Abstract
In the resolution of inflammatory responses, neutrophils rapidly undergo apoptosis. We describe a new proapoptotic pathway in which cathepsin D directly activates caspase-8. Cathepsin D is released from azurophilic granules in neutrophils in a caspase-independent but reactive oxygen species–dependent manner. Under inflammatory conditions, the translocation of cathepsin D in the cytosol is blocked. Pharmacological or genetic inhibition of cathepsin D resulted in delayed caspase activation and reduced neutrophil apoptosis. Cathepsin D deficiency or lack of its translocation in the cytosol prolongs innate immune responses in experimental bacterial infection and in septic shock. Thus, we identified a new function of azurophilic granules that is in addition to their role in bacterial defense mechanisms: to regulate the life span of neutrophils and, therefore, the duration of innate immune responses through the release of cathepsin D.
Neutrophils represent the most common leukocytes in blood and are essential in innate immune responses in response to pathogens (1). However, the many defense mechanisms are also able to destroy normal tissues. Apoptosis is the most common physiological cell death of neutrophils both in vitro and in vivo, and it prevents the release of histotoxic contents from the dying cell and, therefore, limits tissue damage. It has recently been demonstrated that cyclin-dependent kinase inhibitors enhance the resolution of established inflammation by promoting neutrophil apoptosis (2), suggesting that drugs targeting important molecules in the process of neutrophil apoptosis exhibit great pharmacological potential for the treatment of inflammatory disorders.
The induction of neutrophil apoptosis during the resolution of an innate immune response can be mimicked in vitro by culturing the cells in the absence of sufficient amounts of survival factors, a process that is called spontaneous neutrophil apoptosis. Caspases are known to play a key role in this process, but it remains unclear when and how caspases are activated in neutrophils (3). Caspases can be activated by death receptors of the TNF/nerve growth factor receptor family. Interestingly, the initiator or apical caspase-8, which is activated by ligation of death receptors (4), is also activated during spontaneous neutrophil apoptosis (5–13).
However, a functional death ligand does not appear to play a role in this system. For instance, neutrophil apoptosis from Fas receptor– or Fas ligand–deficient mice is normal (14, 15). Moreover, it is unlikely that, in the absence of inflammation, neutrophil apoptosis is regulated via TNF receptors because there is no or only little TNF available. In addition, ∼60% of normal neutrophil populations do not express functional TNF death receptors but still undergo spontaneous apoptosis with a normal kinetic (16). Thus, there is little evidence for death receptor–mediated initiation of neutrophil apoptosis in the absence of inflammation, and the molecular mechanisms leading to caspase-8 activation in these cells are not known.
Although the lysosomal cathepsins have often been considered as intracellular proteases able to mediate caspase-independent death (17), there is also evidence that they act in concert with caspases in apoptotic cell death. In particular, the cysteine protease cathepsin B and the aspartic protease cathepsin D have been reported to be involved in apoptosis regulation (18–20). Genetic evidence for the role of cysteine cathepsins in apoptosis is provided by studies showing resistance against TNF-induced liver apoptosis in mice lacking cathepsin B (19), perhaps because of insufficient cleavage of Bid (21–23). Cathepsin D was shown to activate Bax in T cells (24) and to be involved in the release of cytochrome c from mitochondria in fibroblasts (20, 25). Moreover, pepstatin A (PepA), a pharmacological inhibitor of cathepsin D, blocked mitochondrial cytochrome c release and caspase activation in cardiomyocytes and fibroblasts (25, 26). Collectively, these data suggested a role for lysosomes and cathepsins in proapoptotic pathways proximal to mitochondrial activation in at least some forms of apoptotic cell death.
Because neutrophils rapidly undergo apoptosis after phagocytosis of bacteria (7, 27), we hypothesized that azurophilic granules, in which cathepsins are located and intracellular bacterial killing occurs, might be able to somehow trigger the normal apoptotic program in these cells. To resolve the issue of whether cathepsins are involved in neutrophil apoptosis pathways, we specifically inactivated cathepsin B and D, respectively, by both genetic and pharmacological means. Our studies revealed that cathepsin D is released from azurophilic granules during the initial phase of neutrophil apoptosis, leading to death receptor–independent activation of caspase-8. Importantly, this newly identified alternative proapoptotic pathway of caspase-8 activation observed in neutrophils is blocked under inflammatory conditions and is crucial for the resolution of innate immune responses.
RESULTS
Cathepsin D, but not cathepsin B, deficiency delays neutrophil apoptosis
Neutrophils are known to express cathepsin G in azurophilic granules (28). In initial experiments, we addressed the question of whether the apoptosis-relevant cathepsins B and D are expressed in normal blood neutrophils and obtained evidence that this is the case as assessed by immunoblotting (unpublished data). To determine the possible involvement of these cathepsins in the regulation of neutrophil apoptosis, we purified neutrophils from normal as well as cathepsin B– and cathepsin D–deficient mice. Neutrophils were isolated from bone marrow and blood, and then cultured. Mature neutrophil populations from cathepsin D−/− mice demonstrated more viable cells compared with those isolated from wild-type or cathepsin B−/− mice (Fig. 1 A). In this model, we additionally observed that blood neutrophils die more rapidly than bone marrow neutrophils, perhaps because the mean age of these cell populations is lower compared with blood neutrophils. The delayed death of cathepsin D−/− cells was more pronounced in blood compared with bone marrow neutrophils but was nevertheless also significant in the latter.
Figure 1.
Lack of expression and pharmacological inhibition of cathepsin D, but not cathepsin B, delays neutrophil apoptosis. (A) Viability assay. Mouse neutrophils from cathepsin D−/− (n = 7), cathepsin B−/− (n = 6), and wild-type (n = 6) mice were isolated from bone marrow (left) or blood (right) and cultured in the absence of cytokine support for 24 h before viability was measured. Values are means ± SD. *, P < 0.05; **, P < 0.01. (B) Viability assay. Concentration- and time-dependent effects of CA (left) and PepA (right) on human blood neutrophil death (n = 3). Values are means ± SD. *, P < 0.05; **, P < 0.01. (C) PS redistribution (top) and DNA fragmentation (bottom) assays. Human blood neutrophils were analyzed after 8-h (PS analysis) and 22-h (DNA analysis) cultures. (left) Values are means ± SD (n = 3). *, P < 0.05. (right) The quantitative analysis (percentages) of early and late apoptotic cells is shown. Representative results from three independent experiments.
Similar to mouse neutrophils, human neutrophils also underwent spontaneous cell death in vitro. Cell death, however, occurred somewhat slower in the human compared with the mouse system (∼40 vs. 88% mean cell death in 24-h cultures; Fig. 1, A and B). To investigate the effect of pharmacological inhibition of cathepsin B and D, we used the previously characterized specific cathepsin inhibitors CA-074-ME (CA), a cell-permeable inhibitor of cathepsin B and possibly other cysteine cathepsins (29), and PepA, which, as mentioned in the Introduction, blocks the aspartic protease cathepsin D (20). In initial experiments, we confirmed the specificity of these two inhibitors (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20072152/DC1). In contrast to CA, PepA delayed spontaneous death of human blood neutrophils in a concentration-dependent manner (Fig. 1 B). The optimal concentration of PepA for death inhibition was 100 μM.
We next investigated whether the antideath effect mediated by PepA was caused by inhibition of apoptosis. PepA reduced phosphatidylserine redistribution, a characteristic feature of apoptotic neutrophils (12, 13), with the same efficacy as GM-CSF (Fig. 1 C, top). Agonistic anti-Fas antibody stimulation was used as an additional control and accelerated neutrophil apoptosis in this in vitro system, which was in agreement with previously published work (12, 13). We also analyzed DNA fragmentation, another hallmark of apoptotic cells. Again, PepA and GM-CSF significantly blocked apoptosis, whereas anti-Fas stimulation resulted in increased DNA fragmentation (Fig. 1 C, bottom). Although human and mouse neutrophils appeared to undergo cell death with different kinetics, these data collectively suggested that cathepsin D exerts proapoptotic activities in cultured mature neutrophils.
Cathepsin D translocation precedes cytochrome c release
To identify where cathepsin D is located within neutrophils, we performed double immunofluorescence analysis on mature neutrophils using confocal microscopy. Cathepsin D colocalized with myeloperoxidase (MPO) in freshly isolated neutrophils (Fig. S2 A, available at http://www.jem.org/cgi/content/full/jem.20072152/DC1). MPO is known to be stored in azurophilic granules of neutrophils (30). In contrast, cathepsin D did not colocalize with cytosolic caspase-3 (Fig. S2 B). These data suggested that cathepsin D is stored, like MPO, in azurophilic granules. Using an anti–cathepsin B antibody, we observed the same results (unpublished data). Upon culturing for 3 h, a subpopulation of neutrophils showed a diffuse staining pattern for cathepsin D and MPO (Fig. 2 A, left; see Fig. S2 A for higher magnification; the same data were observed regarding cathepsin B [not depicted]), suggesting the nonspecific permeabilization of azurophilic granules and subsequent cytosolic release of at least some of its components. Both GM-CSF and G-CSF preserved the punctate pattern in the majority of the cells, whereas the pancaspase inhibitor z-VAD and PepA had no effects. In agreement with these immunofluorescence data, GM-CSF and G-CSF, but not z-VAD, suppressed the enzymatic activity of cathepsin D in cultured neutrophils (unpublished data).
Figure 2.
Cathepsin D is released into the cytosol and proceeds mitochondrial cytochrome c liberation during neutrophil apoptosis. (A) Confocal microscopy, cathepsin D colocalization, and staining pattern analysis. Freshly isolated and cultured neutrophils were stained with anti–cathepsin D and anti-MPO antibodies. (left) Representative data. Correlation coefficient and statistical analysis are shown in Fig. S2 A. No detectable staining was observed using control antibodies (not depicted). Bar, 10 μm. (right) Quantitative and statistical analysis of time-dependent cathepsin D release experiments. Values are means ± SD (n = 3). The same data were observed if MPO and cathepsin B releases were investigated (not depicted). (B) Confocal microscopy, cytochrome c colocalization, and staining pattern analysis. Freshly isolated and cultured neutrophils were stained with anti–cytochrome c and anti-Smac antibodies. (left) Representative data. Correlation coefficient and statistical analysis are shown in Fig. S2 C. No detectable staining was observed using control antibodies (not depicted). Bar, 10 μm. (right) Quantitative and statistical analysis of time-dependent cytochrome c release experiments. Values are means ± SD (n = 3). The same data were observed if Smac release was investigated (not depicted). (C) Histology and confocal microscopy using biopsies from patients. Tissue sections were stained with hematoxylin and eosin, anti–caspase-3 antibody/PI, and anti–cathepsin D/anti-MPO antibodies. Cathepsin D localization was analyzed by staining pattern analysis. White arrows indicate apoptotic neutrophils. Boxed areas indicate higher magnifications (far right). The results are representative of three independent experiments. Bars, 10 μm.
Mitochondria have been shown to be essential for optimal apoptosis induction in neutrophils (6, 8, 31). Therefore, we analyzed the kinetics of mitochondrial release of two proapoptotic factors in cultured neutrophils in the presence and absence of PepA by immunofluorescence analysis (Fig. 2 B, left). As expected, cytochrome c and Smac demonstrated a punctate staining pattern and appeared to colocalize in freshly isolated neutrophils (Fig. S2 C). The mitochondrial location of these two proapoptotic factors was confirmed by the demonstration of colocalization with the mitochondrial marker protein cytochrome oxidase subunit I (unpublished data). After culturing for 12 h, we obtained a diffuse staining pattern in a significant proportion of neutrophils. In the presence of PepA and z-VAD, however, the numbers of cells turning into a diffuse pattern were significantly decreased. The same observation was made using GM-CSF and G-CSF.
We systematically counted the cells exhibiting a diffuse staining pattern and examined cathepsin D and cytochrome c releases in a time-dependent manner (Fig. 2, A and B, right). Our data clearly indicated that cathepsin D release occurs earlier than cytochrome c release. Moreover, because PepA and z-VAD blocked cytochrome c release, it can be concluded that both cathepsin D and caspase activities are required for the proapoptotic activation of mitochondria in neutrophils. Collectively, these data suggested that caspase-independent permeabilization of azurophilic granules occurs in early phases of neutrophil apoptosis. This process preceded the caspase-dependent liberation of mitochondrial proapoptotic factors and is blocked by survival cytokines.
To confirm that azurophilic granules undergo permeability changes and that the release of cathepsin D precedes cytochrome c release from mitochondria, we analyzed the kinetics of translocation of these two proteins using cytosolic extracts from cultured neutrophils and immunoblotting (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20072152/DC1). Although cathepsin D was already detected in cytosols from 3-h cultured neutrophils, we obtained evidence for cytochrome c at later time points only (6 and 9 h). These data further supported the concept that membranes of azurophilic granules are permeabilized before those of mitochondria during neutrophil apoptosis. Moreover, transmission electron microscopy of cultured neutrophils demonstrated damaged azurophilic granule membranes, suggesting the permeabilization of these organelles in this process (Fig. S4).
To demonstrate cathepsin D and B expression and their potential release in neutrophils under in vivo conditions, we analyzed neutrophils in tissue sections of patients suffering from inflammatory diseases. Leukocytoclastic vasculitis is a skin disorder known to be associated with apoptotic neutrophils present in skin lesions (32). We confirmed multiple apoptotic neutrophils in the dermis of these patients, as determined by regular histology and activated caspase-3 staining. Immunofluorescence analysis on these tissues demonstrated evidence for cathepsin D and MPO releases in these cells (diffuse staining pattern; Fig. 2 C). In contrast, we detected almost no apoptotic neutrophils in tissue sections from patients suffering from ulcerative colitis and acute appendicitis, in which neutrophils exhibited a punctate staining pattern, suggesting that azurophilic granules were intact. We also performed cathepsin B stainings and obtained the same results (unpublished data). In conclusion, permeabilization of azurophilic granules and the subsequent release of cathepsins and MPO in apoptotic neutrophils is not only an in vitro phenomenon but also occurs in vivo.
Caspase activation triggered by cathepsin D
Because the proapoptotic activation of mitochondria was both cathepsin D and caspase dependent, but the release of cathepsin D (and its enzymatic activation) was caspase independent, we addressed the question of whether active cathepsin D is able to induce caspase activation. In agreement with previously published work (8, 12, 16), we observed caspase-8, caspase-3, and Bax cleavage in a time-dependent manner in association with spontaneous neutrophil apoptosis, as assessed by immunoblotting (Fig. 3 A). Caspase-8 cleavage proceeded caspase-3 cleavage both in spontaneous and Fas receptor–mediated apoptosis. Bax is cleaved by calpain-1 during neutrophil apoptosis (8), an event that is detectable before caspase-3 cleavage. PepA but not CA blocked cleavages of caspase-8, caspase-3, and Bax, confirming that pharmacological inhibition of cathepsin D but not cathepsin B delays neutrophil apoptosis.
Figure 3.
Pharmacological inhibition of cathepsin D, but not cathepsin B, blocks caspase and calpain activation. (A) Immunoblotting. Neutrophils were cultured for the indicated times in the presence and absence of the indicated inhibitors and anti-Fas antibody. Bax cleavage in neutrophils is caused by calpain activation. The same results were obtained in two additional independent experiments. Arrowheads indicate key cleavage products. (B) Caspase activity assays. Human blood neutrophils were analyzed immediately after isolation (fresh) and upon a 9-h culture period. Values are means ± SD (n = 3). *, P < 0.05.
Although we obtained evidence for the generation of active caspases in these experiments (a 15-kD fragment of caspase-8 and a 17-kD fragment of caspase-3), previous work suggested that cleavage does not necessarily indicate caspase activation (33). Therefore, we also analyzed the effect of pharmacological inhibition of cathepsin D on enzymatic caspase activities in cultured neutrophils. Spontaneous neutrophil apoptosis was associated with increases of both caspase-8 and -3 activities, which were completely blocked by PepA (Fig. 3 B). PepA did not directly block the activity of pure caspase-8 or -3 enzymes (Fig. S1 B), suggesting that cathepsin D is indeed able to trigger caspase activation in neutrophils.
Cathepsin D directly activates caspase-8
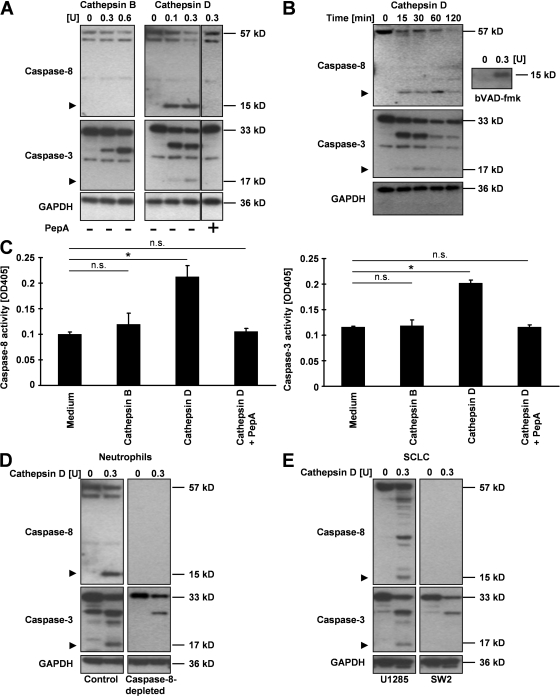
Bid has been described as a target of cathepsin B in vitro (21–23). Therefore, we hypothesized that cathepsin D is also able to cleave cellular substrates involved in apoptosis regulation. In an in vitro cell-free assay using cytosolic extracts of freshly isolated blood neutrophils, we observed that the addition of cathepsin D, but not cathepsin B, was followed by the cleavage of caspase-8 (15-kD fragment) and caspase-3 (17-kD fragment) in a concentration- and time-dependent manner (Fig. 4, A and B). As seen during spontaneous neutrophil apoptosis, caspase-8 cleavage was again detectable before caspase-3 cleavage in this cell-free system. In the presence of PepA, cathepsin D–induced caspase cleavages were completely prevented (Fig. 4 A). In addition, the caspase-8 fragment generated by incubation of cytosolic extracts with cathepsin D could be affinity labeled with the biotinylated caspase substrate VAD-fmk (bVAD-fmk; Fig. 4 B, right), suggesting that the 15-kD fragment of caspase-8 is enzymatically active (34). Additional enzymatic measurements confirmed that cathepsin D, but not cathepsin B, increased the enzymatic activities of both caspase-8 and -3 in the cytosolic extracts of neutrophils (Fig. 4 C).
Figure 4.
Cathepsin D activates caspase-8, which is required for subsequent caspase-3 activation. (A) Immunoblotting. Concentration-dependent cleavage of caspase-8 and -3 proteins by cathepsin D, but not cathepsin B, in cytosolic extracts from freshly isolated human blood neutrophils. Results are representative of three independent experiments. (B) Time-dependent cleavage of caspase-8 and -3 proteins by cathepsin D, but not cathepsin B, in cytosolic extracts from freshly isolated human blood neutrophils. Cytosolic extracts were also used for affinity labeling with bVAD-fmk and analyzed by immunoblotting. Results are representative of three independent experiments. (C) Caspase activity assays in cytosolic extracts of freshly isolated human blood neutrophils after treatment with cathepsins. Values are means ± SD (n = 3). *, P < 0.05. (D) Immunoblotting. Cleavage of caspase-3 (17-kD fragment) mediated by cathepsin D in normal and caspase-8–immunodepleted cytosolic extracts from neutrophils. Results are representative of three independent experiments. (E) Immunoblotting. Cleavage of caspase-3 (17-kD fragment) mediated by cathepsin D in caspase-8–expressing and –deficient cytosolic extracts from SCLC lines. Arrowheads in A, B, D, and E indicate key cleavage products.
Cathepsin D processed and activated both caspase-8 and -3 in vitro, but the cleavage of caspase-8 seemed to be earlier than that of caspase-3. Therefore, we hypothesized that caspase-8 is required for caspase-3 activation in neutrophils. Indeed, cathepsin D was unable to mediate caspase-3 cleavage (17-kD fragment) after immunodepletion of caspase-8 in cytosolic extracts of freshly isolated blood neutrophils (Fig. 4 D). We also used cytosolic extracts of the small cell lung carcinoma (SCLC) lines U1285 (caspase-8 containing) and SW2 (caspase-8 deficient) and confirmed that cathepsin D–mediated caspase-3 cleavage (17-kD fragment) is caspase-8 dependent (Fig. 4 E).
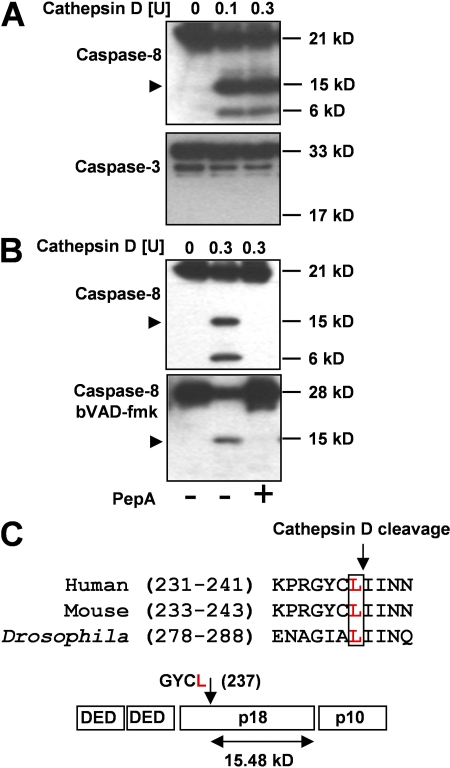
Although cathepsin D activated caspase-8, it remained unclear whether this is a direct or an indirect process. To address this question, we performed identical in vitro experiments using pure recombinant human caspase-8 and -3 proteins instead of cytosolic extracts. Cathepsin D cleaved caspase-8 (15-kD fragment) but not caspase-3 (Fig. 5 A). Moreover, the generated caspase-8 fragment was enzymatically active because it was trapped by bVAD-fmk. In contrast, no active enzyme was generated in the presence of PepA (Fig. 5 B). Sequencing by Edman degradation revealed that cathepsin D cleaves caspase-8 at Leu 237, resulting in a C-terminal fragment of the p18 subunit. The relative molecular mass of this fragment, deduced from mass spectrometry, was 15.48 kD. Comparison of caspase-8 sequences of different species suggested that the cathepsin D cleavage site is highly conserved (Fig. 5 C).
Figure 5.
Cathepsin D directly activates caspase-8. (A) Immunoblotting. (top) Concentration-dependent cleavage of pure recombinant caspase-8 by cathepsin D. (bottom) Cathepsin D did not cleave caspase-3. Results are representative of three independent experiments. (B) Immunoblotting. (top) Cleavage of pure recombinant caspase-8 in the presence and absence of 100 μM PepA. (bottom) The in vitro–cleaved 15-kD fragment of caspase-8 associated with bVAD-FMK. Results are representative of three independent experiments. (C) Sequence analysis. The 15-kD fragment was sequenced by Edman degradation. Cathepsin D cleaved caspase-8 within the p18 subunit at Leu 237. The sequence alignment was performed with ClustalW (http://www.ebi.ac.uk/Tools/clustalw2/index.html) using sequence data obtained from GenBank/EMBL/DDBJ under accession numbers Q14790, O89110, and Q81RY7. Arrowheads in A and B indicate key cleavage products.
Cathepsin D deficiency prolongs innate immune responses
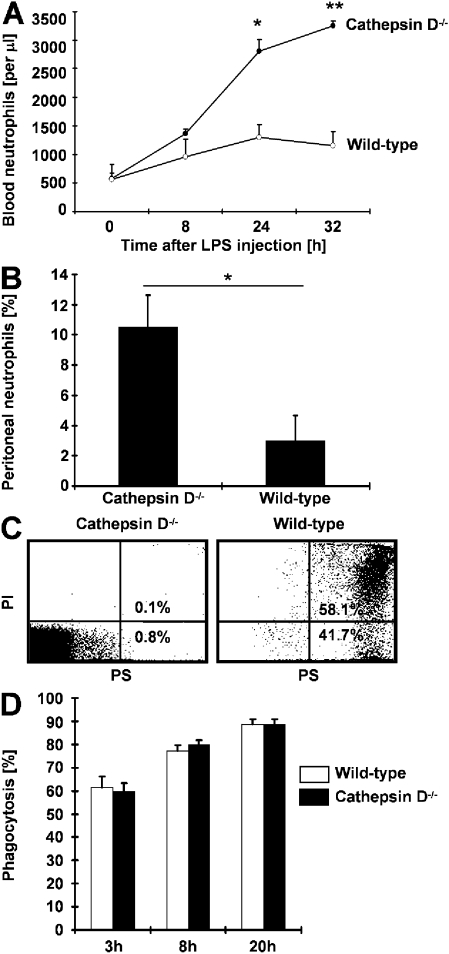
To determine whether the newly identified proapoptotic pathway resulting in caspase-8 activation in the absence of death receptor signaling is relevant in a more physiological setting, we injected wild-type and cathepsin D−/− mice intraperitoneally with low-dose LPS. In this experimental model of bacterial peritonitis, we observed a moderate increase in blood neutrophil numbers peaking at 24 h in wild-type mice (P < 0.05). 32 h after LPS injection, neutrophil numbers started to decline. Compared with wild-type mice, cathepsin D−/− mice demonstrated much higher neutrophil numbers at 24 h that further increased 32 h after LPS administration (Fig. 6 A). We also analyzed the numbers of peritoneal lavage neutrophils 32 h after LPS injection. Although almost no neutrophils were detected in wild-type mice, cathepsin D−/− mice demonstrated evidence for peritoneal neutrophilic infiltration (Fig. 6 B). In addition, although practically all peritoneal lavage neutrophils underwent rapid apoptosis, the neutrophils from cathepsin D−/− mice were apoptosis resistant (Fig. 6 C).
Figure 6.
Lack of cathepsin D amplifies and prolongs inflammation in experimental peritonitis. (A) Blood neutrophil numbers after application of low-dose LPS (30 μg) in wild-type (n = 6) and cathepsin D−/− (n = 7) mice. Values are means ± SD. *, P < 0.05; **, P < 0.01. (B) Neutrophil numbers in peritoneal lavage fluid in wild-type (n = 6) and cathepsin D−/− (n = 7) mice 32 h after LPS application. Values are means ± SD. *, P < 0.05. (C) PS redistribution assay. Peritoneal lavage fluid neutrophils were analyzed by flow cytometry 32 h after LPS application and a subsequent 24-h culture. The quantitative analysis (percentages) is shown in the subpanels. Results are representative of three independent experiments. (D) Phagocytosis assay. The percentages of neutrophils phagocytosed by macrophages are presented. Times indicate how long neutrophils were cultured before the assay. Values are means ± SD (n = 3 for wild-type and cathepsin D−/− mice).
Macrophages have been shown to be key cellular players in the uptake of apoptotic neutrophils and in the resolution of inflammation (35, 36). Therefore, the possibility existed that the increased inflammation seen in cathepsin D−/− mice was also the consequence of a clearance defect, resulting in the accumulation of neutrophils. We isolated both macrophages and neutrophils from wild-type and cathepsin D−/− mice and analyzed the uptake of apoptotic neutrophils by macrophages in both systems in vitro. No difference in the percentage of phagocytosed neutrophils was seen between wild-type and cathepsin D−/− mice (Fig. 6 D). We also observed no difference between wild-type and cathepsin D−/− mice when we intraperitoneally injected low-dose LPS in the mice before performing the same macrophage phagocytosis assays (unpublished data). Collectively, these data excluded a defect in the uptake of apoptotic neutrophils and suggested that the stronger and prolonged inflammatory response in cathepsin D−/− compared with wild-type mice is largely caused by a block of neutrophil apoptosis in this model of experimental bacterial infection.
Because experimental LPS administration in mice reproduces a human bacterial infection only in part, we investigated the newly identified cathepsin D–caspase-8 pathway in neutrophils of patients suffering from septic shock. Analysis of cathepsin D release in the cytosol in neutrophil cultures showed that at least 85% of the sepsis neutrophils maintained intact azurophilic granules with no evidence for permeabilization within the first 9 h. In contrast, at this time point, ∼90% of normal neutrophils released cathepsin D in the cytosol (Fig. 7 A). Moreover, sepsis neutrophil cultures did not show evidence for caspase-8 cleavage within 9 h (Fig. 7 B), further supporting the concept that caspase-8 activation is mediated by cathepsin D in neutrophils. Consequently, spontaneous neutrophil death (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20072152/DC1) and apoptosis (Fig. 7 C) was markedly delayed in sepsis under ex vivo conditions. No difference was observed regarding cathepsin D expression between normal and sepsis neutrophils as assessed by immunoblotting (unpublished data).
Figure 7.
Block of cathepsin D release and subsequent apoptosis defect in blood neutrophils derived from patients with septic shock. (A) Confocal microscopy. Blood neutrophils from patients suffering from septic shock (n = 3) and control individuals (n = 3) were cultured for 3 and 9 h, and analyzed as demonstrated in Fig. 2 A. Values are means ± SD. **, P < 0.01; ***, P < 0.001. The same data were observed if MPO and cathepsin B releases were investigated (not depicted). (B) Immunoblotting. Time-dependent cleavages of caspase-8 and -3 in cultured blood neutrophils from patients suffering from septic shock and control individuals. Arrowheads indicate key cleavage products. Results are representative of three independent experiments. (C) PS redistribution assay. Blood neutrophils from control individuals and patients suffering from septic shock were cultured for 8 h, and analyzed by flow cytometry. The quantitative analysis (percentages) is shown in the subpanels. Results are representative of three independent experiments.
Reactive oxygen species (ROS) deficiency delays cathepsin D release and apoptosis
The absence or inhibition of cathepsin D delays but does not prevent neutrophil apoptosis. This suggests that alternative mechanisms must exist for apoptosis induction. Genetic deficiency of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase or its pharmacological inhibition results in delayed neutrophil apoptosis (12, 37). We therefore investigated the effect of combined inhibition of both ROS production and cathepsin D activity on neutrophil apoptosis. Inhibition of ROS production was performed by diphenyleneiodonium (DPI). Although pharmacological inhibition of both NADPH oxidase and cathepsin D demonstrated additive antideath effects, the complete prevention of neutrophil death was not achieved even under these conditions (Fig. 8 A).
Figure 8.
Delayed cathepsin D release and subsequent apoptosis defect in ROS-deficient neutrophils. (A) Viability assay. Neutrophils were isolated from human control individuals and cultured in the presence and absence of 20 μM DPI and 50 μM PepA for 24 h before analysis. Values are means ± SD from three independent experiments. *, P < 0.05. (B) Confocal microscopy. Blood neutrophils from a patient suffering from CGD were cultured and analyzed as shown in Fig. 2 A and Fig. S2 A. (C) Immunoblotting. In contrast to normal neutrophils (Figs. 3 A and 7 B), no active fragments were detectable in CGD neutrophils in up to 9-h culture periods. (D) PS redistribution assay. Blood neutrophils from a control individual and a CGD patient were cultured for 8 h, and analyzed by flow cytometry. The quantitative analysis (percentages) is shown in the subpanels.
We next investigated whether ROS were required for the translocation of cathepsin D in the cytosol. To have a clean system, we used neutrophils from a patient with chronic granulomatous disease (CGD) that are unable to generate ROS based on a genetic defect in the NADPH oxidase. In the absence of ROS, we observed delayed cathepsin D release (a culture period of 7 h was required to obtain cathepsin D release in 50% of the neutrophils, whereas only 1 h was required in normal neutrophil populations; Figs. 2 A and 8 B). Interestingly, under ROS-deficient conditions, both GM-CSF and G-CSF had only modest additional inhibitory effects on cathepsin D release. The delayed cathepsin D release in ROS-deficient neutrophils was associated with no detectable cleavage of caspases (Fig. 8 C) and reduced apoptosis (Fig. 8 D). These data suggest that ROS are involved in the membrane permeabilization of azurophilic granules.
DISCUSSION
Lysosomal enzymes such as cathepsins and MPO are stored in the azurophilic granules of neutrophils and synthesized early during maturation of these cells in the bone marrow (38). These enzymes are used during phagocytosis and intracellular killing (30), as well as in the extracellular defense against bacteria (39). In this paper, we provide evidence for an important new function of azurophilic granules, namely, the induction of apoptosis in mature neutrophils. Azurophilic granules release cathepsin D, which directly activates the initiator caspase-8, leading to subsequent calpain (as indicated by Bax cleavage) and caspase-3 activation. This proapoptotic pathway is inhibited by the neutrophil survival cytokines GM-CSF and G-CSF in vitro and in vivo. Because phagocytosis of bacteria triggers apoptosis in neutrophils (7, 27), it is also tempting to speculate that this previously reported death mechanism is initiated by the release of lysosomal enzymes from the azurophilic granules in the cytosol. The exact molecular mechanisms regulating the permeabilization of azurophilic granules under the different conditions remain to be investigated.
Apoptosis pathways in neutrophils including caspases have been intensively studied by several groups (3, 5–13). However, the initial events leading to caspase activation have remained obscure in these cells. Besides caspases, it has been recognized that noncaspase proteases play a role in apoptosis pathways in several cell types, including neutrophils (8, 40). Although it has already been suggested that these proteases act in concert with caspases, our finding that cathepsin D acts proximal to caspases and activates an initiator caspase was unexpected. Moreover, the alternative pathway of caspase-8 activation described in this paper resolves the issue of how this caspase is activated in the absence of death receptor ligation in neutrophils. Because cathepsin D is ubiquitously present in lysosomes, it is possible that this pathway can also be used to activate apoptosis in other cells, such as SCLC cells.
To obtain original insights in the permeabilization process of azurophilic granules, we performed experiments in neutrophils derived from a CGD patient. We obtained evidence that ROS are involved, because CGD neutrophils demonstrated delayed cathepsin D release compared with normal neutrophils. These data are in agreement with recent work published by Blomgran et al., who showed that during microbe-induced apoptosis of human neutrophils, ROS-dependent lysosomal destabilization represent an early event (41). This destabilization provoked the release of cathepsin B, which then induced the cleavage of the proapoptotic Bcl-2 protein Bid, mitochondrial damage, and subsequent caspase activation and apoptosis. Our data, however, suggest that cathepsin B is functionally unimportant for apoptosis induction, because its genetic and pharmacological inactivation had no influence on neutrophil death. Clearly, additional work is required to better understand the molecular mechanisms leading to the membrane permeabilization of azurophilic granules during neutrophil apoptosis.
Cathepsin D is inactivated at the pH found in the cytoplasm (42). How then can caspase-8 be activated by cathepsin D in a cell? One possibility is that caspase-8 is cleaved because of the known endopeptidase activity of cathepsin D (i.e., at least partially retained at a neutral pH) (43, 44). On the other hand, because of the nonspecific membrane permeabilization of azurophilic granules, it is likely that acidification of the cytosol may occur, at least in close proximity to the granules, allowing cathepsin D to be completely active. Acidification of the cytosol has previously been noticed in apoptotic cells (45, 46). Although, this study cannot completely explain how cathepsin D maintains its activity in the cytosol, the delayed neutrophil apoptosis in cathepsin D−/− mice represents the most direct demonstration that it is indeed the case.
How is caspase-8 activated by cathepsin D? At present, we do not understand the molecular mechanism of any initiator caspase (47). Two models have been proposed. Initially, it was thought that initiator caspases autoprocess themselves when they are brought into close proximity with each other. Later studies suggested that caspase-8 aggregation serves primarily to facilitate dimerization of the enzyme and that this does not require interchain proteolysis. In contrast, interchain proteolysis, but not enforced dimerization of caspase-8 by Fas-associated protein with death domain, was a prerequisite for its activation by granzyme B or caspase-6 (34, 48). Therefore, the proteolytic processing of caspase-8 by cathepsin D might initiate its activation, but the exact mechanism remains to be investigated.
Most studies of neutrophil accumulation concentrate on adhesion and migration. In contrast, little information is available regarding the functional consequences of delayed neutrophil apoptosis in innate immune responses. Therefore, the demonstration that delayed apoptosis caused by cathepsin D deficiency amplifies and prolongs neutrophilic inflammation in vivo is notable. Future studies may provide new insights into how GM-CSF and G-CSF stabilize the membrane of azurophilic granules, which results in “functional cathepsin D deficiency,” at least in terms of caspase-8 activation and subsequent apoptosis induction. As indicated in this study, the stabilization of azurophilic granules might play an important pathogenic role in sepsis, CGD, ulcerative colitis, and appendicitis. Besides ROS, another potential candidate regulating the permeability of azurophilic granules is Bax, which has been shown to regulate lysosomal membrane permeabilization (49). In addition, heat shock protein 70 has been reported to promote cell survival by inhibiting lysosomal membrane permeabilization (50). Independent from these pathogenic mechanisms, permeabilization of azurophilic granules may provide a new therapeutic strategy to induce neutrophil apoptosis and, consequently, to resolve exaggerated innate immune responses.
MATERIALS AND METHODS
Reagents.
Both cathepsin B and D recombinant enzymes, the specific colorimetric cathepsin B substrate (N-benzyloxycarbonyl (z)-Arg-Arg-para-nitroaniline), and DPI were obtained from EMD. The specific fluorogenic cathepsin D substrate ((7-Methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-N-3-(2,4-Dinitro-phenyl)-L-2,3-diaminopropionyl-Ala-Arg-NH2), human G-CSF, and mouse GM-CSF were obtained from R&D Systems. Human GM-CSF was obtained from Novartis. The caspase inhibitors z-Val-Ala-Asp (VAD)-fluoromethylketone (fmk) (z-VAD), z-Ile-Glu-Thr-Asp (IETD)-fmk (z-IETD), and z-Asp-Glu-Val-Asp (DEVD)-fmk (z-DEVD) were obtained from BD Biosciences. PepA, CA, sucrose, and LPS from Escherichia coli 0111:B4 were purchased from Sigma-Aldrich. Anti-Fas agonistic monoclonal antibody (CH11) was obtained from MBL International. bVAD-fmk was obtained from MP Biomedicals.
Mice.
Cathepsin B−/−, cathepsin D−/−, and wild-type mice were generated in our laboratory as previously described (51, 52). For all experiments, mice with a C57BL/6J background that were 3–4 wk old were used. Mice were maintained under pathogen-free conditions. All animal experiments were reviewed and approved by the Animal Experimentation Review Board of the State of Bern.
Cells.
Peripheral blood neutrophils were purified from healthy normal individuals and patients suffering from septic shock by Ficoll-Hypaque centrifugation (12, 13). Septic shock patients fulfilled the following inclusion criteria: (a) documented or suspected infection; (b) signs of systemic inflammation in response to infection; and (c) systolic arterial blood pressure <70 mm Hg, despite adequate fluid resuscitation, in the absence of other causes of hypotension (53). We also purified neutrophils from the blood of a patient with CGD. We obtained Institutional Review Board approval for the study from the Kantonale Ethikkommission Bern. The purity of the isolated human neutrophil populations was always >95%, as assessed by staining with Diff-Quik (Baxter) and light microscopy analysis. Neutrophils were also isolated from wild-type, cathepsin B−/−, and cathepsin D−/− mice. Mature mouse bone marrow (obtained from femur and tibia of the hind legs) and blood neutrophils (obtained by cardiac puncture) were positively selected using anti–Gr-1 monoclonal antibody (Miltenyi Biotec), as previously described (54). The purity of the resulting mouse neutrophil populations was >90% (bone marrow) and >95% (blood), respectively. For macrophage phagocytosis assays, we injected 2–3 ml of ice-cold 30% sucrose solution in the peritoneal cavity of wild-type and cathepsin D−/− mice, gently palpated for ∼30–60 s, and aspirated the solution, which contained sufficient numbers of peritoneal macrophages. The SCLC cell lines U1285 (expressing caspase-8) and SW2 (lacking caspase-8) (55) were provided by Dr. U. Zangemeister-Wittke (University of Bern, Bern, Switzerland).
Cell cultures.
Human blood and mouse bone marrow neutrophils were cultured at 106 cells per milliliter. Mouse blood neutrophils were used at 25 × 104 cells per milliliter. Neutrophils were cultured in complete culture medium (RPMI 1640 containing 10% fetal calf serum) in the presence and absence of 50 ng/ml GM-CSF, 25 ng/ml G-CSF, 1 μg/ml anti-Fas, 0.5–20 μM CA, 1–300 μM PepA, 20 μM DPI, and 50 μM z-VAD for the times indicated in the figures.
Determination of cell death and apoptosis.
Neutrophil death was assessed by uptake of 1 μM ethidium bromide and flow cytometric analysis (FACSCalibur; Becton Dickinson) (12, 13, 56). To determine whether cell death was apoptosis, redistribution of phosphatidylserine (PS) in the presence of propidium iodide (PI) was measured by flow cytometry (12, 13, 56). Neutrophil apoptosis was also assessed by oligonucleosomal DNA fragmentation (12, 13, 56).
Gel electrophoresis and immunoblotting.
106 cells per milliliter were washed with PBS supplemented with protease inhibitor cocktail (Sigma-Aldrich) and lysed with RIPA buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.25% sodium deoxycholate, 1% Nonidet P-40, 1 mM EGTA supplemented with protease inhibitor cocktail). After a 10-min centrifugation to remove insoluble particles, equal amounts of the cell lysates, cell-free extracts, or subcellular fractionation extracts were loaded on gels (NuPage; Invitrogen). Separated proteins were electrotransferred onto polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore). The filters were incubated overnight at 4°C in TBS/0.1% Tween 20/5% nonfat dry milk with mouse anti–caspase-8 (1:1,000; Cell Signaling Technology), rabbit anti-Bax (1:1,000; BD Biosciences), rabbit anti–caspase-3 (1:1,000; Cell Signaling Technology); mouse anti–cathepsin D (1:1,000; Sigma-Aldrich), or mouse anti–cytochrome c (1:500; BD Biosciences) antibodies. For loading controls, stripped filters were incubated with anti-GAPDH (1:3,000; Chemicon) monoclonal antibody. Filters were washed in TBS/0.1% Tween 20/5% nonfat dry milk for 30 min at room temperature and incubated with the appropriate horseradish peroxidase–conjugated secondary antibody (GE Healthcare) in TBS/0.1% Tween 20/5% nonfat dry milk for 1 h. Filters were developed by an enhanced chemiluminescence technique (ECL kit; GE Healthcare) according to the manufacturer's instructions.
Cell-free assays.
20 × 106 freshly isolated human blood neutrophils were washed with PBS containing 4% BSA and lysed for 35 min on ice in 60 μl of CEB buffer (20 mM Hepes [pH 7.2], 250 mM sucrose, 10 mM KCl, 1.5 mM MgCl2, 2 mM EDTA, 1 mM dithiothreitol (DTT), 100 μM PMSF, 10 μg/ml aprotinin). The lysis was completed with a mechanical homogenization by 25 strokes. After a 1-h centrifugation step at 21,000 g at 4°C, the supernatant (cytosol) was used for the cell-free assay. 10 μg of cytosolic fractions was incubated for the times indicated in the figures at 37°C in buffer B (340 mM NaOAc, 60 mM acetic acid, 4 mM EDTA, 0.1% CHAPS, 8 mM DTT [pH 5.5]) or buffer D (500 mM glycine-HCl [pH 3]) in the presence or absence of the indicated amounts of cathepsin B and D, as well as 100 μM PepA. In some experiments, caspase-8 was removed from neutrophil cytosolic extracts by immunodepletion using 5 μg of anti–human caspase-8 antibody (Cell Signaling Technology). In other experiments, we prepared cytosolic extracts from SCLC cell lines U1285 and SW2, which were incubated for 30 min at 37°C in the presence and absence of 0.3 U cathepsin D. All cell-free extracts were analyzed by immunoblotting.
Enzymatic caspase and cathepsin assays.
Caspase-3 and -8 activities were measured using pure caspase-3 and -8 enzymes in the presence or absence of 100 μM PepA, 10 μM CA, 50 μM z-IETD, and 50 μM z-DEVD, or in total extracts prepared from mature neutrophils cultured in the presence or absence of 100 μM PepA using commercial caspase-3 and -8 cellular activity assay kits (QuantiZyme; BIOMOL International, L.P.), according to the manufacturer's instructions. In addition, caspase-3 and -8 activities were measured in cytosolic extracts from mature granulocytes in the presence and absence of 0.6 U cathepsin B, 0.3 U cathepsin D, and 100 μM PepA for 30 min at 37°C. Activated caspase-8 was also detected by precipitation with bVAD-fmk (34).
Cathepsin B and D activities were measured using pure cathepsin B and D enzymes in the presence or absence of 100 μM PepA and 10 μM CA as enzymatic conversion of the colorimetric cathepsin B substrate or the fluorogenic cathepsin D substrate after a 6-h incubation at 37°C, according to the manufacturer's instructions.
In vitro protease cleavage assays.
To investigate direct cleavage of caspases by cathepsin D, 2 μl of pure caspase-8 (generated from histidine-tagged human caspase-8 cloned in PET15b [57]; provided by K. Schulze-Osthoff, University of Düsseldorf, Düsseldorf, Germany) and caspase-3 (provided by C. Borner, University of Freiburg, Freiburg, Germany) recombinant proteins were incubated with 0.1 and 0.3 U cathepsin D in the presence or absence of 100 μM PepA for the times indicated in the figures at 37°C in buffer D. Recombinant activated human caspase-8 was analyzed by mass spectrometry immediately after purification. Samples for Edman sequencing were prepared by blotting the separated cleavage products onto PVDF membranes and cutting out the bands corresponding to the fragments of interest. Edman sequencing was performed at the Functional Genomics Center operated by the Swiss Federal Institute of Technology Zurich (ETH Zürich) and the University of Zurich, and mass spectrometry analyses were performed by the SVS MS-Plateform of the University of Geneva on a fee-for-service basis.
Histological examination.
Tissue sections from leukocytoclastic vasculitis, ulcerative colitis, and acute appendicitis patients were fixed in 4% paraformaldehyde and embedded in paraffin. 5-μm sections were stained with hematoxylin and eosin and examined by light microscopy (Axiovert 35; Carl Zeiss, Inc.).
Confocal laser scanning microscopy.
Cytospins with 2 × 106 cells per milliliter were made from freshly purified neutrophils or neutrophils cultured in the presence or absence of 100 μM PepA, 50 μM z-VAD, 25 ng/ml G-CSF, or 50 ng/ml GM-CSF on noncoated slides. Cells were fixed in 4% paraformaldehyde for 10 min at room temperature and washed three times in PBS (pH 7.4). Permeabilization of cells was performed with 0.05% saponin in buffer A (3% BSA in PBS) for 5 min at room temperature and with acetone for 15 min at −20°C. To prevent nonspecific binding, slides were incubated in blocking buffer (33% human immunoglobulins, 33% normal goat serum, 33% BSA) for 1 h at room temperature. Indirect immunostainings of cathepsin D, MPO, caspase-3, cytochrome c, CoxI, and Smac were performed by using the following primary antibodies diluted in blocking buffer: monoclonal anti–cathepsin D (1:100; Sigma-Aldrich), polyclonal anti-MPO (1:6,000, Dako), polyclonal anti–caspase-3 (1:100; Cell Signaling Technology), monoclonal anti-CoxI (1:400; Invitrogen), monoclonal anti–cytochrome c (1:100; BD Biosciences), and polyclonal anti-Smac (1:100; Imgenex). Mouse and rabbit control antibodies, respectively, were used at the same concentrations in each experiment.
Immunofluorescent stainings were also performed on 5-μm-thick paraformaldehyde-fixed paraffin-embedded tissue sections from leukocytoclastic vasculitis, ulcerative colitis, and acute appendicitis patients. Slides were dried for 2 h at 52°C and deparaffinized. Slides were blocked and stained as described in the previous paragraph. After incubation with primary antibodies, cells and tissues were incubated with the appropriate TRITC- and FITC-conjugated secondary antibodies (1:500) for 1 h in the dark at room temperature. For active caspase-3 staining, polyclonal anti–caspase-3 antibody (1:200; Cell Signaling Technology) was used. The antifading agent Mowiol (EMD) was added. Slides were covered by coverslips and analyzed by a confocal laser scanning microscope (LSM 510; Carl Zeiss, Inc.) equipped with Ar and HeNe lasers. To quantify the amount of cells with diffuse staining pattern (cathepsin D, MPO, cytochrome c, and Smac experiments), 100 cells were counted in randomly chosen regions, and the mean number of cells demonstrating diffuse staining was calculated.
For colocalization studies, unprocessed, unfiltered and undeconvoluted datasets were analyzed using Imaris software package (Bitplane AG), considering every singular layer of a stack separately. Quantitative data of colocalization events were determined by the statistics modules in the colocalization and Voxelshop software of the Imaris package. Intensities were given as the sum of all colocalizing voxels in a dataset, and a computer image was generated. For quantitative analysis of colocalization, the Pearson's correlation coefficient was calculated, as previously described (58, 59).
Experimental peritonitis.
Cathepsin D−/− and wild-type mice were injected intraperitoneally with 30 μg LPS. Blood was collected at the time points indicated in the figures, and neutrophil numbers were analyzed by differential counts using Türk solution (distributed by Dr. Grogg Chemie AG). After 32 h, mice were killed by CO2 inhalation and injected intraperitoneally with 2 ml PBS. The peritoneal lavage fluid was collected and we performed differential counts according to standard morphological criteria on cytospin preparations stained with Giemsa-May-Grünwald solution (Sigma-Aldrich). In addition, neutrophils were isolated, cultured for 24 h, and subsequently analyzed regarding PS redistribution.
Macrophage phagocytosis assay.
Uptake of apoptotic mouse neutrophils by mouse macrophages was investigated as described previously (16, 35), with slight modifications. In brief, ∼2 × 105 peritoneal macrophages of wild-type and cathepsin D−/− mice were cultured in complete culture medium on a glass coverslip in 24-well tissue culture plates (VWR International AG) in the presence of 50 ng/ml of mouse GM-CSF. 106 mature mouse bone marrow neutrophils of wild-type mice were cultured for the indicated times and added to macrophages at 37°C for 30 min. After coincubation, cells were fixed with 1% acetone-formalin and stained for MPO activity with dimethoxybenzidine in the presence of hydrogen peroxide. Cells were lightly counterstained with Harris' hematoxylin. The proportion of neutrophils phagocytosed by macrophages was determined by two independent investigators by counting in at least five fields (a minimum of 100 neutrophils was evaluated).
Statistical analysis.
Statistical analysis was performed using the Mann-Whitney U test. The figures show mean levels ± SD. For multiple comparisons, differences of the mean levels were analyzed using analysis of variance, followed by Tukey's HSD test. P < 0.05 was considered statistically significant. For colocalization studies, Pearson's correlation coefficient was determined in at least 10 different cells, and means ± SD were calculated.
Online supplemental material.
Fig. S1 demonstrates the specificity of the pharmacological inhibitors PepA and CA. Fig. S2 demonstrates the localization of cathepsin D in azurophilic granules and its translocation in the cytosol. Results of quantitative colocalization analysis are provided. Fig. S3 demonstrates the time-dependent appearance of cathepsin D in the cytosol compared with cytochrome c, as assessed by immunoblotting. Fig. S4 shows a transmission electron micrograph suggesting membrane permeabilization of azurophilic granules during neutrophil apoptosis. Fig. S5 illustrates the delayed spontaneous death of blood neutrophils from septic shock patients compared with control individuals. Supplemental materials and methods describes subcellular fractionation and transmission electron microscopy. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20072152/DC1.
Supplemental Material
Acknowledgments
We are indebted to the participating clinicians (S.M. Jakob, D. Simon, M. Schneider, and R. Seger) who provided blood and tissue samples from patients. We also greatly appreciate the excellent technical assistance of E. Kozlowski, I. Schmid, and V. Winkelmann.
This work was supported by the Swiss National Science Foundation (grant 310000-107526), Jubiläumsstiftung Swiss Life, and the OPO-Foundation.
The authors have no competing financial interests.
Abbreviations used: CA, CA-074-ME; CGD, chronic granulomatous disease; DPI, diphenyleneiodonium; MPO, myeloperoxidase; NADPH, nicotinamide adenine dinucleotide phosphate; PepA, pepstatin A; PI, propidium iodide; PS, phosphatidylserine; ROS, reactive oxygen species; SCLC, small cell lung carcinoma.
References
- 1.Theilgaard-Mönch, K., B.T. Porse, and N. Borregaard. 2006. Systems biology of neutrophil differentiation and immune response. Curr. Opin. Immunol. 18:54–60. [DOI] [PubMed] [Google Scholar]
- 2.Rossi, A.G., D.A. Sawatzky, A. Walker, C. Ward, T.A. Sheldrake, N.A. Riley, A. Caldicott, M. Martinez-Losa, T.R. Walker, R. Duffin, et al. 2006. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat. Med. 12:1056–1064. [DOI] [PubMed] [Google Scholar]
- 3.Simon, H.-U. 2003. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol. Rev. 193:101–110. [DOI] [PubMed] [Google Scholar]
- 4.Kataoka, T. 2005. The caspase-8 modulator c-FLIP. Crit. Rev. Immunol. 25:31–58. [DOI] [PubMed] [Google Scholar]
- 5.Daigle, I., and H.-U. Simon. 2001. Critical role for caspases 3 and 8 in neutrophil but not eosinophil apoptosis. Int. Arch. Allergy Immunol. 126:147–156. [DOI] [PubMed] [Google Scholar]
- 6.Baumann, R., C. Casaulta, D. Simon, S. Conus, S. Yousefi, and H.-U. Simon. 2003. Macrophage migration inhibitory factor delays apoptosis in neutrophils by inhibiting the mitochondria-dependent death pathway. FASEB J. 17:2221–2230. [DOI] [PubMed] [Google Scholar]
- 7.Zhang, B., J. Hirahashi, X. Cullere, and T.N. Mayadas. 2003. Elucidation of molecular events leading to neutrophil apoptosis following phagocytosis: cross-talk between caspase 8, reactive oxygen species, and MAPK/ERK activation. J. Biol. Chem. 278:28443–28454. [DOI] [PubMed] [Google Scholar]
- 8.Altznauer, F., S. Conus, A. Cavalli, G. Folkers, and H.-U. Simon. 2004. Calpain-1 regulates Bax and subsequent Smac-dependent caspase-3 activation in neutrophil apoptosis. J. Biol. Chem. 279:5947–5957. [DOI] [PubMed] [Google Scholar]
- 9.Alvarado-Kristensson, M., F. Melander, K. Leandersson, L. Rönnstrand, C. Wernstedt, and T. Andersson. 2004. p38-MAPK signals survival by phosphorylation of caspase-8 and caspase-3 in human neutrophils. J. Exp. Med. 199:449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouchard, A., C. Ratthe, and D. Girard. 2004. Interleukin-15 delays human neutrophil apoptosis by intracellular events and not via extracellular factors: role of Mcl-1 and decreased activity of caspase-3 and caspase-8. J. Leukoc. Biol. 75:893–900. [DOI] [PubMed] [Google Scholar]
- 11.Maianski, N.A., D. Roos, and T.W. Kuijpers. 2004. Bid truncation, bid/bax targeting to the mitochondria, and caspase activation associated with neutrophil apoptosis are inhibited by granulocyte colony-stimulating factor. J. Immunol. 172:7024–7030. [DOI] [PubMed] [Google Scholar]
- 12.von Gunten, S., S. Yousefi, M. Seitz, S.M. Jakob, T. Schaffner, R. Seger, J. Takala, P.M. Villiger, and H.-U. Simon. 2005. Siglec-9 transduces apoptotic and nonapoptotic death signals into neutrophils depending on the proinflammatory cytokine environment. Blood. 106:1423–1431. [DOI] [PubMed] [Google Scholar]
- 13.Bruno, A., S. Conus, I. Schmid, and H.-U. Simon. 2005. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J. Immunol. 174:8090–8096. [DOI] [PubMed] [Google Scholar]
- 14.Fecho, K., and P.L. Cohen. 1998. Fas ligand (gld) and Fas (lpr) deficient mice do not show alterations in the extravasation or apoptosis of inflammatory neutrophils. J. Leukoc. Biol. 64:373–383. [DOI] [PubMed] [Google Scholar]
- 15.Brown, S.B., and J. Savill. 1999. Phagocytosis triggers macrophage release of Fas ligand and induces apoptosis of bystander leukocytes. J. Immunol. 162:480–485. [PubMed] [Google Scholar]
- 16.Daryadel, A., R.F. Grifone, H.-U. Simon, and S. Yousefi. 2006. Apoptotic neutrophils release macrophage migration inhibitory factor upon stimulation with tumor necrosis factor-alpha. J. Biol. Chem. 281:27653–27661. [DOI] [PubMed] [Google Scholar]
- 17.Lockshin, R.A., and Z. Zakeri. 2004. Caspase-independent cell death? Oncogene. 23:2766–2773. [DOI] [PubMed] [Google Scholar]
- 18.Foghsgaard, L., D. Wissing, D. Mauch, U. Lademann, L. Bastholm, M. Boes, F. Elling, M. Leist, and M. Jäättelä. 2001. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J. Cell Biol. 153:999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guicciardi, M.E., H. Miyoshi, S.F. Bronk, and G.J. Gores. 2001. Cathepsin B knockout mice are resistant to tumor necrosis factor-alpha-mediated hepatocyte apoptosis and liver injury: implications for therapeutic applications. Am. J. Pathol. 159:2045–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberg, K., K. Kågedal, and K. Öllinger. 2002. Microinjection of cathepsin D induces caspase-dependent apoptosis in fibroblasts. Am. J. Pathol. 161:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cirman, T., K. Oresic, G.D. Mazovec, V. Turk, J.C. Reed, R.M. Myers, G.S. Salvesen, and B. Turk. 2004. Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. J. Biol. Chem. 279:3578–3587. [DOI] [PubMed] [Google Scholar]
- 22.Stoka, V., B. Turk, S.L. Schendel, T.H. Kim, T. Cirman, S.J. Snipas, L.M. Ellerby, D. Bredesen, H. Freeze, M. Abrahamson, et al. 2001. Lysosomal protease pathways to apoptosis. Cleavage of bid, not pro-caspases, is the most likely route. J. Biol. Chem. 276:3149–3157. [DOI] [PubMed] [Google Scholar]
- 23.Reiners, J.J., Jr., J.A. Caruso, P. Mathieu, B. Chelladurai, X.M. Yin, and D. Kessel. 2002. Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves Bid cleavage. Cell Death Differ. 9:934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bidere, N., H.K. Lorenzo, S. Carmona, M. Laforge, F. Harper, C. Dumont, and A. Senik. 2003. Cathepsin D triggers Bax activation, resulting in selective apoptosis-inducing factor (AIF) relocation in T lymphocytes entering the early commitment phase to apoptosis. J. Biol. Chem. 278:31401–31411. [DOI] [PubMed] [Google Scholar]
- 25.Johansson, A.-C., H. Steen, K. Öllinger, and K. Roberg. 2003. Cathepsin D mediates cytochrome c release and caspase activation in human fibroblast apoptosis induced by staurosporine. Cell Death Differ. 10:1253–1259. [DOI] [PubMed] [Google Scholar]
- 26.Kägedal, K., U. Johansson, and K. Öllinger. 2001. The lysosomal protease cathepsin D mediates apoptosis induced by oxidative stress. FASEB J. 15:1592–1594. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi, S.D., K.R. Braughton, A.R. Whitney, J.M. Voyich, T.G. Schwan, J.M. Musser, and F.R. DeLeo. 2003. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc. Natl. Acad. Sci. USA. 100:10948–10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pham, C.T. 2006. Neutrophil serine proteases: specific regulators of inflammation. Nat. Rev. Immunol. 6:541–550. [DOI] [PubMed] [Google Scholar]
- 29.Buttle, D.J., M. Murata, C.J. Knight, and A.J. Barrett. 1992. CA074 methyl ester: a proinhibitor for intracellular cathepsin B. Arch. Biochem. Biophys. 299:377–380. [DOI] [PubMed] [Google Scholar]
- 30.Roos, D., and C.C. Winterbourn. 2002. Lethal weapons. Science. 296:669–670. [DOI] [PubMed] [Google Scholar]
- 31.Maianski, N.A., F.P.J. Mul, J.D. van Buul, D. Roos, and T.W. Kuijpers. 2002. Granulocyte colony-stimulating factor inhibits the mitochondria-dependent activation of caspase-3 in neutrophils. Blood. 99:672–679. [DOI] [PubMed] [Google Scholar]
- 32.Shi, Y., M. Honma, and F. Koizumi. 1998. Cutaneous allergic vasculitis: clinicopathological characterization and identification of apoptosis. Pathol. Int. 48:705–716. [DOI] [PubMed] [Google Scholar]
- 33.Boatright, K.M., and G.S. Salvesen. 2003. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 15:725–731. [DOI] [PubMed] [Google Scholar]
- 34.Sohn, D., K. Schulze-Osthoff, and R.U. Jänicke. 2005. Caspase-8 can be activated by interchain proteolysis without receptor-triggered dimerization during drug-induced apoptosis. J. Biol. Chem. 280:5267–5273. [DOI] [PubMed] [Google Scholar]
- 35.Savill, J.S., A.H. Wyllie, J.E. Henson, M.J. Walport, P.M. Henson, and C. Haslett. 1989. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Invest. 83:865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knapp, S., U. Matt, N. Leitinger, and T. van der Poll. 2007. Oxidized phospholipids inhibit phagocytosis and impair outcome in gram-negative sepsis in vivo. J. Immunol. 178:993–1001. [DOI] [PubMed] [Google Scholar]
- 37.Kasahara, Y., K. Iwai, A. Yachie, K. Ohta, A. Konno, H. Seki, T. Miyawaki, and N. Taniguchi. 1997. Involvement of reactive oxygen intermediates in spontaneous and CD95 (Fas/APO-1)-mediated apoptosis of neutrophils. Blood. 89:1748–1753. [PubMed] [Google Scholar]
- 38.Martinelli, S., M. Urosevic, A. Daryadel, P.A. Oberholzer, C. Baumann, M.F. Fey, R. Dummer, H.-U. Simon, and S. Yousefi. 2004. Induction of genes mediating interferon-dependent extracellular trap formation during neutrophil differentiation. J. Biol. Chem. 279:44123–44132. [DOI] [PubMed] [Google Scholar]
- 39.Brinkmann, V., U. Reichard, C. Goosmann, B. Fauler, Y. Uhlemann, D.S. Weiss, Y. Weinrauch, and A. Zychlinsky. 2004. Neutrophil extracellular traps kill bacteria. Science. 303:1532–1535. [DOI] [PubMed] [Google Scholar]
- 40.Knepper-Nicolai, B., J. Savill, and S.B. Brown. 1998. Constitutive apoptosis in human neutrophils requires synergy between calpains and the proteasome downstream of caspases. J. Biol. Chem. 273:30530–30536. [DOI] [PubMed] [Google Scholar]
- 41.Blomgran, R., L. Zheng, and O. Stendahl. 2007. Cathepsin-cleaved Bid promotes apoptosis in human neutrophils via oxidative stress-induced lysosomal membrane permeabilization. J. Leukoc. Biol. 81:1213–1223. [DOI] [PubMed] [Google Scholar]
- 42.Beaujouin, M., S. Baghdiguian, M. Glondu-Lassis, G. Berchem, and E. Liaudet-Coopman. 2006. Overexpression of both catalytically active and inactive cathepsin D by cancer cells enhances apoptosis-dependent chemo-sensitivity. Oncogene. 25:1967–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foghsgaard, L., U. Lademann, D. Wissing, B. Poulsen, and M. Jäättelä. 2002. Cathepsin B mediates tumor necrosis factor-induced arachidonic acid release in tumor cells. J. Biol. Chem. 277:39499–39506. [DOI] [PubMed] [Google Scholar]
- 44.Jäättelä, M., and J. Tschopp. 2003. Caspase-independent cell death in T lymphocytes. Nat. Immunol. 4:416–423. [DOI] [PubMed] [Google Scholar]
- 45.Gottlieb, R.A., J. Nordberg, E. Skowronski, and B.M. Babior. 1996. Apoptosis induced in Jurkat cells by several agents is preceded by intracellular acidification. Proc. Natl. Acad. Sci. USA. 93:654–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuyama, S., J. Llopis, Q.L. Deveraux, R.Y. Tsien, and J.C. Reed. 2000. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat. Cell Biol. 2:318–325. [DOI] [PubMed] [Google Scholar]
- 47.Shi, Y. 2004. Caspase activation, inhibition, and reactivation: A mechanistic view. Protein Sci. 13:1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy, B.M., E.M. Creagh, and S.J. Martin. 2004. Interchain proteolysis, in the absence of a dimerization stimulus, can initiate apoptosis-associated caspase-8 activation. J. Biol. Chem. 279:36916–36922. [DOI] [PubMed] [Google Scholar]
- 49.Kågedal, K., A.C. Johansson, U. Johansson, G. Heimlich, K. Roberg, N.S. Wang, J.M. Jurgensmeier, and K. Ollinger. 2005. Lysosomal membrane permeabilization during apoptosis-involvement of Bax? Int. J. Exp. Pathol. 86:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nylandsted, J., M. Gyrd-Hansen, A. Danielewicz, N. Fehrenbacher, U. Lademann, M. Hoyer-Hansen, E. Weber, G. Multhoff, M. Rohde, and M. Jäättelä. 2004. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J. Exp. Med. 200:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halangk, W., M.M. Lerch, B. Brandt-Nedelev, W. Roth, M. Ruthenbuerger, T. Reinheckel, W. Domschke, H. Lippert, C. Peters, and J. Deussing. 2000. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J. Clin. Invest. 106:773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saftig, P., M. Hetman, W. Schmahl, K. Weber, L. Heine, H. Mossmann, A. Köster, B. Hess, M. Evers, K. von Figura, et al. 1995. Mice deficient for the lysosomal proteinase cathepsin D exhibit progressive atrophy of the intestinal mucosa and profound destruction of lymphoid cells. EMBO J. 14:3599–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levy, M.M., M.P. Fink, J.C. Marshall, E. Abraham, D. Angus, D. Cook, J. Cohen, S.M. Opal, J.-L. Vincent, and G. Ramsay. 2003. 2001 SCCM/ESICM/ACCP/ATS/SIS International sepsis definitions conference. Crit. Care Med. 31:1250–1256. [DOI] [PubMed] [Google Scholar]
- 54.Sasmono, R.T., A. Ehrnsperger, S.L. Cronau, T. Ravasi, R. Kandane, M.J. Hickey, A.D. Cook, S.R. Himes, J.A. Hamilton, and D.A. Hume. 2007. Mouse neutrophilic granulocytes express mRNA encoding the macrophage colony-stimulating factor receptor (CSF-1R) as well as many other macrophage-specific transcripts and can transdifferentiate into macrophages in vitro in response to CSF-1. J. Leukoc. Biol. 82:111–123. [DOI] [PubMed] [Google Scholar]
- 55.Hopkins-Donaldson, S., A. Ziegler, S. Kurtz, C. Bigosch, D. Kandioler, C. Ludwig, U. Zangemeister-Wittke, and R. Stahel. 2003. Silencing of death receptor and caspase-8 expression in small cell lung carcinoma cell lines and tumors by DNA methylation. Cell Death Differ. 10:356–364. [DOI] [PubMed] [Google Scholar]
- 56.Altznauer, F., S. Martinelli, S. Yousefi, C. Thürig, I. Schmid, E.M. Conway, M.H. Schöni, P. Vogt, C. Mueller, M.F. Fey, et al. 2004. Inflammation-associated cell cycle–independent block of apoptosis by survivin in terminally differentiated neutrophils. J. Exp. Med. 199:1343–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lauber, K., H.A. Appel, S.F. Schlosser, M. Gregor, K. Schulze-Osthoff, and S. Wesselborg. 2001. The adapter protein apoptotic protease-activating factor-1 (Apaf-1) is proteolytically processed during apoptosis. J. Biol. Chem. 276:29772–29781. [DOI] [PubMed] [Google Scholar]
- 58.Costes, S.V., D. Daelemans, E.H. Cho, Z. Dobbin, G. Pavlakis, and S. Lockett. 2004. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys. J. 86:3993–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yousefi, S., R. Perozzo, I. Schmid, A. Ziemiecki, T. Schaffner, L. Scapozza, T. Brunner, and H.-U. Simon. 2006. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat. Cell Biol. 8:1124–1132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.