Abstract
There is unequivocal evidence that the combination of an inhaled corticosteroid (ICS)—i.e. glucocorticoid—and an inhaled long-acting β2-adrenoceptor agonist (LABA) is superior to each component administered as a monotherapy alone in the clinical management of asthma. Moreover, Calverley and colleagues (Lancet 2003, 361: 449–456; N Engl J Med 2007, 356: 775–789) reporting for the ‘TRial of Inhaled STeroids ANd long-acting β2-agonists (TRISTAN)' and ‘TOwards a Revolution in COPD Health (TORCH)' international study groups also demonstrated the superior efficacy of LABA/ICS combination therapies over ICS alone in the clinical management of chronic obstructive pulmonary disease. This finding has been independently confirmed indicating that the therapeutic benefit of LABA/ICS combination therapies is not restricted to asthma and may be extended to other chronic inflammatory diseases of the airways. Despite the unquestionable benefit of LABA/ICS combination therapies, there is a vast gap in our understanding of how these two drugs given together deliver superior clinical efficacy. In this article, we review the history of LABA/ICS combination therapies and critically evaluate how these two classes of drugs might interact at the biochemical level to suppress pro-inflammatory responses. Understanding the molecular basis of this fundamental clinical observation is a Holy Grail of current respiratory diseases research as it could permit the rational exploitation of this effect with the development of new ‘optimized' LABA/ICS combination therapies.
Keywords: combination therapy, asthma management, chronic obstructive pulmonary disease, salmeterol/fluticasone (Seretide®/Advair®), formoterol/budesonide (Symbicort®), transactivation, p57kip2, toward a revolution in COPD health, mitogen-activated protein kinase phosphatase, glucocorticoid-inducible leucine zipper
Asthma and chronic obstructive pulmonary disease—a brief introduction
Asthma
Asthma is a chronic inflammatory disease characterized by reversible airways obstruction, airways remodelling and nonspecific airways hyperresponsiveness (AHR) (Johnson et al., 2001; James, 2005). The inflammation in asthma is multifaceted, often allergic and involves both infiltrating leucocytes and structural cells. CD4+ T lymphocytes are believed to play a pivotal pathogenic role; they elicit a Th2-driven inflammatory responses and, through orchestrating pulmonary eosinophil recruitment, may contribute to airways remodelling, which is a cardinal feature of this disease (Cho et al., 2004; Humbles et al., 2004; Williams, 2004). The prevalence of asthma is rising worldwide and this has become the most common chronic disease in industrialized nations with 5–10% of the population being affected (Gendo and Lodewick, 2005). In the United States, asthma attacks are a primary cause of hospitalization—497 000 discharges due to asthma were recorded in 2004—and, in the preceding year, were responsible for 4099 deaths (Krishnan et al., 2006) (see www.lungusa.org/site/apps/s/content.asp?c=dvLUK9O0E&b=34706&ct=3224077).
Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is a nonspecific term that embraces several debilitating inflammatory pathologies that often coexist, and is characterized by a slowly progressive and largely irreversible decrement in lung function (Barnes, 2000; Hogg et al., 2004; Hogg, 2004). Persistent chronic airflow limitation, usually associated with airway collapse, oedema, mucus hypersecretion and fibrosis, is present to a greater or lesser extent and accounts for the wide spectrum of disease (Barnes, 2000; Hogg et al., 2004; Hogg, 2004). COPD afflicts middle-aged and elderly people (Briggs, 2004) and is caused, in approximately 95% of all cases, by chronic cigarette smoking, although other environmental insults such as the burning of biomass fuels are also major risk factors (Barnes et al., 2003; Rabe et al., 2007b). A recent systematic review and meta-analysis of studies performed in 28 countries between 1994 and 2004 provides compelling evidence that the prevalence of COPD is appreciably higher in individuals who smoke or who are ex-smokers when compared to subjects who have never smoked (Halbert et al., 2006). Accordingly, COPD is largely preventable and, in the early stages, its progression can be arrested by smoking cessation. Unfortunately, the highly addictive properties of nicotine in tobacco render most individuals unable to renounce cigarette smoking despite intensive interventions.
COPD is believed to be a neutrophilic inflammatory disorder of the small airways and lungs (cf. asthma) that is perpetuated, in part, by macrophages and possibly CD8+ T lymphocytes, particularly those of the Tc2 subset (Shapiro, 1999; Barnes, 2000; Chung, 2001; Jeffery, 2001a). In addition to airways inflammation, many individuals with COPD also demonstrate systemic inflammation that is positively associated with disease severity (Sin and Man, 2007). As in asthma, airways remodelling and AHR are characteristic features of COPD (Hossain and Heard, 1970; Jeffery, 2001b; Aoshiba and Nagai, 2004; Hogg et al., 2004; Hogg, 2004). COPD has a long latency before symptoms become evident, afflicts 9–10% of the population globally over the age of 40 years (Halbert et al., 2006) and is the only cause of mortality that is increasing worldwide (Jemal et al., 2005). Indeed, by 2020, the Global Burden of Disease Study predicts that COPD will be the third leading cause of death driven by the expanding epidemic of cigarette smoking and changing demographics, with more of the global population living longer (Murray and Lopez, 1997; Lopez et al., 2006). Even in 2007, COPD is under-recognized, under-diagnosed and under-reported (Shahab et al., 2006). Accordingly, COPD is under-treated (Rabe et al., 2007a).
Current treatment options for asthma and COPD
Inhaled corticosteroids (ICSs) or, more correctly, glucocorticoids are the most efficacious therapy currently available for the control of asthma (see www.ginasthma.org; Barnes, 2004). These drugs control symptoms, reduce exacerbations and improve health status in most patients irrespective of disease severity (Barnes, 2006a). Accordingly, ICSs are the first-line therapy for all patients who use a β2-adrenoceptor agonist inhaler more than once a day, and this is reflected in all national and international guidelines for asthma management (see www.ginasthma.org/Guidelineitem.asp??l1=2&l2=1&intId=60; NHLBI, 1997, 2002; BTS/SIGN, 2003; Lemiere et al., 2004). The efficacy of ICSs is due primarily to the suppression of airways inflammation and associated AHR. Acting via the glucocorticoid receptor (GR), ICSs repress the expression of inflammatory cytokines, their receptors, adhesion molecules and other disease-inducing mediators. These effects of ICSs, and their ability to promote apoptosis of many cell types including the eosinophil, act to reduce pulmonary leucocyte burden and attenuate inflammation (Barnes, 2001; Belvisi, 2004; Georas, 2004). Of particular relevance is that ICSs suppress the pro-inflammatory activity of airway epithelial cells and the underlying smooth muscle, for which profound pro-inflammatory roles are now recognized (Barnes, 1996; Schwiebert et al., 1996; Panettieri, 2004). Indeed, the ability of epithelial and smooth muscle cells to elaborate lipids, chemokines, cytokines and pro-fibrotic mediators makes these tissues primary and critical targets for the anti-inflammatory actions of ICSs (Barnes, 1996; Schwiebert et al., 1996).
Unlike in individuals with asthma, ICSs appear to be of relatively limited benefit in subjects with COPD. Although there is some evidence that certain indices of pulmonary inflammation (for example interleukin (IL)-8 levels in bronchoalveolar lavage fluid, inducible nitric oxide synthase expression) are suppressed by ICSs (reviewed in Sin and Man, 2007), the effect on mortality is far from clear (see Sin and Tu, 2001; Soriano et al., 2002; Fan et al., 2003; Sin and Man, 2003; Suissa, 2003; Kiri et al., 2005; Tkacova et al., 2006). Currently, there is only one published prospective trial of ICSs in COPD that has been powered on mortality and, contrary to retrospective analyses (Sin et al., 2005; Macie et al., 2006), that study demonstrated that the rate of death in subjects taking the ICS fluticasone propionate alone did not differ from placebo and, in fact, showed an increased incidence of pneumonia (Calverley et al., 2007). In contrast, ICSs have unequivocal and clinically meaningful anti-inflammatory efficacy in subjects with asthma and also significantly reduce mortality (Panizza et al., 2006).
Despite the utility of ICSs in the management of asthma, there is now persuasive clinical evidence, supported by basic science, that chronic cigarette smoking in someway renders asthmatic individuals relatively refractory to the anti-inflammatory actions of ICSs (Chalmers et al., 2001, 2002; Thomson et al., 2004, 2006; Thomson and Spears, 2005). It has been estimated that 28–30% of individuals with asthma smoke cigarettes on a regular basis and that in these individuals, the decline in lung function with age is more rapid than in asthmatic subjects who do not smoke (Lange et al., 1998; James et al., 2005). Accordingly, disease control in smoking asthmatics is more difficult to manage pharmacotherapeutically (Gilliland et al., 2006). Glucocorticoid insensitivity in asthmatic smokers is also displayed systemically (Livingston et al., 2007). Thus, cutaneous vasoconstriction measured as skin blanching following topical administration of glucocorticoids is significantly reduced in asthmatic individuals who smoke when compared to asthmatic subjects who have never smoked (Livingston et al., 2007). Significantly, both pulmonary and extra-pulmonary insensitivity to glucocorticoids is restored in individuals who stop smoking (Chaudhuri et al., 2006) indicating that cigarette smoke blocks, at least in part, glucocorticoid action in a potentially reversible manner. These data from asthmatic individuals who smoke strongly imply that the relative resistance of subjects with COPD to the anti-inflammatory activity of ICSs is due either to constituents of cigarette smoke per se or, more likely, to the presence of a cigarette smoke-induced neutrophilic (that is, non-eosinophilic) inflammation that is relatively resistant to the beneficial effects of ICSs (Green et al., 2002; Siva et al., 2007). However, it is currently unclear whether the presence of neutrophilic airways inflammation predicts a relative lack of clinical response to ICS/long-acting β2-adrenoceptor agonist (LABA) combination therapies and this requires thorough investigation.
LABA/ICS combination therapies: the seminal clinical observation
In 1994, Greening et al. conducted a double-blind, parallel group trial of 6-months duration in 426 asthmatic subjects who were symptomatic despite maintenance therapy with the ICS, beclomethasone dipropionate (BDP; 200 μg b.i.d.). Subjects were randomized to receive salmeterol xinafoate (50 μg b.i.d.) and BDP (200 μg b.i.d.; n=220) delivered via separate inhaler devices, or BDP alone at a higher dose of 500 μg (b.i.d.; n=206). Both treatment interventions significantly improved lung function (that is, mean morning peak expiratory flow rate (PEFR)), but the LABA/ICS combination therapy was superior at all time points. Other end points that favoured salmeterol/BDP over high-dose BDP alone included diurnal variation in PEFR, daytime and night time symptoms, and rescue bronchodilator consumption. Significantly, there was no difference between the two treatment groups in exacerbation rate indicating that salmeterol, given chronically with BDP, was not associated with any risk of asthma deterioration over the duration of the study. Thus, the addition of salmeterol to a standard dose of BDP was more effective clinically than increasing, by 2.5-fold, the dose of BDP (Greening et al., 1994).
Confirmation of effect
The superior clinical benefit of salmeterol and BDP given in combination was confirmed subsequently in subjects with more severe asthma in whom symptoms were not controlled on BDP (500 μg b.i.d.) or equivalent (Woolcock et al., 1996). In this double-blind, parallel group study of 6-months duration, 738 subjects at 72 centres were randomized to receive either salmeterol (50 or 100 μg b.i.d.) in combination with BDP (500 μg b.i.d.), delivered by separate inhaler devices, or BDP alone at a higher dose (1000 μg b.i.d.). Consistent with the results of Greening et al. (1994), subjects taking either dose of salmeterol showed mean improvements of >45 and >30 l min−1 in their morning and evening PEFR, respectively, which was markedly superior to that achieved in individuals on the higher dose of BDP only (PEFR morning: 16 l min−1; PEFR evening 6 l min−1). Moreover, rescue bronchodilator use and symptoms in those subjects taking either dose of salmeterol were significantly lower when compared with individuals in the BDP (1000 μg b.i.d.) treatment group. There was no significant difference in the clinical benefit afforded by the two doses of salmeterol, suggesting that the higher dose (100 μg b.i.d.) is supra-maximal. Exacerbation rates did not differ among the three treatment groups confirming, again, that there was no deterioration in asthma control in those individuals taking the combination therapy. Thus, the addition of salmeterol to BDP was more effective clinically than doubling the dose of BDP.
A possible criticism of these findings is that the design of many trials that have evaluated the efficacy of ICS/LABA combination therapies was biased towards finding a clinically significant effect. Thus, one of the inclusion criteria required for these clinical studies was the presence of significant (⩾12%) post-bronchodilator reversibility (Pauwels et al., 1997; O'Byrne et al., 2001; Bateman et al., 2004; Rabe et al., 2006). However, many patients with well-controlled asthma have normal or near-normal spirometry, and consequently the results from these studies may not necessarily be applicable to such patients. However, if one accepts that a fundamental characteristic of asthma is the presence of variable airflow limitation, then it is reasonable for investigators to list the presence of post-bronchodilator reversibility as an inclusion criterion for their clinical studies. Accordingly, the results of such trials are generally applicable to the broader population of patients with asthma. Moreover, studies of pharmacotherapies in asthma have generally used post-bronchodilator reversibility as an inclusion criterion, suggesting that any observed benefits, including those with ICS and LABA combination therapies, may only be generalized to those patients with suboptimal asthma control and ongoing evidence of variable airflow limitation. The reported clinical benefits may not necessarily hold true for individuals with well-controlled asthma who have normal or near-normal lung function. Conversely, asthmatic subjects with chronic airflow limitation who do not demonstrate significant post-bronchodilator reversibility will also have been excluded from such studies. Therefore, further targeted clinical trials with this population of asthmatic subjects are warranted.
Superiority of LABA/ICS combination therapy is class specific
Since the seminal report of Greening et al. (1994), many trials have been conducted comparing the clinical effectiveness in asthma of LABA/ICS combination therapies with a higher dose of an ICS alone. What has emerged, unambiguously, is that the clinical superiority of salmeterol and BDP in combination over higher dose ICS alone is unequivocal and class specific (that is, it is not peculiar to salmeterol or BDP, but a generic effect of LABAs and ICSs when used in combination). Indeed, meta-analyses of nine parallel group trials in which the clinical efficacy and safety of salmeterol in combination with either BDP or fluticasone were assessed and compared to a higher dose of ICS given as monotherapy led Shrewsbury et al. (2000) to conclude that ‘….giving salmeterol to patients who have symptoms on at least 400 μg BDP per day will result in better lung function, better control of symptoms, less need for rescue medication, and fewer exacerbations than increased doses of inhaled steroid'. This conclusion was confirmed again in a multicentre, double-blind, parallel group study of 6-months duration involving 496 symptomatic asthmatic patients with a history of exacerbations on ICS (500–800 μg b.i.d.). In that trial, Ind et al. (2003) demonstrated that adding salmeterol (50 μg b.i.d.) to fluticasone propionate (250 μg b.i.d.) was clinically superior to doubling the dose of the glucocorticoid. Thus, the salmeterol/fluticasone combination significantly improved mean morning PEFR by 42.1 l min−1, which was more than twice the improvement achieved with fluticasone given as a monotherapy at either 250 μg (b.i.d.) or 500 μg (b.i.d.). Similar data in favour of the combination therapy were also obtained when symptoms and exacerbation rate were used as end points (Ind et al., 2003). The results of the more recent Gaining Optimal Asthma controL (GOAL) study has also confirmed the superiority of salmeterol/fluticasone in combination in achieving greater asthma control than the glucocorticoid alone (Bateman et al., 2004).
The clinical significance of these finding was endorsed further when the landmark FACET (Formoterol And Corticosteroids Establishing Therapy) study established that combination therapy was also clinically superior to glucocorticoid alone when exacerbation rate was used as the primary outcome measure. Indeed, the exacerbation rate in subjects with moderately severe asthma was lower when the LABA, formoterol (9 μg b.i.d.), was added to low and high dose of another glucocorticoid, budesonide (that is 100 or 400 μg b.i.d.) when compared to the ICS alone (Pauwels et al., 1997). The clinical superiority of LABA/ICS combination therapies also extends to subjects with mild persistent asthma in whom the optimal treatment regime is uncertain. Thus, the OPTIMA (Oxis and Pulmicort Turbuhaler In the Management of Asthma) study convincingly demonstrated that adding formoterol (4.5 μg b.i.d.) to low-dose budesonide (100 μg b.i.d.) for 1 year in subjects with mild asthma was more effective than doubling the dose of ICS in increasing the time to first severe asthma exacerbation (O'Byrne et al., 2001).
Delivery of LABA/ICS combination therapies by a single inhaler device
Following confirmation of the efficacy and safety of LABAs and ICSs administered in combination by separate inhaler devices, a number of studies were performed comparing the efficacy and safety of salmeterol/fluticasone (Seretide/Advair; GlaxoSmithKline, Stevenage, UK) and formotetol/budesonide (Symbicort; AstraZeneca, Lund, Sweden) delivered by single inhaler devices (reviewed by Miller-Larsson and Selroos, 2006). Although differences in the effectiveness of the combination therapies between the different methods of administration were reported in some studies, the balance of evidence demonstrated similar efficacy and safety profiles irrespective of whether the components of the combination were delivered in single or separate inhalers (Zetterstrom et al., 2001; Nelson et al., 2003). Accordingly, these single inhaler devices are now increasingly used in asthma management (Lyseng-Williamson and Plosker, 2003; Goldsmith and Keating, 2004).
Formoterol/budesonide as a maintenance and rescue therapy—a SMART approach to asthma management
In 2005, O'Byrne et al. reported the results of a double-blind, randomized, parallel group study of 12-months duration (the Single Therapy in Adults and Young children (STAY) study), which evaluated, in subjects with moderate to severe asthma, whether replacing a short-acting β2-adrenoceptor agonist rescue medication (terbutaline) with the combination of budesonide/formoterol as reliever would provide both rapid symptom relief and reduce asthma exacerbations (O'Byrne et al., 2005). The results of that study, which involved 2760 subjects, demonstrated that, when formoterol/budesonide was used both as a maintenance therapy and on an as-needed basis for symptom control, the improvement in lung function, symptoms and time to first exacerbation (the primary efficacy outcome measure of the study) were greater than when formoterol/budesonide or high-dose budesonide alone were used as a maintenance therapy, with terbutaline, in both cases, as a rescue medication. Significantly, the clinical benefit was also seen in children with asthma (Bisgaard et al., 2006) where the efficacy of combination therapies is, historically, less convincing (Zimmerman et al., 2004). Subsequent studies have revealed that this beneficial effect of budesonide/formoterol as maintenance and reliever requires both components of the combination (Rabe et al., 2006). Collectively, these data suggest that formoterol/budesonide in a single inhaler can be used for maintenance and rescue and has been described as the SMART (Symbicort for Maintenance And Reliever Therapy) approach to asthma management (Bisgaard et al., 2006).
Towards a revolution in COPD health
In 2000, the first subject was recruited to participate in a large (n=6112), prospective, randomized trial of 3-years duration that was powered to compare against placebo (n=1523) the effect of salmeterol (50 μg b.i.d.; n=1521) and fluticasone (500 μg b.i.d.; n=1534) alone as mono-therapies, and in combination (n=1533), on clinical efficacy and all-cause mortality in COPD (Vestbo, 2004). The results of that study were reported in February 2007 (Calverley et al., 2007). Compared to placebo, the combination therapy significantly reduced exacerbation rates and improved quality of life and spirometry. The combination therapy also reduced the risk of dying from any cause by 17.5%, although the pre-determined level of statistical significance was missed by just 0.2% points (P=0.052). Although it is possible that the salmeterol/fluticasone combination does not prolong life and that the beneficial effects on exacerbation rates, lung function and health status are independent of mortality in COPD, the study may simply have been underpowered to detect a statistically significant difference in the primary end point of patient mortality. Indeed, as a part of the study protocol, interim analyses were performed, which increased the threshold required for significance (Calverley et al., 2007). It is noteworthy that the results of a recent Cochrane review (Nannini et al., 2007) indicating that ICS and LABA combination therapy significantly reduces all-cause mortality in COPD certainly supports the view that the TOwards a revolution in COPD health (TORCH) study was underpowered to detect a statistically significant difference in the primary outcome. Thus, on balance and statistics aside, the results of TORCH should be considered within these limitations and in context with several other studies that have demonstrated the superior clinical efficacy of LABA/ICS combinations relative to either drug alone on lung function, quality of life, dyspnoea, rescue medication consumption, symptoms and exacerbations (see for example, Mahler et al., 2002; Calverley et al., 2003a, 2003b; Hanania et al., 2003; Szafranski et al., 2003).
Implications for treatment
Based upon the results of a large number of clinical trials, the recently updated GINA (Global INitiative for Asthma) guidelines now recommend combination therapies for asthma where symptoms are not adequately controlled on low-dose ICS (see http://www.ginasthma.org/Guidelineitem.asp??l1=2&l2=1&intId=60). Indeed, a significant proportion of asthmatic subjects who are treated with a moderate dose of ICS remain symptomatic (FitzGerald et al., 2006; Peters et al., 2007). In addition, the SMART approach to the treatment of moderate to severe asthma is gaining general acceptance and is already recommended as a treatment option in Canada, Australia and the European Union (Buhl and Vogelmeier, 2007; O'Byrne, 2007). With regard to COPD, the results of the TORCH study found that the combination of fluticasone and salmeterol was clinically superior to the efficacy of either agent given alone as a monotherapy (Calverley et al., 2007). Although the reduction in all-cause mortality just missed statistical significance (see above), it seems likely given the clear benefit on lung function, quality of life, dyspnoea, rescue medication consumption, symptoms and exacerbations that current international guidelines will eventually be amended to endorse the use of LABA/ICS combinations alone or concurrently with the long-acting muscarinic receptor antagonist (aka antimuscarinic), tiotropium bromide (Spiriva) (Aaron et al., 2007) for the treatment of moderate/severe COPD.
The interaction of LABAs and ICSs: additive or synergistic?
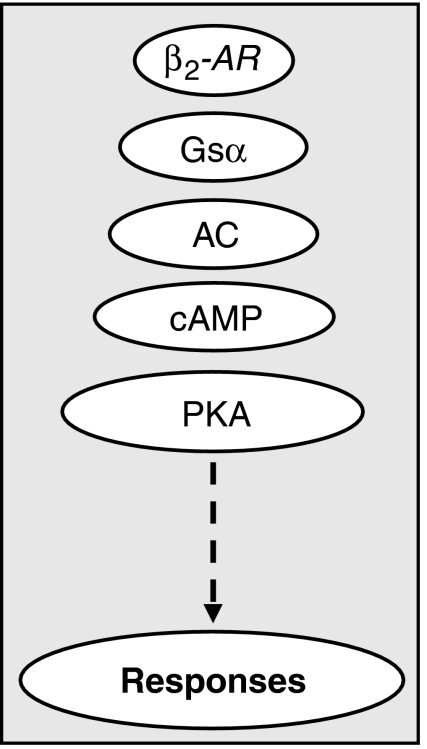
Despite the therapeutic advantages of LABA/ICS combination therapies, the mechanistic basis for their superior efficacy remains vague. According to traditional dogma, LABAs bind to cell surface β2-adrenoceptors and augment the activity of one or more isoforms of adenylyl cyclase by a Gsα-dependent mechanism. This catalysis increases the intracellular concentration of cAMP and activates cAMP-dependent protein kinase (PKA) with ultimate functional consequences that include bronchodilatation (Figure 1) (Giembycz and Newton, 2006). In the context of this classical pathway, there is good evidence that ICSs improve β2-adrenoceptor-mediated signalling in the lung. Indeed, glucocorticoids increase β2-adrenoceptor density (Mak et al., 1995a, 1995b), reduce functional desensitization of the receptor (Chong et al., 1997) and enhance both Gsα expression and coupling to adenylyl cyclase (Kalavantavanich and Schramm, 2000). Glucocorticoids also prevent the ability of pro-inflammatory cytokines (for example IL-1β) to render tissues such as human airways smooth muscle (HASM) hyporesponsive to β2-adrenoceptor agonists. Mechanistically, this is may relate to their ability to block the induction of COX-2 and the subsequent synthesis of prostaglandin E2, which is able to produce heterologous desensitization of several receptors that couple through Gs, including the β2-adrenoceptor (Pang et al., 1998; Moore et al., 1999). In contrast, the mechanism(s) by which LABAs enhance GR-mediated signalling is largely unexplored.
Figure 1.
Classical β2-adrenoceptor (β2-AR) signalling in which the β2-AR couples via Gsα to adenylyl cyclase (AC) increases the formation of cAMP and activates PKA to elicit responses, such as the enhancement of GRE-dependent transcription. GRE, glucocorticoid response element; PKA, cAMP-dependent protein kinase.
Data obtained with several different primary cells and cell lines demonstrate that LABAs and glucocorticoids inhibit a variety of pro-inflammatory responses and that, in combination, additivity is usually, but not exclusively, seen. Thus, salmeterol and formoterol attenuate IL-1β- and tumour necrosis factor (TNF)-α-induced intercellular adhesion molecule-1 and vascular adhesion molecule-1 expression on human lung fibroblast and this effect is enhanced, in an additive manner, by fluticasone and budesonide, respectively (Silvestri et al., 2001; Spoelstra et al., 2002). Formoterol and budesonide also block serum-induced proteoglycan production from human lung fibroblasts and a seemingly synergistic effect is seen when both drugs are used in combination (Todorova et al., 2006). In human airway epithelial cells, budesonide can suppress TNFα-induced granulocyte/macrophage colony-stimulating factor release by a mechanism that is enhanced by formoterol (Korn et al., 2001; Lovén et al., 2007). Similar effects have been reported with salmeterol on the inhibition, by fluticasone, of both IL-8 and eotaxin release from HASM cells (Pang and Knox, 2000, 2001). Budesonide and formoterol have also been shown to inhibit the generation of superoxide anions from human eosinophils and that a combination of both drugs is more effective than budesonide alone (Persdotter et al., 2007). In the cells present in induced sputum, formoterol and salmeterol may enhance beclomethasone-induced translocation of GR to the nucleus and suppress the release of RANTES (regulated upon activation, normal T cell-expressed and -secreted) and IL-8 (aka CCL5 and CXCL8, respectively) (Profita et al., 2005).
These general findings have been extended to studies in which viruses were used as stimuli to assess the potential efficacy of ICS/LABA combination therapies in exacerbations of asthma and COPD. Thus, exposure of BEAS-2B cells (a normal human bronchial epithelial cell line infected with a hybrid virus composed of adenovirus 12 and simian virus 40) to rhinovirus resulted in the elaboration of two factors putatively involved in airway remodelling (vascular endothelial growth factor, fibroblast growth factor-2) by a mechanism that was inhibited by both fluticasone and, to a lesser extent, salmeterol. Significantly, these two drugs acted synergistically when used in combination (Volonaki et al., 2006). Fluticasone and salmeterol have also been shown to inhibit, in an additive or even synergistic manner, rhinovirus-induced chemokine (RANTES, IL-8, IP-10 (interferon-γ-inducible protein of 10 kDa; aka CXCL10), ENA-78 (epithelial cell-derived neutrophil-activating peptide of 78 amino acids, aka CXCL5)) release from BEAS-2B cells (Edwards et al., 2006), and this enhanced efficacy seemingly can be extrapolated to in vivo systems (Singam et al., 2006). Indeed, the AHR and inflammation in sensitized mice exposed to respiratory syncytial virus is more effectively suppressed by the combination of salmeterol and fluticasone than either drug alone. Finally, recent in vitro studies have established that the rate of proliferation of HASM cells is negatively regulated by glucocorticoids and a transcription factor, CAATT-enhancer binding protein-α, that is activated by LABAs (Roth et al., 2002). At low concentrations, the two drugs in combination interact in an apparently synergistic manner to suppress proliferation by increasing the expression of a negative cell cycle regulator, p21(Waf1/Cip1) (Roth et al., 2002). These are intriguing data as the rate of proliferation of HASM cells taken from asthmatic subjects is higher relative to cells retrieved from individuals without asthma (Johnson et al., 2001) and is attributable to a deficiency, in the asthmatic myocyte, of CAATT-enhancer binding protein-α (Roth et al., 2004). Although, collectively, these studies demonstrate that a glucocorticoid and a LABA in combination suppress indices of inflammation in an additive or, possibly, synergistic manner, little information has been published on the underlying molecular mechanism(s). At least two plausible theories that are not, necessarily, mutually exclusive may account for the clinical superiority of LABA/ICS combination therapies over ICSs alone:
LABAs and ICSs activate mechanistically-distinct pathways that combine to produce an additive response
LABAs augment the activity of ICSs through a common mechanism(s) to produce a synergistic response
Evidence for which of these premises might be more important in explaining the enhanced efficacy of LABA/ICS combination therapies comes from clinical studies. Thus, despite the ability of LABAs to suppress in vitro several indices of inflammation (see section above), they do not evoke significant (clinically-relevant) anti-inflammatory effects in vivo when given as a monotherapy (Roberts et al., 1999; Howarth et al., 2000). This fact argues against separate mechanisms that combine to elicit an additive effect as being the only and more important mode of action. Conversely, Pauwels et al. (1997), reporting for the FACET International Study Group, found that formoterol reduced exacerbation rate and asthma severity in patients taking inhaled budesonide to a greater degree that those subjects who received the same dose of budesonide as a single medication. Those data suggest that, contrary to masking the underlying inflammation (a concern when β2-adrenoceptor agonists are administered chronically as a monotherapy (reviewed by Sears and Taylor, 1994)), LABAs enhance the clinical efficacy of ICSs to a level that cannot be achieved by the ICS alone. Although there are few in vivo studies in human asthmatic subjects demonstrating that the combination of an ICS and a LABA delivered by a single inhaler device has additive, or even synergistic, anti-inflammatory effects compared with either compound alone, such data are emerging. For example, Duong et al. (2007) confirmed that the combination of budesonide and formoterol was significantly better than either component alone in attenuating the late asthmatic response and AHR (measured as methacholine provocative concentration increasing airways resistance by 20%) following allergen challenge. These data are consistent with the results of an earlier study conducted by O'Conner et al. (2006), which found that the combination of budesonide and formoterol reduced sputum eosinophilia, measured 7 h post-allergen challenge, by a significantly greater degree than achieved by budesonide alone. Moreover, in COPD, persuasive evidence is available that the superiority of the combination therapy is due to enhanced anti-inflammatory activity (Barnes et al., 2006; Bourbeau et al., 2007). Thus, although ICSs given as a monotherapy to subjects with COPD do not reduce key pro-inflammatory cell numbers resident within the lung (Keatings et al., 1996; Gizycki et al., 2002; Hattotuwa et al., 2002), a significant widespread anti-inflammatory activity has been noted with a LABA/ICS combination therapy in both airway biopsies and in induced sputum (Barnes et al., 2006). Although these results do not exclude the possibility that a component of the enhanced clinical efficacy of ICS/LABA combinations is due to direct bronchodilatation/bronchoprotection, this cannot be the sole mechanism given the increasing evidence that combination therapies provide added clinical benefit, in terms of attenuating both direct and indirect markers of airway inflammation. Thus, collectively, these data favour an interpretation in which positive interactions (synergy) between LABAs and ICSs play a critical role.
Modelling the enhancement of ICS action by LABAs
Repression of inflammatory gene expression by ICSs is believed to occur by at least two general mechanisms. The classical repressive mode of glucocorticoid action is termed transrepression in which the activity of key pro-inflammatory transcription factors, such as nuclear factor-κB and activator protein (AP)-1, is inhibited via direct interactions with ligand-bound GR (Barnes, 2001; De Bosscher et al., 2003). In this paradigm, the GR is thought to recruit histone deacetylase(s) to the promoter regions of target pro-inflammatory genes (Barnes, 2006b). The resulting complex then acts to ‘dampen down' inflammatory gene transcription by deacetylating both the chromatin in the immediate vicinity of the promoter, and possibly of the GR itself, to facilitate interaction with nuclear factor-κB for enhanced transrepression (De Bosscher et al., 2003; Barnes, 2006b; Ito et al., 2006). In this context, there are data to suggest that glucocorticoids may also negatively modulate histone acetylation and that this effect is enhanced by LABAs (Nie et al., 2005). Thus, although the mechanisms that govern glucocorticoid- and/or LABA-dependent modulation of histone structure remain undefined, it is possible that such processes may represent a level of control that is common to both glucocorticoids and LABAs and that can lead to effects on inflammatory gene expression that are greater than that produced by either drug alone.
In simple model systems, glucocorticoids are often relatively weak (partial) inhibitors of inflammatory gene transcription (Chivers et al., 2004), implying that processes in addition to transrepression, including both post-transcriptional and probably translational mechanisms, must be operative to account for the anti-inflammatory effects seen in bona fide models of inflammation (Newton, 2000; Stellato, 2004; Clark, 2007; Newton and Holden, 2007). In this respect, the induction (transactivation) by glucocorticoids of anti-inflammatory genes, which then repress pro-inflammatory processes, is now believed to be a major mechanism of glucocorticoid action (Newton et al., 1998; Newton, 2000; Lasa et al., 2002; Clark, 2003, 2007; Newton and Holden, 2007). Thus, glucocorticoids can rapidly, and very profoundly, induce the expression of genes, such as mitogen-activated protein (MAP) kinase phosphatase (MKP)-1 (aka dual-specificity phosphatase 1). This enzyme is able to dephosphorylate (inactivate) the three core mammalian MAP kinases (p38 MAP kinase; extracellular signal-regulated kinase-1 and extracellular signal-regulated kinase-2; and c-Jun-N-terminal kinase) that are central to the induction of many pro-inflammatory genes. Indeed, MKP-1 is involved in the ability of dexamethasone to repress growth-related oncogene-α expression in HASM cells via inactivation of the c-jun-N-terminal kinase signalling cascade (Issa et al., 2007). Moreover, in vivo, the repression by glucocorticoids of pro-inflammatory genes is significantly impaired in mkp-1-deficient mice when compared to the wild-type control littermates (Abraham et al., 2006). Although such effects may involve MPK-1 acting to reduce the stability of pro-inflammatory gene mRNAs (Clark, 2003), it is also likely that MKP-1 acts at both transcriptional and translational levels (Newton and Holden, 2007) (Figure 2). Another anti-inflammatory gene that is markedly induced by glucocorticoids is glucocorticoid-inducible leucine zipper (GILZ), which effectively represses the transcriptional activity of both nuclear factor-κB and AP-1 (Mittelstadt and Ashwell, 2001; Eddleston et al., 2007). Thus, glucocorticoid-inducible genes, operating at a variety of regulatory levels, have the potential to repress the expression of pro-inflammatory genes and this process of transactivation may operate in concert with the more traditional mechanisms of glucocorticoid action that involve transrepression.
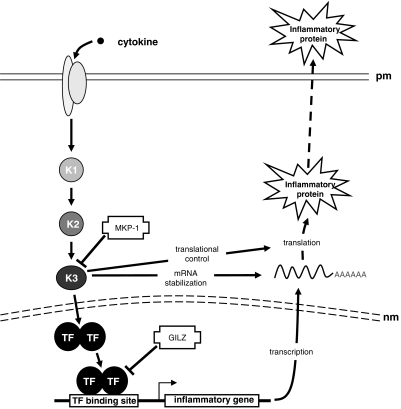
Figure 2.
Enhanced expression of MKP-1, but not GILZ, may explain additive effects of LABA/corticosteroid combination therapies in the repression of inflammatory gene expression. A schematic representation of the signalling cascades leading to inflammatory gene expression is depicted with possible targets and sites of action for putative anti-inflammatory glucocorticoid-inducible genes. Activation of a pro-inflammatory cascade, following binding of cytokine to its cognate receptor in the plasma membrane (pm), is shown occurring via a number of kinases (K1–3). The signal crosses the nuclear membrane (nm) and leads to transcription factor (TF) activation and the production of inflammatory gene mRNAs. Under the influence of further kinase cascades (here K1–3), the mRNA is stabilized and translated into protein. Finally, many proteins (for example cytokines, chemokines) are exported into the extracellular space for biological function. Sites of action of the two glucocorticoid-inducible genes, MKP-1 and GILZ, are indicated. MKP-1 is an inhibitor of the MAP kinase family of protein kinases and may, therefore, impact on numerous cellular mechanisms, including the activation of transcription, mRNA stability and translation. GILZ inhibits key inflammatory transcription factors (NF-κB and AP-1). AP-1, activator protein-1; GILZ, glucocorticoid-inducible leucine zipper; LABA, long-acting β2-adrenoceptor agonist; MAP, mitogen-activated protein; MKP, mitogen-activated protein kinase phosphatase; NF-κB, nuclear factor κB.
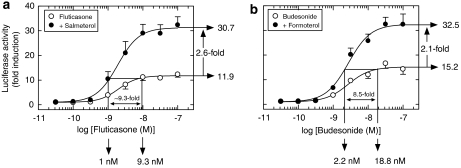
The ability of LABAs to enhance the transactivation of anti-inflammatory genes in human airway epithelial cells was recently investigated in some detail. Kaur et al. (2007) modelled this response by utilizing a classical glucocorticoid response element (GRE)-dependent luciferase reporter construct that was stably transfected into BEAS-2B cells (Chivers et al., 2004). In this simple system, LABAs did not activate the reporter (not shown), but, as is illustrated in Figure 3, markedly potentiated glucocorticoid-induced transcription. Thus, fluticasone activated the GRE-dependent reporter in a concentration-dependent manner with a mean EC50 of 2 nM. Salmeterol, at a concentration (100 nM) that maximally activated cAMP-response element-dependent transcription, had no effect on the potency of fluticasone (mean EC50=1.8 nM), but augmented (2.1-fold at the Emax) the induction of the luciferase gene at all active concentrations of fluticasone tested (Figure 3a). Comparable data were obtained with formoterol and budesonide in combination (Figure 3b; Kaur et al., 2007), demonstrating that in this simple system, LABAs and glucocorticoids can interact synergistically and that this is a class-specific effect. It is noteworthy that this synergism at the level of GRE-dependent transcription is not a peculiar property of LABAs. Indeed, an identical interaction is found between short-acting β2-adrenoceptor agonists (for example procaterol, salbutamol, terbutaline) and dexamethasone (Kaur et al., 2007), indicating that it is the elevation of cAMP per se that, in someway, augments glucocorticoid action. Therefore, in the clinical setting a similar enhancement of glucocorticoid action could be seen with short-acting β2-adrenoceptor agonists, provided they are administered on a regular basis.
Figure 3.
LABA/ICS combinations are both steroid-sparing and enhance GRE-dependent transcription. BEAS-2B cells stably expressing a GRE-reporter construct were treated concurrently with salmeterol (100 nM; a) or formoterol (10 nM; b) in the absence and presence of fluticasone and budesonide, respectively (10 pM–100 nM). After 6 h, cells were harvested for luciferase assay. The data show that the effect of formoterol/budesonide and salmeterol/fluticasone in combination promotes GRE-dependent transcription, above what can be achieved by the glucocorticoid alone, and is steroid sparing. Thus, in this simple system, neither LABA activated the GRE reporter construct (not shown), but markedly potentiated glucocorticoid-induced transcription (2.6- and 2.1-fold for salmeterol and formoterol respectively at the Emax). In addition, salmeterol and formoterol were glucocorticoid sparing in this model. Thus, both glucocorticoids at concentrations that evoked 90% of the maximum response produced a 12- to 15-fold induction of the luciferase gene. However, in the presence of salmeterol (100 nM) or formoterol (10 nM), which were inactive, the same degree of gene induction was achieved at a concentration of glucocorticoid that was significantly (∼10-fold) lower. Note: this measurement was made at the EC90 concentration of glucocorticoid (as the upper asymptote, by definition, is never reached) and so the degree to which the LABAs are steroid sparing is underestimated. See text and Kaur et al. (2007) for further details. GRE, glucocorticoid response element; ICS, inhaled corticosteroid; LABA, long-acting β2-adrenoceptor agonist.
In addition to enhancing GRE-dependent transcription, salmeterol and formoterol were glucocorticoid-sparing in this model (Kaur et al., 2007). Thus, both glucocorticoids at their maximally effective concentration produced an 11.9- to 15.2-fold induction of the luciferase gene. However, in the presence of salmeterol (100 nM) or formoterol (10 nM), which were inactive by themselves, the same degree of gene induction was achieved at a concentration of glucocorticoid that was significantly lower (∼10-fold if the EC90 of the glucocorticoid response alone is taken as reference) (Figure 3). This general effect in BEAS-2B cells is also seen in primary HASM cells harbouring the same reporter construct, indicating that the synergistic interaction between LABAs and ICSs is probably a characteristic of all glucocorticoid-responsive cells that express β2-adrenoceptors (Kaur et al., 2007).
Further studies also found that the enhancement by LABAs of glucocorticoid-induced gene expression was abolished by ICI 118,551 and was mimicked by forskolin, implicating a process that requires β2-adrenoceptor-mediated activation of adenylyl cyclase. Furthermore, the elevation of cAMP is likely to result in the activation of the classical signalling pathway (Figure 1) for the synergy between LABAs and glucocorticoids was abolished in cells infected with an adenovirus vector encoding a highly selective inhibitor of PKA (Meja et al., 2004; Kaur et al., 2007).
Do LABAs enhance GRE-dependent transcription of ‘real' genes?
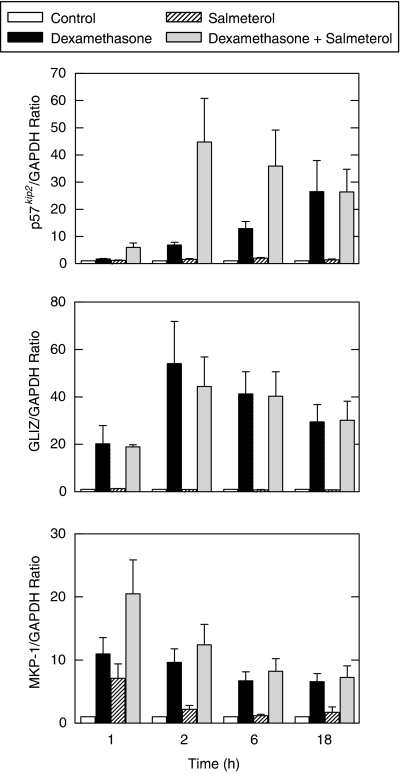
A crucial detail to establish is whether the synergy between LABAs and glucocorticoids obtained with the GRE-reporter construct is representative of the induction, by combination therapies, of real anti-inflammatory genes. If so, then the process of enhanced transactivation, modelled using the GRE-reporter, provides a compelling explanation for the superior clinical efficacy of low-dose ICS/LABA combinations when compared to high-dose ICS alone. This is especially true in those clinical situations where the amount of ICS given therapeutically is close to, or at the top of, the dose–response curve. In this context, Kaur et al. (2007) have examined the effect of LABAs on a number of glucocorticoid-inducible genes expressed by airway epithelial cells to gauge the credibility of the data obtained on the GRE-reporter construct. Three of these are discussed here: GILZ, a gene that when induced produces a protein that suppresses the elaboration of pro-inflammatory cytokines from human airway epithelial cells in response to a variety of stimuli (Mittelstadt and Ashwell, 2001; Eddleston et al., 2007); p57KIP2, which negatively regulates cell proliferation by generating a potent, tight-binding inhibitor of several cyclin-dependent kinase complexes involved in the G1 and S phases of the cell cycle (Lee et al., 1995); and a dual specificity phosphatase, encoded by MKP-1 (see above). As shown in Figure 4a, exposure of BEAS-2B cells to dexamethasone increased p57kip2 mRNA in a time-dependent manner (12.9- and 26.5-fold at 6 and 18 h, respectively), whereas salmeterol had little, if any, effect (1.9- and 1.4-fold induction at the same time points, respectively). When cells were exposed to salmeterol and dexamethasone concurrently, a marked synergistic induction of p57KIP2 was evoked at all time points up to 6 h. Thereafter, the ability of salmeterol to synergize with dexamethasone was lost, which might be related to the relatively transient nature of the cAMP signal. Thus, the effects of dexamethasone and salmeterol on the expression of p57KIP2, alone and in combination, closely resemble the data obtained with the GRE-reporter construct (Figure 2). In contrast, although dexamethasone similarly promoted a robust induction of GILZ and salmeterol was inactive, the combination of both drugs elicited an effect that was the same as the glucocorticoid alone (Figure 4b). In this situation, GILZ does not behave in a manner predicted by the GRE reporter and, therefore, may be subject to regulation by a more complex glucocorticoid-regulatory region in its promoter. The other gene described here is MKP-1. As shown in Figure 4c, MKP-1 was induced (6.6- to 11-fold) by dexamethasone at all time points tested. However, in contrast to p57KIP2 and GILZ, both forskolin and salmeterol induced MKP-1 expression at 1 h (9.0- and 7.1-fold, respectively), although this effect had waned dramatically by 2 h. Dexamethasone in combination with either forskolin or salmeterol produced a significant enhancement of MKP-1 expression over any drug alone (21.3- and 20.5-fold, respectively, vs 11.0-fold for dexamethasone alone), but these interactions were purely additive and transient (that is the effect was lost 2 h post-treatment). Therefore, although MKP-1 is sensitive to glucocorticoids and cAMP-elevating agents, these two classes of drug act independently in the positive regulation of this gene.
Figure 4.
Effect of salmeterol on the induction by dexamethasone of p57KIP2, GILZ and MKP-1. BEAS-2B cells were treated with dexamethasone (1 μM) salmeterol (100 nM), a combination of the two drugs or vehicle for 1–18 h and harvested for real-time PCR analysis of p57KIP2, GILZ, MKP-1 and GAPDH mRNA using SYBR GreenER technology. Data are expressed as a ratio to the housekeeping gene, GAPDH. See text for further details. GILZ, glucocorticoid-inducible leucine zipper; MKP, mitogen-activated protein kinase phosphatase.
In several non-pulmonary tissues, glucocorticoids suppress cell proliferation by a mechanism that involves the induction of p57KIP2 and may influence the expression of vascular endothelial growth factor (Samuelsson et al., 1999; Matsuura et al., 2002). It is possible, therefore, that the ability of LABAs to enhance the induction of this gene by glucocorticoids could be beneficial in asthma and COPD through its ability to oppose or limit the process of airways remodelling where airway epithelial and bronchial smooth muscle cells play an important role (Hogg et al., 2004; James, 2005). However, what is probably more important is the principle that other glucocorticoid-induced genes may be activated in a synergistic manner by LABA/ICS combination therapies. It has been estimated that from 1 to 20% of genes in the human genome are regulated by glucocorticoids and, of these, about half are inducible (Galon et al., 2002; Planey et al., 2003; Wang et al., 2003; Donn et al., 2007). Although a large proportion of these genes are not directly induced by glucocorticoids (that is through the binding of GR to classical GRE site(s) in a promoter region), we suggest that a clinically relevant fraction will, nevertheless, be synergistically regulated by glucocorticoid and cAMP. Indeed, Kaur et al. (2007) found that of nine genes that were dexamethasone inducible and were tested for enhancement by β2-adrenoceptor agonists, two were regulated synergistically in a manner that was similar to that shown by the classical GRE reporter construct. Currently, the identity and function of genes that are regulated in this way are largely unknown. However, it is highly likely that the currently available LABA/ICS combination therapies co-induce many of these genes and that, collectively, this may account for the superior clinical activity of this treatment option in asthmatic subjects in whom ICS alone provide an inadequate therapy.
Additive interactions between LABAs and glucocorticoids
As described above, many investigations have shown that, in the context of inhibiting pro-inflammatory responses, LABAs and glucocorticoids interact in an additive, rather than, synergistic manner. It is noteworthy, therefore, that the data described by Kaur et al. (2007) may also provide an explanation for this discrepancy. The expression of many pro-inflammatory genes is frequently controlled, to a substantial degree, by MAP kinases, in particular p38 MAP kinase and c-jun-N-terminal kinase (Kyriakis and Avruch, 2001). Conversely, these same genes are negatively regulated by glucocorticoids by a mechanism that involves the upregulation of MKP-1. Thus, by dephosphorylating p38 MAP kinase and c-jun-N-terminal kinase, MKP-1 reduces the transcription, mRNA stability and translation of a plethora pro-inflammatory genes that are induced by activators of MAP kinase signalling (Clark, 2007; Newton and Holden, 2007). Thus, the additive interaction between salmeterol and dexamethasone on the expression of MKP-1 in BEAS-2B cells (Kaur et al., 2007) together with a report of a similar effect of a LABA and glucocorticoid on MKP-1 promoter activity (Usmani et al., 2005) provides an attractive explanation for the additivity observed with LABA/corticosteroid combinations in many in vitro studies.
Additivity or synergy?
Collectively, the data presented in this review imply that LABAs are able to synergistically augment the transcription of only a subset of glucocorticoid-sensitive genes, whereas other genes may be unaffected. It is inevitable that the degree to which LABAs enhance GRE-dependent transcription is both time dependent and will vary from gene to gene. Furthermore, there are genes, represented by MKP-1, that are induced by both cAMP and glucocorticoid, and, in such instances, the combination of both stimuli may produce a purely additive response. In the final analysis, the categorization of any particular glucocorticoid-inducible gene is likely to be dependent on the nature of the relevant glucocorticoid-responsive regions in the promoter of that gene. In this context, only a relatively small fraction of genuine glucocorticoid-inducible genes feature classical GRE sites, whereas the remainder is regulated via composite sites where GR binds DNA, often as a monomer, but also in partnership with other transcription factors (So et al., 2007). It is important to state that such interactions may be highly complex (Newton and Holden, 2007; So et al., 2007) and will not be adequately modelled by the simple GRE-reporter construct described by Kaur et al. (2007). Thus, it is highly likely that LABAs and glucocorticoids may interact in the regulation of gene expression in ways that remain unexplored.
How do LABAs enhance the action of ICSs?
Arguably, a major objective of combination therapy research is to discover the molecular mechanism(s) by which LABAs augment glucocorticoid action. LABAs have been shown to enhance the translocation of the GR from the cytosol to the nucleus, even in the absence of exogenous glucocorticoid (Eickelberg et al., 1999; Roth et al., 2002; Profita et al., 2005; Usmani et al., 2005) (although this effect is contentious; see Lovén et al., 2007 and discussion below). This, so-called, ligand-independent translocation, which is a well-recognized action of cAMP on other steroid hormone receptors (Weigel and Zhang, 1998; Cenni and Picard, 1999), has been reported in a variety of cell types relevant to the treatment of respiratory diseases, including airways smooth muscle and epithelial cells (Eickelberg et al., 1999; Roth et al., 2002; Usmani et al., 2005). It has also been observed in vivo in human subjects (Usmani et al., 2005). Historically, therefore, it has been suggested that enhanced translocation of GR to the nucleus may account for the superior clinical benefit of LABA/ICS combination therapies (see Miller-Larsson and Selroos, 2006). Further support for this assertion is that the combination therapy is associated with increased binding of the GR to GREs on target genes (Korn et al., 1998; Roth et al., 2002), which could be consistent with the enhanced GR/DNA binding seen in cells overexpressing PKA (Rangarajan et al., 1992).
Despite these data, there is evidence from a number of studies that question the overall importance of this mechanism. Reference to Figure 3 shows that, although the LABAs enhanced GRE-dependent transcription, they did not, by themselves, activate the reporter and had no effect on the potency of glucocorticoid (that is, the EC50 values in the absence and presence of LABA were not significantly different). This finding supports the data obtained from clinical studies where one would predict that the ligand-independent translocation of GR to the nucleus evoked by LABAs would be anti-inflammatory. However, this is not the case (Roberts et al., 1999; Howarth et al., 2000). In addition, the model depicted in Figure 3 shows that LABAs enhanced GRE-dependent transcription in the presence of concentrations of glucocorticoids that will promote the translocation of all GR to the nucleus (Chivers et al., 2004; Lewis-Tuffin et al., 2007). This finding is clinically relevant as there are asthmatic subjects who are not well controlled on high doses of ICSs where all GR is predicted to be in the nuclear compartment. Indeed, >75% of the GR in airway epithelial cells is found in the nucleus following the administration of a moderate dose (800 μg) of inhaled BDP to asthmatic subjects (Usmani et al., 2005). In contrast, asthma control in many of these same individuals may be achieved following the administration of a LABA in combination with the same ICS at a lower dose. Based on these data, it is difficult to rationalize further GR translocation as the primary mechanism for the improvement in asthma (and COPD) control produced by the addition of a LABA, especially in those individuals receiving high-dose ICS. Alternative explanations are required.
One attractive possibility is that LABAs, by virtue of their ability to elevate cAMP, increase the expression of functional GRs. Indeed, pretreatment of human skin fibroblasts with dibutyryl-cAMP increases, 2.6-fold, the number of specific [3H]dexamethasone binding sites (Oikarinen et al., 1984). Comparable data have been derived from rat hepatoma cells treated with 8-Br-cAMP or forskolin in which GR number was significantly increased by a mechanism attributable, at least in part, to GR mRNA stabilization (Dong et al., 1989). Of significance is that an increase in GR density is paralleled by enhanced GRE-dependent transcription (Hirst et al., 1990; Szapary et al., 1996; Zhang et al., 2007).
Another highly likely possibility, which is not mutually exclusive with the mechanisms described in the previous sections, is that LABAs enhance GR-mediated transcription by acting predominantly within the nucleus (that is on the transcriptional process itself). This will be highly complex involving numerous transcriptional cofactors and co-activators, changes in chromatin structure, acetylation, methylation and/or other modifications that impact on transcription. More daunting, perhaps from a research perspective, is that such effects are probably gene specific. Nevertheless, in hepatoma cells, PKA has been shown to stabilize the interaction of glucocorticoid-bound GR and relevant transcription factors with DNA (Espinas et al., 1995).
Concluding remarks
The ability of LABAs to augment the efficacy of ICSs in asthma to a level that cannot be achieved by a glucocorticoid alone is a landmark clinical observation that may equally well apply to other chronic airways inflammatory diseases, such as COPD. Evidence is now available that LABAs, at least in part, enhance the anti-inflammatory effect of ICSs possibly by facilitating the GRE-dependent transcription of anti-inflammatory genes. It is conceivable that LABAs may act as powerful positive ‘allosteric' modulators, which in some way modify the conformation of glucocorticoid-bound GR (and/or the binding of necessary cofactors and co-activators) such that it interacts with DNA in a manner that is optimized for the transcription of anti-inflammatory genes. Clearly, an understanding of the molecular basis of this fundamental clinical observation is a Holy Grail of current respiratory diseases research, as it could permit the rational exploitation of this effect with the development of new ‘optimized' LABA/ICS and, possibly, other novel combination therapies.
Acknowledgments
MAG and RN are Alberta Heritage Foundation for Medical Research (AHFMR) Senior Scholar and Scholar, respectively. RN is a Canadian Institutes of Health Research (CIHR) New Investigator. RL is a CIHR Clinician Scientist and an AHFMR Clinical Investigator; he currently holds the GSK-CIHR Professorship in Inflammatory Lung Diseases at the University of Calgary. We acknowledge the CIHR, AHFMR, AstraZeneca, Nycomed and GlaxoSmithKline for financial support.
Abbreviations
- AHR
airways hyperresponsiveness
- AP-1
activator protein-1
- BDP
beclomethasone dipropionate
- COPD
chronic obstructive pulmonary disease
- GILZ
glucocorticoid-inducible leucine zipper
- GR
glucocorticoid receptor
- GRE
glucocorticoid response element
- HASM
human airways smooth muscle
- ICS
inhaled corticosteroid
- IL
interleukin
- LABA
long-acting β2-adrenoceptor agonist
- MAP
mitogen-activated protein
- MKP
mitogen-activated protein kinase phosphatase
- PEFR
peak expiratory flow rate
- PKA
cAMP-dependent protein kinase
- SMART
Symbicort for maintenance and reliever therapy
Conflict of interest
The authors state no conflict of interest.
References
- Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146:545–555. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, et al. Anti-inflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med. 2006;203:1883–1889. doi: 10.1084/jem.20060336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoshiba K, Nagai A. Differences in airway remodeling between asthma and chronic obstructive pulmonary disease. Clin Rev Allergy Immunol. 2004;27:35–44. doi: 10.1385/CRIAI:27:1:035. [DOI] [PubMed] [Google Scholar]
- Barnes NC, Qiu YS, Pavord ID, Parker D, Davis PA, Zhu J, et al. Anti-inflammatory effects of salmeterol/fluticasone propionate in chronic obstructive lung disease. Am J Respir Crit Care Med. 2006;173:736–743. doi: 10.1164/rccm.200508-1321OC. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Pathophysiology of asthma. Br J Clin Pharmacol. 1996;42:3–10. doi: 10.1046/j.1365-2125.1996.03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Molecular mechanisms of corticosteroids in allergic diseases. Allergy. 2001;56:928–936. doi: 10.1034/j.1398-9995.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. New drugs for asthma. Nat Rev Drug Discov. 2004;3:831–844. doi: 10.1038/nrd1524. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Corticosteroids: the drugs to beat. Eur J Pharmacol. 2006a;533:2–14. doi: 10.1016/j.ejphar.2005.12.052. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. How corticosteroids control inflammation. Br J Pharmacol. 2006b;148:245–254. doi: 10.1038/sj.bjp.0706736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170:836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- Belvisi MG. Regulation of inflammatory cell function by corticosteroids. Proc Am Thorac Soc. 2004;1:207–214. doi: 10.1513/pats.200402-002MS. [DOI] [PubMed] [Google Scholar]
- Bisgaard H, Le Roux P, Bjamer D, Dymek A, Vermeulen JH, Hultquist C. Budesonide/formoterol maintenance plus reliever therapy: a new strategy in pediatric asthma. Chest. 2006;130:1733–1743. doi: 10.1378/chest.130.6.1733. [DOI] [PubMed] [Google Scholar]
- Bourbeau J, Christodoulopoulos P, Maltais F, Yamauchi Y, Olivenstein R, Hamid Q. Effect of salmeterol/fluticasone propionate on airway inflammation in COPD: a randomised controlled trial. Thorax. 2007;62:938–943. doi: 10.1136/thx.2006.071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs DD. Chronic obstructive pulmonary disease overview: prevalence, pathogenesis, and treatment. J Manag Care Pharm. 2004;10:S3–S10. [PubMed] [Google Scholar]
- BTS/SIGN British guideline on the management of asthma. Thorax. 2003;58 Suppl 1:i1–i94. doi: 10.1136/thorax.58.suppl_1.1i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl R, Vogelmeier C. Budesonide/formoterol maintenance and reliever therapy: a new treatment approach for adult patients with asthma. Curr Med Res Opin. 2007;23:1867–1878. doi: 10.1185/030079907X210769. [DOI] [PubMed] [Google Scholar]
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003a;361:449–456. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003b;22:912–919. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- Cenni B, Picard D. Ligand-independent activation of steroid receptors: new roles for old players. Trends Endocrinol Metab. 1999;10:41–46. doi: 10.1016/s1043-2760(98)00121-0. [DOI] [PubMed] [Google Scholar]
- Chalmers GW, Macleod KJ, Little SA, Thomson LJ, McSharry CP, Thomson NC. Influence of cigarette smoking on inhaled corticosteroid treatment in mild asthma. Thorax. 2002;57:226–230. doi: 10.1136/thorax.57.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers GW, Macleod KJ, Thomson L, Little SA, McSharry C, Thomson NC. Smoking and airway inflammation in patients with mild asthma. Chest. 2001;120:1917–1922. doi: 10.1378/chest.120.6.1917. [DOI] [PubMed] [Google Scholar]
- Chaudhuri R, Livingston E, McMahon AD, Lafferty J, Fraser I, Spears M, et al. Effects of smoking cessation on lung function and airway inflammation in smokers with asthma. Am J Respir Crit Care Med. 2006;174:127–133. doi: 10.1164/rccm.200510-1589OC. [DOI] [PubMed] [Google Scholar]
- Chivers JE, Cambridge LM, Catley MC, Mak JC, Donnelly LE, Barnes PJ, et al. Differential effects of RU486 reveal distinct mechanisms for glucocorticoid repression of prostaglandin E release. Eur J Biochem. 2004;271:4042–4052. doi: 10.1111/j.1432-1033.2004.04342.x. [DOI] [PubMed] [Google Scholar]
- Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, et al. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest. 2004;113:551–560. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong LK, Drury DE, Dummer JF, Ghahramani P, Schleimer RP, Peachell PT. Protection by dexamethasone of the functional desensitization to β2-adrenoceptor-mediated responses in human lung mast cells. Br J Pharmacol. 1997;121:717–722. doi: 10.1038/sj.bjp.0701185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2001;34:50s–59s. [PubMed] [Google Scholar]
- Clark AR. MAP kinase phosphatase 1: a novel mediator of biological effects of glucocorticoids. J Endocrinol. 2003;178:5–12. doi: 10.1677/joe.0.1780005. [DOI] [PubMed] [Google Scholar]
- Clark AR. Anti-inflammatory functions of glucocorticoid-induced genes. Mol Cell Endocrinol. 2007;275:79–97. doi: 10.1016/j.mce.2007.04.013. [DOI] [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-κB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- Dong Y, Aronsson M, Gustafsson JA, Okret S. The mechanism of cAMP-induced glucocorticoid receptor expression. Correlation to cellular glucocorticoid response. J Biol Chem. 1989;264:13679–13683. [PubMed] [Google Scholar]
- Donn R, Berry A, Stevens A, Farrow S, Betts J, Stevens R, et al. Use of gene expression profiling to identify a novel glucocorticoid sensitivity determining gene, BMPRII. FASEB J. 2007;21:402–414. doi: 10.1096/fj.06-7236com. [DOI] [PubMed] [Google Scholar]
- Duong M, Gauvreau G, Watson R, Obminski G, Strinich T, Evans M, et al. The effects of inhaled budesonide and formoterol in combination and alone when given directly after allergen challenge. J Allergy Clin Immunol. 2007;119:322–327. doi: 10.1016/j.jaci.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Eddleston J, Herschbach J, Wagelie-Steffen AL, Christiansen SC, Zuraw BL. The anti-inflammatory effect of glucocorticoids is mediated by glucocorticoid-induced leucine zipper in epithelial cells. J Allergy Clin Immunol. 2007;119:115–122. doi: 10.1016/j.jaci.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Edwards MR, Johnson MW, Johnston SL. Combination therapy: synergistic suppression of virus-induced chemokines in airway epithelial cells. Am J Respir Cell Mol Biol. 2006;34:616–624. doi: 10.1165/rcmb.2005-0385OC. [DOI] [PubMed] [Google Scholar]
- Eickelberg O, Roth M, Lorx R, Bruce V, Rudiger J, Johnson M, et al. Ligand-independent activation of the glucocorticoid receptor by β2-adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. J Biol Chem. 1999;274:1005–1010. doi: 10.1074/jbc.274.2.1005. [DOI] [PubMed] [Google Scholar]
- Espinas ML, Roux J, Pictet R, Grange T. Glucocorticoids and protein kinase A coordinately modulate transcription factor recruitment at a glucocorticoid-responsive unit. Mol Cell Biol. 1995;15:5346–5354. doi: 10.1128/mcb.15.10.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan VS, Bryson CL, Curtis JR, Fihn SD, Bridevaux PO, McDonell MB, et al. Inhaled corticosteroids in chronic obstructive pulmonary disease and risk of death and hospitalization: time-dependent analysis. Am J Respir Crit Care Med. 2003;168:1488–1494. doi: 10.1164/rccm.200301-019OC. [DOI] [PubMed] [Google Scholar]
- FitzGerald JM, Boulet LP, McIvor RA, Zimmerman S, Chapman KR. Asthma control in Canada remains suboptimal: the Reality of Asthma Control (TRAC) study. Can Respir J. 2006;13:253–259. doi: 10.1155/2006/753083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, et al. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002;16:61–71. doi: 10.1096/fj.01-0245com. [DOI] [PubMed] [Google Scholar]
- Gendo K, Lodewick MJ. Asthma economics: focusing on therapies that improve costly outcomes. Curr Opin Pulm Med. 2005;11:43–50. doi: 10.1097/01.mcp.0000146782.11092.d6. [DOI] [PubMed] [Google Scholar]
- Georas SN. Inhaled glucocorticoids, lymphocytes, and dendritic cells in asthma and obstructive lung diseases. Proc Am Thorac Soc. 2004;1:215–221. doi: 10.1513/pats.200402-004MS. [DOI] [PubMed] [Google Scholar]
- Giembycz MA, Newton R. Beyond the dogma: novel β2-adrenoceptor signalling in the airways. Eur Respir J. 2006;27:1286–1306. doi: 10.1183/09031936.06.00112605. [DOI] [PubMed] [Google Scholar]
- Gilliland FD, Islam T, Berhane K, Gauderman WJ, McConnell R, Avol E, et al. Regular smoking and asthma incidence in adolescents. Am J Respir Crit Care Med. 2006;174:1094–1100. doi: 10.1164/rccm.200605-722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizycki MJ, Hattotuwa KL, Barnes N, Jeffery PK. Effects of fluticasone propionate on inflammatory cells in COPD: an ultrastructural examination of endobronchial biopsy tissue. Thorax. 2002;57:799–803. doi: 10.1136/thorax.57.9.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Keating GM. Budesonide/formoterol: a review of its use in asthma. Drugs. 2004;64:1597–1618. doi: 10.2165/00003495-200464140-00006. [DOI] [PubMed] [Google Scholar]
- Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57:875–879. doi: 10.1136/thorax.57.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Allen & Hanburys Limited UK Study Group. Lancet. 1994;344:219–224. doi: 10.1016/s0140-6736(94)92996-3. [DOI] [PubMed] [Google Scholar]
- Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28:523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, et al. The efficacy and safety of fluticasone propionate (250 μg)/salmeterol (50 μg) combined in the Diskus inhaler for the treatment of COPD. Chest. 2003;124:834–843. doi: 10.1378/chest.124.3.834. [DOI] [PubMed] [Google Scholar]
- Hattotuwa KL, Gizycki MJ, Ansari TW, Jeffery PK, Barnes NC. The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease: a double-blind, placebo-controlled biopsy study. Am J Respir Crit Care Med. 2002;165:1592–1596. doi: 10.1164/rccm.2105025. [DOI] [PubMed] [Google Scholar]
- Hirst MA, Northrop JP, Danielsen M, Ringold GM. High level expression of wild type and variant mouse glucocorticoid receptors in Chinese hamster ovary cells. Mol Endocrinol. 1990;4:162–170. doi: 10.1210/mend-4-1-162. [DOI] [PubMed] [Google Scholar]
- Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- Hossain S, Heard BE. Hyperplasia of bronchial muscle in chronic bronchitis. J Pathol. 1970;101:171–184. doi: 10.1002/path.1711010212. [DOI] [PubMed] [Google Scholar]
- Howarth PH, Beckett P, Dahl R. The effect of long-acting β2-agonists on airway inflammation in asthmatic patients. Respir Med. 2000;94 Suppl F:S22–S25. doi: 10.1016/s0954-6111(00)90129-x. [DOI] [PubMed] [Google Scholar]
- Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- Ind PW, Dal Negro R, Colman NC, Fletcher CP, Browning D, James MH. Addition of salmeterol to fluticasone propionate treatment in moderate-to-severe asthma. Respir Med. 2003;97:555–562. doi: 10.1053/rmed.2003.1483. [DOI] [PubMed] [Google Scholar]
- Issa R, Xie S, Khorasani N, Sukkar M, Adcock IM, Lee KY, et al. Corticosteroid inhibition of growth-related oncogene protein-α via mitogen-activated kinase phosphatase-1 in airway smooth muscle cells. J Immunol. 2007;178:7366–7375. doi: 10.4049/jimmunol.178.11.7366. [DOI] [PubMed] [Google Scholar]
- Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, et al. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-κB suppression. J Exp Med. 2006;203:7–13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A. Airway remodeling in asthma. Curr Opin Pulm Med. 2005;11:1–6. doi: 10.1097/01.mcp.0000146779.26339.d8. [DOI] [PubMed] [Google Scholar]
- James AL, Palmer LJ, Kicic E, Maxwell PS, Lagan SE, Ryan GF, et al. Decline in lung function in the Busselton Health Study: the effects of asthma and cigarette smoking. Am J Respir Crit Care Med. 2005;171:109–114. doi: 10.1164/rccm.200402-230OC. [DOI] [PubMed] [Google Scholar]
- Jeffery PK. Lymphocytes, chronic bronchitis and chronic obstructive pulmonary disease. Novartis Found Symp. 2001a;234:149–161. [PubMed] [Google Scholar]
- Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001b;164:S28–S38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294:1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, King G, et al. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med. 2001;164:474–477. doi: 10.1164/ajrccm.164.3.2010109. [DOI] [PubMed] [Google Scholar]
- Kalavantavanich K, Schramm CM. Dexamethasone potentiates high-affinity β-agonist binding and Gsα protein expression in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1101–L1106. doi: 10.1152/ajplung.2000.278.5.L1101. [DOI] [PubMed] [Google Scholar]
- Kaur M, Chivers JE, Giembycz MA, Newton R.Long-acting β2-adrenoceptor agonists synergistically enhance glucocorticoid-dependent transcription in human airway epithelial and smooth muscle cells Mol Pharmacol 2007[e-pub ahead of print] [DOI] [PubMed]
- Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-α in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153:530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- Kiri VA, Pride NB, Soriano JB, Vestbo J. Inhaled corticosteroids in chronic obstructive pulmonary disease: results from two observational designs free of immortal time bias. Am J Respir Crit Care Med. 2005;172:460–464. doi: 10.1164/rccm.200502-210OC. [DOI] [PubMed] [Google Scholar]
- Korn SH, Jerre A, Brattsand R. Effects of formoterol and budesonide on GM-CSF and IL-8 secretion by triggered human bronchial epithelial cells. Eur Respir J. 2001;17:1070–1077. doi: 10.1183/09031936.01.00073301. [DOI] [PubMed] [Google Scholar]
- Korn SH, Wouters EF, Wesseling G, Arends JW, Thunnissen FB. Interaction between glucocorticoids and β2-agonists: α and β glucocorticoid-receptor mRNA expression in human bronchial epithelial cells. Biochem Pharmacol. 1998;56:1561–1569. doi: 10.1016/s0006-2952(98)00179-8. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Diette GB, Rand CS, Bilderback AL, Merriman B, Hansel NN, et al. Mortality in patients hospitalized for asthma exacerbations in the United States. Am J Respir Crit Care Med. 2006;174:633–638. doi: 10.1164/rccm.200601-007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339:1194–1200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- Lasa M, Abraham SM, Boucheron C, Saklatvala J, Clark AR. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol Cell Biol. 2002;22:7802–7811. doi: 10.1128/MCB.22.22.7802-7811.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Reynisdottir I, Massague J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- Lemiere C, Bai T, Balter M, Bayliff C, Becker A, Boulet LP, et al. Adult asthma consensus guidelines update 2003. Can Respir J. 2004;11:9A–18A. doi: 10.1155/2004/271362. [DOI] [PubMed] [Google Scholar]
- Lewis-Tuffin LJ, Jewell CM, Bienstock RJ, Collins JB, Cidlowski JA. Human glucocorticoid receptor β binds RU-486 and is transcriptionally active. Mol Cell Biol. 2007;27:2266–2282. doi: 10.1128/MCB.01439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston E, Chaudhuri R, McMahon AD, Fraser I, McSharry CP, Thomson NC. Systemic sensitivity to corticosteroids in smokers with asthma. Eur Respir J. 2007;29:64–71. doi: 10.1183/09031936.06.00120505. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- Lovén J, Svitacheva N, Jerre A, Miller-Larsson A, Korn SH. Anti-inflammatory activity of β2-agonists in primary lung epithelial cells is independent of glucocorticoid receptor. Eur Respir J. 2007;30:848–856. doi: 10.1183/09031936.00129606. [DOI] [PubMed] [Google Scholar]
- Lyseng-Williamson KA, Plosker GL. Inhaled salmeterol/fluticasone propionate combination: a pharmacoeconomic review of its use in the management of asthma. Pharmacoeconomics. 2003;21:951–989. doi: 10.2165/00019053-200321130-00004. [DOI] [PubMed] [Google Scholar]
- Macie C, Wooldrage K, Manfreda J, Anthonisen NR. Inhaled corticosteroids and mortality in COPD. Chest. 2006;130:640–646. doi: 10.1378/chest.130.3.640. [DOI] [PubMed] [Google Scholar]
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:1084–1091. doi: 10.1164/rccm.2112055. [DOI] [PubMed] [Google Scholar]
- Mak JC, Nishikawa M, Barnes PJ. Glucocorticosteroids increase β2-adrenergic receptor transcription in human lung. Am J Physiol. 1995a;268:L41–L46. doi: 10.1152/ajplung.1995.268.1.L41. [DOI] [PubMed] [Google Scholar]
- Mak JC, Nishikawa M, Shirasaki H, Miyayasu K, Barnes PJ. Protective effects of a glucocorticoid on downregulation of pulmonary β2-adrenergic receptors in vivo. J Clin Invest. 1995b;96:99–106. doi: 10.1172/JCI118084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura T, Takahashi K, Nakayama K, Kobayashi T, Choi-Miura NH, Tomita M, et al. Increased expression of vascular endothelial growth factor in placentas of p57KIP2 null embryos. FEBS Lett. 2002;532:283–288. doi: 10.1016/s0014-5793(02)03681-5. [DOI] [PubMed] [Google Scholar]
- Meja K, Catley MC, Cambridge LM, Barnes PJ, Lum H, Newton R, et al. Adenovirus-mediated delivery and expression of a cAMP-dependent protein kinase inhibitor gene to BEAS-2B epithelial cells abolishes the anti-inflammatory effects of rolipram, salbutamol and prostaglandin E2: a comparison with H-89. J Pharmacol Exp Ther. 2004;309:833–844. doi: 10.1124/jpet.103.060020. [DOI] [PubMed] [Google Scholar]
- Miller-Larsson A, Selroos O. Advances in asthma and COPD treatment: combination therapy with inhaled corticosteroids and long-acting β2-agonists. Curr Pharm Des. 2006;12:3261–3279. doi: 10.2174/138161206778194187. [DOI] [PubMed] [Google Scholar]
- Mittelstadt PR, Ashwell JD. Inhibition of AP-1 by the glucocorticoid-inducible protein GILZ. J Biol Chem. 2001;276:29603–29610. doi: 10.1074/jbc.M101522200. [DOI] [PubMed] [Google Scholar]
- Moore PE, Laporte JD, Gonzalez S, Moller W, Heyder J, Panettieri RA, et al. Glucocorticoids ablate IL-1β-induced β-adrenergic hypo-responsiveness in human airway smooth muscle cells. Am J Physiol. 1999;277:L932–L942. doi: 10.1152/ajplung.1999.277.5.L932. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- Nannini LJ, Cates CJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting β-agonist in one inhaler versus long-acting β-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007;17:CD006829. doi: 10.1002/14651858.CD006829. [DOI] [PubMed] [Google Scholar]
- Nelson HS, Chapman KR, Pyke SD, Johnson M, Pritchard JN. Enhanced synergy between fluticasone propionate and salmeterol inhaled from a single inhaler versus separate inhalers. J Allergy Clin Immunol. 2003;112:29–36. doi: 10.1067/mai.2003.1558. [DOI] [PubMed] [Google Scholar]
- Newton R. Molecular mechanisms of glucocorticoid action: what is important. Thorax. 2000;55:603–613. doi: 10.1136/thorax.55.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton R, Holden NS. Separating transrepression and transactivation: a distressing divorce for the glucocorticoid receptor. Mol Pharmacol. 2007;72:799–809. doi: 10.1124/mol.107.038794. [DOI] [PubMed] [Google Scholar]
- Newton R, Seybold J, Kuitert LM, Bergmann M, Barnes PJ. Repression of cyclooxygenase-2 and prostaglandin E2 release by dexamethasone occurs by transcriptional and post-transcriptional mechanisms involving loss of polyadenylated mRNA. J Biol Chem. 1998;273:32312–32321. doi: 10.1074/jbc.273.48.32312. [DOI] [PubMed] [Google Scholar]
- Nie M, Knox AJ, Pang L. β2-Adrenoceptor agonists, like glucocorticoids, repress eotaxin gene transcription by selective inhibition of histone H4 acetylation. J Immunol. 2005;175:478–486. doi: 10.4049/jimmunol.175.1.478. [DOI] [PubMed] [Google Scholar]
- NHLBI 1997National asthma education and prevention program. Expert panel report 2: guidelines for the diagnosis and management of asthma
- NHLBI 2002Global initiative for asthma. Global strategy for asthma management and prevention
- O'Byrne PM. Acute asthma intervention: insights from the STAY study. J Allergy Clin Immunol. 2007;119:1332–1336. doi: 10.1016/j.jaci.2007.03.007. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM, Barnes PJ, Rodriguez-Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med. 2001;164:1392–1397. doi: 10.1164/ajrccm.164.8.2104102. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005;171:129–136. doi: 10.1164/rccm.200407-884OC. [DOI] [PubMed] [Google Scholar]
- O'Conner TM, Kelly MM, Leigh R, Otis J, Gauvreau GM, Gauldie J, et al. Additional anti-inflammatory effects of inhaled budesonide/formoterol over inhaled budesonide in subjects with atopic asthma. Eur Respir J. 2006;28:441s. [Google Scholar]
- Oikarinen J, Hamalainen L, Oikarinen A. Modulation of glucocorticoid receptor activity by cyclic nucleotides and its implications on the regulation of human skin fibroblast growth and protein synthesis. Biochim Biophys Acta. 1984;799:158–165. doi: 10.1016/0304-4165(84)90290-3. [DOI] [PubMed] [Google Scholar]
- Panettieri RA. Effects of corticosteroids on structural cells in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:231–234. doi: 10.1513/pats.200402-021MS. [DOI] [PubMed] [Google Scholar]
- Pang L, Holland E, Knox AJ. Role of cyclo-oxygenase-2 induction in interleukin-1β-induced attenuation of cultured human airway smooth muscle cell cyclic AMP generation in response to isoprenaline. Br J Pharmacol. 1998;125:1320–1328. doi: 10.1038/sj.bjp.0702193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang L, Knox AJ. Synergistic inhibition by β2-agonists and corticosteroids on tumor necrosis factor-α-induced interleukin-8 release from cultured human airway smooth-muscle cells. Am J Respir Cell Mol Biol. 2000;23:79–85. doi: 10.1165/ajrcmb.23.1.3985. [DOI] [PubMed] [Google Scholar]
- Pang L, Knox AJ. Regulation of TNFα-induced eotaxin release from cultured human airway smooth muscle cells by β2-agonists and corticosteroids. FASEB J. 2001;15:261–269. doi: 10.1096/fj.00-0103com. [DOI] [PubMed] [Google Scholar]
- Panizza JA, James AL, Ryan G, de Klerk N, Finucane KE. Mortality and airflow obstruction in asthma: a 17-year follow-up study. Intern Med J. 2006;36:773–780. doi: 10.1111/j.1445-5994.2006.01214.x. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Lofdahl CG, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997;337:1405–1411. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- Persdotter S, Lindahl M, Malm-Erjefält M, von Wachenfeldt K, Korn SH, Stevens T, et al. Cooperative inhibitory effects of budesonide and formoterol on eosinophil superoxide production stimulated by bronchial epithelial cell conditioned medium. Int Arch Allergy Immunol. 2007;143:201–210. doi: 10.1159/000099463. [DOI] [PubMed] [Google Scholar]
- Peters SP, Jones CA, Haselkorn T, Mink DR, Valacer DJ, Weiss ST. Real-world evaluation of asthma control and treatment (REACT): findings from a national Web-based survey. J Allergy Clin Immunol. 2007;119:1454–1461. doi: 10.1016/j.jaci.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Planey SL, Abrams MT, Robertson NM, Litwack G. Role of apical caspases and glucocorticoid-regulated genes in glucocorticoid-induced apoptosis of pre-B leukemic cells. Cancer Res. 2003;63:172–178. [PubMed] [Google Scholar]
- Profita M, Gagliardo R, Di Giorgi R, Pompeo F, Gjomarkaj M, Nicolini G, et al. Biochemical interaction between effects of beclomethasone dipropionate and salbutamol or formoterol in sputum cells from mild to moderate asthmatics. Allergy. 2005;60:323–329. doi: 10.1111/j.1398-9995.2005.00702.x. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet. 2006;368:744–753. doi: 10.1016/S0140-6736(06)69284-2. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Beghe B, Luppi F, Fabbri LM. Update in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007a;175:1222–1232. doi: 10.1164/rccm.200704-586UP. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of COPD—2006 update. Am J Respir Crit Care Med. 2007b;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- Rangarajan PN, Umesono K, Evans RM. Modulation of glucocorticoid receptor function by protein kinase A. Mol Endocrinol. 1992;6:1451–1457. doi: 10.1210/mend.6.9.1435789. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Bradding P, Britten KM, Walls AF, Wilson S, Gratziou C, et al. The long-acting β2-agonist salmeterol xinafoate: effects on airway inflammation in asthma. Eur Respir J. 1999;14:275–282. doi: 10.1034/j.1399-3003.1999.14b07.x. [DOI] [PubMed] [Google Scholar]
- Roth M, Johnson PR, Borger P, Bihl MP, Rudiger JJ, King GG, et al. Dysfunctional interaction of C/EBPα and the glucocorticoid receptor in asthmatic bronchial smooth-muscle cells. N Engl J Med. 2004;351:560–574. doi: 10.1056/NEJMoa021660. [DOI] [PubMed] [Google Scholar]
- Roth M, Johnson PR, Rudiger JJ, King GG, Ge Q, Burgess JK, et al. Interaction between glucocorticoids and β2-agonists on bronchial airway smooth muscle cells through synchronised cellular signalling. Lancet. 2002;360:1293–1299. doi: 10.1016/S0140-6736(02)11319-5. [DOI] [PubMed] [Google Scholar]
- Samuelsson MK, Pazirandeh A, Davani B, Okret S. p57Kip2, a glucocorticoid-induced inhibitor of cell cycle progression in HeLa cells. Mol Endocrinol. 1999;13:1811–1822. doi: 10.1210/mend.13.11.0379. [DOI] [PubMed] [Google Scholar]
- Schwiebert LM, Stellato C, Schleimer RP. The epithelium as a target of glucocorticoid action in the treatment of asthma. Am J Respir Crit Care Med. 1996;154:S16–S19. doi: 10.1164/ajrccm/154.2_Pt_2.S16. [DOI] [PubMed] [Google Scholar]
- Sears MR, Taylor DR. The β2-agonist controversy. Observations, explanations and relationship to asthma epidemiology. Drug Saf. 1994;11:259–283. doi: 10.2165/00002018-199411040-00005. [DOI] [PubMed] [Google Scholar]
- Shahab L, Jarvis MJ, Britton J, West R. Prevalence, diagnosis and relation to tobacco dependence of chronic obstructive pulmonary disease in a nationally representative population sample. Thorax. 2006;61:1043–1047. doi: 10.1136/thx.2006.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SD. The macrophage in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:S29–S32. doi: 10.1164/ajrccm.160.supplement_1.9. [DOI] [PubMed] [Google Scholar]
- Shrewsbury S, Pyke S, Britton M. Meta-analysis of increased dose of inhaled steroid or addition of salmeterol in symptomatic asthma (MIASMA) BMJ. 2000;320:1368–1373. doi: 10.1136/bmj.320.7246.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri M, Fregonese L, Sabatini F, Dasic G, Rossi GA. Fluticasone and salmeterol downregulate in vitro, fibroblast proliferation and ICAM-1 or H-CAM expression. Eur Respir J. 2001;18:139–145. doi: 10.1183/09031936.01.00067901. [DOI] [PubMed] [Google Scholar]
- Sin DD, Man SF. Inhaled corticosteroids and survival in chronic obstructive pulmonary disease: does the dose matter. Eur Respir J. 2003;21:260–266. doi: 10.1183/09031936.03.00040803. [DOI] [PubMed] [Google Scholar]
- Sin DD, Man SF. Do chronic inhaled steroids alone or in combination with a bronchodilator prolong life in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2007;13:90–97. doi: 10.1097/MCP.0b013e3280142021. [DOI] [PubMed] [Google Scholar]
- Sin DD, Tu JV. Inhaled corticosteroids and the risk of mortality and readmission in elderly patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:580–584. doi: 10.1164/ajrccm.164.4.2009033. [DOI] [PubMed] [Google Scholar]
- Sin DD, Wu L, Anderson JA, Anthonisen NR, Buist AS, Burge PS, et al. Inhaled corticosteroids and mortality in chronic obstructive pulmonary disease. Thorax. 2005;60:992–997. doi: 10.1136/thx.2005.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singam R, Jena PK, Behera S, Hellermann GR, Lockey RF, Ledford D, et al. Combined fluticasone propionate and salmeterol reduces RSV infection more effectively than either of them alone in allergen-sensitized mice. Virol J. 2006;3:32. doi: 10.1186/1743-422X-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siva R, Green RH, Brightling CE, Shelley M, Hargadon B, McKenna S, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Resp J. 2007;29:906–913. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- So AY, Chaivorapol C, Bolton EC, Li H, Yamamoto KR. Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLoS Genet. 2007;3:e94. doi: 10.1371/journal.pgen.0030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano JB, Vestbo J, Pride NB, Kiri V, Maden C, Maier WC. Survival in COPD patients after regular use of fluticasone propionate and salmeterol in general practice. Eur Respir J. 2002;20:819–825. doi: 10.1183/09031936.02.00301302. [DOI] [PubMed] [Google Scholar]
- Spoelstra FM, Postma DS, Hovenga H, Noordhoek JA, Kauffman HF. Additive anti-inflammatory effect of formoterol and budesonide on human lung fibroblasts. Thorax. 2002;57:237–241. doi: 10.1136/thorax.57.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellato C. Post-transcriptional and nongenomic effects of glucocorticoids. Proc Am Thorac Soc. 2004;1:255–263. doi: 10.1513/pats.200402-015MS. [DOI] [PubMed] [Google Scholar]
- Suissa S. Effectiveness of inhaled corticosteroids in chronic obstructive pulmonary disease: immortal time bias in observational studies. Am J Respir Crit Care Med. 2003;168:49–53. doi: 10.1164/rccm.200210-1231OC. [DOI] [PubMed] [Google Scholar]
- Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- Szapary D, Xu M, Simons SS. Induction properties of a transiently transfected glucocorticoid-responsive gene vary with glucocorticoid receptor concentration. J Biol Chem. 1996;271:30576–30582. doi: 10.1074/jbc.271.48.30576. [DOI] [PubMed] [Google Scholar]
- Thomson NC, Chaudhuri R, Livingston E. Asthma and cigarette smoking. Eur Respir J. 2004;24:822–833. doi: 10.1183/09031936.04.00039004. [DOI] [PubMed] [Google Scholar]
- Thomson NC, Shepherd M, Spears M, Chaudhuri R. Corticosteroid insensitivity in smokers with asthma: clinical evidence, mechanisms and management. Treat Respir Med. 2006;5:467–481. doi: 10.2165/00151829-200605060-00010. [DOI] [PubMed] [Google Scholar]
- Thomson NC, Spears M. The influence of smoking on the treatment response in patients with asthma. Curr Opin Allergy Clin Immunol. 2005;5:57–63. doi: 10.1097/00130832-200502000-00011. [DOI] [PubMed] [Google Scholar]
- Tkacova R, Toth S, Sin DD. Inhaled corticosteroids and survival in COPD patients receiving long-term home oxygen therapy. Respir Med. 2006;100:385–392. doi: 10.1016/j.rmed.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Todorova L, Gürcan E, Miller-Larsson A, Westergren-Thorsson G. Lung fibroblast proteoglycan production induced by serum is inhibited by budesonide and formoterol. Am J Respir Cell Mol Biol. 2006;34:92–100. doi: 10.1165/rcmb.2005-0048OC. [DOI] [PubMed] [Google Scholar]
- Usmani OS, Ito K, Maneechotesuwan K, Ito M, Johnson M, Barnes PJ, et al. Glucocorticoid receptor nuclear translocation in airway cells after inhaled combination therapy. Am J Respir Crit Care Med. 2005;172:704–712. doi: 10.1164/rccm.200408-1041OC. [DOI] [PubMed] [Google Scholar]
- Vestbo J. The TORCH (towards a revolution in COPD health) survival study protocol. Eur Respir J. 2004;24:206–210. doi: 10.1183/09031936.04.00120603. [DOI] [PubMed] [Google Scholar]
- Volonaki E, Psarras S, Xepapadaki P, Psomali D, Gourgiotis D, Papadopoulos NG. Synergistic effects of fluticasone propionate and salmeterol on inhibiting rhinovirus-induced epithelial production of remodelling-associated growth factors. Clin Exp Allergy. 2006;36:1268–1273. doi: 10.1111/j.1365-2222.2006.02566.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Malone MH, He H, McColl KS, Distelhorst CW. Microarray analysis uncovers the induction of the pro-apoptotic BH3-only protein Bim in multiple models of glucocorticoid-induced apoptosis. J Biol Chem. 2003;278:23861–23867. doi: 10.1074/jbc.M301843200. [DOI] [PubMed] [Google Scholar]
- Weigel NL, Zhang Y. Ligand-independent activation of steroid hormone receptors. J Mol Med. 1998;76:469–479. doi: 10.1007/s001090050241. [DOI] [PubMed] [Google Scholar]
- Williams TJ. The eosinophil enigma. J Clin Invest. 2004;113:507–509. doi: 10.1172/JCI21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med. 1996;153:1481–1488. doi: 10.1164/ajrccm.153.5.8630590. [DOI] [PubMed] [Google Scholar]
- Zetterstrom O, Buhl R, Mellem H, Perpina M, Hedman J, O'Neill S, et al. Improved asthma control with budesonide/formoterol in a single inhaler, compared with budesonide alone. Eur Respir J. 2001;18:262–268. doi: 10.1183/09031936.01.00065801. [DOI] [PubMed] [Google Scholar]
- Zhang S, Jonklaas J, Danielsen M. The glucocorticoid agonist activities of mifepristone (RU486) and progesterone are dependent on glucocorticoid receptor levels but not on EC50 values. Steroids. 2007;72:600–608. doi: 10.1016/j.steroids.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Zimmerman B, D'Urzo A, Berube D. Efficacy and safety of formoterol Turbuhaler when added to inhaled corticosteroid treatment in children with asthma. Pediatr Pulmonol. 2004;37:122–127. doi: 10.1002/ppul.10404. [DOI] [PubMed] [Google Scholar]