild-type −1000FSHβluc reporter or a 3xSBE mutant was transiently transfected into LβT2 cells along with PR or AR. After overnight starvation in serum-free media, cells were treated with vehicle, 10 ng/μl activin, 100 nm R5020 or R1881, or both activin and steroid hormones for 24 h. *, Significant differences from the vehicle-treated control; †, synergistic interaction as defined by a two-way ANOVA (P < 0.05).
Second, we measured expression of a luciferase reporter containing three mutated SBEs at −267, −153, and −120 in the proximal FSHβ promoter (3xSBE). These elements have been shown previously to play an essential role in activin responsiveness on the FSHβ promoter (29,53,54). Activin treatment did not induce transcription of the 3xSBE mutant (Fig. 9, C and D). Unlike activin, both progestins and androgens increased expression of the wild-type and mutant FSHβ-luciferase reporter gene; the induction was greater on the 3xSBE mutant than the wild-type promoter. Significantly, there was no synergy observed after treatment of the cells with both activin and the gonadal steroid hormones (Fig. 9, C and D). We further confirmed the necessity of Smad proteins binding to activin-responsive elements in the FSHβ promoter by testing whether overexpression of Smad3 could induce a synergistic interaction on the mutant 3xSBE promoter after treatment of the cells with the relevant steroid hormone. In the context of both ligand-bound PR and AR, Smad3 was unable to promote synergy on the mutated FSHβ promoter (data not shown).
Discussion
The experimental results presented herein provide strong evidence of a cooperative interaction between gonadal steroid hormones and activin in gonadotrope cells. In particular, the synergy between progestin or androgen and activin regulation of FSHβ gene expression involves a direct protein-protein interaction between PR or AR and Smad proteins while also requiring DNA binding of both steroid receptors and Smads to their cognate DNA-binding elements within the FSHβ promoter. Our results also complement recent studies showing a synergistic interaction between activin and testosterone on ovine FSHβ gene expression (22) as well as synergy between activin and glucocorticoids or GnRH on rodent FSHβ promoters (29,55,56).
There are several ways in which gonadal steroid hormones could interact with activin to regulate FSHβ gene expression. One prevalent hypothesis is that testosterone interacts with activin by reducing the amount of bioavailable activin or by modifying components of the activin signaling pathway. If activin signaling were globally altered in the gonadotrope by gonadal steroid hormones, a change in the induction of any activin-responsive promoter would be expected. However, we did not observe any synergy between activin and progesterone or testosterone on a GRAS element derived from the GnRH receptor promoter or the LHβ promoter. This indicates that the mechanism of synergy does not involve a global modulation of activin signaling but instead points to a direct interaction of the steroid and activin signaling pathways on the proximal FSHβ promoter. We cannot rule out a role for modulation of activin signaling by steroid hormones, especially because testosterone appears to alter components of activin signaling including the levels of follistatin mRNA (17,18). However, we propose that this regulation may be more important in other pituitary cells, such as folliculostellate cells rather than in pituitary gonadotrope cells. Winters and Moore (57) have also suggested that the differences in the pituitary cellular environment, especially in the levels of follistatin and inhibin, may be responsible for the species-specific differences in FSH regulation by gonadal steroid hormones among mammals. It should also be noted that steroid receptor signaling was not generally affected because progestin and androgen responsiveness of the MMTV promoter did not change after cotreatment with gonadal steroid hormones and activin.
Because the synergistic interaction between gonadal steroid hormones and activin does not appear to be due to a global response in gonadotrope cells, we examined whether this cross-talk occurs at the level of transcription factor binding to the proximal FSHβ promoter. We demonstrated that a functional steroid receptor DBD was essential for the synergism. Because the DBD of steroid receptors has been reported to interact with other transcription factors such as AP-1 (58,59,60), we also showed that when the −381 HRE was mutated in the FSHβ promoter, the synergy between gonadal hormones and activin disappeared, providing additional evidence that binding of PR and AR to the proximal FSHβ promoter is crucial for synergy to occur.
In analyzing the role of the classical activin-signaling pathway in this synergy, we initially showed that follistatin can block the synergy between gonadal steroid hormones and activin by reducing the amount of bioavailable activin. Interestingly, follistatin also blunted the response to progesterone or androgen treatment on its own; probably due to the presence of autocrine activin in the LβT2 cells. However, in contrast to our previous report on synergy between testosterone and activin on the ovine FSHβ promoter (22), follistatin did not completely abrogate the gonadal steroid hormone induction, indicating that the autocrine activin loop is not a required component for progesterone and testosterone signaling. The fact that Smad7 and the pharmacological inhibitor SB-431542 blocked Smad-dependent signaling but not the steroid hormone induction of FSHβ also supports this conclusion.
The next important issue that we addressed was whether the activin signaling required for the synergy occurred through a Smad-dependent or -independent mechanism. TGFβ, a member of the activin family, can signal through different pathways in multiple cell types (51). Activin has been shown to regulate several kinases in gonadotrope cells including PI3K/Akt, ERK1/2, MAPK kinase 1/2 (MEK), and p38 MAPK. Inhibition of these pathways has been reported to have no effect on FSHβ gene expression in several studies (55,61), whereas in another study, TGFβ-activated kinase 1 signaling through p38 MAPK was suggested to play a role in activin responsiveness on the ovine FSHβ promoter (62). We initially chose to address this question by showing that Smad7 completely blocked the synergy between activin and progestins or androgens. However, because Smad7 has been reported to have additional cellular functions in addition to preventing the phosphorylation of Smad2/3, we were cautious about interpreting our results. To further address whether the synergy occurs through a Smad-dependent mechanism, we used a pharmacological approach to inhibit the kinase activity of the activin receptor. The inhibitor SB-431542 completely blocked the synergy between gonadal steroid hormones and activin, further supporting the hypothesis that the synergy occurs through Smad-dependent signaling. In our experiments, the inhibitor SB-202190 (a close analog of SB-203580) reduced but did not abolish the cooperation interaction between activin and gonadal steroid hormones, suggesting that although p38 MAPK signaling may play a role in activin responsiveness, it is not required for the synergism.
Having demonstrated that Smad-dependent signaling was necessary for the synergy between gonadal steroid hormones and activin, we tested whether there was direct interaction between Smads and PR or AR. Using GST-interaction assays, we showed that Smad proteins can physically interact with PR in addition to AR. Our data expand the list of steroid and nuclear receptors that directly partner with Smads and suggest that a steroid receptor-Smad complex bound to DNA may be crucial for the synergy. We then addressed whether Smad proteins are sufficient for the synergy. Overexpression of Smad3 elicited a cooperative response, in the presence of ligand-activated PR or AR. Finally, we determined whether Smad proteins need to bind to the FSHβ promoter or whether they can be tethered to the promoter via protein-protein interactions with steroid receptors. Using a Smad3 protein lacking the MH1 DBD and a FSHβ promoter containing three mutated activin-responsive regions, we showed that binding of Smads to their cognate DNA-binding elements is necessary for synergy. Altogether, these results provide strong evidence for synergy between gonadal steroid hormones and activin modulating FSHβ gene expression that involves direct interaction of the steroid receptors and Smad proteins at the level of the FSHβ promoter.
These findings have important physiological implications for the differential regulation of LH and FSH. During the ovulatory cycle, GnRH levels peak right before ovulation, and this surge is immediately followed by an increase in both LH and FSH. There is also a secondary rise in FSH, independent of GnRH, that is not accompanied by an increase in LH levels (63,64). This differential regulation of LH and FSH can also be seen at the transcriptional level of the LH and FSH β-subunits (65,66). Several hypotheses have been put forth to explain how the secondary FSH surge is regulated, for example 1) a change in GnRH pulse frequency that favors FSHβ transcription (5), 2) a higher level of bioavailable activin due to a drop in follistatin and inhibin levels that selectively favor FSHβ synthesis (67,68), or 3) a rise in steroid hormone levels, such as progestins and androgens, that induce FSHβ expression but inhibit LHβ (14,15). Our results support a combination of the second and third hypotheses and show that they are not mutually exclusive but rather complementary. Activin may act as a tonic hormone to maintain FSHβ at basal levels, whereas gonadal steroid hormones such as progesterone and testosterone synergistically cooperate with activin to induce FSHβ mRNA levels and produce the secondary FSH surge. A decrease of inhibitors like follistatin and inhibin mediated by gonadal steroid hormones in folliculostellate cells in the pituitary may also play an important role. Thus, it is unlikely that any one hormone is responsible for generating the secondary FSH surge. Instead, it is probably the interactions among multiple hormonal signaling pathways in the pituitary gonadotrope that are responsible for regulating FSH synthesis.
In summary, we have established that cross-talk between gonadal steroid hormones and activin signaling pathways can occur directly on the murine FSHβ promoter at the level of the gonadotrope. Moreover, our data refute the idea that the synergy is due solely to steroid hormone modulation of the activin autocrine loop. Rather, we demonstrate that this cooperation cannot be accomplished without the binding of both steroid receptors and Smad proteins to their HREs in the FSHβ promoter and suggest that a direct protein-protein interaction between steroid receptors and Smads may also play a role. Further examination of the mechanisms that the two types of transcription factors employ in their synergistic enhancement of FSHβ gene expression will lead to a better comprehension of the physiological role this interaction plays in the maintenance of reproductive fitness.
Acknowledgments
We thank Djurdjica Coss, Shauna McGillivray, and other members of the Mellon lab for helpful discussions and comments. We also thank Scott Kelley for his suggestions and critical reading of the manuscript. We thank Susan Mayo for technical assistance. We are grateful to Jorma Palvimo, Benita Katzenellenbogen, Dean Edwards, and Rik Derynck for providing plasmids. We also thank the UCSD Cancer Center DNA Sequencing Shared Resource for dideoxynucleotide sequencing.
Footnotes
First Published Online December 13, 2007
Abbreviations: AR, Androgen receptor; DBD, DNA-binding domain; DHT, dihydrotestosterone; GFP, green fluorescent protein; GST, glutathione-S-transferase; HRE, hormone response element; MMTV, mouse mammary tumor virus; PR, progesterone receptor; SBE, Smad-binding element.
This work was supported by National Institute of Child Health and Human Development, National Institutes of Health (NIH) through a cooperative agreement (U54 HD12303) as part of the Specialized Cooperative Centers Program in Reproduction Research (P.L.M.). This work was also supported by NIH Grant R01 HD20377 (to P.L.M.). V.G.T. was supported by NIH F32 DK065437 and NIH T32 HD07203.
Disclosure Statement: The authors have nothing to disclose.
References
- Kumar TR, Wang Y, Lu N, Matzuk MM 1997 Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 15:201–204 [DOI] [PubMed] [Google Scholar]
- Ma X, Dong Y, Matzuk MM, Kumar TR 2004 Targeted disruption of luteinizing hormone β-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci USA 101:17294–17299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KH, Matzuk MM 2002 Minireview: genetic models for the study of gonadotropin actions. Endocrinology 143:2823–2835 [DOI] [PubMed] [Google Scholar]
- Pierce JG, Parsons TF 1981 Glycoprotein hormones: structure and function. Annu Rev Biochem 50:465–495 [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Jakubowiak A, Steinberger A, Chin WW 1997 Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology 138:1224–1231 [DOI] [PubMed] [Google Scholar]
- Papavasiliou SS, Zmeili S, Khoury S, Landefeld TD, Chin WW, Marshall JC 1986 Gonadotropin-releasing hormone differentially regulates expression of the genes for luteinizing hormone α and β subunits in male rats. Proc Natl Acad Sci USA 83:4026–4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Rivier C, Brown M 1977 Regulatory peptides of the hypothalamus. Annu Rev Physiol 39:473–527 [DOI] [PubMed] [Google Scholar]
- Ying SY 1988 Inhibins, activins, and follistatins: gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr Rev 9:267–293 [DOI] [PubMed] [Google Scholar]
- Carroll RS, Corrigan AZ, Gharib SD, Vale W, Chin WW 1989 Inhibin, activin, and follistatin: regulation of follicle-stimulating hormone messenger ribonucleic acid levels. Mol Endocrinol 3:1969–1976 [DOI] [PubMed] [Google Scholar]
- Attardi B, Miklos J 1990 Rapid stimulatory effect of activin-A on messenger RNA encoding the follicle-stimulating hormone β-subunit in rat pituitary cell cultures. Mol Endocrinol 4:721–726 [DOI] [PubMed] [Google Scholar]
- Blumenfeld Z, Ritter M 2001 Inhibin, activin, and follistatin in human fetal pituitary and gonadal physiology. Ann NY Acad Sci 943:34–48 [DOI] [PubMed] [Google Scholar]
- Yamada Y, Yamamoto H, Yonehara T, Kanasaki H, Nakanishi H, Miyamoto E, Miyazaki K 2004 Differential activation of the luteinizing hormone β-subunit promoter by activin and gonadotropin-releasing hormone: a role for the mitogen-activated protein kinase signaling pathway in LβT2 gonadotrophs. Biol Reprod 70:236–243 [DOI] [PubMed] [Google Scholar]
- Coss D, Thackray VG, Deng CX, Mellon PL 2005 Activin regulates luteinizing hormone β-subunit gene expression through smad-binding and homeobox elements. Mol Endocrinol 19:2610–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC 2004 Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol 33:559–584 [DOI] [PubMed] [Google Scholar]
- Thackray VG, McGillivray SM, Mellon PL 2006 Androgens, progestins and glucocorticoids induce follicle-stimulating hormone β-subunit gene expression at the level of the gonadotrope. Mol Endocrinol 20:2062–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Takio K, Eto Y, Shibai H, Titani K, Sugino H 1990 Activin-binding protein from rat ovary is follistatin. Science 247:836–838 [DOI] [PubMed] [Google Scholar]
- Bilezikjian LM, Corrigan AZ, Blount AL, Vale WW 1996 Pituitary follistatin and inhibin subunit messenger ribonucleic acid levels are differentially regulated by local and hormonal factors. Endocrinology 137:4277–4284 [DOI] [PubMed] [Google Scholar]
- Burger LL, Haisenleder DJ, Aylor KW, Dalkin AC, Prendergast KA, Marshall JC 2004 Regulation of luteinizing hormone-β and follicle-stimulating hormone (FSH)-β gene transcription by androgens: testosterone directly stimulates FSH-β transcription independent from its role on follistatin gene expression. Endocrinology 145:71–78 [DOI] [PubMed] [Google Scholar]
- Burger LL, Haisenleder DJ, Wotton GM, Aylor KW, Dalkin AC, Marshall JC 2007 The regulation of FSHβ transcription by gonadal steroids: testosterone and estradiol modulation of the activin intracellular signaling pathway. Am J Physiol Endocrinol Metab 293:E277–E285 [DOI] [PubMed] [Google Scholar]
- Leal AM, Blount AL, Donaldson CJ, Bilezikjian LM, Vale WW 2003 Regulation of follicle-stimulating hormone secretion by the interactions of activin-A, dexamethasone and testosterone in anterior pituitary cell cultures of male rats. Neuroendocrinology 77:298–304 [DOI] [PubMed] [Google Scholar]
- Bohnsack BL, Szabo M, Kilen SM, Tam DH, Schwartz NB 2000 Follistatin suppresses steroid-enhanced follicle-stimulating hormone release in vitro in rats. Biol Reprod 62:636–641 [DOI] [PubMed] [Google Scholar]
- Spady TJ, Shayya R, Thackray VG, Ehrensberger L, Bailey JS, Mellon PL 2004 Androgen regulates FSHβ gene expression in an activin-dependent manner in immortalized gonadotropes. Mol Endocrinol 18:925–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarid ET, Windle JJ, Whyte DB, Mellon PL 1996 Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 122:3319–3329 [DOI] [PubMed] [Google Scholar]
- Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang H-J, Miller WL, Mellon PL 2001 Cell-specific transcriptional regulation of FSHβ by activin and GnRH in the LβT2 pituitary gonadotrope cell model. Endocrinology 142:2284–2295 [DOI] [PubMed] [Google Scholar]
- Graham KE, Nusser KD, Low MJ 1999 LβT2 gonadotroph cells secrete follicle stimulating hormone (FSH) in response to activin A. J Endocrinol 162:R1–R5 [DOI] [PubMed] [Google Scholar]
- Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, Vale W 2000 Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature 404:411–414 [DOI] [PubMed] [Google Scholar]
- Lawson MA, Li D, Glidewell-Kenney CA, Lopez FJ 2001 Androgen responsiveness of the pituitary gonadotrope cell line LβT2. J Endocrinol 170:601–607 [DOI] [PubMed] [Google Scholar]
- Kawakami S, Fujii Y, Okada Y, Winters SJ 2002 Paracrine regulation of FSH by follistatin in folliculostellate cell-enriched primate pituitary cell cultures. Endocrinology 143:2250–2258 [DOI] [PubMed] [Google Scholar]
- McGillivray SM, Thackray VG, Coss D, Mellon PL 2007 Activin and glucocorticoids synergistically activate follicle-stimulating hormone β-subunit gene expression in the immortalized LβT2 gonadotrope cell line. Endocrinology 148:762–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval DL, Nelson SE, Clay CM 1997 The tripartite basal enhancer of the gonadotropin-releasing hormone (GnRH) receptor gene promoter regulates cell-specific expression through a novel GnRH receptor activating sequence. Mol Endocrinol 11:1814–1821 [DOI] [PubMed] [Google Scholar]
- Ikonen T, Palvimo JJ, Janne OA 1998 Heterodimerization is mainly responsible for the dominant negative activity of amino-terminally truncated rat androgen receptor forms. FEBS Lett 430:393–396 [DOI] [PubMed] [Google Scholar]
- Slinker BK 1998 The statistics of synergism. J Mol Cell Cardiol 30:723–731 [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Waring DW 2006 Differential expression and regulation of progesterone receptor isoforms in rat and mouse pituitary cells and LβT2 gonadotropes. J Endocrinol 190:837–846 [DOI] [PubMed] [Google Scholar]
- Curtin D, Jenkins S, Farmer N, Anderson AC, Haisenleder DJ, Rissman E, Wilson EM, Shupnik MA 2001 Androgen suppression of GnRH-stimulated rat LHβ gene transcription occurs through Sp1 sites in the distal GnRH-responsive promoter region. Mol Endocrinol 15:1906–1917 [DOI] [PubMed] [Google Scholar]
- Takimoto GS, Tasset DM, Eppert AC, Horwitz KB 1992 Hormone-induced progesterone receptor phosphorylation consists of sequential DNA-independent and DNA-dependent stages: analysis with zinc finger mutants and the progesterone antagonist ZK98299. Proc Natl Acad Sci USA 89:3050–3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P 1997 Identification of Smad7, a TGFβ-inducible antagonist of TGF-β signalling. Nature 389:631–635 [DOI] [PubMed] [Google Scholar]
- Souchelnytskyi S, Nakayama T, Nakao A, Moren A, Heldin CH, Christian JL, ten Dijke P 1998 Physical and functional interaction of murine and Xenopus Smad7 with bone morphogenetic protein receptors and transforming growth factor-β receptors. J Biol Chem 273:25364–25370 [DOI] [PubMed] [Google Scholar]
- Landstrom M, Heldin NE, Bu S, Hermansson A, Itoh S, ten Dijke P, Heldin CH 2000 Smad7 mediates apoptosis induced by transforming growth factor β in prostatic carcinoma cells. Curr Biol 10:535–538 [DOI] [PubMed] [Google Scholar]
- Edlund S, Bu S, Schuster N, Aspenstrom P, Heuchel R, Heldin NE, ten Dijke P, Heldin CH, Landstrom M 2003 Transforming growth factor-β1 (TGF-β)-induced apoptosis of prostate cancer cells involves Smad7-dependent activation of p38 by TGF-β-activated kinase 1 and mitogen-activated protein kinase kinase 3. Mol Biol Cell 14:529–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S, Cao X 2002 A nuclear antagonistic mechanism of inhibitory Smads in transforming growth factor-beta signaling. J Biol Chem 277:4176–4182 [DOI] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS 2002 SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol 62:65–74 [DOI] [PubMed] [Google Scholar]
- Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL 2002 p38 mitogen-activated protein kinase is required for TGFβ-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci 115:3193–3206 [DOI] [PubMed] [Google Scholar]
- Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL 2000 Phosphatidylinositol 3-kinase function is required for transforming growth factor β-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem 275:36803–36810 [DOI] [PubMed] [Google Scholar]
- Zawel L, Dai JL, Buckhaults P, Shou S, Kinzler KW, Vogelstein B, Karn SE 1998 Human Smad3 and Smad 4 are sequence-specific transcription activators. Mol Cell 1:611–617 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Feng XH, Derynck R 1998 Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-β-induced transcription. Nature 394:909–913 [DOI] [PubMed] [Google Scholar]
- Hua X, Liu X, Ansari DO, Lodish HF 1998 Synergistic cooperation of TFE3 and smad proteins in TGF-β-induced transcription of the plasminogen activator inhibitor-1 gene. Genes Dev 12:3084–3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SA, Zarnegar M, Sharma M, Yang F, Peehl DM, ten Dijke P, Sun Z 2001 SMAD3 represses androgen receptor-mediated transcription. Cancer Res 61:2112–2118 [PubMed] [Google Scholar]
- Kang HY, Huang KE, Chang SY, Ma WL, Lin WJ, Chang C 2002 Differential modulation of androgen receptor-mediated transactivation by Smad3 and tumor suppressor Smad4. J Biol Chem 277:43749–43756 [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Cornelius SC, Pultz NJ, Jorgensen JS, Bonham MJ, Kim SJ, Danielpour D 2002 The androgen receptor represses transforming growth factor-β signaling through interaction with Smad3. J Biol Chem 277:1240–1248 [DOI] [PubMed] [Google Scholar]
- Bernard DJ 2004 Both SMAD2 and SMAD3 mediate activin-stimulated expression of the follicle-stimulating hormone β-subunit in mouse gonadotrope cells. Mol Endocrinol 18:606–623 [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE 2003 Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425:577–584 [DOI] [PubMed] [Google Scholar]
- Suszko MI, Balkin DM, Chen Y, Woodruff TK 2005 Smad3 mediates activin-induced transcription of follicle-stimulating hormone β-subunit gene. Mol Endocrinol 19:1849–1858 [DOI] [PubMed] [Google Scholar]
- Suszko MI, Lo DJ, Suh H, Camper SA, Woodruff TK 2003 Regulation of the rat follicle-stimulating hormone β-subunit promoter by activin. Mol Endocrinol 17:318–332 [DOI] [PubMed] [Google Scholar]
- Bailey JS, Rave-Harel N, Coss D, McGillivray SM, Mellon PL 2004 Activin regulation of the follicle-stimulating hormone β-subunit gene involves Smads and the TALE homeodomain proteins Pbx1 and Prep1. Mol Endocrinol 18:1158–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SJ, Lacza CT, Detz AA, Xu S, Petrillo LA, Kaiser UB 2005 Synergy between activin A and gonadotropin-releasing hormone in transcriptional activation of the rat follicle-stimulating hormone-β gene. Mol Endocrinol 19:237–254 [DOI] [PubMed] [Google Scholar]
- Coss D, Hand CM, Yaphockun KK, Ely HA, Mellon PL 2007 p38 mitogen-activated kinase is critical for synergistic induction of the FSHβ gene by gonadotropin-releasing hormone and activin through augmentation of c-Fos induction and Smad phosphorylation. Mol Endocrinol 21:3071–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters SJ, Moore JP 2004 Intra-pituitary regulation of gonadotrophs in male rodents and primates. Reproduction 128:13–23 [DOI] [PubMed] [Google Scholar]
- Heck S, Kullmann M, Gast A, Ponta H, Rahmsdorf HJ, Herrlich P, Cato AC 1994 A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO J 13:4087–4095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schutz G 1998 DNA binding of the glucocorticoid receptor is not essential for survival. Cell 93:531–541 [DOI] [PubMed] [Google Scholar]
- Kallio PJ, Poukka H, Moilanen A, Janne OA, Palvimo JJ 1995 Androgen receptor-mediated transcriptional regulation in the absence of direct interaction with a specific DNA element. Mol Endocrinol 9:1017–1028 [DOI] [PubMed] [Google Scholar]
- Dupont J, McNeilly J, Vaiman A, Canepa S, Combarnous Y, Taragnat C 2003 Activin signaling pathways in ovine pituitary and LβT2 gonadotrope cells. Biol Reprod 68:1877–1887 [DOI] [PubMed] [Google Scholar]
- Safwat N, Ninomiya-Tsuji J, Gore AJ, Miller WL 2005 Transforming growth factor-β activated kinase1 (TAK1) is a key mediator of ovine follicle stimulating hormone β-subunit expression. Endocrinology 146:4814–4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daane TA, Parlow AF 1971 Periovulatory patterns of rat serum follicle stimulating hormone and luteinizing hormone during the normal estrous cycle as revealed by radioimmunoassays: effects of pentobarbital. Endocrinology 88:653–663 [DOI] [PubMed] [Google Scholar]
- Gay VL, Midgley Jr AR, Niswender GD 1970 Patterns of gonadotrophin secretion associated with ovulation. Fed Proc 29:1880–1887 [PubMed] [Google Scholar]
- Ortolano GA, Haisenleder DJ, Dalkin AC, Iliff-Sizemore SA, Landefeld TD, Maurer RA, Marshall JC 1988 Follicle-stimulating hormone β-subunit messenger ribonucleic acid concentrations during the rat estrous cycle. Endocrinology 123:2946–2948 [DOI] [PubMed] [Google Scholar]
- Zmeili SM, Papavasiliou SS, Thorner MO, Evans WS, Marshall JC, Landefeld TD 1986 Alpha and luteinizing hormone beta subunit messenger ribonucleic acids during the rat estrous cycle. Endocrinology 119:1867–1869 [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Besecke LM, Groome N, Draper LB, Schwartz NB, Weiss J 1996 Inhibin A and inhibin B are inversely correlated to follicle-stimulating hormone, yet are discordant during the follicular phase of the rat estrous cycle, and inhibin A is expressed in a sexually dimorphic manner. Endocrinology 137:5463–5467 [DOI] [PubMed] [Google Scholar]
- Besecke LM, Guendner MJ, Sluss PA, Polak AG, Woodruff TK, Jameson JL, Bauer-Dantoin AC, Weiss J 1997 Pituitary follistatin regulates activin-mediated production of follicle-stimulating hormone during the rat estrous cycle. Endocrinology 138:2841–2848 [DOI] [PubMed] [Google Scholar]