Abstract
Study Objectives:
Primary Sjögren's syndrome is an autoimmune disease typified by xerostomia (dry mouth) that, in turn, could lead to increased saliva surface tension (γ) and increased upper airway collapsibility. Fatigue, of unknown etiology, is also frequently reported by patients with primary Sjögren's syndrome. Recent preliminary data indicate a high prevalence of obstructive sleep apnea in healthy-weight women with primary Sjögren's syndrome. Concurrent research highlights a significant role of γ in the maintenance of upper airway patency. The aim of this study was to compare oral mucosal wetness, saliva γ, and upper airway collapsibility during wake and sleep between women with primary Sjögren's syndrome and matched control subjects.
Setting:
Participants slept in a sound-insulated room with physiologic measurements controlled from an adjacent room.
Participants:
Eleven women with primary Sjögren's syndrome and 8 age- and body mass index-matched control women.
Interventions:
Upper airway collapsibility index (minimum choanal-epiglottic pressure expressed as a percentage of delivered choanal pressure) was determined from brief negative-pressure pulses delivered to the upper airway during early inspiration in wakefulness and sleep.
Measurements and Results:
Patients with primary Sjögren's syndrome had significantly higher saliva γ (“pull-off” force method) compared with control subjects (67.2 ± 1.1 mN/m versus 63.2 ± 1.7 mN/m, P < 0.05). Upper airway collapsibility index significantly increased from wake to sleep (Stage 2 and slow wave sleep) but was not different between groups during wake (primary Sjögren's syndrome versus controls; 36.3% ± 8.0% vs 46.0 ± 13.8%), stage 2 sleep (53.1% ± 11.9% vs 63.4% ± 7.2%), or slow-wave sleep (60.8% ± 12.2% vs 60.5% ± 9.3%).
Conclusions:
Despite having a significantly “stickier” upper airway, patients with primary Sjögren's syndrome do not appear to have abnormal upper airway collapsibility, at least as determined from upper airway collapsibility index.
Citation:
Hilditch CJ; McEvoy RD; George KE; Thompson CC; Ryan MK; Rischmueller M; Catcheside PG. Upper airway surface tension but not upper airway collapsibility is elevated in primary Sjögren's syndrome. SLEEP 2008;31(3):367-374.
Keywords: Sjögren's syndrome, obstructive sleep apnea, surface tension, oral mucosal wetness
SJÖGREN'S SYNDROME IS A CHRONIC AUTOIMMUNE DISEASE CHARACTERIZED BY INFLAMMATION OF THE LACRIMAL AND SALIVARY GLANDS.1,2 Sjögren's syndrome can present either alone (primary) or concurrent with rheumatic diseases such as rheumatoid arthritis (secondary). Although reports of primary Sjögren's syndrome prevalence vary (0.04%-4.8%), current literature suggests it is one of the most common autoimmune diseases.2,3 The onset of Sjögren's syndrome generally occurs during middle age and is far more prevalent in females (female:male ratio 9:1).1–3 The cardinal symptoms of Sjögren's syndrome include xerostomia (dry mouth) and keratoconjunctivitis (dry eyes). These symptoms are the result of lymphocytic infiltration and subsequent hypofunction of the salivary and lacrimal exocrine glands.1,3,4
Debilitating fatigue and daytime sleepiness are also common complaints of patients with primary Sjögren's syndrome.1,4–8 Studies show that up to 70% of patients with Sjögren's syndrome report higher fatigue levels, as compared with control subjects without Sjögren's syndrome.1,4,5,7 Gudbjörnsson et al7 found that patients with primary Sjögren's syndrome reported that they felt sleepy during the day, suffered from fatigue, and napped more frequently than controls.
A pilot study from our laboratory (Hlavac, personal communication, 2006), designed to investigate potential causes of sleepiness and fatigue in patients with primary Sjögren's syndrome, found a higher prevalence of obstructive sleep apnea (OSA) in a group of 28 healthy-weight women with primary Sjögren's syndrome, compared with 15 age- and body mass index-matched healthy women. OSA is a sleep-related breathing disorder characterized by repetitive airway obstruction and arousal during sleep and is an established cause of daytime hypersomnolence.9 Together with airway dryness in primary Sjögren's syndrome, these findings may indicate altered upper airway lining liquid (UAL) surface tension (γ) as a potential contributor to sleep- disordered breathing and ultimately daytime sleepiness in patients with primary Sjögren's syndrome. The role of γ in maintaining airway patency for gas exchange in the alveoli and, to some extent, in bronchioles is well described.10 However, the role of γ in the upper airway has only recently been investigated. Several recent studies have found that the intraluminal pressure required to reopen the collapsed airway (opening pressure) is higher than that at which the airway collapses (closing pressure).11–14 Animal11 and human15,16 studies have shown that greater γ in the upper airway can predispose the airway to collapse and that this propensity for collapse is significantly reduced upon application of a surfactant.13,15,17 Van der Touw and colleagues13 found that application of a surfactant to reduce γ in awake healthy humans lowered opening pressure, whereas saline treatment still required greater intraluminal pressure to reopen the airway. This observed hysteretic pattern suggests that adhesion between the upper airway walls attributable to surface forces may be instrumental in determining the length of apneic events.10–12,17 Several studies15,17,18 have also directly assessed the role of γ in patients with OSA and found that lowering γ by means of a surfactant significantly reduces the frequency of obstructive events by up to 42% in patients with OSA.18 Kirkness et al15 employed a more direct approach in their night study of patients with OSA by quantitatively assessing UAL γ and its effect on sleep-disordered breathing. The results showed a reduction in respiratory disturbance index from 51 ± 8 events per hour to 35 ± 8 per hour (mean ± SEM), which correlated with a fall in UAL γ with surfactant. In a separate study, these investigators also reported that, although patients with OSA did not have abnormal salivary flow rates, UAL γ was significantly higher in patients with OSA compared with control subjects (59.9 [53.8, 58.8] mN/m OSA patients, 56.3 [57.7, 62.1] mN/m healthy subjects; mean [95% confidence interval]).19
Another key finding from previous studies is a strong inverse correlation between oral mucosal wetness and UAL γ20 In route-of-breathing studies, nasal breathing has been shown to be associated with increased oral mucosal wetness,20 overnight drops in UAL γ, and an approximately 50% reduction in respiratory disturbance index.19 Nasal turbinates efficiently heat and humidify nasal-breathed air such that mouth-breathed air is comparatively colder and drier. Mouth breathing therefore heightens evaporation from upper airway mucosal surfaces, leading to upper airway drying.19 Hence, oral dryness, and concurrent increased UAL γ observed in mouth breathing20 may serve as a model for dry airways in patients with primary Sjögren's syndrome.
The overarching hypothesis that upper airway dryness contributes to fatigue observed in patients with primary Sjögren's syndrome warrants further investigation. Firstly, however, it remains to be established whether airway surface tension and the mechanical behavior of the upper airway are indeed abnormal in this patient group. Available evidence suggests that upper airway dryness may alter upper airway surface forces in patients with primary Sjögren's syndrome and could potentially lead to abnormal upper airway function, disordered breathing in sleep, and ultimately daytime fatigue. Consequently, the aims of this study were to assess oral mucosal wetness, saliva surface tension, and the collapsibility of the upper airway during wakefulness and sleep in patients with primary Sjögren's syndrome compared with control subjects without Sjögren's syndrome. It was hypothesized that, when compared with results from age-, body mass index-, and sex-matched controls, oral mucosal dryness, saliva surface tension, and upper airway collapsibility would be greater in patients with primary Sjögren's syndrome during both wakefulness and sleep.
METHODS
Subject Selection
All participants were women. Eleven patients with primary Sjögren's syndrome were recruited from South Australian rheumatology outpatient clinics. All patients with primary Sjögren's syndrome satisfied the American-European classification criteria for primary Sjögren's syndrome.21 Eight controls without primary Sjögren's syndrome matched for age and body mass index were recruited on a “bring-a-friend” basis and through local advertisements. Subjects did not have any history of respiratory, craniofacial, or cardiovascular disorders, had not recently had respiratory tract infections (< 1 month ago), and were not using any xerogenic drugs. A supplementary polysomnography visit was conducted for those subjects who had not had a standard diagnostic polysomnography within the past 24 months. Subjects with severe OSA (respiratory disturbance index > 40 events/h) were excluded from the study to allow for negative-pressure-pulse delivery during stable periods of breathing. The study was approved by the Repatriation General Hospital and Adelaide University Human Research and Ethics Committees. All participants provided informed written consent.
Equipment and Instrumentation
Wetness and Surface Tension Measurements
Oral mucosal wetness measurements were obtained using standardized, gravimetric, absorbent paper strips (SialoPaper Strips, Oraflow Inc, Plain View, NY) placed on the posterior midline of the tongue for 5 seconds. The volume of absorbed fluid, as a measure of wetness, was calculated as the difference in weight of the strip before and after fluid absorption.20,22 Saliva was collected for γ analysis using a small polyethylene tube (OD 0.80 mm, ID 0.50 mm, Biocorp, Huntingdale, Victoria, Australia) positioned under the tongue and attached to a needle (23 gauge) and 1-mL syringe to draw approximately 1.0 μL of fluid into the tubing. Samples were stored frozen (-80°C) and sent on dry ice to the Department of Respiratory Medicine, Westmead Hospital, NSW within 2 weeks of collection for γ analysis using the “pull off force” technique.23,24 Briefly, γ was determined by the amount of force required to separate 2 curved silica surfaces bridged by approximately 0.2 μL of the test fluid. Reference normal samples drawn from laboratory staff were sent with each batch to assess the impact of the transportation process.
Sleep, Respiratory, and Pressure Measurements
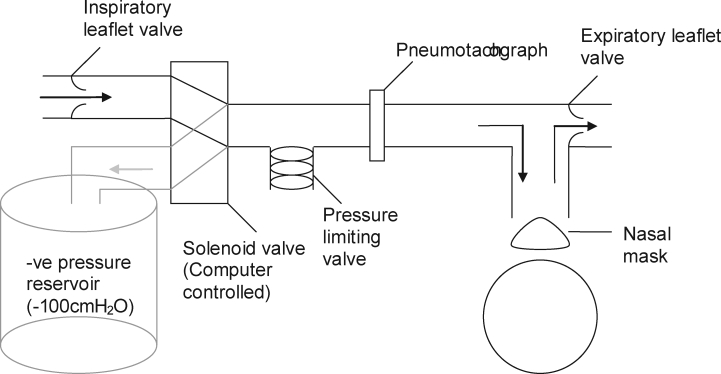
Ventilatory (inspiratory flow, end-tidal co2 (ETco2), mask leak) and sleep data (electroencephalogram, electrooculogram, submental electromyogram) were recorded as described by Hlavac et al.25 A nasal mask (ComfortGel Nasal Mask, Respironics, Murrysville, PA) was attached, via a flexible tube, to a negative-pressure pulse delivery system in the adjacent observation room (see Figure 1). Breath-by-breath ventilation was measured with a pneumotachograph (PT36, Jaeger, Hoechberg, Germany) attached to the nasal mask. Breathing effort was measured using respiratory inductive plethysmography bands fitted to the chest and abdomen (Respitrace system, Ambulatory Monitoring Inc, Ardsley, NY). Mask (Pmask), epiglottic (Pepi), and choanal (Pcho) pressure were measured via pressure transducers (Validyne Engineering, Northridge, CA). Pepi and Pcho were connected to air-perfused catheters (PE tubing, Microtube Extrusions, North Rocks, NSW, Australia) advanced via the nares under local anesthesia (lignocaine hydrochloride, 50 mg/mL) to the level of the epiglottis and posterior nasopharyngeal wall, respectively. For more detail see Jordan et al.26
Figure 1.
Schematic of the respiratory circuit. Arrows indicate direction of airflow: black during normal breathing, grey during brief negative pressure pulses.
Negative Pressure Pulses
Upper airway collapsibility was measured using brief negative-pressure pulses (−8 to −12 cm h2o at the choanae) delivered to the mask every 2 to 6 breaths at early inspiration during wakefulness, stable Stage 2 sleep, and slow-wave sleep (SWS) (> 20 pulses in each state).27 Pulse generation was achieved using a partially evacuated (∼−100 cm h2o) 50-L plastic barrel connected to a computer controlled, pneumatically driven solenoid valve (SXE9575-A70-00, IsoStar, Norgren, Switzerland). Custom software was used to monitor inspiratory flow and trigger brief (∼200 msec) pulses of negative pressure by rapid switching of the inspiratory circuit from room air to the negative-pressure source. An adjustable spring-loaded pressure-limiting valve (Threshold inspiratory muscle trainer, Healthscan Products Inc, Cedar Grove, NJ, USA) was used to regulate the pressure delivered to the subject (see Figure 1).
Protocol
Subjects presented to the laboratory approximately 2 hours prior to their normal sleep time and at least 1 hour postprandial, having abstained from caffeine, alcohol, and strenuous exercise for 12 hours. Upon arrival, written consent was obtained, and the subject was familiarized with the procedures. Oral wetness and γ measurements were taken in the evening and again in the morning immediately after the overnight sleep study. Subjects were asked to drink water 30 minutes prior to these measurements and to perform nasal breathing for 15 minutes prior to these measurements.
The supine position was adopted and confirmed visually via video monitoring during all wake and sleep collapsibility measurements. Subjects were asked to stay awake for 20 minutes, 10 minutes for baseline wakefulness data collection and 10 minutes during which approximately 30 wake pulses were delivered. Subjects were then allowed to fall asleep. In the event of discomfort in the supine position during the night, subjects were allowed to stretch and, if necessary, briefly sleep in a lateral position, before recommencing supine sleep. After any cortical arousal, 1 minute of stable Stage 2 sleep, or SWS was required before another pulse was delivered. If full awakening occurred, further pulses were withheld until at least 2 minutes of stable Stage 2 or SWS had occurred.
Data Analysis
Analogue signals were recorded using a 32-channel data-collection system (Windaq DI 720, DataQ Instruments Inc, Akron, OH) sampling at 200 Hz per channel. Sleep parameters, plethysmography, oxygen saturation (Sao2) and flow were simultaneously recorded using a second computerized data-acquisition system (Compumedics Ltd, E series, Abbotsford, Vic, Australia). Both systems were time locked using an event marker recorded simultaneously on both systems at the onset of each negative pressure pulse.25 Sleep staging, arousal, and respiratory event scoring were performed by a qualified sleep technician, blinded to subject group.
Pulses delivered during swallows or transient catheter blockages were removed from analysis. Flow, Pmask, Pepi, and Pcho were ensemble averaged for each subject from 500 milliseconds before to 1000 milliseconds after each replicate pulse. Upper airway collapsibility index was calculated for each subject from ensemble averaged minimum Pcho − Pepi (at minimum Pcho) expressed as a percentage of the delivered pulse pressure (minimum Pcho).27
Ventilatory parameters, including inspiratory and expiratory time (TI, TE), inspiratory tidal volume (VTI), inspiratory minute ventilation (VI), and upper airway resistance (RUA, slope of Pmask to Pepi versus flow relationship during inspiration) were calculated from the breath preceding each pulse delivered and averaged within each sleep stage (wake, stage 2 sleep, and SWS).
Statistical Analysis
Upper airway collapsibility index, ventilatory parameters, saliva γ, and oral wetness measures were compared between groups (primary Sjögren's syndrome vs controls with Sjögren's syndrome), and across states (wake vs Stage 2 vs SWS) or time (evening vs morning) using analysis of variance for repeated measures. Significant Greenhouse-Geisser adjusted analysis of variance effects were examined using posthoc Student paired t-tests corrected for multiple comparisons using the Dunn-Sidak procedure.28 Statistical significance was inferred when P < 0.05. All data are reported as mean ± SEM.
RESULTS
Of the 11 patients with primary Sjögren's syndrome who were recruited, 2 were unable to sleep and thus contributed only wake data. Four patients and 1 control subject did not achieve SWS during the study night, such that only 7 patients with primary Sjögren's syndrome and 7 control subjects contributed SWS data. Due to the paucity of saliva in patients with primary Sjögren's syndrome, samples for surface tension measurements could be obtained from only 7 patients with primary Sjögren's syndrome in the evening and 8 patients in the morning.
There were no significant between-group differences in age (primary Sjögren's syndrome, 61.0 ± 2.8 years vs control subjects, 55.9 ± 3.2 years), body mass index (primary Sjögren's syndrome, 23.2 ± 1.1 kg/m2 vs control subjects, 25.9 ± 0.8 kg/m2), or respiratory disturbance index (primary Sjögren's syndrome, 16.6 ± 2.8 events/h vs control subjects, 17.9 ± 4.4 events/h).
Sleep Characteristics
On the study night, there were no between-group differences in the total sleep time (overall mean ± SEM; 200 ± 20 minutes), time awake after sleep onset (171 ± 10 minutes), sleep efficiency (44% ± 4%), or the percentage of sleep time spent in stage 2 sleep, SWS, or rapid eye movement sleep (56% ± 3%, 13% ± 3% and 4% ± 2%, respectively). However, patients with primary Sjögren's syndrome demonstrated a greater percentage of stage 1 sleep (33% ± 5% vs 20% ± 3%, P = 0.038) and a trend for a higher arousal index (51 ± 6 vs 36 ± 5 events/h, P = 0.06) compared with control subjects.
Ventilatory Parameters
There were no group or group-by-state effects found for any of the respiratory variables examined. However, VTI and VI significantly decreased from wake to sleep with a corresponding increase in upper airway resistance (see Table 1).
Table 1.
Ventilatory Parameters in Patients with Primary Sjögren's Syndrome and Control Subjects without Primary Sjögren's Syndrome During Wake, Stage 2 Sleep, and Slow-Wave Sleep
| Wake |
Stage 2 |
SWS |
||||
|---|---|---|---|---|---|---|
| Control | SS | Control | SS | Control | SS | |
| TI, sec | 2.2 ± 0.2 | 2.0 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.1 | 1.9 ± 0.1 | 2.0 ± 0.1 |
| TE, sec | 3.0 ± 0.3 | 2.8 ± 0.3 | 2.5 ± 0.3 | 2.7 ± 0.2 | 2.5 ± 0.2 | 2.8 ± 0.3 |
| FB, breaths/min | 12.5 ± 0.9 | 14.2 ± 1.4 | 13.8 ± 0.9 | 13.3 ± 1.0 | 14.1 ± 1.1 | 13.1 ± 1.0 |
| VTI, L | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.4 ± 0.0 | 0.4 ± 0.0* | 0.4 ± 0.0 | 0.4 ± 0.0* |
| VI, L/min | 6.8 ± 0.6 | 7.3 ± 0.7 | 5.4 ± 0.6 | 4.9 ± 0.5* | 5.1 ± 0.6 | 4.9 ± 0.7* |
| Rupper airway, cm h2o/(L/sec) | 5.3 ± 0.7 | 5.9 ± 1.1 | 20.5 ± 3.4 | 13.6 ± 3.2* | 17.2 ± 3.2 | 16.4 ± 4.3* |
| Sao2, % | 97.9 ± 0.4 | 98.2 ± 0.6 | 97.4 ± 0.5 | 97.9 ± 0.4 | 97.6 ± 0.2 | 98.0 ± 0.4 |
| ETco2, mm Hg | 41.2 ± 1.4 | 39.0 ± 1.6 | 43.6 ± 0.7 | 38.7 ± 5.1 | 45.1 ± 0.5 | 37.9 ± 6.7 |
Values are mean ± SEM. Inspiratory time, TI; expiratory time, TE; frequency of breathing, FB; inspiratory tidal volume, VTI; inspiratory minute ventilation, VI; upper airway resistance, RUA; O2 saturation, SaO2; and end-tidal CO2, ETCO2. Primary Sjögren's syndrome (SS), wake, n = 11; primary Sjögren's syndrome stage 2 sleep, n = 9; primary Sjögren's syndrome slow-wave sleep (SWS), n = 7; controls wake and stage 2 sleep, n = 8; controls SWS, n = 7. *P < 0.05 compared to wake conditions when groups were combined.
Oral mucosal wetness
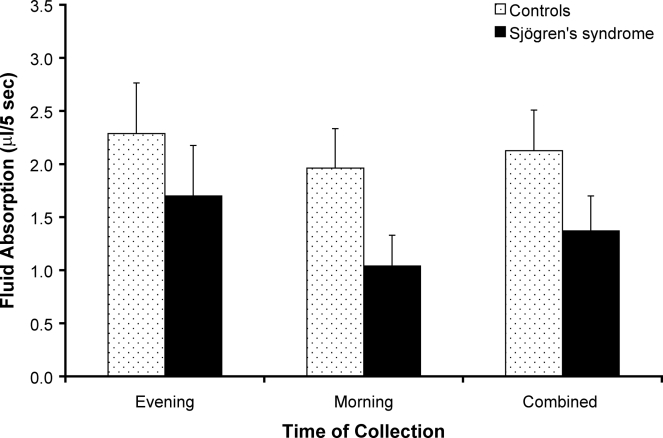
Oral mucosal wetness was not significantly different between the groups with and without primary Sjögren's syndrome (P = 0.101) or across collection times (P = 0.178). Figure 2 shows the amount of gravimetric fluid absorption after sample collection.
Figure 2.
Comparison of gravimetric fluid absorption after 5-sec absorbent paper contact on the posterior midline of the tongue (μL/5 sec) in subjects with primary Sjögren's syndrome (n = 11) and control subjects without Sjögren's syndrome (n = 8) at evening, morning, and combined collection times. There were no significant differences between groups or across collection times. Values are mean ± SEM.
Saliva Surface Tension
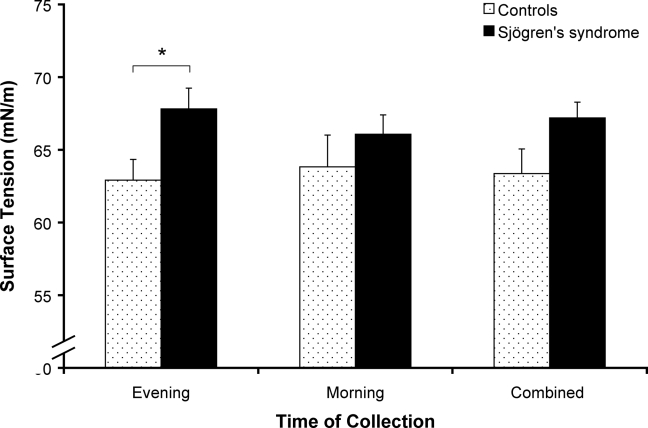
The evening saliva surface tension of patients with primary Sjögren's syndrome was significantly higher than that of the control group (P < 0.05). There were no significant differences between collection times (see Figure 3).
Figure 3.
Saliva surface tension (mN/m) in subjects with primary Sjögren's syndrome and control subjects without Sjögren's syndrome. Patients with primary Sjögren's syndrome had significantly higher saliva surface tension than controls (*P < 0.05). Values are mean ± SEM. Primary Sjögren's syndrome evening, n = 7; primary Sjögren syndrome's morning, n = 8; controls at both collection times, n = 8.
Collapsibility Index
Collapsibility index data were averaged from 326 wake pulses, 449 stage 2 pulses, and 482 SWS pulses for patients with primary Sjögren's syndrome and from 195 wake, 491 stage 2, and 309 SWS pulses for control subjects. Given the pulse-exclusion criteria, 178 of the 1435 pulses delivered to patients with primary Sjögren's syndrome were excluded from primary Sjögren's syndrome analysis and 128 pulses, from a total of 1123, were excluded from control analysis due to swallows or transient catheter blockages.
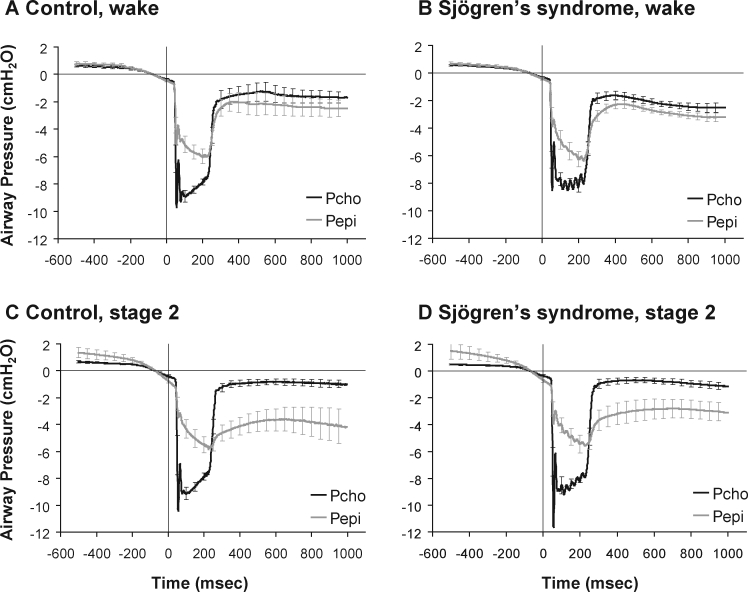
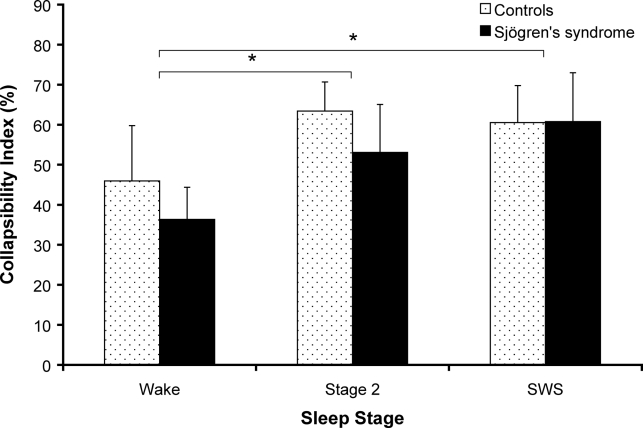
Figure 4 shows Pepi and Pcho traces, ensemble averaged within each subject during multiple negative pressure pulses in wakefulness and stage 2 sleep. The collapsibility index significantly increased from wake to sleep (stage 2 and SWS, P < 0.05), as displayed in Figure 5. However, the collapsibility index was not significantly different between groups (primary Sjögren's syndrome vs control subjects) under any condition. No group-by-stage interaction effects were found.
Figure 4.
Ensemble averaged choanal (Pcho) and epiglottic (Pepi) pressures during negative pressure pulses applied during wake (A, B) and stage 2 sleep (C, D) in patients with primary Sjögren's syndrome (B, D) and control subjects without Sjögren's syndrome (A, C). Raw data averaged from 500 msec before to 1,000 msec after the pulse. Values are mean ± SEM. Primary Sjögren's syndrome wake, n = 11; primary Sjögren's syndrome stage 2 sleep, n = 9; controls in both states, n = 8.
Figure 5.
Comparison of collapsibility index, as a measure of upper airway collapsibility, between primary Sjögren's syndrome and non-primary Sjögren's syndrome controls during wake, stage 2 and SWS. Collapsibility index was calculated from ensemble averaged pulses for each state using the formula: minimum Pcho − Pepi pressure (at minimum Pcho)/ minimum Pcho; expressed as a percentage. *P < 0.05 compared to wake. Values are mean ± SEM. Primary Sjögren's syndrome wake, n = 11; primary Sjögren's syndrome stage 2 sleep, n = 9; primary Sjögren's syndrome slow-wave sleep (SWS), n = 7; controls wake and stage 2 sleep, n = 8; controls SWS, n = 7.
DISCUSSION
The present study was designed to assess, for the first time, the surface active forces and collapsibility of the upper airway in primary Sjögren's syndrome. Saliva γ was found to be significantly higher in patients with primary Sjögren's syndrome compared with control subjects. However, oral wetness and upper airway collapsibility, at least as assessed using negative pressure pulses applied during early inspiration, were not significantly different in patients with primary Sjögren's syndrome compared with well-matched controls without Sjögren's syndrome, nor were there time-of-collection effects detected. A state difference was detected in upper airway collapsibility and for several ventilatory parameters, including VTI, VI, and upper airway resistance, consistent with previous studies,29 but none of these parameters showed between-group differences.
The non-significant trend for reduced oral mucosal wetness in patients with primary Sjögren's syndrome likely reflects a Type II error, given that the criteria for a Sjögren's syndrome diagnosis includes a subjective perception of dryness or an objective measure of salivary flow reduction. Similarly, the lack of a between-group difference in upper airway collapsibility index may also reflect inadequate statistical power. Given the sample size, a significance level of 0.05, and within-group standard deviations in the order of 25%, we estimate that a difference in the collapsibility index of approximately 35% would have been detected between groups with a power of 0.8. In the absence of between-group trends, coupled with significant sleep-stage effects on upper airway collapsibility and upper airway resistance, it appears unlikely that an important effect of primary Sjögren's syndrome on upper airway mechanics, at least of the patent airway during inspiration, has been missed due to Type II error.
Oral Mucosal Wetness
Although our oral mucosal wetness results do not show a significant decrease in wetness in the primary Sjögren's syndrome group compared with the control group, as shown in previous literature, we are confident that our group selection criteria were stringently adhered to and that the trend toward drier mouths in the primary Sjögren's syndrome group is indicative of this. It may be that differences between groups are relatively small due to concomitant age-related mild xerostomia in the control group30 or a relative insensitivity of our mucosal wetness measurements to detect oral dryness in patients with primary Sjögren's syndrome.
Our absorbent paper strip method, adapted from Verma et al,20 although not a direct measure of salivary flow rate, is a novel approach to assessing xerostomia in primary Sjögren's syndrome. The collection of whole or specific gland saliva is a preferred method of objective dry mouth assessment,31 but several studies have recommended the use of absorbent paper strips in various health and disease states, including Sjögren's syndrome.32 Furthermore, Wolff and Kleinberg33 claim that oral mucosal wetness can be directly related to salivary flow rate. Given that the experimental group had already been diagnosed with primary Sjögren's syndrome, the gravimetric absorbent paper strip technique was employed as a fast, simple, and reproducible test to confirm this biologically significant change in salivary gland function. However, the lack of a between-group difference in oral mucosal wetness suggests that direct salivary flow measurements may have provided a more sensitive assessment of oral dryness.
Saliva Surface Tension
Patients with Sjögren's syndrome were found to have significantly higher saliva γ values than control subjects. This is the first time that saliva γ values have been reported in this patient group. It is interesting to note that the mean values were considerably higher, in both patient and control groups, than those previously reported for human saliva (59.2 ± 0.6 mN/m)24 and upper airway lining liquid in healthy normal subjectss (56.3 [53.7, 59.5]; mean [95% Confidence Interval]) and patients with OSA (59.9 [53.8, 58.8]).19 These data were derived from collection and analysis methods identical to those employed in this study. There is no current literature addressing the potential influence of age and sex on upper airway lining liquid γ, but such variables may account for our elevated results in this relatively older, female population. Despite the relatively high values in both groups, the primary Sjögren's syndrome group still maintained significantly higher values than those reported in the control group.
Although human saliva is approximately 95% water, it contains phospholipids that act as a biologic surfactant to maintain a γ lower than water (71.2 mN/m).34 One may speculate that, as well as an elevated saliva γ in patients with primary Sjögren's syndrome, perhaps it is also the lack of saliva and, thus, a reduced total volume of its natural surfactant properties, that may influence upper airway patency. However, Hill et al35 claim that airway mechanics are independent of γ if the fluid lining layer is less than 2% luminal volume, indicative of a relatively dry airway. This study did not, however, venture whether a low liquid volume of the upper airway lining had a protective or destabilizing effect on upper airway patency.
It could be argued that sampling sublingual saliva does not reflect upper airway lining liquid γ. Sampling of upper airway lining liquid from patients with primary Sjögren's syndrome was attempted in this study but was abandoned because the volume of fluid that could be recovered was insufficient for analysis. However, Kirkness et al24 found no significant difference in γ between fluid sampled from under the tongue and the posterior oropharyngeal wall, consistent with the view that swallowing moves saliva to the back of the throat and coats the upper airway such that saliva is the primary constituent of upper airway lining liquid. Although the pharyngeal airway is known to contain secretory cells,36 the quantitative contribution of upper airway glands versus salivary glands to upper airway lining liquid is unknown in healthy humans and may be different in patients with primary Sjögren's syndrome.
Upper Airway Collapsibility
The absence of differences in upper airway collapsibility between groups, despite between-group differences in γ, suggests that any increased tendency to OSA in primary Sjögren's syndrome reflects surface tension and/or airway drying effects not captured by early inspiratory airway collapsibility measurements or other underlying upper airway or respiratory control pathophysiology. Surface forces are likely more important when airway caliber is reduced and in the passive airway, such as near end-expiration. Consequently, we cannot rule out important effects of increased surface tension and mucosal dryness on the propensity for passive airway collapse and potentially more difficult airway opening following collapse in patients with primary Sjögren's syndrome. Further assessment of upper airway critical closing pressure,37 closing38 and opening pressures, or airway behavior during respiratory loading39 may provide more useful indices of airway mechanics in future studies in patients with primary Sjögren's syndrome. In addition, abnormal respiratory events in sleep are defined as a reduction in air flow persisting for at least 10 seconds.9 Thus, increased frequency of obstructive respiratory events in primary Sjögren's syndrome may be due to a prolongation of events10,12 that would normally be counteracted within 10 seconds. Higher luminal γ may require greater intraluminal force (i.e. dilator muscle activity) to restore upper airway patency. OSA is also thought to be multifactorial in etiology,40 and arousal responses or central respiratory control instability might potentially contribute to OSA in this population.
Although the limited sample size of this study warrants caution when interpreting the results, these data support the hypothesis that saliva γ is elevated in patients with primary Sjögren's syndrome compared with well-matched control subjects. Patients with Sjogren syndrome were not, however, found to have abnormal upper airway collapsibility or airflow resistance during inspiration. The apparent increased propensity for OSA in primary Sjögren's syndrome is likely due to alternative mechanisms such as an increased propensity for passive upper airway collapse, compromised upper airway re-opening, or other manifestations of primary Sjögren's syndrome affecting upper airway stability or respiratory control. Further investigation is required to isolate these mechanisms.
ACKNOWLEDGMENTS
The surface liquid lining surface tension measurements were undertaken in the Department of Respiratory Medicine, Westmead Hospital, under the supervision of Dr. Terry Amis. Marni Ahmer scored the sleep studies. Finally, the patients and control subjects are acknowledged for generously donating their time and enduring considerable inconvenience and some discomfort.
Institution at which work was performed: Adelaide Institute for Sleep Health, Repatriation General Hospital, Daw Park, Australia
Financial support: The study was supported in part by NHMRC project grant #324732
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. McEvoy has received equipment and research support from Respironics, ResMed, and Masimo and has consulted for Respironics. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjogren syndrome. Arch Intern Med. 2004 Jun 28;164:1275–1284. doi: 10.1001/archinte.164.12.1275. [DOI] [PubMed] [Google Scholar]
- 2.Venables PJ. Sjogren's syndrome. Best Pract Res Clin Rheumatol. 2004;18:313–329. doi: 10.1016/j.berh.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Thomas E, Hay EM, Hajeer A, Silman AJ. Sjogren's syndrome: a community-based study of prevalence and impact. Br J Rheumatol. 1998;37:1069–1076. doi: 10.1093/rheumatology/37.10.1069. [DOI] [PubMed] [Google Scholar]
- 4.Barendregt PJ, Visser MR, Smets EM, et al. Fatigue in primary Sjogren's syndrome. Ann Rheum Dis. 1998;57:291–295. doi: 10.1136/ard.57.5.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giles I, Isenberg D. Fatigue in primary Sjogren's syndrome: is there a link with the fibromyalgia syndrome? Ann Rheum Dis. 2000;59:875–878. doi: 10.1136/ard.59.11.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godaert GL, Hartkamp A, Geenen R, et al. Fatigue in daily life in patients with primary Sjogren's syndrome and systemic lupus erythematosus. Ann N Y Acad Sci. 2002;966:320–326. doi: 10.1111/j.1749-6632.2002.tb04232.x. 320–6. [DOI] [PubMed] [Google Scholar]
- 7.Gudbjornsson B, Broman JE, Hetta J, Hallgren R. Sleep disturbances in patients with primary Sjogren's syndrome. Br J Rheumatol. 1993;32:1072–1076. doi: 10.1093/rheumatology/32.12.1072. [DOI] [PubMed] [Google Scholar]
- 8.Walker J, Gordon T, Lester S, et al. Increased severity of lower urinary tract symptoms and daytime somnolence in primary Sjogren's syndrome. J Rheumatol. 2003;30:2406–2412. [PubMed] [Google Scholar]
- 9.Stradling JR, Davies RJ. Sleep. 1: Obstructive sleep apnoea/hypopnoea syndrome: definitions, epidemiology, and natural history. Thorax. 2004;59:73–78. doi: 10.1136/thx.2003.007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaver DP, III, Samsel RW, Solway J. Effects of surface tension and viscosity on airway reopening. J Appl Physiol. 1990;69:74–85. doi: 10.1152/jappl.1990.69.1.74. [DOI] [PubMed] [Google Scholar]
- 11.Kirkness JP, Christenson HK, Garlick SR, et al. Decreased surface tension of upper airway mucosal lining liquid increases upper airway patency in anaesthetised rabbits. J Physiol. 2003 Mar 1;547:603–611. doi: 10.1113/jphysiol.2002.031013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson LG, Strohl KP. Airway secretions influence upper airway patency in the rabbit. Am Rev Respir Dis. 1988;137:1379–1381. doi: 10.1164/ajrccm/137.6.1379. [DOI] [PubMed] [Google Scholar]
- 13.Van der TT, Crawford AB, Wheatley JR. Effects of a synthetic lung surfactant on pharyngeal patency in awake human subjects. J Appl Physiol. 1997;82:78–85. doi: 10.1152/jappl.1997.82.1.78. [DOI] [PubMed] [Google Scholar]
- 14.Wilson SL, Thach BT, Brouillette RT, Abu-Osba YK. Upper airway patency in the human infant: influence of airway pressure and posture. J Appl Physiol. 1980;48:500–504. doi: 10.1152/jappl.1980.48.3.500. [DOI] [PubMed] [Google Scholar]
- 15.Kirkness JP, Madronio M, Stavrinou R, Wheatley JR, Amis TC. Relationship between surface tension of upper airway lining liquid and upper airway collapsibility during sleep in obstructive sleep apnea hypopnea syndrome. J Appl Physiol. 2003;95:1761–1766. doi: 10.1152/japplphysiol.00488.2003. [DOI] [PubMed] [Google Scholar]
- 16.Kirkness JP, Eastwood PR, Szollosi I, et al. Effect of surface tension of mucosal lining liquid on upper airway mechanics in anesthetized humans. J Appl Physiol. 2003;95:357–363. doi: 10.1152/japplphysiol.01198.2002. [DOI] [PubMed] [Google Scholar]
- 17.Morrell MJ, Arabi Y, Zahn BR, Meyer KC, Skatrud JB, Badr MS. Effect of surfactant on pharyngeal mechanics in sleeping humans: implications for sleep apnoea. Eur Respir J. 2002;20:451–457. doi: 10.1183/09031936.02.00273702. [DOI] [PubMed] [Google Scholar]
- 18.Jokic R, Klimaszewski A, Mink J, Fitzpatrick MF. Surface tension forces in sleep apnea: the role of a soft tissue lubricant: a randomized double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 1998;157:1522–1525. doi: 10.1164/ajrccm.157.5.9708070. [DOI] [PubMed] [Google Scholar]
- 19.Kirkness JP, Madronio M, Stavrinou R, Wheatley JR, Amis TC. Surface tension of upper airway mucosal lining liquid in obstructive sleep apnea/hypopnea syndrome. Sleep. 2005 Apr 1;28:457–463. doi: 10.1093/sleep/28.4.457. [DOI] [PubMed] [Google Scholar]
- 20.Verma M, Seto-Poon M, Wheatley JR, Amis TC, Kirkness JP. Influence of breathing route on upper airway lining liquid surface tension in humans. J Physiol. 2006 Aug 1;574:859–866. doi: 10.1113/jphysiol.2005.102129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciantar M, Caruana DJ. Periotron 8000: calibration characteristics and reliability. J Periodontal Res. 1998;33:259–264. doi: 10.1111/j.1600-0765.1998.tb02198.x. [DOI] [PubMed] [Google Scholar]
- 23.Kirkness JP, Amis TC, Wheatley JR, Christenson HK. Determining the Surface Tension of Microliter Amounts of Liquid. J Colloid Interface Sci. 2000 Dec 15;232:408–409. doi: 10.1006/jcis.2000.7146. [DOI] [PubMed] [Google Scholar]
- 24.Kirkness JP, Christenson HK, Wheatley JR, Amis TC. Application of the ‘pull-off’ force method for measurement of surface tension of upper airway mucosal lining liquid. Physiol Meas. 2005;26:677–688. doi: 10.1088/0967-3334/26/5/009. [DOI] [PubMed] [Google Scholar]
- 25.Hlavac MC, Catcheside PG, McDonald R, Eckert DJ, Windler S, McEvoy RD. Hypoxia impairs the arousal response to external resistive loading and airway occlusion during sleep. Sleep. 2006 May 1;29:624–631. [PubMed] [Google Scholar]
- 26.Jordan AS, Catcheside PG, O'Donoghue FJ, McEvoy RD. Long-term facilitation of ventilation is not present during wakefulness in healthy men or women. J Appl Physiol. 2002;93:2129–2136. doi: 10.1152/japplphysiol.00135.2002. [DOI] [PubMed] [Google Scholar]
- 27.Malhotra A, Pillar G, Fogel R, Beauregard J, Edwards J, White DP. Upper-airway collapsibility: measurements and sleep effects. Chest. 2001;120:156–161. doi: 10.1378/chest.120.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludbrook J. Repeated measurements and multiple comparisons in cardiovascular research. Cardiovasc Res. 1994;28:303–311. doi: 10.1093/cvr/28.3.303. [DOI] [PubMed] [Google Scholar]
- 29.Wiegand L, Zwillich CW, White DP. Collapsibility of the human upper airway during normal sleep. J Appl Physiol. 1989;66:1800–1808. doi: 10.1152/jappl.1989.66.4.1800. [DOI] [PubMed] [Google Scholar]
- 30.Pedersen W, Schubert M, Izutsu K, Mersai T, Truelove E. Age-dependent decreases in human submandibular gland flow rates as measured under resting and post-stimulation conditions. J Dent Res. 1985;64:822–825. doi: 10.1177/00220345850640050801. [DOI] [PubMed] [Google Scholar]
- 31.Sreebny LM, Zhu WX. The use of whole saliva in the differential diagnosis of Sjogren's syndrome. Adv Dent Res. 1996;10:17–24. doi: 10.1177/08959374960100010201. [DOI] [PubMed] [Google Scholar]
- 32.Shern RJ, Fox PC, Cain JL, Li SH. A method for measuring the flow of saliva from the minor salivary glands. J Dent Res. 1990;69:1146–1149. doi: 10.1177/00220345900690050501. [DOI] [PubMed] [Google Scholar]
- 33.Wolff M, Kleinberg I. Oral mucosal wetness in hypo- and normosalivators. Arch Oral Biol. 1998;43:455–462. doi: 10.1016/s0003-9969(98)00022-3. [DOI] [PubMed] [Google Scholar]
- 34.Lide D. CRC Handbook of Chemistry and Physics: a Ready-Reference Book of Chemical and Physical Data. Boca Raton: CRC Press; 2001. [Google Scholar]
- 35.Hill MJ, Wilson TA, Lambert RK. Effects of surface tension and intraluminal fluid on mechanics of small airways. J Appl Physiol. 1997;82:233–239. doi: 10.1152/jappl.1997.82.1.233. [DOI] [PubMed] [Google Scholar]
- 36.Gray H. Anatomy, descriptive and surgical. 1901 Edition ed. Philadelphia: Running Press; 1974. [Google Scholar]
- 37.Boudewyns A, Punjabi N, Van de Heyning PH, et al. Abbreviated method for assessing upper airway function in obstructive sleep apnea. Chest. 2000;118:1031–1041. doi: 10.1378/chest.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 38.Issa FG, Sullivan CE. Upper airway closing pressures in obstructive sleep apnea. J Appl Physiol. 1984;57:520–527. doi: 10.1152/jappl.1984.57.2.520. [DOI] [PubMed] [Google Scholar]
- 39.Pillar G, Malhotra A, Fogel R, Beauregard J, Schnall R, White DP. Airway mechanics and ventilation in response to resistive loading during sleep: influence of gender. Am J Respir Crit Care Med. 2000;162:1627–1632. doi: 10.1164/ajrccm.162.5.2003131. [DOI] [PubMed] [Google Scholar]
- 40.White DP. The pathogenesis of obstructive sleep apnea: advances in the past 100 years. Am J Respir Cell Mol Biol. 2006;34:1–6. doi: 10.1165/rcmb.2005-0317OE. [DOI] [PubMed] [Google Scholar]