Abstract
Human immunodeficiency virus (HIV)-1 infection causes progressive impairment of the immune system in humans, characterized by depletion of CD4 T cells and loss of T cell function. Increased markers of T cell activation and lymphoid hyperplasia suggest that chronic T cell activation persists in immunocompromised hosts, and contributes to the exhaustion of immune functions. Here we propose a revision of this hypothesis, in which we suggest that chronic activation of innate immunity may negatively affect adaptive T cell-mediated responses. We hypothesize that constant exposure of the effector cells of innate immunity to HIV results in their chronic hyperactivation, with deleterious effects on T cells. In particular, plasmacytoid dendritic cells (pDC) may be highly susceptible to HIV-induced activation due to its interaction with the cellular receptor CD4, expressed by pDC. Subsequent production of type I interferon and indoleamine 2,3-dioxygenase may exert suppressive and cytotoxic effects on T cells.
Introduction
The advances made during the last 25 years toward understanding the biology of the human immunodeficiency virus (HIV) have not yet clarified the causes of the immunopathogenesis associated with this infection. Several dysfunctions of the immune system have been reported to be associated with HIV infection, and multiple pathogenic mechanisms are considered to contribute to the immunodeficiency syndrome [1; 2; 3; 4]. A frequently-cited hypothesis that summarizes these dysfunctions as a causative mechanism is that a state of chronic immune activation contributes to the loss of CD4 T cells and to the alterations of immune responses, ultimately leading to disease progression [5; 6]. In particular, the increased expression of different activation markers, such as HLA-DR and CD38, on CD4 and CD8 T cell of HIV-infected patients, suggests that these lymphocyte subsets may be chronically stimulated by the lymphotropic HIV virus [6; 7; 8]. Despite a general consensus on the association between T cell activation and HIV disease progression, the understanding of the mechanisms by which chronic immune activation results in the apparently paradoxical effect of chronic immune suppression is still limited.
A number of reports have recently suggested that HIV may trigger immune mechanisms that are normally associated with innate immune responses, not only during early phases of infection but also through the course of the chronic phase [9]. Innate immune responses fulfill the double function of limiting viral replication in the initial stages of infection and enhancing the generation of efficient adaptive immune responses [10]. In particular, production of interferon (IFN)-α and IFN-β by plasmacytoid dendritic cells (pDC) may be triggered through Toll-like receptor (TLR) engagement, and have both immune-stimulating and anti-viral activity, including against HIV [11]. Recent evidence demonstrates that some of these mechanisms may have negative regulatory effects on T cell function [12; 13; 14]. Thus, it is possible that during the early stages of HIV infection, stimulation of innate immunity associated with pDC activation leads to the enhancement of adaptive immune responses. Conversely, continuous pDC activation for a prolonged period of time may result in long-term suppression of T cell responses, causing a state of immune deficiency, similar to that observed during chronic HIV-disease.
We propose a revised version of the chronic immune activation hypothesis, in which HIV infection primarily induces chronic activation of innate immune responses, which subsequently results in the functional impairment of T cells, despite the persistence of an activated T cell phenotype. This imbalanced response has two major consequences: 1) progressive depletion of T cell subsets due to dysregulated production of pro-apoptotic cytokines; and 2) progressive loss of T cell function due to immune suppressive mechanisms associated with innate immunity. Thus, we view chronic innate immune activation to be mainly responsible for the dysregulation of adaptive immunity and immune deficiency.
Chronic immune activation in immunocompromised hosts: an HIV paradox
HIV infection in humans is, by definition, associated with the development of progressive immune deficiency, characterized by impaired adaptive responses in vitro and in vivo, and by increased susceptibility to opportunistic infections [15; 16; 17; 18]. This inability of the immune system to respond to different stimuli is, however, associated with an apparent state of basal immune hyperactivation in the infected host [5; 6]. In particular, signs of chronic T cell activation, such as increased expression of activation markers (for example CD38, HLA-DR and Ki67) are detected in HIV-infected patients and simian immunodeficiency virus (SIV)-infected macaques [5; 6; 7]. This immunological profile contrasts with that observed in natural hosts of HIV/SIV (chimpanzees for HIV and sooty mangabeys for SIV, for example), in which the infection does not cause pathogenesis, despite elevated virus replication [5; 6; 19; 20]. It is noteworthy that the activated state of T cells from HIV/SIV-infected susceptible hosts is “incomplete”, in the sense that these cells show impaired ability to progress through the cell cycle, which can be corrected by administration of exogenous IL-2 [2; 21; 22].
Thus, during pathogenic HIV/SIV infection, the emergence of phenotypic signs of T cell activation appears to be detached from the development of functional T cell responses. Such dichotomy is likely the consequence of aberrant immune response to HIV/SIV, rather than a direct effect of infection, as the same infectious agent replicates at high levels in its natural hosts that show moderate T cell responses, despite ineffective clearance of the virus [19; 20; 23].
Plasmacytoid dendritic cells during HIV infection
IFN-α-producing pDC represent the first line of immune defenses against viral infections [24]. The effect of HIV infection on the function and dynamics of pDC is still debated. Upon in vitro stimulation with TLR agonists, IFN-α production is diminished in blood leukocytes from HIV-infected patients [25; 26; 27; 28]. However, this apparent defect is probably an artifact due to the reduced frequency of pDC in the peripheral blood of HIV-infected patients [25; 26]. Recent evidence suggests that pDC from HIV-infected patients are normally activated by TLR agonists, despite their reduction in number [26].Two different hypothesis have been proposed to account for the diminished proportion of blood pDC in HIV-infected patients: 1) depletion of pDC, due to apoptosis or cytopatic effect of HIV; and 2) activation and migration of pDC from peripheral blood into lymphoid tissues. Two recent studies failed to detect the classical markers of pDC in lymphoid tissues of HIV-infected patients or SIV-infected macaques [29; 30], and concluded that pDC are depleted, rather than redistributed in different tissues during HIV infection. However, type I IFN production, the hallmark of pDC activity, was not analyzed in these two reports [29; 30]. In contrast, other studies have shown high levels of IFN-α in the serum of chronically HIV-infected patients, as well as in tonsils during both chronic and acute HIV infection [31; 32; 33]. Similar, increased levels of the tryptophan-catabolizing enzyme indoleamine 2,3-dyoxigenase (IDO), expressed at high levels by activated pDC, are found in lymphoid tissues during HIV/SIV infection [34; 35; 36; 37]. Furthermore, HIV-mediated activation of pDC in vitro has been demonstrated, which results in IFN-α production, IDO activity and expression of the chemokine receptor CCR7, normally associated with migration to lymphoid tissues [33; 36; 37; 38; 39; 40]. Thus, although phenotypic markers of pDC are not detectable in lymphoid tissues, high levels of their functional markers, type I IFN and IDO, are detected at both peripheral and tissue level in HIV-infected patients, suggesting that the activity of these cells may influence the immunologic environment during HIV infection.
Immune down-regulatory function of plasmacytoid dendritic cells
Studies performed in both murine and human systems have revealed new functions of pDC, in addition to that of anti-viral type I IFN production [12; 14; 40]. In particular, TLR agonists that upregulate IFN-α production also induce the expression of IDO by pDC [41; 42]. IDO catalyzes the degradation of the essential amino acid tryptophan (Trp) into the kynurenine (Kyn) pathway, exerting a powerful suppressive effect on T cell proliferation and activity [14]. IDO-expressing pDC have a tolerizing function under physiologic conditions such as prevention of autoimmune reactions and of maternal-fetal recognition during pregnancy [12; 14]. During chronic pathologic disorders, such as persistent infections and cancer, IDO has been suggested to play a role in preventing efficient immune responses, favoring the maintenance of the chronic condition [13; 14; 36].
An increased rate of Trp degradation, detected as augmented plasma Kyn-to-Trp concentration ratio (Kyn/Trp), has been widely reported in HIV-infected patients (reviewed in [36; 43]). More recently IDO expression has been directly analyzed in both blood cells and lymphoid tissues and found to be increased during HIV-infection [34; 35; 40; 44]. TLR- and HIV-activated pDC express high levels of IDO [40; 41; 42], and inhibition of HIV-induced IDO improved CD4 T cell proliferative responses in vitro [40] and enhanced the clearance of HIV-infected macrophages in a mouse model of HIV-related encephalitis [45]. In addition, IDO activity is strictly associated with the function of regulatory T cells (Treg), in that Treg induce IDO expression in APC, and IDO-expressing APC induce a Treg phenotype in naïve T cells [46; 47; 48; 49]. Treg accumulate in lymphoid tissues where IDO is overexpressed during HIV infection [34; 35; 37; 44], suggesting a tight functional link between these two mechanisms in HIV infection.
The reasons for the simultaneous production of anti-viral type I IFN and T cell-suppressive IDO in the economy of innate anti-viral immune responses are unknown. One possibility is that T cells are protected from the pro-apoptotic effect of type I IFN when their proliferation is prevented by IDO. In fact, IFN-α induces expression of the nuclear protein p53, which regulates apoptotic pathways in cycling cells [50; 51]. Thus, arresting the T cell cycle by Trp catabolism may freeze T cells in a stage in which IFN-α-induced p53 is not detrimental.
Is chronic innate immune activation harmful during HIV infection?
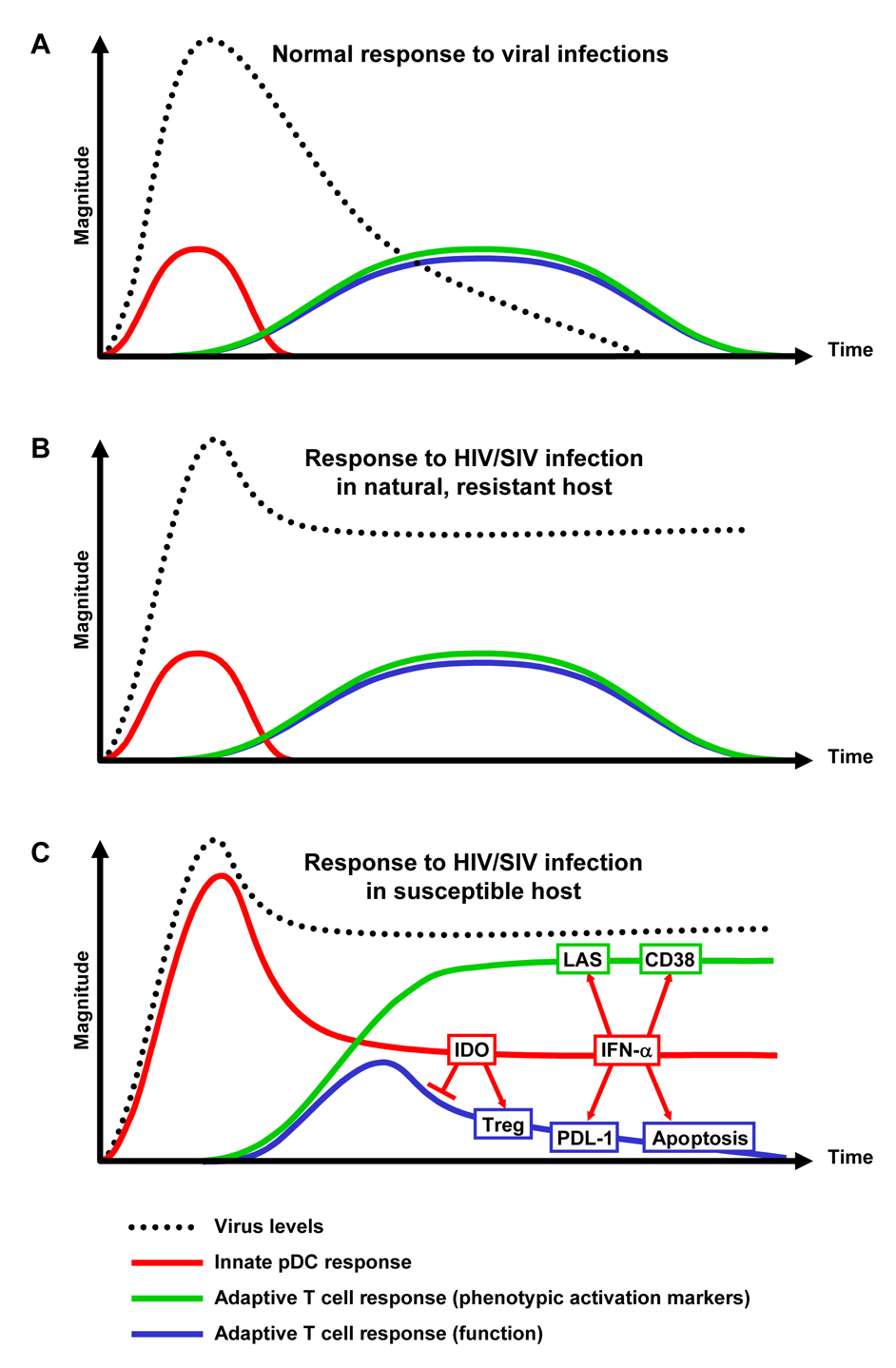
Innate immune responses to viral infections are usually initiated within hours of the first encounter with the virus [52]. This non-antigen-specific response limits viral replication for a short period of time, and is normally replaced by an adaptive, antigen-specific response within days after infection [52; 53], ultimately resulting in the efficient clearance or control of the infectious agent (Fig. 1A). HIV/SIV does not activate pDC of natural resistant hosts in vitro (reported in [54]), and neither signs of T cell activation nor T cell functional impairment are observed in HIV/SIV-infected natural hosts, despite chronically high levels of viral replication [6; 19; 20], suggesting that the profile of innate/adaptive immune response is similar to that observed during viral infections other than HIV/SIV (Fig. 1B). However, in the case of HIV/SIV infection of susceptible hosts, elevated expression of markers associated with pDC activation, such as type I IFN and IDO, are detected during acute infection and are maintained during the chronic phase, concomitant with the lack of an efficient adaptive anti-HIV response [31; 32; 33; 34; 35; 36; 40; 43; 44]. Thus, because of its chronic nature, HIV infection results in prolonged continuous stimulation of pDC, which is maintained beyond the acute-early phase and throughout the entire course of infection. This uncontrolled chronic innate immune activation may lead to a dysregulated adaptive immune response, characterized by functionally impaired T cells and increased levels of phenotypic markers of activation (Fig. 1C).
Figure 1. Chronic innate immune activation as a cause of chronic T cell activation and functional impairment.
(A) During most viral infections, early innate immune responses promote and are replaced by efficient adaptive T cell responses, ultimately resulting in the clearance or control of the infectious agent. (B) HIV/SIV infection of natural resistant hosts is not efficiently cleared by immune responses, but the immune system appears to respond normally to the infection, and no signs of chronic immune activation or immune deficiency are observed. (C) We hypothesize that HIV/SIV infection of susceptible hosts results in abnormal and prolonged activation of innate immune responses, resulting in: 1) induction of phenotypic markers of T cell activation, such as CD38 and the lymphoadenopathy syndrome (LAS); 2) progressive T cell depletion through apoptotic mechanisms; and 3) suppression of functional T cell responses by mechanisms such as IDO/Treg or PDL-1.
Therefore, the dysregulation of innate immune mechanisms could contribute in different ways to both the numerical depletion of CD4 T cells and to the progressive loss of functional responsiveness of T lymphocytes. Activation of IFN-α-induced apoptotic pathways has been reported to contribute to CD4 T cell depletion in HIV-infected humans and SIV-infected macaques, but not in non-human primates in which infection is not pathogenic [9; 33; 55; 56]. Similarly, work by Mark Fienberg’s laboratory demonstrated that the ability of pDC to produce type I IFN in response to SIV is lower in sooty mangabeys, in which SIV infection is not pathogenic, compared to rhesus macaques (reported in [54]).
IDO-expressing pDC inhibit both CD4 and CD8 T cell responses and, in the setting of HIV infection, T cell responses and antiviral activity were improved by competitive inhibition of IDO activity [40; 45] or interference with other IDO-inducing mechanisms such as CTLA-4 [57]. It should be noted that HIV-induced IDO may also contribute to the accumulation of Treg into lymphoid tissues and/or increase Treg activity, as suggested in mouse models and in the case of leukemia [47; 49]. Thus, Fallarino and colleagues reported that, after prolonged exposure to an environment enriched of Kyn and depleted of Trp, naïve CD4 T cells assumed Treg phenotype and function upon T cell receptor engagement [47]. We recently proposed that such cascade of events may also occur during HIV infection [36], leading to the sequential loss of T cell responses to recall antigens, allo-antigens and mitogens [16; 17]. Furthermore, the downregulatory T cell coreceptor CTLA-4, which is constitutively expressed by Treg, is permanently expressed in HIV-infected patients, in contrast to its transient expression characteristic of normal T cell responses [34; 35; 58].
The programmed death (PD)-1 receptor and its ligand (PDL-1) represent another immune regulatory mechanism that dampens anti-viral reponses in HIV-infected individuals [59; 60; 61; 62; 63]. Upregulation of PDL-1 has been reported in pDC and mDC upon stimulation with TLR agonists and type I IFN, respectively [64; 65]. Interestingly, increased PDL-1 also correlated with impaired T cell function in patients with chronic hepatitis B [65].
The “chronic T cell activation” hypothesis is supported by a series of observations which collectively suggest that increased expression of T cell activation markers, such as CD38 and HLA-DR, correlates with disease progression and CD4 T cell depletion better than plasma virus levels [6; 7; 8]. CD38 expression by CD8 T cells is considered the most reliable surrogate marker for immune activation and HIV-disease progression [8]. Notably, in vitro treatment with IFN-α has been shown to induce CD38 upregulation in CD8 T cells, particularly in cells from HIV-infected patients [66]. In the same report, in vitro incubation with blocking antibodies against the cellular receptor for IFN-α diminished CD38 expression on CD8 T cells from HIV-infected patients [66]. These data provide an important link between mediators of innate immunity and expression of the T cell activation marker CD38 on CD8 T cells.
Brenchley and colleagues recently proposed that the mobilization of microbial products from the gut mucosa, due to traumatic virologic and immunologic events that occur in the gut during HIV infection, may be a cause of chronic, systemic immune activation [67]. They found that plasma levels of lipopolisaccaride (LPS), a common structural component of bacteria and strong activator of innate immune responses, were increased in chronically HIV-infected patients and SIV-infected macaques and correlated with markers of immune activation [67]. Although LPS may not directly stimulate pDC, they found that LPS levels correlated with plasma IFN-α and suggested that other factors, originating in the intestinal flora could induce IFN-α production. The mechanisms proposed by Brenchely and colleagues [67] may therefore contribute directly to hyperactivation of innate immunity and the consequent impairment of T cell responses.
The strongest support of our hypothesis comes however from a non-HIV-related study conducted in mice, that investigated the effects of repeated CpG oligodeoxynucleotides (ODN) administration, which activate pDC through TLR9 [68]. Thus, mice that received daily injection of CpG ODN displayed dramatic alterations in the morphology and function of lymphoid organs [68], which resembled those that are seen during HIV infection in humans. For example, lymph node hyperplasia, splenomegaly and disruption of follicle microarchitecture in spleen and lymph nodes were observed within 7 days of initiating CpG ODN-treatment [68]. These changes were accompanied by a temporary increase in the total cellularity in spleen and lymph node, followed, after 20 days of CpG ODN administration, by decreases in the number of CD4 and CD8 T cells, which displayed markers typically associated with an activated phenotype [68]. These alterations were dependent on type I IFN signaling, because CpG ODN treatment showed significantly milder effects in animals lacking the receptor for IFN-α or for both IFN-α and IFN-γ [68]. Considering the kinetic differences in the occurrence of these events in different species, the alterations of lymphoid tissue architecture and cellular dynamics observed in CpG ODN-treated mice appear remarkably similar to those described for HIV infection [6; 32; 69]. This interesting study may represent an in vivo model of acquired immunodeficiency syndrome that does not involve viral infection but approximates the alterations observed in pathogenic HIV/SIV infection.
What makes the HIV-pDC interaction unique?
Activation of pDC occurs as part of the normal innate response to different viral insults [52; 53]. Most viral infections are limited by innate immunity and are eventually cleared by efficient adaptive responses [52; 53]. In some cases, including HIV infection, the combination of innate and adaptive immunity fails to eliminate the infectious agent, which persists in the system as a chronic infection [70]. In the case of HIV, the persistence of the infection is likely a result of both intrinsic characteristics of the virus and ineffective immune response [70; 71], including: 1) ability of the virus to integrate into the host’s cellular genome; 2) hypervariability of HIV which escapes immune recognition; and 3) impairment of cell-mediated responses intrinsic to the disease itself.
Once HIV has established a chronic infection, newly produced virions interact preferentially with cells expressing CD4, including pDC [72]. HIV can directly activate pDC, similar to other viruses, when viral RNA is recognized by intravescicular TLR, most likely TLR7, which recognizes single stranded RNA [73]. CD4 molecules expressed on the cell surface are susceptible to endocytosys, particularly on DC, that do not express the p56lck protein which modulates clathrin-dependent internalization of CD4 in T cells [74; 75]. Thus, the internalization of HIV by pDC may be particularly rapid and efficient due to the peculiar interaction of the virus with a cellular receptor (CD4) that is directly associated with the endocytotic machinery. Alternatively, the endocytotic process might not be as efficient for other viruses, which do not interact directly with endocytosis-competent receptors. Consistent with this hypothesis are the findings that either antibodies against CD4 or soluble CD4 molecules are efficient in inhibiting both HIV-induced IFN-α production and IDO expression by pDC [40; 55].
Our hypothesis predicts that in naturally-resistant HIV/SIV hosts, part of the machinery for HIV/SIV-mediated activation of pDC differs from species in which the infection is pathogenic. These differences may involve different stages of HIV/SIV-mediated pDC activation: 1) CD4-gp120 interaction; 2) CD4-mediated endocytosis; 3) TLR expression or sensitivity to HIV/SIV nucleic acids; or 4) signal transduction and gene expression pattern following TLR-engagement. As a consequence, the strength of the innate immune response to HIV/SIV during both acute and chronic infection could be different between susceptible and resistant species. Unfortunately, no information is available comparing IFN-α and IDO during acute infection in resistant versus susceptible primates. However, sooty mangabeys were reported to develop an adaptive cell-mediated response to SIV which is of lower magnitude than that observed in rhesus macaques or HIV-infected humans [76]. Because innate responses are pivotal in sustaining and enhancing the generation of T cell responses [10], it is possible that stronger activation of innate immunity occurs in the acute phase of SIV infection in rhesus macaques compared to sooty mangabeys (and HIV in humans compared to chimpanzees), thus leading to an apparently stronger adaptive T cell response. This hypothesis is consistent with the kinetics of plasma IFN-α and Kyn levels matching those of plasma viral load during acute infection in SIV-infected cynomologous macaques (Mallaret B. et al, Keystone symposia – HIV pathogenesis 2007 – Abstract 444). Interestingly, an immunologic profile that resembles the model we propose for HIV/SIV infection, was described for classical swine fever infection in pigs, in which high levels of IFN-α production correlated with depletion of blood lymphocytes, beginning in the early stages of acute infection [77].
Conclusions
Advances have been made in the recent years acquiring knowledge about the pathogenic mechanisms that result in the progressive immune deficiency characteristic of HIV infection. The long list of functional alterations of the immune system collected during 25 years of research has given rise to different hypotheses to account for these pathogenic changes. The paradoxical appearance of signs of increasing immune activation in patients who progressively become more functionally immune-compromised has generated the hypothesis that chronic stimulation of T cells may ultimately lead to their malfunction, exhaustion and death. Here we suggest an alternative hypothesis of chronic immune activation. Therefore, we propose that the persistence of HIV particles provides a chronic stimulus for mechanisms normally associated with innate immune responses, such as those mediated by pDC, and that chronic innate immune activation suppresses functional T cell-mediated adaptive immune responses while sustaining the activated phenotype of T cells. In accordance with our hypothesis, repeated administration of CpG ODN in mice, which activate pDC through TLR9, resulted in lymph node hyperplasia, with disruption of lymphoid architecture and multifactorial immune suppression, including decreased CD4 and CD8 T cells [68], which resembles many of the alterations observed during HIV infection.
In addition, we propose that the key molecular events required for the chronic innate immune activation result from the binding of HIV to its cellular receptor CD4. The inhibition of this gp-120-CD4 interaction may provide a therapeutic approach aimed not only at limiting the virus-cell interactions resulting in new infections, but importantly at preventing the interaction of the virus with key components of the innate immune system which leads to qualitative and quantitative aberrations of T cell-mediated responses. Such a strategy might prevent the appearance of the dichotomy between persistent expression of T cell activation markers and T cell functional deficiency characteristic of AIDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Letvin NL, Walker BD. Immunopathogenesis and immunotherapy in AIDS virus infections. Nat Med. 2003;9:861–866. doi: 10.1038/nm0703-861. [DOI] [PubMed] [Google Scholar]
- 2.Paiardini M, Cervasi B, Dunham R, Sumpter B, Radziewicz H, Silvestri G. Cell-cycle dysregulation in the immunopathogenesis of AIDS. Immunol Res. 2004;29:253–268. doi: 10.1385/IR:29:1-3:253. [DOI] [PubMed] [Google Scholar]
- 3.Picker LJ. Immunopathogenesis of acute AIDS virus infection. Curr Opin Immunol. 2006;18:399–405. doi: 10.1016/j.coi.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 5.Derdeyn CA, Silvestri G. Viral and host factors in the pathogenesis of HIV infection. Curr Opin Immunol. 2005;17:366–373. doi: 10.1016/j.coi.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Moanna A, Dunham R, Paiardini M, Silvestri G. CD4+ T-cell depletion in HIV infection: killed by friendly fire? Curr HIV/AIDS Rep. 2005;2:16–23. doi: 10.1007/s11904-996-0004-3. [DOI] [PubMed] [Google Scholar]
- 7.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, Wolinsky SM, Detels R. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Herbeuval JP, Shearer GM. HIV-1 immunopathogenesis: how good interferon turns bad. Clin Immunol. 2007;123:121–128. doi: 10.1016/j.clim.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medzhitov R, Janeway CA., Jr Innate immune recognition and control of adaptive immune responses. Semin Immunol. 1998;10:351–353. doi: 10.1006/smim.1998.0136. [DOI] [PubMed] [Google Scholar]
- 11.Tailor P, Tamura T, Ozato K. IRF family proteins and type I interferon induction in dendritic cells. Cell Res. 2006;16:134–140. doi: 10.1038/sj.cr.7310018. [DOI] [PubMed] [Google Scholar]
- 12.Fallarino F, Gizzi S, Mosci P, Grohmann U, Puccetti P. Tryptophan catabolism in IDO+ plasmacytoid dendritic cells. Curr Drug Metab. 2007;8:209–216. doi: 10.2174/138920007780362581. [DOI] [PubMed] [Google Scholar]
- 13.Mellor AL, Munn DH. Tryptophan catabolism and regulation of adaptive immunity. J Immunol. 2003;170:5809–5813. doi: 10.4049/jimmunol.170.12.5809. [DOI] [PubMed] [Google Scholar]
- 14.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 15.Amendola A, Boschini A, Colzani D, Anselmi G, Oltolina A, Zucconi R, Begnini M, Besana S, Tanzi E, Zanetti AR. Influenza vaccination of HIV-1-positive and HIV-1-negative former intravenous drug users. J Med Virol. 2001;65:644–648. doi: 10.1002/jmv.2085. [DOI] [PubMed] [Google Scholar]
- 16.Clerici M, Stocks NI, Zajac RA, Boswell RN, Lucey DR, Via CS, Shearer GM. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest. 1989;84:1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolan MJ, Clerici M, Blatt SP, Hendrix CW, Melcher GP, Boswell RN, Freeman TM, Ward W, Hensley R, Shearer GM. In vitro T cell function, delayed-type hypersensitivity skin testing, and CD4+ T cell subset phenotyping independently predict survival time in patients infected with human immunodeficiency virus. J Infect Dis. 1995;172:79–87. doi: 10.1093/infdis/172.1.79. [DOI] [PubMed] [Google Scholar]
- 18.Fowke KR, D'Amico R, Chernoff DN, Pottage JC, Jr, Benson CA, Sha BE, Kessler HA, Landay AL, Shearer GM. Immunologic and virologic evaluation after influenza vaccination of HIV-1-infected patients. Aids. 1997;11:1013–1021. doi: 10.1097/00002030-199708000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch VM. What can natural infection of African monkeys with simian immunodeficiency virus tell us about the pathogenesis of AIDS? AIDS Rev. 2004;6:40–53. [PubMed] [Google Scholar]
- 20.Silvestri G. Naturally SIV-infected sooty mangabeys: are we closer to understanding why they do not develop AIDS? J Med Primatol. 2005;34:243–252. doi: 10.1111/j.1600-0684.2005.00122.x. [DOI] [PubMed] [Google Scholar]
- 21.Paiardini M, Cervasi B, Sumpter B, McClure HM, Sodora DL, Magnani M, Staprans SI, Piedimonte G, Silvestri G. Perturbations of cell cycle control in T cells contribute to the different outcomes of simian immunodeficiency virus infection in rhesus macaques and sooty mangabeys. J Virol. 2006;80:634–642. doi: 10.1128/JVI.80.2.634-642.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paiardini M, Galati D, Cervasi B, Cannavo G, Galluzzi L, Montroni M, Guetard D, Magnani M, Piedimonte G, Silvestri G. Exogenous interleukin-2 administration corrects the cell cycle perturbation of lymphocytes from human immunodeficiency virus-infected individuals. J Virol. 2001;75:10843–10855. doi: 10.1128/JVI.75.22.10843-10855.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur A, Alexander L, Staprans SI, Denekamp L, Hale CL, McClure HM, Feinberg MB, Desrosiers RC, Johnson RP. Emergence of cytotoxic T lymphocyte escape mutations in nonpathogenic simian immunodeficiency virus infection. Eur J Immunol. 2001;31:3207–3217. doi: 10.1002/1521-4141(200111)31:11<3207::aid-immu3207>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 24.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 25.Hosmalin A, Lebon P. Type I interferon production in HIV-infected patients. J Leukoc Biol. 2006;80:984–993. doi: 10.1189/jlb.0306154. [DOI] [PubMed] [Google Scholar]
- 26.Martinson JA, Tenorio AR, Montoya CJ, Al-Harthi L, Gichinga CN, Krieg AM, Baum LL, Landay AL. Impact of class A, B and C CpG-oligodeoxynucleotides on in vitro activation of innate immune cells in human immunodeficiency virus-1 infected individuals. Immunology. 2007;120:526–535. doi: 10.1111/j.1365-2567.2007.02530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldman S, Stein D, Amrute S, Denny T, Garcia Z, Kloser P, Sun Y, Megjugorac N, Fitzgerald-Bocarsly P. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin Immunol. 2001;101:201–210. doi: 10.1006/clim.2001.5111. [DOI] [PubMed] [Google Scholar]
- 28.Soumelis V, Scott I, Gheyas F, Bouhour D, Cozon G, Cotte L, Huang L, Levy JA, Liu YJ. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–912. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 29.Biancotto A, Grivel JC, Iglehart SJ, Vanpouille C, Lisco A, Sieg SF, Debernardo R, Garate K, Rodriguez B, Margolis LB, Lederman MM. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–4279. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown KN, Trichel A, Barratt-Boyes SM. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J Immunol. 2007;178:6958–6967. doi: 10.4049/jimmunol.178.11.6958. [DOI] [PubMed] [Google Scholar]
- 31.Abel K, Alegria-Hartman MJ, Rothaeusler K, Marthas M, Miller CJ. The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFN-alpha/beta) and IFN-alpha/beta-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. J Virol. 2002;76:8433–8445. doi: 10.1128/JVI.76.16.8433-8445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grundstrom S, Andersson J. Studies of HIV-associated immune responses in lymphoid compartments. Curr HIV/AIDS Rep. 2006;3:32–38. doi: 10.1007/s11904-006-0006-1. [DOI] [PubMed] [Google Scholar]
- 33.Herbeuval JP, Nilsson J, Boasso A, Hardy AW, Kruhlak MJ, Anderson SA, Dolan MJ, Dy M, Andersson J, Shearer GM. Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc Natl Acad Sci U S A. 2006;103:7000–7005. doi: 10.1073/pnas.0600363103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersson J, Boasso A, Nilsson J, Zhang R, Shire NJ, Lindback S, Shearer GM, Chougnet CA. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol. 2005;174:3143–3147. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- 35.Nilsson J, Boasso A, Velilla PA, Zhang R, Vaccari M, Franchini G, Shearer GM, Andersson J, Chougnet C. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–3817. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boasso A, Shearer GM. How does indoleamine 2,3-dioxygenase contribute to HIV-mediated immune dysregulation. Curr Drug Metab. 2007;8:217–223. doi: 10.2174/138920007780362527. [DOI] [PubMed] [Google Scholar]
- 37.Boasso A, Vaccari M, Nilsson J, Shearer GM, Andersson J, Cecchinato V, Chougnet C, Franchini G. Do regulatory T-cells play a role in AIDS pathogenesis? AIDS Rev. 2006;8:141–147. [PubMed] [Google Scholar]
- 38.Fonteneau JF, Larsson M, Beignon AS, McKenna K, Dasilva I, Amara A, Liu YJ, Lifson JD, Littman DR, Bhardwaj N. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol. 2004;78:5223–5232. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yonezawa A, Morita R, Takaori-Kondo A, Kadowaki N, Kitawaki T, Hori T, Uchiyama T. Natural alpha interferon-producing cells respond to human immunodeficiency virus type 1 with alpha interferon production and maturation into dendritic cells. J Virol. 2003;77:3777–3784. doi: 10.1128/JVI.77.6.3777-3784.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boasso A, Herbeuval JP, Hardy AW, Anderson SA, Dolan MJ, Fuchs D, Shearer GM. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–3359. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fallarino F, Puccetti P. Toll-like receptor 9-mediated induction of the immunosuppressive pathway of tryptophan catabolism. Eur J Immunol. 2006;36:8–11. doi: 10.1002/eji.200535667. [DOI] [PubMed] [Google Scholar]
- 42.Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol. 2005;175:5601–5605. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- 43.Schroecksnadel K, Zangerle R, Bellmann-Weiler R, Garimorth K, Weiss G, Fuchs D. Indoleamine-2, 3-dioxygenase and other interferon-gamma-mediated pathways in patients with human immunodeficiency virus infection. Curr Drug Metab. 2007;8:225–236. doi: 10.2174/138920007780362608. [DOI] [PubMed] [Google Scholar]
- 44.Estes JD, Li Q, Reynolds MR, Wietgrefe S, Duan L, Schacker T, Picker LJ, Watkins DI, Lifson JD, Reilly C, Carlis J, Haase AT. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J Infect Dis. 2006;193:703–712. doi: 10.1086/500368. [DOI] [PubMed] [Google Scholar]
- 45.Potula R, Poluektova L, Knipe B, Chrastil J, Heilman D, Dou H, Takikawa O, Munn DH, Gendelman HE, Persidsky Y. Inhibition of indoleamine 2,3-dioxygenase (IDO) enhances elimination of virus-infected macrophages in an animal model of HIV-1 encephalitis. Blood. 2005;106:2382–2390. doi: 10.1182/blood-2005-04-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 47.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The Combined Effects of Tryptophan Starvation and Tryptophan Catabolites Down-Regulate T Cell Receptor {zeta}-Chain and Induce a Regulatory Phenotype in Naive T Cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 48.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 49.Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, Salvestrini V, Bonanno G, Rutella S, Durelli I, Horenstein AL, Fiore F, Massaia M, Colombo MP, Baccarani M, Lemoli RM. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25− into CD25+ T regulatory cells. Blood. 2007;109:2871–2877. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- 50.Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H, Sasaki S, Imai K, Shibue T, Honda K, Taniguchi T. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516–523. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- 51.Vilcek J. Boosting p53 with interferon and viruses. Nat Immunol. 2003;4:825–826. doi: 10.1038/ni0903-825. [DOI] [PubMed] [Google Scholar]
- 52.Kirchner H. The interferon system as an integral part of the defense system against infections. Antiviral Res. 1986;6:1–17. doi: 10.1016/0166-3542(86)90035-5. [DOI] [PubMed] [Google Scholar]
- 53.Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Heeney JL, Plotkin SA. Immunological correlates of protection from HIV infection and disease. Nat Immunol. 2006;7:1281–1284. doi: 10.1038/ni1206-1281. [DOI] [PubMed] [Google Scholar]
- 55.Herbeuval JP, Hardy AW, Boasso A, Anderson SA, Dolan MJ, Dy M, Shearer GM. Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: role of type I IFN-producing plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2005;102:13974–13979. doi: 10.1073/pnas.0505251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim N, Dabrowska A, Jenner RG, Aldovini A. Human and Simian immunodeficiency virus-mediated upregulation of the apoptotic factor TRAIL occurs in antigen presenting cells from AIDS-susceptible but not from AIDS-resistant species. J Virol. 2007 doi: 10.1128/JVI.02616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hryniewicz A, Boasso A, Edghill-Smith Y, Vaccari M, Fuchs D, Venzon D, Nacsa J, Betts MR, Tsai WP, Heraud JM, Beer B, Blanset D, Chougnet C, Lowy I, Shearer GM, Franchini G. CTLA-4 blockade decreases TGF-beta, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood. 2006;108:3834–3842. doi: 10.1182/blood-2006-04-010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leng Q, Bentwich Z, Magen E, Kalinkovich A, Borkow G. CTLA-4 upregulation during HIV infection: association with anergy and possible target for therapeutic intervention. Aids. 2002;16:519–529. doi: 10.1097/00002030-200203080-00002. [DOI] [PubMed] [Google Scholar]
- 59.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 60.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V, Vaccari M, Tryniszewska E, Gostick E, Roederer M, Douek DC, Morgan SH, Davis SJ, Franchini G, Koup RA. SIV-specific CD8+T-cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007 doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trabattoni D, Saresella M, Biasin M, Boasso A, Piacentini L, Ferrante P, Dong H, Maserati R, Shearer GM, Chen L, Clerici M. B7-H1 is up-regulated in HIV infection and is a novel surrogate marker of disease progression. Blood. 2003;101:2514–2520. doi: 10.1182/blood-2002-10-3065. [DOI] [PubMed] [Google Scholar]
- 63.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 64.Abe M, Wang Z, de Creus A, Thomson AW. Plasmacytoid dendritic cell precursors induce allogeneic T-cell hyporesponsiveness and prolong heart graft survival. Am J Transplant. 2005;5:1808–1819. doi: 10.1111/j.1600-6143.2005.00954.x. [DOI] [PubMed] [Google Scholar]
- 65.Chen L, Zhang Z, Chen W, Zhang Z, Li Y, Shi M, Zhang J, Chen L, Wang S, Wang FS. B7-h1 up-regulation on myeloid dendritic cells significantly suppresses T cell immune function in patients with chronic hepatitis B. J Immunol. 2007;178:6634–6641. doi: 10.4049/jimmunol.178.10.6634. [DOI] [PubMed] [Google Scholar]
- 66.Rodriguez B, Lederman MM, Jiang W, Bazdar DA, Garate K, Harding CV, Sieg SF. Interferon-alpha differentially rescues CD4 and CD8 T cells from apoptosis in HIV infection. Aids. 2006;20:1379–1389. doi: 10.1097/01.aids.0000233571.51899.ab. [DOI] [PubMed] [Google Scholar]
- 67.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 68.Heikenwalder M, Polymenidou M, Junt T, Sigurdson C, Wagner H, Akira S, Zinkernagel R, Aguzzi A. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat Med. 2004;10:187–192. doi: 10.1038/nm987. [DOI] [PubMed] [Google Scholar]
- 69.Fauci AS, Pantaleo G, Stanley S, Weissman D. Immunopathogenic mechanisms of HIV infection. Ann Intern Med. 1996;124:654–663. doi: 10.7326/0003-4819-124-7-199604010-00006. [DOI] [PubMed] [Google Scholar]
- 70.Lieberman J, Shankar P, Manjunath N, Andersson J. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood. 2001;98:1667–1677. doi: 10.1182/blood.v98.6.1667. [DOI] [PubMed] [Google Scholar]
- 71.Munier ML, Kelleher AD. Acutely dysregulated, chronically disabled by the enemy within: T-cell responses to HIV-1 infection. Immunol Cell Biol. 2007;85:6–15. doi: 10.1038/sj.icb.7100015. [DOI] [PubMed] [Google Scholar]
- 72.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD, Bhardwaj N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pelchen-Matthews A, da Silva RP, Bijlmakers MJ, Signoret N, Gordon S, Marsh M. Lack of p56lck expression correlates with CD4 endocytosis in primary lymphoid and myeloid cells. Eur J Immunol. 1998;28:3639–3647. doi: 10.1002/(SICI)1521-4141(199811)28:11<3639::AID-IMMU3639>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 75.Pitcher C, Honing S, Fingerhut A, Bowers K, Marsh M. Cluster of differentiation antigen 4 (CD4) endocytosis and adaptor complex binding require activation of the CD4 endocytosis signal by serine phosphorylation. Mol Biol Cell. 1999;10:677–691. doi: 10.1091/mbc.10.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dunham R, Pagliardini P, Gordon S, Sumpter B, Engram J, Moanna A, Paiardini M, Mandl JN, Lawson B, Garg S, McClure HM, Xu YX, Ibegbu C, Easley K, Katz N, Pandrea I, Apetrei C, Sodora DL, Staprans SI, Feinberg MB, Silvestri G. The AIDS resistance of naturally SIV-infected sooty mangabeys is independent of cellular immunity to the virus. Blood. 2006;108:209–217. doi: 10.1182/blood-2005-12-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Summerfield A, Alves M, Ruggli N, de Bruin MG, McCullough KC. High IFN-alpha responses associated with depletion of lymphocytes and natural IFN-producing cells during classical swine fever. J Interferon Cytokine Res. 2006;26:248–255. doi: 10.1089/jir.2006.26.248. [DOI] [PubMed] [Google Scholar]