Abstract
Many xenobiotics have been associated with endocrine effects in a wide range of biological systems. These associations are usually between small nonsteroid molecules and steroid receptor signaling systems. In this report, triclocarban (TCC; 3,4,4′-trichlorocarbanilide), a common ingredient in personal care products that is used as an antimicrobial agent was evaluated and found to represent a new category of endocrine-disrupting substance. A cell-based androgen receptor-mediated bioassay was used to demonstrate that TCC and other urea compounds with a similar structure, which have little or no endocrine activity when tested alone, act to enhance testosterone (T)-induced androgen receptor-mediated transcriptional activity in vitro. This amplification effect of TCC was also apparent in vivo when 0.25% TCC was added to the diet of castrated male rats that were supported by exogenous testosterone treatment for 10 d. All male sex accessory organs increased significantly in size after the T+TCC treatment, compared with T or TCC treatments alone. The data presented here suggest that the bioactivity of endogenous hormones may be amplified by exposure to commercial personal care products containing sufficient levels of TCC.
AN INCREASING NUMBER of experimental and epidemiological studies demonstrate that a variety of exogenous compounds, designated as endocrine-disrupting substances (EDS), have the ability to alter the signaling and function of endogenous hormones (1,2,3). Reports have revealed associations between EDS exposures and reduced fecundity and fertility (1,4), abnormal fetal development (5), timing of the onset of puberty (6,7), altered steroid hormone biosynthesis (8), disruption of ovarian function (9,10), abnormal lactation (11), early onset of reproductive senescence (1), and cancer (12,13,14). Whereas it remains a topic of debate, it is often speculated that exposures to persistent contaminants and lifestyle choices are key determinants in the pathogenesis of certain specific forms of developmental defects and reproductive failure with EDS being a principal contributor (3,15).
The increase of EDS exposures in daily life has raised public concern relating to their potential ecological and human health impacts. Efforts to identify and characterize EDS have revealed that a relatively large number of them have estrogenic, antiestrogenic, or antiandrogenic activity (16). In contrast, comparatively few EDS have been associated with androgenic activity despite increasing public concern regarding the influence of environmental factors on male reproductive health (17,18). Triclocarban (TCC; 3,4,4′-trichlorocarbanilide), an antimicrobial compound, is commonly added to a wide range of household and personal care products including bar soaps, detergents, body washes, cleansing lotions, and wipes for its sanitizing properties (19). TCC-containing products have been marketed broadly for more than 45 yr and thus have a long history of use in Europe and the United States. It is estimated that approximately 1 million pounds of TCC are produced for the U.S. market per year, and recent reports suggest widespread TCC contamination of U.S. water resources (19). Available research data on TCC’s potential impact on reproductive health, however, are scarce and outdated, having been collected approximately 20 yr ago at a time when little attention was devoted to the influence of EDS in disturbances of hormonal homeostasis (20). In this report, we show that TCC does not compete with the endogenous hormone for receptor binding but amplifies the androgen receptor-mediated, native androgen-induced transcriptional activity in vitro and in vivo. Thus, TCC should be considered as a new type of EDS (21).
Materials and Methods
Chemicals and cell culture reagents
TCC (reported purity of 99.3%), carbanilide (reported purity of 99.9%), flutamide (nonsteroid antiandrogen), and testosterone propionate (TP) were purchased from Sigma-Aldrich (St. Louis, MO). Other TCC analogs (purity > 99%) were synthesized in the laboratory of Dr. Bruce D. Hammock by the condensation of the appropriate isocyanate and amine (22,23). 17β-Hydroxy-4-androsten-3-one [testosterone (T)] and 5α-androstan-17β-ol-3-one (dihydrotestosterone) were purchased from Steraloids (Newport, RI). MDA-kb2, a cell line expressing endogenous androgen receptor (AR) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from American Type Culture Collection (Manassas, VA). The T and dihydrotestosterone were dissolved in absolute ethyl alcohol, and all other compounds were dissolved in dimethylsulfoxide. Phenol-red-free DMEM, L-15 (Leibovitz) medium, fetal bovine serum (FBS), l-glutamine, penicillin/streptomycin sulfate, blasticidin, and geneticin sulfate (G418) were obtained from Invitrogen (Carlsbad, CA). Dextran-coated charcoal-treated FBS was purchased from Hyclone (Logan, UT). Cell lysis buffer was purchased from Promega (Madison, WI). AR (441) mouse monoclonal IgG raised against human AR amino acids 299–315 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Human chorionic gonadotropin (CG) standard (CR 121) was provided by Dr. John O’Connor (Columbia University, NY) and diluted in DMEM.
Cell-based human AR-mediated bioassay
A full detailed description of the development and application of the cell-based human AR-mediated bioassay has been published by Chen et al. (24). Briefly, the bioassay system uses human embryonic kidney 293 cells that lack critical steroid metabolizing enzymes. The cells are stably transfected with PCDNA6-human AR and an MMTV-Luc.neo plasmid containing a luciferase reporting gene (24). The cells (designated as 2933Y) are highly responsive to endogenous steroids as well as synthetic compounds. The signal induction is stable for more than 60 passages under double antibiotic selection conditions (24).
The details of the in vitro procedures to evaluate the androgenic/antiandrogenic activity of the EDS as well as the concentration selection for T in the AR-mediated cell system have been previously described (25). The lower limit of detection of this assay is 15 pm T in cell culture medium (blank + 3 sd) with intra- and interassay coefficients of variation of 7.4 and 7.5% at 0.25 nm T and 4.9 and 6.4% at 0.03 nm T, respectively (24).
AR competitor assay
The competition of TCC with endogenous hormone for AR binding was evaluated using the PolarScreen AR fluorescence polarization (FP) assay with a Beacon 2000 fluorescence polarization system according to the manufacturer’s instructions (Invitrogen; catalog no. P3018).
Western blot analysis
The expression of AR protein in MDA-kb2 and 2933Y cells was analyzed by Western blot. Briefly, after treatment with T (1.0 nm), TCC (1.0 μm), or T and TCC in combination for 48 h, the cells were lysed, and whole-cell lysates were prepared and subjected to 7.5% SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membrane was then blocked in 20 mm Tris-HCl, 137 mm NaCl, and 0.1% (vol/vol) Tween 20 (pH 7.4) containing 5% nonfat milk. The membrane was immunoblotted with AR (441) mouse antihuman monoclonal antibody overnight, followed by secondary antibody (donkey antimouse antibody) coupled to horseradish peroxidase from Amersham Biosciences (Piscataway, NJ) for 1 h. The membrane was exposed on x-ray film (Eastman Kodak Co., Rochester, NY) using enhanced chemiluminescence Western blot detection reagents (Amersham Biosciences). To reprobe with β-actin, the membrane was stripped in stripping buffer at 53 C for 30 min.
cAMP/protein kinase A (PKA)-mediated luciferase transcriptional activity
Luciferase transcriptional activity mediated by the cAMP/PKA pathway was measured by the in vitro bioassay described by Jia et al. (26) and modified as described below. This assay uses human embryonic kidney 293 cells stably transfected with the human luteinizing/chorionic gonadotropin receptor gene and the luciferase reporter gene (pCRE-luc) (JK293) (27). JK293 cells were cultured in DMEM containing 10% FBS, 2 mm l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, and 100 μg/ml geneticin sulfate. After the JK293 cells were cultured to 80–100% confluence in 100- × 20-mm cell culture dishes, cells were counted and incubated in 96-well plates. Each well contained 105 cells in 100 μl DMEM. Then, 50 μl human CG standard CR 121 at a concentration of 3.2 ng/ml and/or test compounds at designated concentrations were added to each well containing 150 μl dextran-coated charcoal-treated FBS containing phenol red-free DMEM. Cells were further incubated for 16 h. The media were then removed and 100 μl of cell lysis buffer was added to each well and allowed to incubate for 30 min. Cell lysates (60 μl) were transferred to 96-well Microfluor II plates (Fisher Scientific, Santa Clara, CA). Luciferin substrate was then injected into each well, and the luciferase activity induced by test compounds was measured by a Veritas Luminometer (Turner Biosystems, Sunnyvale, CA) (25).
To compensate for any organic solvent effects, the final content of ethyl alcohol in both the AR-mediated and cAMP/PKA-mediated assay systems was 0.1% (vol/vol) for all studies, and the total DMSO concentration in the final culture media was no more than 0.2% (vol/vol). The total concentration of organic solvent (vol/vol) was maintained at the same level for both controls and test compounds.
MTT assay
The MTT assay for cell proliferation or cytotoxicity testing under varying concentrations of test compounds was performed according to the manufacturer’s instructions (American Type Culture Collection; catalog no. 30-1010K) and has been described previously (25).
In vivo effect of TCC on accessory sex organ weight
Forty-eight male Sprague Dawley rats 48–52 d old (castrated at 42–46 d old) were randomly assigned to four treatment groups with 12 rats in each group. All animals were maintained on their respective treatment regimen for 10 d. Animals in group 1 served as controls and received sham treatments of sesame oil (no androgen support) and normal diet (no TCC supplement). Animals in group 2 were treated with TP injection (0.2 mg/kg, sc in sesame oil) and received a normal diet. Animals in group 3 received vehicle control injections (no androgen support) and TCC-supplemented diet [0.25% TCC (wt/wt) mixed in rat chow] for the 10-d treatment period. Group 4 animals received TP injection (0.2 mg/kg, sc in sesame oil) and TCC-supplemented diet [0.25% TCC (wt/wt) mixed in rat chow]. At the end of treatment, the animals were euthanized by carbon dioxide asphyxiation, and the liver, kidney, levator anibulbocavernosus muscle (LABC), glans penis, ventral prostate, seminal vesicles, and Cowper’s gland were surgically removed and weighed. All experiments were conducted in accordance with the regulations of the Animal Care and Use Committee of Yale University in facilities fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Statistical analysis
For in vitro studies, the values shown are mean ± sd from three independent experiments for each dose tested, and for the in vivo study, the values shown are mean ± sd of each group. For both in vitro and in vivo data, one-way ANOVA was applied followed by multiple comparisons test when appropriate, using SigmaStat (Systat Software, San Jose, CA). The level of significance was set at P < 0.05. For the in vitro study, treatments were compared with the negative control group containing vehicle only to test for agonist properties, and for androgen antagonist properties, treatments were compared with the T-positive control group.
Results
Effect of TCC on cell proliferation and cytotoxicity
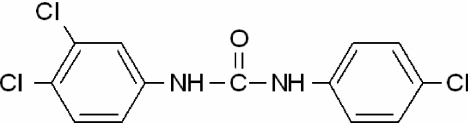
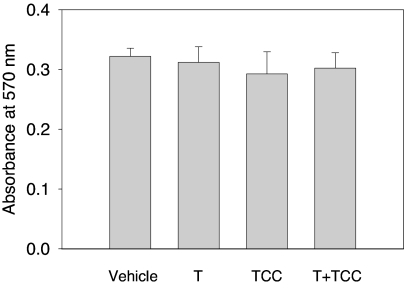
The structure of TCC is shown in Fig. 1. It is a polychlorinated diphenyl urea. Concentrations of TCC up to 1.0 μm did not result in cytotoxicity in 2933Y cells when tested alone or in combination with 0.125 nm of T (Fig. 2). Vehicle-treated or TCC-treated cells did not demonstrate statistically significant differences with respect to proliferation at the concentrations used in this study.
Figure 1.
Structure of TCC.
Figure 2.
The effect of TCC on cell proliferation. 2933Y cells were treated for 16 h with TCC (1.0 μm) alone or in combination with T (0.125 nm). The cytotoxicity or cell proliferation was evaluated by the MTT assay. The absorbance was measured at 570 nm with a reference wavelength of 650 nm using an EMax spectrophotometer (Molecular Devices, Sunnyvale, CA). Absorbance (OD) at 570 nm is expressed as mean ± sd (n = 6). No significant difference in cell proliferation was observed in cells with TCC alone or in combination with T when compared with the vehicle control.
Effect of TCC on AR-mediated transcriptional activity
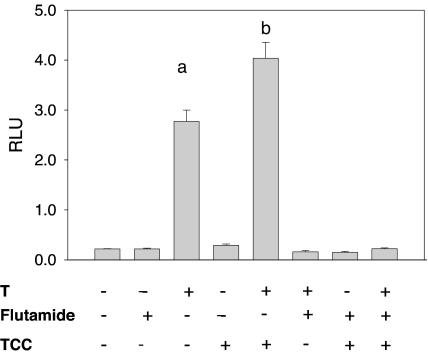
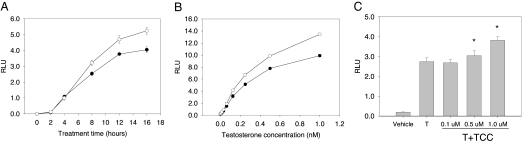
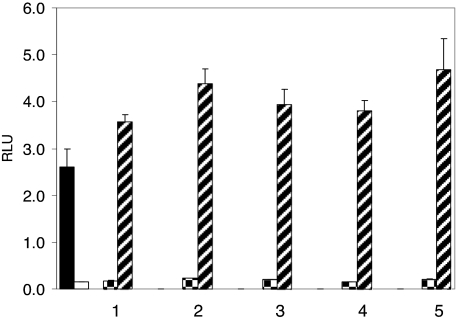
At a concentration of 1.0 μm, TCC revealed little or no androgenicity when tested alone. In contrast, in the presence of a native androgen, such as T (0.125 nm), a 45% increase of the T-induced signal was observed (P < 0.05, Fig. 3) and this amplification of the T-induced transcriptional activity by TCC was both time dependent (Fig. 4A) and dose dependent (Fig. 4, B and C). This amplification of the T-induced signal transcriptional activity was also detected in other urea compounds structurally similar to TCC (Fig. 5). To further assess the mechanism by which TCC mediates the enhancement of the T signal, flutamide was used. This known antiandrogen functions as a competitive inhibitor for androgen binding to the AR (28), and at 10 μm, flutamide dramatically suppressed the amplification effect of 1.0 μm TCC (P < 0.05, Fig. 3).
Figure 3.
The effect of TCC on AR-mediated transcriptional activity induced by T. 2933Y cells were treated for 16 h with and without TCC (1.0 μm) and in combination with T (0.125 nm) and/or flutamide (10 μm). Cell lysates were assessed for luciferase activity, which is expressed as mean ± sd (n = 4) of relative light units (RLU). a, Significantly different from vehicle control; b, significantly different from vehicle control and T treatment.
Figure 4.
A, The time course effect of TCC on AR-mediated transcriptional activity induced by T. 2933Y cells were treated with T (0.125 nm) alone (•) or with a combination of TCC (1.0 μm) (○) for different time periods. B, The dose-response effect of TCC on AR-mediated transcriptional activity induced by T. 2933Y cells were treated with T (0–1.0 nm) alone (•) or with a combination of TCC (1.0 μm) (○) for 16 h. C, The dose-response effect of TCC on AR-mediated transcriptional activity induced by T. 2933Y cells were treated with T (0.125 nm) alone or with a combination of various concentrations of TCC for 16 h. In each experiment, cell lysates were measured for luciferase activity, which is expressed as mean ± sd (n = 3) of relative light units (RLU). *, Significantly different from T treatment.
Figure 5.
The augmentation of TCC analogs on AR-mediated transcriptional activity. Closed bar, T at 0.125 nm alone; open bar, vehicle control; checkered bar, TCC analogs at 1.0 μm alone; hatched bar, TCC analogs at 1.0 μm in the presence of 0.125 nm of T. 1, Carbanilide; 2, 4,4′-dichlorocarbanilide; 3, TCC; 4, 3,3′,4,4′-tetrachlorocarbanilide; 5, 4′-methoxy-3,4-dichlorocarbanilide.
Competitive binding of TCC for AR
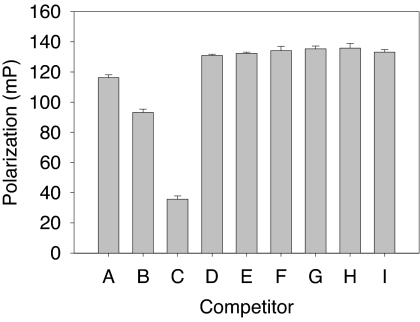
To investigate the potential of TCC to mimic the native hormone by binding to the AR, a competitive binding assay was conducted. As shown in Fig. 6, TCC did not compete for T binding to the AR at tested concentrations up to 200 μm. In contrast, the polarization value was reduced by 20 and 70% at DHT concentrations of 10 and 100 nm, respectively.
Figure 6.
Competitive binding of TCC in AR FP assay. Rat AR ligand binding domain/fluormone complex was incubated with TCC at various concentrations. A, Maximum FP in the absence of any competitor. B and C, FP values in the presence of DHT, a strong AR competitor (B: 10 nm and C: 100 nm). D-I, FP values in the presence of increasing concentrations of TCC (D: 2 nm; E: 20 nm; F: 200 nm; G: 2 μm; H: 20 μm; I: 200 μm). Data presented as mean ± sd of triplicates.
The effect of TCC treatment on AR protein
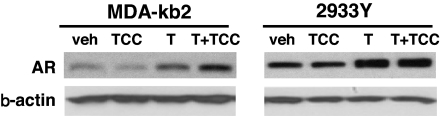
We investigated whether TCC increases the expression of the AR protein in cells that express endogenous AR. Western blot analysis indicated that, compared with vehicle control, an increase of immunoreactive AR protein was detected in MDA-kb2 cells treated with T or T+TCC with the latter treatment yielding more AR protein (Fig. 7). Similarly, T or T+TCC combination treatment increased immunoreactive AR expression in 293 cells, compared with vehicle control; however, unlike the MDA-kb2 cells, there was no difference between the amount of protein observed in the T+TCC combination treatment and the T-only treatment.
Figure 7.
Effect of TCC on the amount of immunoreactive AR protein. MDA-kb2 or 2933Y cells were treated with vehicle, T (1.0 nm), TCC (1.0 μm), or a combination of T+TCC for 48 h. Whole-cell lysates were probed by Western blot analysis with antibody against amino acids 299–315 of human AR. Each lane contained either 60 μg (for MDA-kb2) or 15 μg (for 2933Y) of protein. Veh, Vehicle control.
Effect of TCC on cAMP/PKA-mediated transcriptional activity
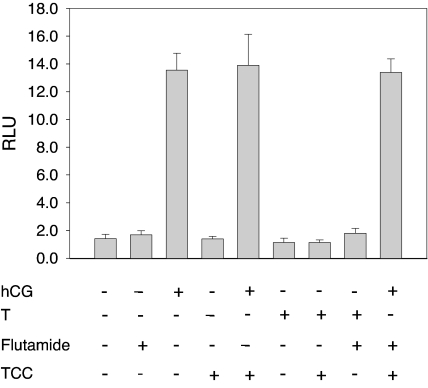
Numerous studies have indicated that AR-mediated signaling is affected by an array of cytokines and growth factors that act through a web of complex signaling cascades (29). Of these, cAMP/PKA are particularly interesting because of the ability to phosphorylate AR in vivo and stimulate the expression of the AR-regulated gene expression (29,30,31). The concept of cAMP as an intracellular second messenger to a wide range of hormones, neurotransmitters, and other signaling substances is well developed (32). The target for cAMP has been identified as cAMP-dependent protein kinase (PKA). In the absence of cAMP, PKA is an enzymatically inactive tetrameric holoenzyme (33). To investigate TCC’s potential to activate cAMP/PKA signaling, the ability of TCC to stimulate the transactivation of luciferase controlled by the cAMP/PKA pathway in a cAMP/PKA-mediated assay system was studied (27,34). As shown in Fig. 8, TCC alone did not activate cAMP/PKA-mediated luciferase activity beyond control levels nor did it enhance the signal transduction induced by the presence of human CG (hCG), which is a strong stimulus for cAMP production in this system.
Figure 8.
The effect of TCC on cAMP/PKA-mediated transcriptional activity induced by hCG. JK293 cells were treated for 16 h with and without TCC (1.0 μm) and in combination with hCG (3.2 ng/ml), T (0.125 nm), and/or flutamide (10 μm). Cell lysates were measured for luciferase activity, which is expressed as mean ± sd (n = 4) of relative light units (RLU). Neither T nor flutamide induced any effect on cAMP/PKA-mediated transcriptional activity. No significant differences in luciferase activities were observed in TCC and hCG combinations when compared with hCG treatment alone.
The effect of TCC treatment on organ weight after 10 d of treatment in castrated rats
To investigate TCC’s potential amplification of native androgen ligands in vivo, we evaluated the effects of TCC in castrated male sd rats aged 48–52 d (castrated at age 42–46 d). This model has been well established and widely used to study the androgenic/antiandrogenic effects of AR ligands, EDS, and/or AR modulators on accessory sex tissues (35). In this model, the change in the weight of accessory sex organs after various treatments is used to indicate the amount of androgenic support. TP (0.2 mg/kg) was used as the positive control due to its superior pharmacokinetic properties and enhanced efficacy both in humans and animal models (35). A suboptimal dose of 0.2 mg/kg TP was selected for use to ensure the ability to observe an amplification effect of TCC. No statistically significant differences were observed for total body or kidney weights between any groups; however, there was a slight increase in the mean liver weight in the group of animals treated with TCC alone (Table 1). No significant differences were observed for the weights of the seminal vesicles, Cowper’s gland, LABC muscle, and glans penis between sham-treated rats and rats receiving TCC only in the diet; however, an increase in ventral prostate weight was observed in rats treated with TCC only, compared with sham-treated rats. In contrast and as hypothesized, TP treatment alone significantly increased the weights of accessory sex organs, compared with controls and TCC alone (Table 1). The cotreatment of TP with TCC revealed a substantial and significant increase in the weights of all accessory sex organs, compared with TP treatment alone, indicating a synergism between TP and TCC in vivo (Table 1).
Table 1.
The in vivo effect of TCC treatment on organ weight (mean ± sd) of male Sprague Dawley rats
| Vehicle control | TCC | T | T+TCC | |
|---|---|---|---|---|
| Body weight (g) | 217.75 ± 8.62 | 222.75 ± 11.07 | 217.08 ± 6.42 | 217.00 ± 8.15 |
| Kidney (g) | 2.07 ± 0.26 | 1.98 ± 0.26 | 1.96 ± 0.27 | 1.91 ± 0.21 |
| Liver (g) | 10.73 ± 0.94 | 12.53 ± 1.21a | 11.4 ± 1.29 | 11.68 ± 1.20 |
| Seminal vesicles (mg) | 105.42 ± 28.18 | 132.17 ± 39.7 | 323.08 ± 69.97a | 576.5 ± 73.41b |
| Ventral prostate (mg) | 58.5 ± 18.92 | 85.83 ± 22.25a | 136.67 ± 8.49a | 228.00 ± 23.54b |
| Glans penis (mg) | 72.25 ± 12.46 | 78.08 ± 8.53 | 83.75 ± 8.53 | 113.3 ± 14.13b |
| Cowper’s gland (mg) | 15.08 ± 3.23 | 18.08 ± 2.47 | 22.00 ± 3.77a | 36.33 ± 4.46b |
| LABC muscle (mg) | 129.25 ± 5.99 | 133.92 ± 7.35 | 323.92 ± 7.28a | 366.92 ± 12.23b |
Values are mean ± sd of 12 rats in each group.
P < 0.05, compared with vehicle control.
P < 0.05, compared with vehicle control and T treatment.
Discussion
Recent reports relating to several nonsteroidal compounds indicate that a number of compounds have the ability to modulate, activate, and/or bind to the human AR (36). These compounds are of particular public concern because human exposures to many of these compounds are ubiquitous and can accumulate in the environment, and human exposures to some of them are possibly constant (36,37). We therefore investigated the EDS properties of a subset of these compounds by using a cell-based AR-mediated reporting system (24) to determine whether any of these compounds are able to interfere with the natural action of endogenous androgens. In that investigation, most EDS were identified as weak antagonists (25), but a small group of polychlorinated biphenyls were found to have enhancing properties and seemed worthy of further investigation.
TCC is an antimicrobial agent commonly added to personal care products. The present data indicate that TCC has little or no androgenic activity alone but has an amplification effect on strong native androgens such as T. This amplification effect is characterized by an increased transcriptional activity transduced through the AR as the cotreatment of flutamide significantly suppressed the signal in vitro (Fig. 3). It has been reported that 0.39% of an average 138 mg of triclocarban (or 0.54 mg) applied to the entire body was absorbed after a typical whole-body shower lather (38). Therefore, the actual systemic dose of TCC would be approximately 0.1 mg/liter (or 0.1 μg/ml) for an adult of 60 kg with 5 liters of blood. The concentration of TCC used in the in vitro study was 1.0 μm, which is equal to approximately 0.3 μg/ml. Thus, this in vitro dose represents only a 3-fold increase above that of a typical human exposure after a whole-body shower. Existing evidence also indicates that percutaneous penetration of similar compounds varies with the anatomic site of application. With chlorinated hydrocarbon pesticides, for example, the forearm allowed relatively less penetration, whereas the abdomen, scalp, and postauricular area and the scrotum allowed almost total absorption (39).
One of the aims of the present study was to determine whether the in vitro endocrine disrupting effects of TCC could be supported in vivo. The use of 0.25% (wt/wt) TCC in the study was based on reports of extended TCC exposure in the rat (20). Whereas many EDS seem to be less potent than the natural ligands in both in vitro and in vivo assays, comparable effects were observed when these compounds were administered at critical time points at doses that were several orders of magnitude lower (40). Available data have demonstrated that TCC exposures by dermal or oral routes in rats and humans lead to similar metabolic profiles and that the administration of TCC in the diet is considered an appropriate way of assessing the toxicity of TCC (41).
It is particularly noteworthy that in vivo, TCC in combination with TP resulted in a significant increase in accessory sex organ weights, compared with TP treatment alone, using the castrated male Sprague Dawley rat animal model (Table 1). Our data strongly suggest that TCC has a positive AR modulatory effect in tissues or cells that are androgen targets. These observations open the possibility that other nuclear receptor signal transduction systems could also be modulated by TCC in a similar fashion. This possibility was confirmed in vitro by demonstrating that TCC also potentiated the estrogen receptor-α-mediated signal transcriptional activity induced by estradiol as well as amplifying the cortisol-induced signal transduction in cells with endogenous expression of the glucocorticoid receptor (data not shown).
It should also be noted that the seminal vesicle, ventral prostate, and Cowper’s gland weights increased additively with the TCC and T combination treatment. In contrast, only a marginal increase in the LABC muscle weight was observed with the combination treatment (Table 1). Further investigation will be required to determine whether TCC acts to preferentially enhance T’s ability to increase reproductive organ weight over that of muscle mass.
The concentration of 1.0 μm TCC tested in vitro was orders of magnitude in excess of the T concentration used. It is clear therefore that the relative binding efficiencies, if any, of TCC for the AR are orders of magnitude below that of the natural ligands. This conclusion is supported by the results of the AR competition assay in which TCC did not compete for T binding to the AR at concentrations up to 200 μm (Fig. 6). These results also support the concept that TCC is not a typical hormone mimic because it shows minimal receptor activation in the absence of cognate ligand (Fig. 3).
Nuclear receptor-mediated signaling is affected by an array of cytokines and growth factors that act through a web of complex signaling cascades (32). We found TCC alone did not activate cAMP/PKA-mediated luciferase activity nor did it enhance the signal transduction induced by hCG. These data indicate that the cAMP/PKA pathway may not be involved in the amplification of the T-induced transcriptional activity by TCC.
Recent evidence points to the potential role of MAPK pathways in the nuclear receptor mediated signal augmentation for certain EDS (21). The prolonged half-life of the nuclear receptor, recruitment of novel coactivators, and involvement of a secondary binding domain in the nuclear receptor may also contribute to the signal potentiation phenomenon of TCC (42,43,44,45,46).
The synergistic increase of immunoreactive AR protein with T+TCC treatment in MDA-kb2 cells, which express endogenous AR (Fig. 7), could be the result of T and TCC on AR transcription and/or AR protein stability (43). Whereas the synergistic effect of TCC+T treatment on luciferase activity was observed in 2933Y cells, no synergistic effect on the amount of immunoreactive AR was detected. This lack of a pronounced increase of AR in the T+TCC combination treatment in 2933Y cells could be due to the inherent differences between the exogenous AR in 2933Y cells and endogenous AR in MDA-kb2 cells (47). Because much of the AR expression regulation is believed to occur at the posttranscriptional level, in which untranslated regions play a central role, the lack of both 5′- and 3′-untranslated regions in the exogenous AR transcripts in the 2933Y cells could result in different patterns of posttranscriptional gene regulation (47). In addition, the synergistic effects of T+TCC on AR-mediated transcriptional activity in 2933Y cells could arise from the altered DNA-binding activity of the receptor (48,49). Clearly, comprehensive investigations are required to identify the potential mechanisms of sex steroid amplification by TCC.
This report identifies a new category of EDS for androgens and other steroid hormones. The data presented here indicate that TCC and its urea analogs should be categorized as steroid hormone amplifiers or enhancers rather than simple agonists or antagonists because these compounds demonstrate the novel EDS property of synergism with the native androgen hormone receptor ligand (21). To our knowledge, this is the first report regarding the synergistic effect of TCC on native sex hormones in vitro and in vivo. Given the scarcity of toxicological data in humans and laboratory animal models with respect to TCC and related compounds, the properties exhibited here by TCC may have more significance than for previously identified EDS. In terms of modulating steroid hormone action, TCC and its analogs elicit a positive biological effect rather than an inhibitory or weakly agonistic effect and have the potential to act through multiple nuclear receptors. This effect would be more likely to induce hyperstimulation rather than the attenuation of normal stimulation. Furthermore, the amplification effect of TCC on endogenous sex steroids may have an array of widespread subtle physiological alterations in both males and females. For example, because the amplification of androgens by TCC occurs at the target cell, there is the likelihood that such exposures may be associated with idiopathic hyperandrogenism. Thus, despite seemingly normal native circulating androgen levels, virilization may occur.
TCC exposure may also result in defects in development (i.e. cryptorchidism, hypospadias) or decreased reproductive function (decrease in sperm quality) in adults because compensation through the long-loop feedback would occur with the effect of lowering gonadotropin drive in response to TCC exposure. In females, increased androgenic feedback could disrupt the normal female-specific positive feedback loop associated with ovulation and derange ovarian function. The exposure to these EDS may also change the balance between estrogen signaling and androgen signaling in breast homeostasis. Depending on the level that hormone signaling pathways are disrupted (10,50), in utero exposure to TCC could also impair neurogenesis and sexually dimorphic neurobehavioral development. Because TCC has the potential to amplify synthetic steroidal compounds, further investigation of the interaction of TCC with oral contraceptives, hormone replacement therapy, synthetic androgens, and glucocorticoid therapy is also warranted.
Acknowledgments
We thank Dr. Dan Chang (University of California, Davis) for critical review of the manuscript, Dr. C. S. Chang (University of Rochester) for providing the PSG5 AR plasmid, and Dr. V. S. Wilson (USEPA) for providing the MMTV-Luc.neo plasmid.
Footnotes
This work was supported by the National Institute of Environmental Health Sciences (NIEHS) Superfund Basic Research Program Grant P42ES04699, National Institutes of Health Grant P51 RR000169, and NIEHS Grants P01ES06198, R37ES02710, and P30ES005707.
Disclosure Statement: J.C., K.C.A., N.A.G., S.J.G., B.D.H., and B.L.L. have filed a patent for the University of California for TCC as a sex steroid amplifier. B.D.H. has equity interests in Arête Therapeutics. L.Z., M.I.A., and A.J.D. have no competing disclosures to declare.
First Published Online November 29, 2007
Abbreviations: AR, Androgen receptor; CG, chorionic gonadotropin; EDS, endocrine-disrupting substances; FBS, fetal bovine serum; FP, fluorescence polarization; hCG, human CG; LABC, levator anibulbocavernosus muscle; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PKA, protein kinase A; T, testosterone; TCC, triclocarban; TP, testosterone propionate.
References
- Buck Louis GM, Lynch CD, Cooney MA 2006 Environmental influences on female fecundity and fertility. Semin Reprod Med 24:147–155 [DOI] [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, Snyder RJ, Phillips TM, Jefferson WN 2007 Developmental exposure to endocrine disruptors and the obesity epidemic. Reprod Toxicol 23:290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM, Irvine DS 2004 How strong is the evidence of a link between environmental chemicals and adverse effects on human reproductive health? BMJ 328:447–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusani L, Della Seta D, Dessi-Fulgheri F, Farabollini F 2007 Altered reproductive success in rat pairs after environmental-like exposure to xenoestrogen. Proc Biol Sci 274:1631–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokanson R, Hanneman W, Hennessey M, Donnelly KC, McDonald T, Chowdhary R, Busbee DL 2006 DEHP, bis(2)-ethylhexyl phthalate, alters gene expression in human cells: possible correlation with initiation of fetal developmental abnormalities. Hum Exp Toxicol 25:687–695 [DOI] [PubMed] [Google Scholar]
- Blanck HM, Marcus M, Tolbert PE, Rubin C, Henderson AK, Hertzberg VS, Zhang RH, Cameron L 2000 Age at menarche and tanner stage in girls exposed in utero and postnatally to polybrominated biphenyl. Epidemiology 11:641–647 [DOI] [PubMed] [Google Scholar]
- Nebesio TD, Eugster EA 2007 Current concepts in normal and abnormal puberty. Curr Probl Pediatr Adolesc Health Care 37:50–72 [DOI] [PubMed] [Google Scholar]
- Sanderson JT 2006 The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals. Toxicol Sci 94:3–21 [DOI] [PubMed] [Google Scholar]
- Bretveld RW, Thomas CM, Scheepers PT, Zielhuis GA, Roeleveld N 2006 Pesticide exposure: the hormonal function of the female reproductive system disrupted? Reprod Biol Endocrinol 4:30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savabieasfahani M, Kannan K, Astapova O, Evans NP, Padmanabhan V 2006 Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology 147:5956–5966 [DOI] [PubMed] [Google Scholar]
- McLachlan JA, Simpson E, Martin M 2006 Endocrine disrupters and female reproductive health. Best Pract Res 20:63–75 [DOI] [PubMed] [Google Scholar]
- Fenton SE 2006 Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology 147:S18–S24 [DOI] [PubMed] [Google Scholar]
- Maffini MV, Rubin BS, Sonnenschein C, Soto AM 2006 Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol 254–255:179–186 [DOI] [PubMed] [Google Scholar]
- Rayner JL, Enoch RR, Fenton SE 2005 Adverse effects of prenatal exposure to atrazine during a critical period of mammary gland growth. Toxicol Sci 87:255–266 [DOI] [PubMed] [Google Scholar]
- Lipworth L 1995 Epidemiology of breast cancer. Eur J Cancer Prev 4:7–30 [DOI] [PubMed] [Google Scholar]
- Guillette Jr LJ 2006 Endocrine disrupting contaminants—beyond the dogma. Environ Health Perspect 114(Suppl 1):9–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallinga JW, Moonen EJ, Dumoulin JC, Evers JL, Geraedts JP, Kleinjans JC 2002 Decreased human semen quality and organochlorine compounds in blood. Hum Reprod 17:1973–1979 [DOI] [PubMed] [Google Scholar]
- Korner W, Vinggaard AM, Terouanne B, Ma R, Wieloch C, Schlumpf M, Sultan C, Soto AM 2004 Interlaboratory comparison of four in vitro assays for assessing androgenic and antiandrogenic activity of environmental chemicals. Environ Health Perspect 112:695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota A, Heidler J, Halden RU 2007 Detection of triclocarban and two cocontaminating chlorocarbanilides in U.S. aquatic environments using isotope dilution liquid chromatography tandem mass spectrometry. Environ Res 103:21–29 [DOI] [PubMed] [Google Scholar]
- Nolen GA, Dierckman TA 1979 Reproduction and teratogenic studies of a 2:1 mixture of 3,4,4′-trichlorocarbanilide and 3-trifluoromethyl-4,4′-dichlorocarbanilide in rats and rabbits. Toxicol Appl Pharmacol 51:417–425 [DOI] [PubMed] [Google Scholar]
- Jansen MS, Nagel SC, Miranda PJ, Lobenhofer EK, Afshari CA, McDonnell DP 2004 Short-chain fatty acids enhance nuclear receptor activity through mitogen-activated protein kinase activation and histone deacetylase inhibition. Proc Natl Acad Sci USA 101:7199–7204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C, Goodrow MH, Dowdy D, Zheng J, Greene JF, Sanborn JR, Hammock BD 1999 Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc Natl Acad Sci USA 96:8849–8854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JW, Denton DL, Morisseau C, Koger CS, Wheelock CE, Hinton DE, Hammock BD 2001 Evaluation of fish models of soluble epoxide hydrolase inhibition. Environ Health Perspect 109:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sowers MR, Moran FM, McConnell DS, Gee NA, Greendale GA, Whitehead C, Kasim-Karakas SE, Lasley BL 2006 Circulating bioactive androgens in midlife women. J Clin Endocrinol Metab 91:4387–4394 [DOI] [PubMed] [Google Scholar]
- Chen J, Ahn KC, Gee NA, Gee SJ, Hammock BD, Lasley BL 2007 Antiandrogenic properties of parabens and other phenolic containing small molecules in personal care products. Toxicol Appl Pharmacol 221:278–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia XC, Oikawa M, Bo M, Tanaka T, Ny T, Boime I, Hsueh AJ 1991 Expression of human luteinizing hormone (LH) receptor: interaction with LH and chorionic gonadotropin from human but not equine, rat, and ovine species. Mol Endocrinol 5:759–768 [DOI] [PubMed] [Google Scholar]
- Chen J, Thirkill TL, Overstreet JW, Lasley BL, Douglas GC 2003 Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on chorionic gonadotropin secretion by human trophoblasts. Reprod Toxicol 17:87–93 [DOI] [PubMed] [Google Scholar]
- Yin D, He Y, Perera MA, Hong SS, Marhefka C, Stourman N, Kirkovsky L, Miller DD, Dalton JT 2003 Key structural features of nonsteroidal ligands for binding and activation of the androgen receptor. Mol Pharmacol 63:211–223 [DOI] [PubMed] [Google Scholar]
- Kim J, Jia L, Stallcup MR, Coetzee GA 2005 The role of protein kinase A pathway and cAMP responsive element-binding protein in androgen receptor-mediated transcription at the prostate-specific antigen locus. J Mol Endocrinol 34:107–118 [DOI] [PubMed] [Google Scholar]
- Gioeli D, Ficarro SB, Kwiek JJ, Aaronson D, Hancock M, Catling AD, White FM, Christian RE, Settlage RE, Shabanowitz J, Hunt DF, Weber MJ 2002 Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J Biol Chem 277:29304–29314 [DOI] [PubMed] [Google Scholar]
- Sadar MD, Hussain M, Bruchovsky N 1999 Prostate cancer: molecular biology of early progression to androgen independence. Endocr Relat Cancer 6:487–502 [DOI] [PubMed] [Google Scholar]
- Robinson GA, Sutherland EW 1971 On the relation of cyclic AMP to adrenergic receptors and sympathin. Adv Cytopharmacol 1:263–272 [PubMed] [Google Scholar]
- Skalhegg BS, Tasken K 1997 Specificity in the cAMP/PKA signaling pathway. differential expression, regulation, and subcellular localization of subunits of PKA. Front Biosci 2:d331–d342 [DOI] [PubMed] [Google Scholar]
- Jia XC, Perlas E, Su JG, Moran F, Lasley BL, Ny T, Hsueh AJ 1993 Luminescence luteinizing hormone/choriogonadotropin (LH/CG) bioassay: measurement of serum bioactive LH/CG during early pregnancy in human and macaque. Biol Reprod 49:1310–1316 [DOI] [PubMed] [Google Scholar]
- Ostrowski J, Kuhns JE, Lupisella JA, Manfredi MC, Beehler BC, Krystek Jr SR, Bi Y, Sun C, Seethala R, Golla R, Sleph PG, Fura A, An Y, Kish KF, Sack JS, Mookhtiar KA, Grover GJ, Hamann LG 2007 Pharmacological and x-ray structural characterization of a novel selective androgen receptor modulator: potent hyperanabolic stimulation of skeletal muscle with hypostimulation of prostate in rats. Endocrinology 148:4–12 [DOI] [PubMed] [Google Scholar]
- Bisson WH, Cheltsov AV, Bruey-Sedano N, Lin B, Chen J, Goldberger N, May LT, Christopoulos A, Dalton JT, Sexton PM, Zhang XK, Abagyan R 2007 Discovery of antiandrogen activity of nonsteroidal scaffolds of marketed drugs. Proc Natl Acad Sci USA 104:11927–11932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hwang DJ, Bohl CE, Miller DD, Dalton JT 2005 A selective androgen receptor modulator for hormonal male contraception. J Pharmacol Exp Ther 312:546–553 [DOI] [PubMed] [Google Scholar]
- Scharpf Jr LG, Hill ID, Maibach HI 1975 Percutaneous penetration and disposition of triclocarban in man: body showering. Arch Environ Health 30:7–14 [DOI] [PubMed] [Google Scholar]
- Maibach HI, Feldman RJ, Milby TH, Serat WF 1971 Regional variation in percutaneous penetration in man. Pesticides. Arch Environ Health 23:208–211 [DOI] [PubMed] [Google Scholar]
- Soto AM, Maffini MV, Schaeberle CM, Sonnenschein C 2006 Strengths and weaknesses of in vitro assays for estrogenic and androgenic activity. Best Pract Res 20:15–33 [DOI] [PubMed] [Google Scholar]
- Hiles RA 1977 Metabolism and toxicity of halogenated carbanilides: absorption, distribution and excretion of radioactivity from 3,4,4′-trichloro[14C]carbanilide (TCC) and 3-trifluoromethyl-4,4′-dichloro[14C]carbanilide (TFC) in rats. Food Cosmet Toxicol 15:205–211 [DOI] [PubMed] [Google Scholar]
- Chang CY, McDonnell DP 2005 Androgen receptor-cofactor interactions as targets for new drug discovery. Trends Pharmacol Sci 26:225–228 [DOI] [PubMed] [Google Scholar]
- Gregory CW, Johnson Jr RT, Mohler JL, French FS, Wilson EM 2001 Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res 61:2892–2898 [PubMed] [Google Scholar]
- Heinlein CA, Chang C 2002 Androgen receptor (AR) coregulators: an overview. Endocr Rev 23:175–200 [DOI] [PubMed] [Google Scholar]
- Syms AJ, Norris JS, Panko WB, Smith RG 1985 Mechanism of androgen-receptor augmentation. Analysis of receptor synthesis and degradation by the density-shift technique. J Biol Chem 260:455–461 [PubMed] [Google Scholar]
- Wang Y, Chirgadze NY, Briggs SL, Khan S, Jensen EV, Burris TP 2006 A second binding site for hydroxytamoxifen within the coactivator-binding groove of estrogen receptor β. Proc Natl Acad Sci USA 103:9908–9911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov IV, Chang C, Isaacs JT 2004 Molecular characterization of the commonly used human androgen receptor expression vector, pSG5-AR. Prostate 58:319–324 [DOI] [PubMed] [Google Scholar]
- Dai J, Shen R, Sumitomo M, Stahl R, Navarro D, Gershengorn MC, Nanus DM 2002 Synergistic activation of the androgen receptor by bombesin and low-dose androgen. Clin Cancer Res 8:2399–2405 [PubMed] [Google Scholar]
- Ikonen T, Palvimo JJ, Kallio PJ, Reinikainen P, Janne OA 1994 Stimulation of androgen-regulated transactivation by modulators of protein phosphorylation. Endocrinology 135:1359–1366 [DOI] [PubMed] [Google Scholar]
- Moorman WJ, Cheever KL, Skaggs SR, Clark JC, Turner TW, Marlow KL, Schrader SM 2000 Male adolescent exposure to endocrine-disrupting pesticides: vinclozolin exposure in peripubertal rabbits. Andrologia 32:285–293 [DOI] [PubMed] [Google Scholar]