Abstract
The renin–angiotensin system (RAS) mediates several classic physiologies including body water and electrolyte homeostasis, blood pressure, cyclicity of reproductive hormones and sexual behaviors, and the regulation of pituitary gland hormones. These functions appear to be mediated by the angiotensin II (AngII)/AT1 receptor subtype system. More recently, the angiotensin IV (AngIV)/AT4 receptor subtype system has been implicated in cognitive processing, cerebroprotection, local blood flow, stress, anxiety and depression. There is accumulating evidence to suggest an inhibitory influence by AngII acting at the AT1 subtype, and a facilitory role by AngIV acting at the AT4 subtype, on neuronal firing rate, long-term potentiation, associative and spatial learning, and memory. This review initially describes the biochemical pathways that permit synthesis and degradation of active angiotensin peptides and three receptor subtypes (AT1, AT2 and AT4) thus far characterized. There is vigorous debate concerning the identity of the most recently discovered receptor subtype, AT4. Descriptions of classic and novel physiologies and behaviors controlled by the RAS are presented. This review concludes with a consideration of the emerging therapeutic applications suggested by these newly discovered functions of the RAS.
Keywords: Renin–angiotensin system, Angiotensin receptor subtypes, Angiotensin II, Angiotensin III, Angiotensin IV, Angiotensin(1–7), Cardiovascular control, Thirst, Sodium appetite, Long-term potentiation, Learning and memory, Cerebroprotection, Seizure, Stress, Depression
1. Introduction
Well over 100 years ago Tiegerstedt and Bergman (1898) discovered a pressor agent extracted from the kidney that they called “renin”. Some 40 years later this finding led to the isolation of a vasoconstrictor agent from the ischemic kidneys of Goldblatt hypertensive dogs (Braun-Menendez et al., 1940). Page and Helmer (1940) independently isolated the same agent after injecting renin into intact animals and they also identified a “renin activator”, later determined to be angiotensinogen (de Gasparo et al., 2000). The vasoconstrictor agent was determined to be an octapeptide and was variously called “renin substrate”, “angiotonin”, and “hypertensin” but was later termed angiotensin II (AngII; Bumpus et al., 1957, 1958; Elliott and Peart, 1956; Skeggs et al., 1957). From this beginning many additional findings have been made including the observation that intracerebroventricular (icv) AngII produced brain-mediated pressor (Bickerton and Buckley, 1961) and drinking responses (Epstein et al., 1970). Ganten et al. (1971a,b) isolated renin in the dog brain; while Fisher-Ferraro et al. (1971) identified both renin and AngII in the dog brain. Sirrett et al. (1977) developed a radio-receptor binding assay permitting the identification and localization of angiotensin receptors in the brain and throughout the body. Taken together these findings suggested the presence of an independent brain renin–angiotensin system (RAS). Confirmation of this hypothesis required several additional years of laboratory work utilizing a variety of techniques including radioimmunoassay, immunohistochemistry, radio-receptor binding assays, and Northern blots of renin and angiotensinogen mRNAs (Dzau et al., 1986; Ganten et al., 1983; Harding et al., 1981; Hermann et al., 1984; Lynch et al., 1986; Phillips et al., 1979).
Two recent discoveries have further extended our understanding of the RAS: (1) the angiotensin receptor proteins AT1 and AT2 were cloned and sequenced (Chiu et al., 1989; Whitebread et al., 1989; Iwai et al., 1991; Murphy et al., 1991; Kambayashi et al., 1993; Mukoyama et al., 1993). (2) A third angiotensin receptor subtype, AT4, was discovered (Harding et al., 1992) that appears to mediate a number of novel functions including memory consolidation, blood flow, renal tubular reabsorption, and cellular proliferation (Llorens-Cortes and Mendelsohn, 2002; Wright and Harding, 1997, 2004). These discoveries have rekindled interest in the brain RAS and its role in additional physiologies and pathologies.
The present paper briefly describes the biochemistry of the RAS including the formation of active angiotensin ligands, and the three receptor subtypes thus far identified. Next, the controversy over the identity of the AT4 receptor subtype is presented, followed by descriptions of classic and novel functions of the RAS. We conclude with a consideration of potential clinical targets resulting from these new discoveries.
2. Biochemistry of the renin–angiotensin system
The RAS mediates a number of classic physiologies and behaviors including blood pressure, sodium and body water balance, cyclicity of reproductive hormones and sexual behaviors, and pituitary gland hormones. These functions appear to be under the control of the AT1 receptor subtype (Allen et al., 2000; de Gasparo et al., 2000; Gard, 2002; McKinley et al., 2003; Thomas and Mendelsohn, 2003). A second subtype, AT2, has also been implicated in the regulation of blood pressure, renal function, and vascular growth (de Gasparo and Siragy, 1999; de Gasparo et al., 2000; Speth et al., 1995). AngII has traditionally been considered the end-product of the RAS and therefore the active ligand at these receptors subtypes. Accumulating evidence indicates that additional shorter chain angiotensins also serve as effector peptides in this system. These peptides include the heptapeptide des Asp1-AngII, referred to as angiotensin III (AngIII) (Vauquelin et al., 2002; Wright and Harding, 1997), the hexapeptide des Asp1, des Arg2-AngII, referred to as AngIV (Albiston et al., 2003; de Gasparo et al., 2000; Thomas and Mendelsohn, 2003; Wright and Harding, 1994, 1997; Wright et al., 1995), and the heptapeptide des Phe8-AngII, referred to as Ang(1–7) (Ferrario, 2003; Ferrario et al., 1997; Santos et al., 2000). The proposed functions mediated by AngIV include influences upon blood flow (Coleman et al., 1998; Kramár et al., 1997; Mǿeller et al., 1997; Slinker et al., 1999), kidney natriuresis (Hamilton et al., 2001; Handa et al., 1998), expression of plasminogen activator inhibitor (PAI-1) in endothelial cells (Kerins et al., 1995: Mehta et al., 2002) and in epithelial cells of the kidney proximal tubule (Gesualdo et al., 1999), and memory facilitation (reviewed in Albiston et al., 2003; von Bohlen und Halbach, 2003; Wright et al., 2002a). The functions thus far identified for Ang(1–7) include vasopressin, nitric oxide (NO), and prostaglandin release, and facilitation of baroreceptor reflex sensitivity (Ferrario and Chappell, 2004; Kucharewicz et al., 2002; Santos et al., 2000).
2.1. Formation of angiotensin ligands
The protein angiotensinogen serves as a precursor to angiotensin peptides (Fig. 1). The decapeptide angiotensin I (AngI) is formed by renin (EC 3.4.23.15) acting upon the amino terminal of angiotensinogen. AngI is a substrate for angiotensin converting enzyme (ACE: EC 3.4.15.1), a zinc metalloprotease that hydrolizes the carboxy terminal dipeptide His-Leu to form AngII (Johnston, 1990), and the chymotrypsin-like serine protease, chymase (Unger, 2004). AngII is converted to AngIII by glutamyl aminopeptidase A (AP-A: EC 3.4.11.7, or A-like activity) that cleaves the Asp residue at the N-terminal (Chauvel et al., 1994; Ramírez et al., 1990; Rich et al., 1984; Wilk and Healy, 1993). Membrane alanyl aminopeptidase N (AP-N: EC 3.4.11.2) cleaves Arg at the N-terminal of AngIII to form AngIV. AngIV can be further converted to Ang(3–7) by carboxypeptidase P (Carb-P) and propyl oligopeptidase (PO) cleavage of the Pro-Phe bond. Endopeptidases such as chymotrypsin are capable of cleaving the Val, Tyr, and Ile residues along with dipeptidyl carboxypeptidase that cleaves the His–Pro bond, reducing AngIV and Ang(3–7) to inactive peptide fragments and amino acid constituents (Banegas et al., 2006; Johnston, 1990; Reudelhuber, 2005; Saavedra, 1992; Speth et al., 2003; Unger et al., 1988).
Fig. 1.
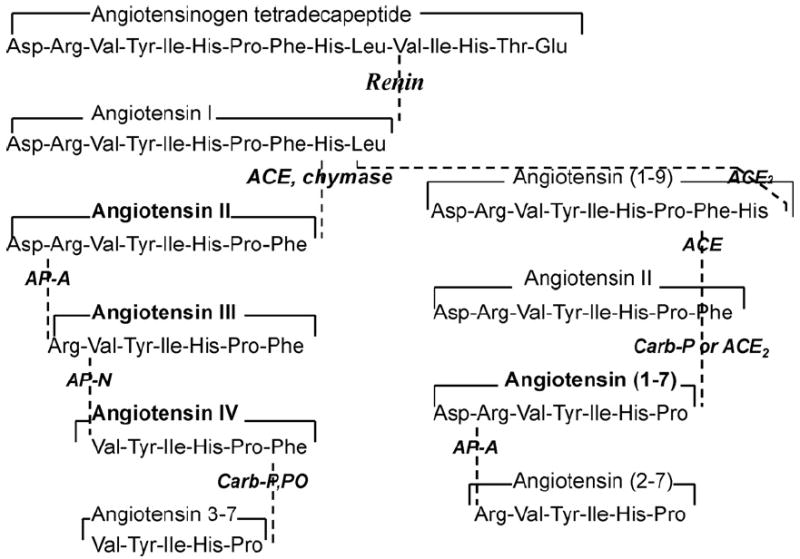
Description of the peptide structures and enzymes involved in the conversion of angiotensinogen to angiotensin I through shorter fragments. The biologically active forms include angiotensin II, III, IV and (1–7). Adapted from Wright and Harding (2004).
AngII can also be converted to Ang(1–7) by Carb-P cleavage of Phe (Wright and Harding, 1997), by the newly discovered mono-peptidase ACE2 (Ferrario and Chappell, 2004) or by ACE cleavage of the dipeptide Phe-His from Ang(1–9) (Vauquelin et al., 2002), and can be further converted to Ang(2–7) by AP-A acting at the Asp-Arg bond (Mentlein and Roos, 1996).
AngI is considered inactive while AngII and AngIII are full agonists at the AT1 and AT2 receptor subtypes (reviewed in de Gasparo et al., 2000). AngIV binds with low affinity at the AT1 and AT2 receptor subtypes (Bennett and Snyder, 1976; Glossmann et al., 1974; Harding et al., 1992; Swanson et al.,1992), but with high affinity and specificity at the AT4 receptor subtype (Bernier et al., 1994; Harding et al., 1992; Jarvis et al., 1992; Swanson et al., 1992). A specific binding site for Ang(1–7) has been reported (Ferrario, 2003; Neves et al., 2003; Santos et al., 1994, 2000), but not fully elucidated.
2.2. Angiotensin receptor subtypes
As described above, at present there are three recognized angiotensin receptor subtypes (de Gasparo et al., 1995), two structurally similar, and a third that is different (Table 1). The AT1 and AT2 subtypes are G-protein coupled. In contrast the AT4 subtype is a much larger protein insensitive to guanine nucleotides, suggesting that it is not G-protein-linked (de Gasparo et al., 2000). There is presently a dispute over the identy of this sybytpe as insulin-regulated aminopeptidase (IRAP) or the growth factor receptor c-Met (Yamamoto et al., submitted for publication). This controversy is further described below.
Table 1.
Characteristics of the angiotensin receptor subtypes
| Characteristics | AT1 | AT2 | AT4–c-Met |
|---|---|---|---|
| Affinity | AngII > AngIII > AngI | AngIII > AngII > AngI | Nle1, Leual3-AngIV > HGF = AngIV > LVV-H7a |
| Selective antagonists | CGP46027, DuP753, DuP 532, EXP3174, L158809, GR117289, SK/F108566, SC51316, UP269-6, LR-B/081 | CGP42112A, PD123177, PD121981, PD123319, PD124125 | Nle1, Leual3-AngIV, Divalinal-AngIV |
| Coupling to | G-protein | G-protein | Tyrosine kinase |
| Signal transduction | ↑Ca2+, ↑IP3, DAG, ↓Adenylyl cyclase, Src, JAK/STAT, ↑Prostaglandins, PL-A, -C, and -D | ↓cGMP/↑cGMP, ↑Prostaglandins PL-A2, NO | Gab1, Grb2, Grb10, PI3K, PLC-8, SHP2, Shc |
| Structure | 359 amino acids; 7 transmembrane domains | 363 amino acids; 7 transmembrane domains | dimer linked by disulfide bonds |
| Molecular size | 41–42 kDa | 40–41 kDa | α 50 kDa; β 140 kDa |
Adapted from Birchmeier et al. (2003), de Gasparo et al. (2000), Ma et al. (2003), Mehta and Griendling (2007), Speth et al. (2003) and Wright and Harding (1997, 2004).
Tentative order regarding relative affinities.
2.2.1. AT1 and AT2 receptor subtypes
The AT1 receptor subtype is a G-protein coupled receptor with signaling via phospholipase-C and calcium. Thus, the angiotensin ligand binds to the AT1 receptor and induces a conformational change in the receptor protein that activates G proteins, and in turn, mediate signal transduction. This transduction involves several plasma membrane mechanisms including phospholipase-C, -A2, and -D-adenylate cyclase, plus L-type and T-type voltage sensitive calcium channels (de Gasparo et al., 2000; Sayeski et al., 1998). This AT1 receptor (now designated AT1A) is also coupled to intracellular signaling cascades that regulate gene transcription and the expression of proteins that mediate cellular proliferation and growth in many target tissues. Expression cloning was used to isolate the cDNAs encoding this receptor protein (Murphy et al., 1991; Sasaki et al., 1991) and it was found to be a seven-transmembrane domain protein consisting of 359 amino acids with a mass of approximately 41 kDa (Sandberg et al., 1994). Subsequently, a second AT1 subtype was discovered and designated AT1B that was also cloned in the rat (Iwai and Inagami, 1992; Kakar et al., 1992), mouse (Sadamura et al., 1992), and human (Konoshi et al., 1994). This subtype is approximately 92–95% homologous with the amino acid sequence of the AT1A subtype (Guo and Inagami, 1994; Speth et al., 1995). Of these two isoforms the AT1A subtype appears to be responsible for the classic functions associated with the brain angiotensin system (reviewed in Saavedra, 1999; Thomas and Mendelsohn, 2003).
The AT2 receptor subtype has also been cloned and sequenced using a rat fetus expression library (Bottari et al., 1991; Kambayashi et al., 1993). In common with the AT1 subtype, this receptor protein also evidences a seven-transmembrane domain characteristic of G-protein coupled receptors, however, it shows only about 32–34% amino acid sequence identity with the rat AT1 receptor. The AT2 receptor protein includes a 363 amino acid sequence (40 kDa) with 99% sequence agreement between rat and mouse, and 72% homology with human (de Gasparo et al., 2000). Even though this AT2 receptor possesses structural features in common with members of the 7-transmembrane family of receptors, it displays few if any functional similarities with this group, although it does appear to be G-protein coupled (Bottari et al., 1991; Kambayashi et al., 1993; Mukoyama et al., 1993).
2.2.2. AT4 receptor subtype
Prior to 1988 angiotensins shorter than AngIII were considered biologically inactive and therefore of little physiological importance. This assumption was based on two facts: (1) AngIV reveals a very poor affinity for the AT1 and AT2 sites (Bennett and Snyder, 1976; Glossmann et al., 1974; Harding et al., 1992; Swanson et al., 1992). (2) AngIV and shorter fragments are considerably less potent than Ang II and AngIII in eliciting classic angiotensin-dependent functions (Blair-West et al., 1971; Fitzsimons, 1971; Tonnaer et al., 1982; Unger et al., 1988; Wright et al., 1989). Two discoveries changed this assumption. First, Braszko et al. (1988) reported that AngIV facilitated acquisition of a conditioned avoidance response in rats. Second, a separate and distinct binding site for AngIV was identified (Harding et al., 1992; Swanson et al., 1992) and subsequently classified as the AT4 subtype (de Gasparo et al., 1995). This subtype was originally isolated using bovine adrenal membranes (Bernier et al., 1994; Harding et al., 1992; Jarvis et al., 1992; Swanson et al., 1992). These characterization studies indicated that the AT4 receptor subtype is distinct from the AT1 and AT2 sites given that ligands known to bind to these sites do not bind at the AT4 site (Harding et al., 1992; Swanson et al., 1992). It was determined that [125I]AngIV binds at the AT4 site reversibly, saturably, and with high affinity. This AT4 site has been found in a variety of mammalian tissues including adrenal gland, bladder, colon, heart, kidney, prostate, brain, and spinal cord (Wright et al., 1995).
Given that a small peptide is capable of activating the AT4 site, and that the vast majority of small peptide receptors are G protein-linked, it was logical to predict that the AT4 receptor would be a serpentine G protein-linked receptor. However, this is not the case since binding to this site was found to be insensitive to guanine nucleotides. In addition, the AT4 receptor subunit exhibited a molecular weight in the 100+ kDa range as determined by reduced SDS-polyacrylamide gel electrophoresis. An equivalent molecular weight has been reported for this receptor in other bovine tissues including heart, thymus, kidney, bladder, aorta, and hippocampus (Zhang et al., 1999). Further, Bernier et al. (1995, 1998) established a similar molecular weight for the binding subunit of the AT4 receptor in bovine aortic endothelial cells. The lack of linkage to G proteins is also supported by the observation that GTPγS failed to alter [125I]AngIV binding in rabbit heart (Hanesworth et al., 1993), guinea pig brain (Miller-Wing et al., 1993), and rat vascular smooth muscle (Hall et al., 1993). A single report by Dulin et al. (1995) indicated that GTPγS inhibited AT4 receptor binding in opossum kidney cells. Thus, to date there is little evidence linking the AT4 receptor to G proteins, however, as experienced with the AT2 receptor, a definitive conclusion must await sequencing of this receptor protein.
2.2.3. Is the AT4 receptor c-Met or insulin-regulated aminopeptidase (IRAP)?
A potentially important advance in our understanding of the AT4 receptor system was the identification of the AT4 receptor as insulin-regulated aminopeptidase (Albiston et al., 2001), a membrane associated aminopeptidase that co-distributes with the GLUT4 transporter (Kandror and Pilch, 1994; Keller et al.,1995). This initial identification was based on sequence homology between a tryptic fragment derived from the human brain AT4 receptor and human IRAP, and the near identical masses of IRAP and the AT4 receptor binding subunit protein. Subsequent expression of IRAP in HEK293T cells yielded an AT4 receptor-like binding site with an affinity for AngIV that was similar to the native receptor (Lee et al., 2003). These investigators further proposed that the multiple physiological actions of AT4 receptor ligands are due to their ability to competitively inhibit the peptidase activity of IRAP, thus potentiating the actions of endogenous peptides that are normally degraded by IRAP (Lew et al., 2003). This model predicts that the efficacy of all AT4 receptor ligands should be qualitatively equivalent since their action is due to binding to IRAP and competitive interference with IRAP’s ability to catabolize endogenous peptides.
There are several problems with this proposal. (1) This notion is difficult to reconcile with results from agonist and antagonist compounds that exhibit opposite physiological actions (Hamilton et al., 2001; Kramár et al., 1997, 2001; Wright et al., 1999). (2) The onset of physiological effects should be slow since this action requires an accumulation of endogenous AT4 ligand. This prediction does not agree with the observation that AT4 ligands have very rapid effects on signaling molecules (Chen et al., 2001; Handa, 2001; Li et al.,2002). For example, AT4 receptor activation can lead to a 20-fold increase in ERK activation in C6 glioma cells within 30 s (Harding, Anderson and Meighan, unpublished). Similarly, in vivo studies indicate rapid AT4-mediated changes in blood flow (Kramár et al., 1997), renal oxygen consumption (Handa et al., 1998), and long-term potentiation (LTP; Kramár et al., 2001; Wayner et al., 2001), typically manifesting in less than 1 min. It is unlikely that sufficient peptide accumulates to impact physiological responses in such a short period of time. More typical time frames for in vivo peptidase inhibitors are hours or days, not seconds. (3) The concentrations of AT4 ligands required to effect changes in physiological function are subpicomolar or subnanomolar (Chen et al., 2001; Handa, 2001; Li et al., 2002); concentrations well below those reported for any known enzyme inhibitor. This concern is relevant for IRAP given that the reported Ki of Nle1-AngIV for IRAP (>0.3 μM, Lew et al., 2003) is several orders of magnitude higher than the biologically effective doses of AT4 ligands. (4) Also casting doubt on the hypothesis that AT4 ligands function as competitive substrates is a study by Caron et al. (2003) indicating that AngIV ligands interact allosterically with IRAP at a site distinct from the active site. The precise characteristics concerning the structure of AngIV, its analogs, and other angiotensins such as AngIII, that render them nonsubstrates for IRAP, but still able to bind, are presently unclear (Lew et al., 2003). (5) This proposal is in opposition to earlier work by Tsujimoto et al. (1992) demonstrating that AngIII is an excellent substrate for human placental leucine aminopeptidase (homolog of rat IRAP, Keller et al., 1995).
The discordance between the IRAP inhibitor model and laboratory observations suggests two likely possibilities. First, IRAP is not the signal transducing AT4 receptor but is instead involved with regulating the extracellular levels of endogenous AT4 receptor ligands. Second, IRAP may be the signal transducing receptor but relies on activities beyond its abilities as an aminopeptidase. If the second possibility is correct then IRAP should possess within its short 109-amino acid hydrophilic N-terminal segment the information required for signal transduction. Lending credibility to this possibility are prevous studies, one indicating that the N-terminus of IRAP contains two dileucine motifs and several acidic regions, that play important roles in vesicular trafficking (Keller et al., 1995; Waters et al., 1997). A peptide consisting of residues 55–82 of the N-terminus, containing one of the dileucine motifs and acidic clusters, was sufficient to cause GLUT4 translocation (Waters et al., 1997). Correspondingly, Ryu et al. (2002) showed in vitro phosphorylation of IRAP Ser80, which is involved in the regulation of insulin stimulated GLUT4 translocation. The poly (ADP-ribose) polymerase tankyrase was identified in a yeast two-hybrid system and interacted with 96–101 amino acids of IRAP (Chi and Lodish, 2000). Interestingly, acyl-coenzyme A dehydrogenases (ACDs), identified by glutathione-S-transferase (GST) fusion-IRAP (GST-IRAP55–82) is probably involved in retention of GLUT4 vesicles to designated intracellular compartments (Katagiri et al., 2002). Similar mechanisms might exist for IRAP at the plasma membrane resulting in signal transduction given that several signaling events have been associated with activation of AT4 receptor by AT4 ligands (Handa, 2001; Li et al., 2002). No matter the exact role played by IRAP in AT4 ligand signaling, the affinity of IRAP for AT4 receptor ligands suggests that its function is in some way important.
The above considerations led members of our laboratory to the conclusion that the physiological actions of angiotensin IV-related ligands are not mediated by IRAP. Furthermore, we made the assumption that Ang IV, and related analogs, must have structural homology with one or more naturally occurring ligands that work through their cognate receptors to mediate physiological actions reminiscent of those initiated by the Ang IV analogs. Based on this thinking we carried out a homology search that yielded a partial match to the anti-angiogenic protein, angiostatin, and the related plasminogen family member hepatocyte growth factor (HGF). HGF is a powerful mitogenic, morphogenic, and motogenic growth factor that acts via the type I tyrosine kinase receptor, c-Met (Fig. 2; Ma et al., 2003). Classically, c-Met has been noted for its ability to direct stem cell proliferation and differentiation (Nakamura et al., 1989; Stoker et al., 1987), induce tubular morphogenesis in many organs including kidney, and support angiogenesis by activating vascular endothelial cells (Birchmeier et al., 2003; Liao et al., 2005). More recently, c-Met has attracted considerable attention because of its role in multiple cancers (Jiang et al., 2005; Shinomiya and Vande Woude, 2003), its ability to blunt neurodegenerative changes, and its potential involvement in learning and memory consolidation (Akimoto et al., 2004; Date et al., 2004; Shimamura et al., 2006; Tada et al., 2006). The known ability of AngIV analogs to alter cognitive function, augment neurite outgrowth, and activate vascular endothelial cells (Wright and Harding, 1997, 2004), which direct the angiogenic process, encouraged us to investigate the possibility that Ang IV-like analogs exert their biological activity through the HGF/c-Met system. These studies demonstrated that the AT4 receptor antagonist, norleual (Nle-Tyr-Leu-(CH2-NH2)3–4-His-Pro-Phe), is capable of inhibiting HGF-dependent proliferation, invasion, and scattering in several cell lines at picomolar concentrations (Yamomoto et al., submitted). Moreover, norleual was shown to block [125I]-HGF binding to c-Met with a Ki of 3 pM; while conversely HGF was found to effectively block [125I]-norleual to HEK cell membranes. As anticipated from these results, norleual exhibits potent anti-angiogenic and anti-tumor activities. Specifically, norleual inhibited the in vivo growth of B-16 melanomas, a c-Met-dependent murine cancer, and induced apoptosis of the U-87 human glioblastoma cells, the survival of which is strictly dependent on an a active c-Met signaling system.
Fig. 2.
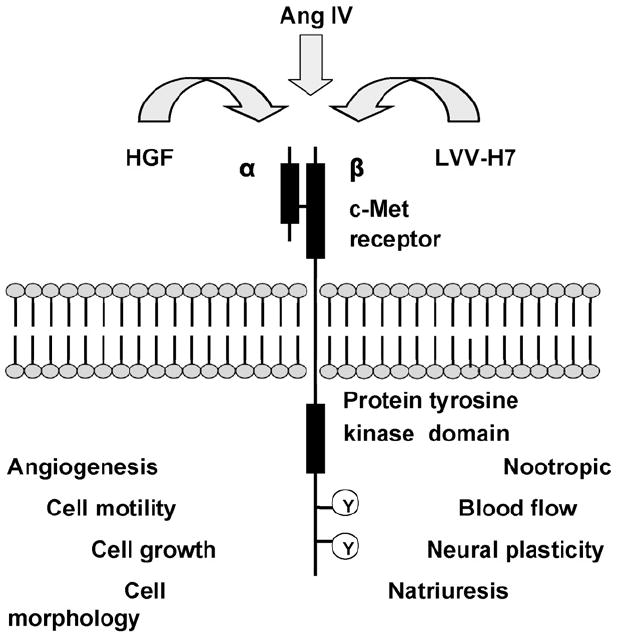
Hepatocyte growth factor (HGF), angiotensin IV (AngIV) and leucine–valine–valine–hemorphin-7 (LVV–H7) appear to each be capable of activating the c-Met receptor. The functions to the bottom left are known to be mediated by the c-Met receptor. Those functions listed to the right are tentative. Adapted from Birchmeier et al. (2003) and Jiang et al. (2005).
2.2.4. Angiotensin(1–7)
Ferrario et al. (1988; Schiavone et al., 1988) were the first to report biological activity by Ang(1–7) in the form of vasopressin release from the posterior pituitary gland. In the years since that discovery many investigators have confirmed the biological importance of this peptide (reviewed in Carey and Saragy, 2003; Kucharewicz et al., 2002; Santos et al., 2000). Ang(1–7) opposes several actions of AngII and AngIII. Specifically, Ang(1–7) stimulates the release of NO and vasodilator prostaglandins (Brosnihan et al., 1996; Li et al., 1997; Meng and Busija, 1993; Osei et al., 1993; Paula et al., 1995). Ang(1–7) stimulated release of NO appears to be primarily from vascular endothelial and smooth muscle cells (Jaiswal et al., 1992; Muthalif et al., 1998) thus opposing AngII-induced vasoconstriction (Ueda et al., 2000). This peptide also appears to protect cardiac and endothelium function as well as coronary perfusion as demonstrated in a heart failure model (Loot et al., 2002). Further, Ang(1–7) has been shown to facilitate baroreceptor reflex sensitivity and modulate circadian rhythm influences on heart rate and blood pressure (Campagnole-Santos et al., 1992; Silva-Barcellos et al., 2001). Recent evidence suggests a role for this peptide in kidney diuresis and natriuresis associated with increased glomerular filtration rate, and protection against preeclampsia during pregnancy (Ferrario and Chappell, 2004).
It is well established that AngII promotes thrombosis primarily via expression of plasminogen activator inhibitor 1 (PAI-1) (Feener et al., 1995; Vaughan et al., 1995); although this effect could be via AngIV (Kerins et al., 1995). Kucharewicz et al. (2000, 2002) have shown that Ang(1–7) functions as an antithrombotic compound when administered to renal hypertensive rats that served as a venous thrombosis model. A putative binding site with high affinity for Ang(1–7) has been identified but not characterized (Santos et al., 1994). Tallant et al. (1997) reported that Ang(1–7) binding to this site cannot be inhibited by AT1 or AT2 receptor subtype antagonists, but can be blocked by sarile (Sar1,Ile8-AngII) in bovine aortic endothelial cells. On the other hand, Santos et al. (2000) have noted the action of Ang(1–7) to be inhibited by losartan and AT2 receptor antagonists. The Mas oncogene has been suggested as the Ang(1–7) receptor (reviewed in Reudelhuber, 2005; Roks and Henning, 2003). The intracellular signaling mechanisms are presently undetermined (reviewed in Santos et al., 2000).
2.3. Cerebral distributions of angiotensin receptor subtypes
Circumventricular organs (CVOs) were initially investigated for the presence of angiotensin receptors because they possess a reduced blood–brain-barrier due to fenestrated capillaries. Binding sites for AngII were discovered within the CVOs, specifically in the subfornical organ (SFO), organum vasculosum of the lamina terminalis (OVLT), area postrema (AP), median eminence, and anterior pituitary gland (Landas et al., 1980; Phillips, 1987; Van Houten et al., 1980). Subsequent investigations localized AngII receptor sites in several brain structures within the blood–brain-barrier. The distribution of brain structures possessing receptor sites is reasonably consistent among the mammalian species examined using quantitative autoradiography and homogenate tissue preparations. These species include rat, mouse, hamster, dog, monkey, and human (reviewed in Chai et al., 2000; von Bohlen und Halbach, 2003; Wright and Harding, 1997, 2004). The above studies have now been extended and indicate that the AT1 subtype is localized with especially high densities in the anterior pituitary, area postrema, median eminence, lateral geniculate, nucleus of the solitary tract (NTS), the anterior ventral third ventricle region (including OVLT), paraventricular (PVN), supraoptic (SON), and ventral medial nuclei (VMN), and the preoptic region of the hypothalamus, SFO, ventral tegmental area (VTA), and the inferior olivary nucleus of the medulla (Table 2).
Table 2.
Predominant mammalian brain distributions of the three angiotensin receptor subtypes identified to date
| Subtype | AT1 | AT2 | AT4 |
|---|---|---|---|
| Structures | |||
| Amygdala | M | H | M |
| Anterior pituitary | H | H | |
| Area postrema (AP) | H | ||
| Caudate putamen | H | H | H |
| Cerebellum | M | M | H |
| Cerebral cortex | H | ||
| Claustrum | H | ||
| Dentate gyrus | M | ||
| Geniculate, lateral | H | H | |
| Geniculate, medial | H | M | |
| Globus pallidus | H | H | |
| Habenula | M | H | H |
| Hippocampus | H | ||
| Hypoglossal nucleus | H | ||
| Inferior colliculus | H | M | |
| Inferior olivary nucleus | H | H | H |
| Lateral olfactory tract | M | M | |
| Locus coeruleus | M | H | M |
| Mammillary body | M | ||
| Medial preoptic nucleus | M | ||
| Median eminence | H | ||
| Nucleus accumbens | M | ||
| Nucleus basalis of Meynert | H | ||
| Nucleus of lateral olfactory tract | M | ||
| Nucleus of solitary tract (NTS) | H | ||
| Organum vasculosum of the lateral terminalis (OVLT) | H | ||
| Paraventricular nucleus (PVN) | H | ||
| Periaqueductal gray | H | ||
| Piriform cortex | L | H | |
| Preoptic nucleus | H | L | |
| Red nucleus | L | ||
| Septum | M | M | M |
| Subfornical organ (SFO) | H | ||
| Substantia nigra | M | ||
| Superior colliculus | M | H | |
| Suprachiasmatic nucleus | M | ||
| Supraoptic nucleus (SON) | H | ||
| Thalamus | H | H | |
| Ventromedial nucleus (VMN) | M | ||
| Ventral tegmental area (VTA) | H | H | |
| Zona incerta | M |
L: low density; M: moderate; H: heavy. Adapted from Chai et al. (2000), Gard (2002), Wright and Harding (1997, 2004) and Wright et al. (1995).
The highest levels of the AT2 site are found in the amygdala, medial geniculate, hypoglossal nucleus, inferior olivary nucleus, lateral habenula, caudate putamen, globus pallidus, locus coeruleus, thalamus, inferior colliculus, and VTA.
Structures with the greatest densities of the AT4 subtype include anterior pituitary gland, caudate-putamen, cerebellum, cerebral and piriform cortices, claustrum, globus pallidus, habenula, hippocampus, inferior olivary nucleus, lateral geniculate, periaquaductal gray, superior colliculi, thalamus, and VTA. Lesser densities of the AT4 subtype have been identified in the lateral olfactory tract and nucleus accumbens.
Comparing the distributions of AT1, AT2, and AT4 receptors, the AT4 site is expressed in reasonably high densities and rather uniquely in the cerebellum, cerebral and piriform cortices, claustrum, hippocampus, caudate putamen, nucleus accumbens, medial habenula, nucleus basalis of Meynert, and periaquaductal gray. Several of these brain structures are intimately involved in cognitive processing.
In summary, the functions mediated by the brain RAS have been assumed to include classic mechanisms concerned with body water balance, blood pressure maintenance, and cyclicity of reproductive hormones and behaviors. The discovery of the AT4 receptor subtype and accumulating evidence concerning its role in neural plasticity, strongly indicate that angiotensin peptides are also involved in the mediation of learning and memory processes.
3. Classic angiotensin-mediated physiologies and behaviors
The RAS is well known as a mediator of systemic blood pressure and the maintenance of volume and electrolyte homeostasis (Table 3). These functions appear to be under the control of the AngII/AT1 receptor subtype system. AngIII and Ang(1–7) also appear to contribute to the regulation of these physiologies. Angiotensins have been implicated in additional nonclassical functions including learning and memory, cerebroprotection, renal blood flow and natriuresis, stress and depression (von Bohlen und Halbach, 2003; Gard, 2002; Saavedra, 2005; Thomas and Mendelsohn, 2003; Wright and Harding, 1997, 2004). This and the following section describe the well-established functions of this system and newly emerging novel physiologies and behaviors, respectively.
Table 3.
Summary of angiotensin mediated physiologies and behaviors
| AT1 | AT2 | AT4–c-Met | Ang(1–7)/Mas? |
|---|---|---|---|
| Receptor subtype | |||
| Blood pressure | Blood pressure | Blood flow | Blood pressure |
| Thirst | Thirst | Kidney natriuresis | Vasopressin release |
| Sodium appetite | PAI-expression | NO release | Prostaglandin release |
| Renal function | Vascular growth | Enhance LTP | NO release |
| Cyclicity of reproductive | Memory consolidation | Baroreceptor reflex | |
| Hormones and behaviors | Cognitive affect | Modulation | |
| Sympathetic activation | Cerebroprotection | Antithrombosis | |
| ACTH release | Anti-preeclampsia | ||
| Vasopressin release | |||
| Baroreceptor reflex | |||
| Memory inhibition |
3.1. Cardiovascular control
The peripheral RAS contributes to cardiovascular functioning by direct inotropic influences upon the heart and via increased vascular resistance (reviewed in Johnston, 1990; Wright and Harding, 1997). The increase in vascular resistance occurs due to direct action on vascular smooth muscle and indirect action via the brain resulting in sympathetic nervous system arousal, the release of the powerful vasoconstrictor vasopressin, and inhibition of the baroreceptor reflex (Culman et al., 2002; Phillips and Sumners, 1998; Unger et al., 1988).
The discovery of RAS components in the brain led to the notion of a local and independent brain RAS. Considerable evidence now supports the existence of two primary brain angiotensinergic pathways (Llorens-Cortes and Mendelsohn, 2002). A forebrain pathway integrates CVOs with the PVN, SON and median preoptic nuclei. A second pathway links the hypothalamus and medulla (including AP, and NTS). Since CVOs possess fenestrated capillaries and are heavily distributed with angiotensin receptors, activation of these receptors by blood–borne angiotensins is thought to impact central cardiovascular circuits, thus permitting interaction between the peripheral and central RASs. Given that the AT1 receptor subtype binds AngII and AngIII with approximately the same affinity, and similarly provoke changes in blood pressure, thirst, and vasopressin release, it has been postulated that either AngII and AngIII are equivalently potent at the AT1 subtype, or AngII must be converted to AngIII in order to activate this receptor subtype (Llorens-Cortes and Mendelsohn, 2002; Stragier et al., 2004; Wright and Harding, 1997). Thus, there is continuing debate over the identity of the active ligand(s) at the AT1 receptor subtype.
This section summarizes the influences of AngII and AngIII upon blood pressure and vasopressin release.
3.2. Angiotensin II
Intracerebroventricular (icv) injections of AngII produce reliable pressor responses via activation of AT1 receptors located in the CVOs (SFO, OVLT, and AP) that directly or indirectly project to the PVN and SON to induce vasopressin release (reviewed in Phillips and Sumners, 1998; Wright and Harding, 1992). The primary mechanism mediating vasopressin release from these nuclei appears to be norepinephrine activation of α-adrenergic receptors located on PVN and SON neurons (Culman et al., 1995). Microinjections of AngII into the SFO, OVLT, and PVN also elicit elevations in blood pressure (reviewed in Wright and Harding, 1992). The pressor response induced by circulating AngII appears to be mediated primarily by the SFO and AP. The absence of a blood–brain barrier at these CVO sites permits penetration by other circulating hormones. AngII also activates AT1 receptors at cardiovascular centers in the medulla. Target structures include NTS, AP, and anterior ventrolateral medulla (Culman et al., 2002). In particular, the AP appears to detect blood–borne AngII; while AngII activation of the NTS influences the baroreceptor reflex. Thus, circulating levels of AngII impact the baroreceptor reflex via a pathway from the AP to the NTS (Muratami, 1996; Phillips, 1987). In addition, AngII activation of AT1 receptors in the anterior ventralateral medulla increases blood pressure by facilitating the sympathetic nervous system, tachycardia, and catecholamine release from the adrenal medulla (Allen et al., 2001; Dampney et al., 1996; Head, 1996; Muratami, 1996; Unger et al., 1985).
3.3. Angiotensin III and shorter fragments
In the 1970s and 1980s Fitzsimons and colleagues investigated the pressor potency of centrally applied AngIII and found it to possess 50% or less the potency of AngII depending upon the infusion site (reviewed in Fitzsimons, 1998; Wright and Harding, 1992, 1997). Tonnaer et al. (1982) reported the greatest pressor activity to icv injected AngII followed by AngI and AngIII (picomol range), with less activity induced by AngII(3–8), (4–8), (5–8), and (6–8) (nmol range). The C-terminal dipeptide AngII(7–8) and other dipeptites were inactive. Studies by Fink and Bruner (1985) and Wright et al. (1985) reevaluated the potency of AngIII and corrected potential shortcomings by siliconizing all glassware to discourage adherence of peptides, reduced the doses in order to minimize the half-life advantage of AngII over AngIII, and utilized degradation resistant analogs in an effort to reduce the in vivo conversion of AngII to AngIII, and AngIII to AngIV. Under these conditions pressor responses induced by icv infused AngII, AngIII and successively shortened C-terminal fragments through AngII(5–8) were compared (Wright et al., 1985, 1989). The results indicated that AngII, AngIII, Sar1-AngII, and Sar1-AngIII were comparable with respect to pressor responses in the alert rat, while AngIV and Sar1-AngIV revealed approximately 70% of the activity of the above compounds. The activity of the shorter C-terminal fragments dropped to below 35%.
Zini et al. (1996) took a different approach to this issue by developing selective inhibitors of AP-A and AP-N (Fig. 3). The AP-A inhibitor (3-amino-4-thio-butyl-sulfonate: EC33) has been shown to increase the half-life of AngII by 2.6-fold as measured in hypothalamic homogenates, and completely blocked the formation of AngIII. An AP-N inhibitor (2-amino-pentane-1,5-dithiol: EC27) increased the half-life of AngIII by 2.3-fold. When AngII was icv injected in mice, plasma vasopressin levels were increased by 2-fold, however, the co-application of EC33 inhibited this AngII-induced vasopressin response in a dose-dependent fashion. In contrast, the icv injection of EC27 alone increased plasma vasopressin levels in a dose-dependent fashion. This EC27 stimulation of vasopressin release could be blocked by the accompanying injection of the nonspecific angiotensin receptor antagonist saralasin (Sar1, Ala8-AngII). These results suggest that central angiotensin-induced vasopressin release is dependent upon the conversion of AngII to AngIII, and therefore AngIII may be the main effector peptide in the brain with respect to the mediation of vasopressin release. Consistent with these findings are results utilizing an antiserum with anti-catalytic activity against AP-A (Song et al., 1997). When icv infused this antiserum reduced both drinking and blood pressure responses to the subsequent icv delivery of AngII by 73 and 59%, respectively. This same antiserum had no effect on icv AngIII-induced drinking and blood pressure responses.
Fig. 3.
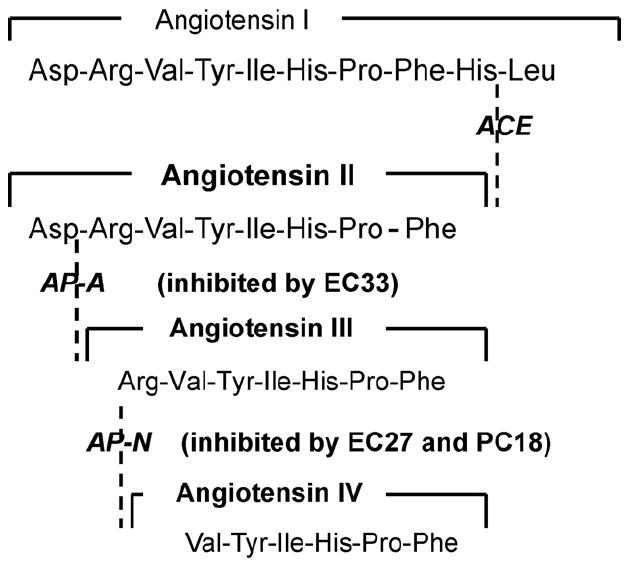
The metabolic conversion pathway of angiotensin II to angiotensin III can be blocked by the specific AP-A inhibitor, [S]-3-amino-4-mercaptobutyl sulfonic acid (EC33). The conversion of angiotensin III to angiotensin IV can be blocked by the specific AP-N inhibitors, [S]-2-amino-pentan-1,5-dithiol (EC27) and 2-amino-4-methylsulfonyl butane thiol (PC18). EC33 is 100-fold better at inhibiting AP-A (Ki = 0.29 μM) than AP-N (Ki = 25 μM). EC27 is approximately 100-fold better at inhibiting AP-N (Ki = 0.03 μM) than AP-A (Ki = 2.4 μM). Adapted from Llorens-Cortes and Mendelsohn (2002).
Our laboratory has utilized icv infused EC33 or PC18 (an AP-N inhibitor with similar structure to EC27) followed by the metabolically stable analogs d-Asp1-AngII and d-Arg1-AngIII in an attempt to sort out relative contributions by AngII and AngIII to pressor responding in rats (Wright et al., 2002b). Pretreatment icv infusion with EC33 blocked the pressor activity induced by the subsequent infusion of d-Asp1-AngII; while EC33 had no effect on the pressor response to subsequent infusion of d-Arg1-AngIII. In contrast, pretreatment infusion with PC18 extended the duration of the d-Asp1-AngII pressor effect by approximately two to three times, and the duration of d-Arg1-AngIII’s effect by approximately 10–15 times. Pretreatment with the specific AT1 receptor antagonist losartan blocked the pressor responses induced by the subsequent infusion of both analogs indicating that they act via the AT1 receptor subtype. These results suggest that the brain AT1 receptor may be designed to preferentially bind AngIII in mediating systemic blood pressure.
3.3.1. Thirst
One of the most dramatic behavioral phenomena associated with the central injection of angiotensin is its ability to produce robust drinking. Linazasoro et al. (1954) and Nairn et al. (1956) first demonstrated that peripherally infused renal extracts produced drinking in rats and postulated that this response was angiotensin-induced. Since then many investigators have confirmed and extended these initial observations.
3.4. Angiotensin II
Booth (1968) discovered that microinjections of AngII into the rostral hypothalamus produced drinking. Soon after Epstein et al. (1970) observed dose-response water intake to icv injected AngII. Simpson and Routtenberg (1973) showed that microinjection of AngII into the SFO produced drinking. Buggy et al. (1975) and Buggy and Fisher (1976) reported that AngII infused into the third ventricle in the proximity of the OVLT, but not permitted to reach the SFO, was also dipsogenic. Following additional efforts to establish the respective contributions of the SFO and OVLT it was generally agreed that the OVLT detects angiotensins in both the cerebrospinal fluid and blood, while drinking induced by elevations in blood–borne angiotensins is primarily mediated by the highly vascularized SFO (reviewed in Lind, 1988; Wright and Harding, 1992).
3.5. Angiotensin III and shorter fragments
AngIII was originally found to possess about 50% of the dipsogenic activity of AngII when delivered into the diencephalon of the rat; while AngIV, AngII(4–8) and Ang(5–8) produced only slight dipsogenic acivitity (Fitzsimons, 1971, 1980). With the removal of phenylalanine from the C-terminal [i.e., Ang(1–7)] a complete loss of drinking was noted. Tonnaer et al. (1982) examined AngI, AngII, and several C-terminal fragments for dipsogenic activity when injected icv in rats. The greatest intakes occurred to AngII, AngI, and AngIII (picomol range) in that order, followed by AngII(4–8), AngIV, AngII(5–8), and AngII(6–8) (nmol range). The C-terminal dipeptide AngII(7–8), and other dipeptide fragments, were relatively ineffective. Pretreatment with the ACE inhibitor, captopril, greatly reduced the drinking induced by AngI suggesting that conversion of AngI to AngII and/or AngIII is necessary for biological activity in the brain. In addition, pretreatment with saralasin blocked drinking to AngI and AngII(4–8); while the other angiotensins and fragments were not similarly tested. More recent investigations have established reasonably equivalent drinking responses in rats to icv infusions of AngII and AngIII, particularly at low doses if precautions are taken to avoid peptide adherence to glass mixing and storage containers, adjustments are made for differences in the purities of the compounds, and the angiotensins are infused rather than bolus injected (Wright et al., 1985).
3.5.1. Sodium appetite
Central application of angiotensins also produces a sodium appetite that follows a slower time course to develop than the drinking response (reviewed in Fitzsimons, 1998). Body sodium conservation is primarily controlled by the renin–angiotensin–aldosterone system and these hormones are elevated during sodium deficiency, and in turn, act directly or indirectly on the brain to stimulate a sodium appetite (Epstein, 1982; Richter, 1936). The salt appetite that develops in a sodium-depleted rat can be suppressed by central application of angiotensin receptor antagonists (Buggy and Jonklaas, 1984; Weiss et al., 1986) or ACE inhibitors (Moe et al., 1984). In fact, sodium appetite induced by adrenalectomy can be suppressed by interruption of the brain RAS (Sakai and Epstein, 1990). AngII-induced sodium intake appears to be a function of activation of forebrain angiotensin receptors, but is not dependent on the SFO. Microinfusion of AngII near the OVLT produced both water and sodium intake in rats, while injections into the SFO elicited only water consumption (Fitts and Masson, 1990).
Peripheral infusions of high doses of AngII are required to provoke a sodium appetite that results in increases in plasma aldosterone, thus facilitating central AngII-elicited sodium intake (Summy-Long et al., 1983). Peripheral aldosterone can penetrate the blood–brain-barrier and has been shown to elevate brain AngII receptor numbers (Wong et al., 1990). There have also been attempts to determine whether this sodium appetite is primary or secondary to an immediate and sustained natriuresis (reviewed in Fitzsimons, 1998; Unger et al., 1988). Intracerebroventricular injections of AngII produced an immediate increase in urinary sodium excretion in alert rats prepared with a chronic indwelling urethral catheter that lasted for at least 1 h (Unger et al., 1989). Thus, it appears that icv AngII-induced sodium loss stimulates sodium appetite as a compensatory response.
Little evidence exists concerning AngIII’s potential involvement in sodium appetite, and what is available is conflicting. Peripheral administration of AngIII has been shown to be ineffective in eliciting a salt appetite in Fischer 344 and Sprague–Dawley rats; while AngII was capable of producing a sodium appetite (Caputo et al., 1992). Acute AngIII infusions into the preoptic area failed to increase sodium appetite in rats, whereas AngII stimulated the appetite (Avrith and Fitzsimons, 1980). However, icv infused AngIII is equipotent to AngII in stimulating sodium consumption in baboons (Blair-West et al., 2001). Our laboratory has utilized the AP-A and AP-N inhibitors, EC33 and PC18, respectively, to investigate the roles of AngII and AngIII in salt appetite (Wilson et al., 2005). Rats were sodium depleted with furosemide, followed by endogenous angiotensin blockade with the ACE inhibitor captopril. [d-Asp1,d-Arg2]AngII and [d-Arg1]AngIII were then icv infused in order to evaluate the relative roles of AngII and AngIII in provoking sodium appetite. Both forms were effective in eliciting water and sodium solution (0.3 M NaCl) intakes. AngII analog-induced intakes of water and NaCl were decreased following pretreatment with EC33. Use of PC18 produced increased intakes of both fluids following treatment with the AngIII analog. These findings support a role for both peptides in eliciting and mediating sodium appetite.
4. Novel angiotensin-mediated physiologies and behaviors
4.1. Long-term potentiation
The phenomenon of LTP was originally described in the rabbit hippocampal slice preparation by Bliss and colleagues (Bliss and Garner-Medwin, 1973; Bliss and Lomo, 1973). Excitatory postsynaptic potentials evoked in the dentate gyrus could be progressively enhanced by short bursts of electrical stimulation. LTP is now thought by many researchers to represent a basic physiological mechanism of memory storage (Eichenbaum and Oto, 1992; Lynch et al., 1991; Martinez and Derrick, 1996). Wayner’s laboratory was the first to report a role for AngII inhibition of hippocampal and dentate LTP (Armstrong et al., 1996; Denny et al., 1991; Wayner et al., 1973, 1993, 1995). This AngII-induced blockade could be prevented by co-injection with saralasin or losartan suggesting that the effect was via activation of the AT1 receptor subtype; while the AT2 receptor antagonist, PD123319, had no effect (Wayner et al., 1993). AngIII was found to be much less effective than AngII at inhibiting LTP (Denny et al., 1991). Activation of the AT4 receptor by Nle1-AngIV significantly increased hippocampal LTP (Kramár et al., 2001). The application of an AT4 receptor antagonist prior to tetanization disrupted the maintenance phase of LTP. This Nle1-AngIV facilitation of LTP was subsequently shown to be NMDA-independent; however, this effect was dependent upon increased intracellular calcium via altered voltage dependent calcium channels (Davis et al., 2006).
Recently, Ang(1–7) has been shown to enhance hippocampal LTP independent of the AT1 and AT4 receptor subtypes (Hellner et al., 2005). These authors present convincing evidence that the Ang(1–7) receptor is the G-protein-coupled Mas receptor, encoded by the mas protooncogene.
There is one paper that evaluated HGF interaction with the hippocampal c-Met receptor as related to LTP and long-term depression (LTD; Akimoto et al., 2004). These investigators first established the presence of the c-Met receptor and HGF in the brain of adult rats. HGF is processed by proteases, including tissue plasminogen activator that is released in a neuronal activity-dependent fashion (Thewke and Seeds, 1999). HGF facilitated LTP in the CA1 region of hippocampal slices taken from 4 to 5 week old rats. This LTP appeared to be NMDA receptor mediated. HGF did not influence LTD. These results agree with the pattern of facilitation seen with Nle1-AngIV, however, they are not consistent with an NMDA-independent phenomenon as observed in our laboratory.
4.2. Learning, memory and cognition
Memory acquisition can be measured in animals using several protocols including passive and active avoidance conditioning and spatial recognition tasks. Passive and active avoidance conditioning procedures are typically used to assess associative learning, the process of attaching meaning (consequences) to a previously neutral object or event where the consequence is often a foot shock (a punisher). With passive avoidance conditioning (variations are called step-through or step-down tasks) the animal is placed in the lighted side of a two-compartment apparatus and permitted to move to the more preferred dark compartment during several pre-conditioning trials. Once the animal has habituated to the compartment it typically moves to the dark side within 10–20 s. The final “conditioning trial” consists of placing the animal into the illuminated compartment and allowing it to enter the dark compartment. Once in the dark compartment a guillotine door closes off the entrance and a mild foot shock is applied for a short duration, usually 0.5–2 s. Thus, the conditioning paradigm consists of an association among the cues that denote the preferred dark compartment (conditioned stimuli) and the noxious foot shock (unconditioned stimulus). The conditioned response takes the form of an increased latency (reluctance) to move from the lighted compartment to the dark compartment on subsequent retention trials, i.e. passive avoidance conditioning. Retention trials are usually placed at 24-h intervals following conditioning in order to measure the subsequent strength of the conditioned response. Active avoidance protocols usually make use of a two compartment apparatus (shuttlebox) each side equipped with a cue light (or tone). The light comes on in one compartment to signal an impending foot shock at a latency of a few seconds. This permits the animal time to move into the opposite chamber thus avoiding foot shock. Following a brief inter-trial interval the light in the second compartment comes on to cue an impending foot shock in that compartment. Trials proceed until the animal anticipates and thus avoids each foot shock. This anticipatory behavior is considered to be the conditioned response.
Circular water and 8-arm radial mazes are used to measure the acquisition of spatial memory by rodent models. The Morris water maze task (Morris, 1984) requires that the animal be placed into the water at a different location next to the wall of the tank on each training trial. The goal is to locate a submerged platform (2–3 cm below the surface typically fixed in position) using extra-maze visual cues placed on the walls surrounding the maze. If the animal is unsuccessful at the end of a trial it is placed on the platform for a short rest period permitting an opportunity to orient using these cues. The number of trials per day can vary from one to as many as 20 or 25. The number of days of training may vary from 1 or 2, to a week or longer. The dependent measures may include the latency and distance required to find the platform on each trial, swim speed and efficiency of search patterns. The 8-arm radial maze protocol requires food reward to motivate the animal. Several arms of the maze are baited with food. The animal is placed at the start point in the middle of the maze (origin of all arms) and must run down each arm to determine whether food is present at the end of the arm. The important measures include the latency required to locate the arms that contain food and avoid those arms not baited with food, and the number of errors due to re-entry into a previously visited arm.
4.2.1. Angiotensin converting enzyme (ACE) inhibitors and cognition
Accompanying the therapeutic benefits of ACE inhibitors (captopril, enalapril, ramipril, ceranapril) in treating hypertension, congestive heart failure, and following mild cardiac infarction, there appears to be facilitated cognitive functioning and feelings of well-being. Croog et al. (1986) employed 626 mild to moderated hypertensive male patients in randomized double-blind trials over a 24-week study. Patient self-reports indicated improved mental acuity at work, less sexual dysfunction, and increased sense of well-being on captopril. There was no change with propranolol treatment, and a decline in those patients placed on methyldopa. Blood pressure was equivalently controlled in all three treatment groups. Deicken (1986) and Zubenko and Nixon (1984) have reported captopril-induced mood elevating effects in depressed patients. Barnes et al. (1992) posited that elevated brain AngII levels may interfere with acetylcholine (Ach) release that in turn interferes with cognitive processing (Bartus et al., 1982). According to this hypothesis ACE inhibitors may facilitate cognitive functioning by reducing the synthesis of AngII, thus removing an inhibitory influence upon Ach release (Barnes et al., 1990). In support of this hypothesis Costall et al. (1989) treated mice with captopril or ceranapril and measured an habituatory response of moving from a brightly lit area to the darker area of a light/dark box. The muscarinic acetylcholine receptor antagonist scopolamine impaired habituation, while captopril and ceranapril were both effective at countering this scopolamine effect (Barnes et al., 1992).
Scopolamine has been shown to delay the time required for rats to locate a submerged platform in the Morris water maze task. Treatment with ceranapril offset this scopolamine-induced impairment such that escape times were equivalent with controls. In further support, Barnes et al. (1991a,b) reported high binding densities for [3H]-ceranapril in rat striatum and hippocampus, and human caudate, attributed to ACE in the mirovasculature and perhaps at extravascular sites. Intravenous pretreatment with captopril reduced subsequent [3H]-ceranapril binding in most areas of the brain, except the striatum and brain stem. Barnes et al. (1989) have also reported AngII-induced interference with potassium-mediated release of [3H]-Ach from rat entorhinal cortex slices. This AngII effect could be blocked by sarthran. Along these lines, Mondadori and Etienne (1990) found that captopril and enalapril reduced electroshock-induced amnesia in mice. These animals were trained to avoid the dark compartment of a two-chamber passive avoidance apparatus by the application of foot shock in the dark side immediately following an electroconvulsive shock. Recall of the conditioned response was facilitated in those mice given ACE inhibitors 1 h prior to the conditioning trial. Flood and Morley (1993) reported similar results using an active avoidance task in mice. Barnes et al. (1990, 1991b) have shown that reasonably low doses of losartan and the AT2 receptor antagonist P123177, improved scopolamine-impaired performance in the previously described habituation test. Similarly, DeNoble et al. (1991) measured impaired performance on a passive avoidance task in rats icv treated with renin. This impairment could be offset with ACE inhibitor treatment, or by the application of the AT1 receptor antagonists EXP3312 or EXP3880, but not PD123177. The proposal that ACE inhibitors enhance learning has been challenged by Chen and Mendelsohn (1992) who found that a high oral dose of ceranapril in rats inhibited ACE at the CVOs, but not within blood–brain barrier protected structures. This suggests that ceranapril does not cross the blood–brain barrier.
At present evidence favoring improved cognitive functioning by ACE inhibitors and AT1 receptor antagonists is stronger in animal tests of habituation and active or passive avoidance tasks than for animals evaluated using spatial learning paradigms. Our laboratory has measured facilitated Morris water maze performance in scopolamine, or nicotinic acetylcholine receptor antagonist mecamylamine pretreated rats with icv treatment of AngIV analogs (Olson et al., 2004; Pederson et al., 1998; Wright et al., 1999). This suggests a role for AngIV in the facilitation of cognitive processing noted during treatment with ACE inhibitors. It has also been established that Ang(1–7) and AngI(3–10) levels are elevated during treatment with ACE inhibitors (Lawrence et al., 1990). Both AngII(2–7) and AngI(3–10) bind at the AT4 receptor subtype with affinities generally comparable to that of native AngIV (Harding et al., unpublished observations; Sardinia et al., 1993). Also, conversion of Ang(1–7) to a ligand that acts at the AT4 receptor is possible.
4.2.2. Angiotensin II
Thirty-five years ago Rolls et al. (1972) placed rats on a progressive ratio schedule-bar press response for food and water, and found that motivational levels were approximately equal following 24 h of water deprivation and when provided water and prepared with an injection of AngII into the preoptic area of the hypothalamus. Graeff et al. (1973) conditioned water-deprived rats to press a bar for water, and then injected AngII into the septal area and measured equivalent bar pressing when rats were satiated. These results suggested that AngII injections may simulate the motivational characteristics present while water-deprived. It was also reported that icv-infused AngII interfered with performance on a variable interval operant task in rabbits (Melo and Graeff, 1975). Similarly, icv renin infused 1 min prior to the initiation of acquisition training on a passive avoidance task in rats interfered with recall of that task 1 and 2 days later (Köller et al., 1979). (Note that renin is responsible for the conversion of angiotensinogen to AngI, thus providing additional substrate for ACE mediated conversion to AngII.) Angiotensin II was assumed to be responsible for disruption of recall given that this performance deficit was attenuated by icv infusion of the ACE inhibitor captopril. It was also reported that AngII injected into the dorsal neostriatum 5 min following passive avoidance conditioning interfered with the recall of the conditioned response 24 h later (Morgan and Routtenberg, 1977). Along these lines, DeNoble et al. (1991) observed that icv infused renin dose-dependently disrupted performance of a passive avoidance task, i.e. as the renin dose was increased the level of retention decreased. The co-application of an AT1 receptor antagonist (EXP3312 or EXP3880) and the ACE inhibitor captopril, attenuated this renin-induced deficit. Since co-application of an AT2 receptor antagonist (PD123177) failed to influence the performance deficit produced by renin, it was concluded that the AT1 receptor subtype mediated this deficit. It follows that compounds that decrease AT1 receptor activation would be expected to facilitate cognitive processing.
In contrast with the above findings, central injections of AngII have been reported to improve acquisition and recall by some investigators. For example, Baranowska et al. (1983) injected AngII (icv: 1 and 2 μg) 15 min prior to active avoidance conditioning trials in rats. A buzzer served as a conditioned stimulus and foot shock as the unconditioned stimulus. Angiotensin II facilitated acquisition of the response but did not influence extinction. A low icv dose of AngII (0.5 mg) inhibited the acquisition of this conditioned response. Pretreatment with saralasin or sarile (Sar1, Ile8-AngII) failed to block these AngII effects. The authors suggest that AngII exerts a bimodal action upon learning, that is, an inhibitory influence at low doses and a facilitory effect at higher doses. Subsequent reports from this laboratory indicated that icv delivered AngII and AngIV (1 nmol ≈ 1 μg), 15 min prior to testing for retention, facilitated recall of a passive avoidance conditioned response (Braszko et al., 1987; Georgiev et al., 1988). These treatments also facilitated the acquisition of a shuttlebox active avoidance task (Braszko et al., 1987, 1988; Georgiev et al., 1988). Further, such treatments facilitated T-maze performance when delivered immediately following acquisition training. However, if AngII and AngIV were administered 15 min prior to testing for recall of T-maze performance, no facilitation of performance was noted (Braszko et al., 1987, 1988).
Along these lines, microinjection of AngII into the CA1 hippcampal field has also been shown to facilitate acquisition of an active avoidance (shuttle box) task in rats (Belcheva et al., 2000). Kulakowska et al. (1996) extended this work to an object recognition task in which AngII facilitation could be blocked by pretreatment with losartan. These results suggest that the AT1 receptor mediated this AngII-induced improvement in object recognition. However, Braszko (2002) has recently reported that icv AngII-induced facilitation of passive avoidance conditioning, conditioned avoidance responding, and open field locomotor behavior. This improvement in behavior could be blocked by combined pretreatment with losartan plus an AT2 receptor antagonist (PD123319), but not by each alone. Further, Braszko et al. (2003) have attempted to explain these variable AngII effects upon acquisition by measuring changes in motor and anxiety responses to icv infusion of AngII. They found significant increases in anxiety as measured using an elevated “plus” maze, and impaired motor coordination as measured with the “chimney” test. Pretreatment with either losartan or PD123319 counteracted the AngII-induced heightened anxiety effects, but only losartan offset the impaired motor coordination effects.
The vast majority of the above studies utilized native angiotensins rather than analogs that resist conversion to shorter chain peptides. Thus, it is likely that these results are due to a combination of effects resulting from the conversion of AngII to AngIII and perhaps to Ang(1–7), Ang(2–7), Ang(3–7), and AngIV.
4.2.3. Angiotensin IV
The often noted failure of AT1 and AT2 receptor antagonists to influence performance on cognitive tasks, or block subsequent AngII facilitation of a conditioned response, may indicate that AngII is converted to AngIII, and then to AngIV (or an AngIV-like compound), and it is this ligand that acts at the AT4 receptor subtype to improve performance. Our laboratory has discovered that the icv infusion of AngIV leads to c-Fos expression in the hippocampal and piriform cortices; while similar injection of AngII failed to induce c-Fos-like immunoreactivity in these structures, but did activate c-Fos expression in circumventricular organs (Roberts et al., 1995) and the hypothalamus (Zhu and Herbert, 1996). Pretreatment with losartan prevented this AngII-induced c-Fos immunoreactivity, while pretreatment with the AT4 receptor antagonist, divalinal-AngIV [Val1(CH2-NH2)1–2,Tyr2(CH2-NH2)2–3,Val3-AngIV], blocked AngIV-induced c-Fos expression. There were no crossover effects exhibited by these antagonists. Along these lines, Braszko et al. (1988, 1991) were the first to report that icv injected AngII and AngIV were equivalent at facilitating exploratory behavior in rats tested in an open field, improved recall of passive avoidance conditioning, and the acquisition of active avoidance conditioning. Our laboratory confirmed and extended the above findings in that icv infused AngIV improved the recall of a passive avoidance response in a dose-dependent fashion, with the most prominent facilitation at the highest dose (1 nmol, Wright et al., 1993, 1995). We also found that icv treatment with divalinal, disrupted recall of this response (Wright et al., 1995). Along these lines osmotic pump icv delivery of divalinal during 6 days of training significantly impaired acquisition of the Morris water maze task (Wright et al., 1999). We further determined that icv injected metabolically resistant analogs of AngIV could be utilized to facilitate acquisition of successful search patterns in spatial memory tasks as compared with control animals treated with artificial cerebrospinal fluid, or a pentapeptide that did not bind at the AT4 receptor subtype (Stubley-Weatherly et al., 1996; Wright et al., 1999). A similar facilitation of acquisition by AngIV analogs (eg. Nle1-AngIV) has been observed in scopolamine pretreated rats (Pederson et al., 1998, 2001), and perforant path knife-cut damaged rats (Wright et al., 1999).
Recently Olson et al. (2004) reported that icv treatment with the nicotinic receptor antagonist, mecamylamine, disrupted acquisition of the Morris water maze task (Olson et al., 2004). Once again the icv application of Nle1-AngIV overcame this deficit in spatial learning. However, Nle1-AngIV could not compensate for impaired acquisition resulting from the combined application of scopolamine plus mecamylamine. These results suggest that Nle1-AngIV-induced compensation via the AT4 receptor subtype may be dependent upon the brain cholinergic system. This notion is supported by the observation that AngIV and leucine-valine-valine-hemorphin-7 (LVV-H7) induced the release of Ach from rat hippocampal slices in a dose-dependent fashion (Lee et al., 2001). This release of Ach could be blocked by divalinal. These investigators have also reported AngIV- and LVV-H7-induced facilitation of spatial learning using the Barnes circular maze in which the animal must locate one escape tunnel among eight possible locations (Lee et al., 2004). The rats were tested three trials per day for 10 training days, but received only one icv bolus injection of AngIV or LVV-H7 on day 1, 5 min prior to testing.
Braszko et al. (2006) have recently demonstrated that icv infused AngII must be converted to AngIV in order to facilitate performance on passive avoidance conditioning and object recognition tasks in rats. AngII injected at 5 or 10 min prior to testing was ineffective; while at 15 min prior to testing it was effective at improving performance. AngIV facilitated performance at 5, 10 and 15 min prior to testing. The authors concluded that degradation of AngII to AngIV is necessary for cognitive facilitation. This laboratory has argued that D2 dopamine (DA) receptors at least partially mediate this AngIV-induced cognitive effect (Braszko, 2006).
Taken together, these results suggest an important role for the AngIV/AT4 receptor system in learning and memory processes.
4.3. Cerebroprotection
4.3.1. Ischemia-induced damage
A number of studies have investigated the potential positive effects of angiotensins as cerebroprotective agents against ischemia-induced damage. It has been known for some time that systemically infused AngII influences regional cerebral blood flow (Reynier-Rebuffel et al., 1987) especially as related to CVO versus non-CVO structures (Tuor et al., 1988). Tamake et al. (1992) reported that AngII increased cerebral blood flow in the rabbit. Haberl et al. (1990) evaluated cerebral arteriole responses to AngII in anesthetized rats prepared with a closed cranial window and found Ang II to dilate cerebral arteries. These investigators also used this closed cranial window technique in rabbits and noted that topical application of renin to the surface of the brain induced dilation of pial arterioles within a few minutes, with blood flow increasing and peaking at 50 min following application (Haberl et al., 1996). These changes were inhibited by intravenous or topical captopril.
The gerbil has an incomplete anastomotic Circle of Willis that permits ipsilateral focal brain ischemia with unilateral carotid artery ligation. Mortality rate is about 50% at 48 h post-surgery. The infusion of AngII has been shown to reduce this rate to approximately 15% (Fernandez et al., 1986). In contrast, treatment with losartan or candesartan failed to change the survival rate; while enalapril or lisinopril reduced the survival rate to approximately 18–25% (Dalmay et al., 2001a). These AngII protective effects appear to be mediated by the AT2 receptor subtype (Brix and Haberl, 1992; Kagiyama et al., 2003; Li et al., 2005; Stromberg et al., 1992). Along these lines, Iwai et al. (2004) have tested AT2-null transgenic mice with permanent middle cerebral artery occlusion and found more severe cerebral damage than in wild-type controls. Pretreatment with an AT1 receptor blocker (valsartan) for 10 days reduced the area of ischemic-induced damage and this protective effect was weaker in the AT2-null than in wild-type mice.
The observation that the protective effect of valsartan only diminished cerebral damage in the AT2-null mice suggests that an additional receptor subtype could be involved in mediating the neuroprotective effect. Our laboratory has shown that AngIV infusion yields a dose-dependent increase in cerebral blood flow without significantly influencing systemic blood pressure (Kramár et al., 1997). This effect was not changed by pretreatment with AT1 or AT2 receptor antagonists, but was abolished by divalinal. In addition, AngIV infusion was shown to restore cerebral blood flow following subarachnoidal hemorrhage (Naveri et al., 1994). Dalmay et al. (2001b) reported that AngIV infusion in the candesartan pretreated gerbil model of unilateral carotid artery ligation, only slightly decreased mortality at post-surgery day 3, but significantly decreased the lisinopril-induced increase in mortality. These results support the notion that AT4 receptor activation contributes to cerebroprotection.
HGF has been shown to positively impact ischemic-induced injuries such as cardiac ischemia (Nakamura et al., 2000) and hind limb ischemia (Morishita et al., 1999; Van Belle et al., 1998). HGF has also been shown to eliminate hippocampal neuronal cell loss in transient global cerebral ischemic gerbils (Miyazawa et al., 1998) and transient focal ischemic rats (Tsuzuki et al., 2001). Date et al. (2004) have reported HGF-induced improvements in escape latencies by microsphere embolism-induced cerebral ischemic rats using a circular water maze task. The authors measured reduced damage to cerebral endothelial cells in ischemic animals treated with HGF. Nagayama et al. (2004) have observed up-regulation of HGF and c-Met mRNAs in the peri-infarct region at 14 and 28 days, respectively, following permanent middle cerebral artery occlusion in mice. Along these lines, Shimamura et al. (2006) have recently shown that over-expression of HGF following permanent middle cerebral artery occlusion resulted in significant recovery of performance using the circular water maze and passive avoidance conditioning tasks. Treatment with HGF was also found to increase the number of arteries in the neocortex some 50+ days following the onset of ischemia.
Taken together these findings suggest a role for the AT4–c-Met receptor in cerebroprotection and are consistent with the notion that AngIV increases blood flow by a NO-dependent mechanism (Kramár et al., 1997). In agreement with this hypothesis is recent work by Faure et al. (2006) indicating that increasing internal carotid artery doses of AngIV significantly decreased mortality and cerebral infarct size in rats 24 h following embolic stroke due to the intracarotid injection of calibrated microspheres. Pretreatment with either divalinal or nomega-nitro-1-arginine methylester (L-NAME) abolished this protective effect. Sequential cerebral arteriography indicated that AngIV caused the redistribution of blood flow to the ischemic areas within a few minutes. Thus, AngIV may yield its cerebroprotective effect against acute cerebral ischemia via an intracerebro-hemodynamic AT4–c-Met receptor-mediated NO-dependent mechanism. These HGF results offer exciting possibilities concerning the treatment of ischemia-induced damage.
4.3.2. Seizure
There is a growing literature suggesting that angiotensins mediate seizure susceptibility. Georgiev and Kambourova (1983; Georgiev et al., 1986) were the first to show that AngII elevated the threshold of pentylenetetrazol, bicuculline, or picrotoxin-induced seizures in mice. They further reported that the AngII analog, sarmesin (Sar1,methyl Tyr4-AngII; Matsoukas et al., 1985) also provided some protection against seizuring in these models (Tchekalarova et al., 2003). Such AngII and sarmesin effects appear to be via the AT1 receptor subtype given that pretreatment with losartan blocked their protective ability (Tchekalarova and Georgiev, 1999). Angiotensin III and AngIV were also shown to dose-dependentally increase the threshold to pentylenetetrazol-induced brain excitability in mice (Tchekalarova et al., 2001a). Nearly equivalent increases in seizure threshold in these seizure models has been observed with the tetrapeptide AngII (3–6) and the tripeptide AngII (1–3) (Klusha et al., 1986; Svirskis et al., 1991).
A functional relationship between angiotensins and dopamine neurotransmission has been proposed by Tchekalarova and Georgiev (2005). In support of this notion activation of AT1 receptors potentiated depolarization-dependent DA release in rat brain (Simonnet and Giorguieff-Chesselet, 1979; Georgiev et al., 1990; Brown et al., 1996). It is also clear that vasopressinergic neurons may be activated by AngII to synthesize DA (Rossi, 1998). Most recently Tchekalarova and Georgiev (2006) have suggested an important role for norepinephrine in the neural protection offered by AngII and AngIII. Along these lines, Tchekalarova and Georgiev (2005) have recently noted anticonvulsive effects accompanying pretreatment with AngIV in the pentylenetetrazol kindling model that revealed increased dopamine D1 and D2 receptor subtype binding. These authors concluded that the cerebroprotective ability of AngIV could be via a modulatory effect on DA receptors in the basal ganglia given the high densities of the AT4 receptor subtype in the caudate putamen and nucleus accumbens (Wright and Harding, 2004). Further, Stragier et al. (2006) have reported that icv infusion of either AngIV or somatostatin-14 in rats produced an elevation in hippocampal DA and serotonin levels, as measured by microdialysis, and protected against pilocarpine-induced seizures. Such neural protective effects could be blocked by simultaneous treatment with the somatostatin receptor antagonist, Cyanamid 154806. These results were interpreted to indicate an AngIV-induced anti-convulsive effect mediated via somatostatin receptor-2 activation.
4.4. Renal blood flow and natriuresis
In mammalian species there is generally a high level of circulating angiotensinogen such that the limiting factor concerning AngI production is the availability of plasma renin (Campbell et al., 1991). The level of renin release is governed by the kidney juxtaglomerular cells, and the formation of AngII is dependent upon the availability of ACE on endothelial cells of vascular beds (Erdos, 1990). AngII acts to constrict vascular smooth muscle cells, facilitate myocardial contractility, promote the release of catecholamines from the adrenal medulla and sympathetic nerve endings, stimulate aldosterone release as well as thirst and sodium appetite. AngII also regulates sodium transport in epithelial cells in the kidney and intestine (Navar et al., 2000).
The AT1 receptor subtype is distributed throughout the renal microvasculature, the proximal tubule brush border, the basolateral membranes and the cortical collecting duct (Burns et al., 1993; Harrison-Bernard et al., 1997). In rats and rabbits intravenous infusion of AngII reduces renal blood flow and cortex blood flow at doses that do not effect medullary blood flow (Evans et al., 2000a, b; Walker et al., 1999). In dogs intravenous AngII somewhat reduces medullar blood flow (Chou et al., 1990). It has been suggested that AngII binds at AT1 receptors to constrict the vasculature of the renal cortex; however such constriction in the medulla is countered by prostaglandin E2 (Sadowski and Badzynska, 2006), and perhaps by NO (Patzak and Persson, 2007). The AT2 receptor subtype has also been implicated in this blunting of AT1 mediated vasoconstriction (Arima, 2003; Rajapakse et al., 2006). Thus, medullary blood flow is key to tubular salt and water reabsorption, and in turn, the regulation of systemic blood pressure. In this regard medullary ischemia may produce salt and water retention and result in elevated blood pressure (Evans et al., 2004).
Autoradiographic and radioligand binding experiments have localized the AT4 receptor subtype on microvilli and cell bodies of rat proximal convoluted and straight tubules (Handa et al., 1998), cultured rat mesangial cells (Chansel et al., 1998), cultured opossum proximal tubule cells (Dulin et al., 1995), apical and basolateral membranes of rabbit cortical tubules (Dulin et al., 1994), cultured rabbit (Garreau et al., 1998) and human (Czekalski et al., 1996) collecting duct cells, and cultured human proximal tubule epithelial cells (Handa, 2001).
Several studies have compared the influences of AngII and AngIV on renal blood flow. Our laboratory reported that renal artery infusion of AngII in the anesthetized rat produced significant elevations in systemic blood pressure, decreases in cortical blood flow, glomerular filtration rate, urine volume and urine sodium excretion (Hamilton et al., 2001). Similar infusion of AngIV produced increases in renal cortical blood flow, and urine sodium excretion, with minimal influence on glomerular filtration rate, urine volume and systemic blood pressure. Thus, in our hands AngIV acted as a natriuretic agent. Li et al. (2006) noted AngII and AngIV to cause dose-dependent elevations in systemic blood pressure in the rat, accompanied by a decrease in renal cortical blood flow. In addition, AngII produced a reduction in renal medullary blood flow; while AngIV did not. These AngIV effects could be blocked by the AT1 receptor antagonist losartan. The authors concluded that at the doses used (nM concentrations) AngIV influenced renal cortical blood flow by interacting with the AT1 receptor subtype. Other laboratories have confirmed that renal artery infusion of AngIV in anesthetize rats produces a decrease in regional renal blood flow that is mediated by the AT1 receptor subtype (Fitzgerald. et al., 1999; Li et al., 2006). Similar results have been obtained via direct measurement of renal microvasculature in the isolated rat kidney (Van Rodijnen et al., 2002). However, Handa (2006) has recently measured total renal blood flow in anesthetized rats using transient doses of AngII, AngIII, and AngIV infused into the renal artery of anesthetized rats. Both AngII and AngIII induced a dose-dependent vasoconstriction; while AngIV produced an immediate dose-dependent vasoconstriction followed by a prolonged vasodilation. This biphasic response to AngIV could be attenuated by losartan but not by divalinal, suggesting that it was AT1 receptor dependent. The vasodilation phase of this AngIV response was not influenced by indomethacin. Thus, it was concluded that this AngIV induced renal vasodilatory effect was not prostaglandin mediated.
Despite its name, HGF is found at higher levels in the adult kidney and lungs with about equivalent levels in the liver and spleen (Liu, 2002). The level of HGF protein measured in the kidney is approximately four times that in the liver. This organ distribution is also true of the c-Met receptor. While HGF is found in glomerular mesangial cells, endothelial cells, interstitial fibroblasts and macrophages, the c-Met receptor is more widespread in the kidney, particularly in tubular epithelial cells and collecting duct (Liu et al., 1996). The expression of HGF is triggered by acute renal failure induced by ischemia or nephrotoxic agents (Liu et al., 1999; Matsumoto and Nakamura, 2001). It has been hypothesized that this upregulation of HGF is designed to encourage recovery from the damage caused by acute renal failure given its known ability to stimulate cell proliferation, migration and differentiation (Liu, 2002; Matsumoto and Nakamura, 2001). Nakatani et al. (2002) have shown that the addition of HGF to the preservation medium of an excised dog kidney improved recovery of renal blood flow and glomerular filtration rate following 3 h of cold ischemia. The authors concluded that such an approach may counter the ischemic injury that occurs with transplanted human kidneys and thus improve graft survival.
The importance of AngIVand HGF in the kidney remains an open question. The resolution of this issue is particularly important given that both are locally synthesized in the kidney (Handa, 2000; Liu, 2002) and the AT4–c-Met receptor is prominently represented in the kidney (Coleman et al., 1998) and renal artery (Handa, 2001).
4.5. Stress
Considerable evidence supports an important role for the brain RAS in the control of stress-induced physiologies. AT1 receptors are prominent in structures that control stress responses including median eminence, PVN, anterior pituitary gland, adrenal medulla and zona glomerulosa (reviewed in Armando et al., 2003; Wright and Harding, 1992). Elevations in brain AngII have been shown to facilitate the release of norepinephrine in vivo and in cell culture (reviewed in Gard, 2002; Phillips, 1987). In turn, elevations in norepineprine were shown to block AngII release and down-regulation of angiotensin receptors. The application of stressors elevated circulating and brain levels of renin and AngII (Peng and Phillips, 2001; Yang et al., 1996). Stress also up-regulates the expression of AT1 receptors within the PVN where corticotrophin-releasing hormone (CRH) cell bodies are located (Castren and Saavedra, 1988; Jezova et al., 1998), and in the anterior pituitary (Leong et al., 2002). During stress synthesized AngII within the anterior pituitary facilitates release of adrenocorticotrophin hormone (ACTH; Ganong and Murakami, 1987). Thus, stress-induced upregulation of PVN AT1 receptors appears to provoke CRH synthesis that precludes the facilitation of ACTH release and elevated adrenal corticoid secretion. Short periods of isolation stress have been shown to elevate AT1 receptor expression in the PVN, along with correlated elevations in pituitary ACTH, adrenal corticosterone, catecholamines, and aldosterone. Nishimura et al. (2000) have shown that peripheral treatment with the AT1 receptor antagonist, candesartan, prevented AT1 receptor binding following isolation both in the anterior pituitary and adrenal glands, and in the PVN. This treatment also interfered with expected elevations of pituitary ACTH and adrenal corticosterone.
A second model that has been examined is gastric ulceration induced by cold restraint. This procedure induces gastric mucosa damage (Overmier and Murison, 2000) due to reduced blood flow and elevated free radical formation (Tuncel et al., 1998). Of particular interest, AngII mediated constriction of the stomach vasculature via AT1 receptor stimulation appears to be an important mediator of this reduction in blood flow (Heinemann et al., 1999). Bregonzio et al. (2003) reported that administration of candesartan significantly decreased the occurrence of gastric ulcerations induced by cold restraint stress.
Taken together these results point to an important role for the AngII/AT1 receptor system in the etiology of stress response. Saavedra and colleagues (Armando et al., 2003) have recently recommended that AT1 receptor antagonists be evaluated for clinical efficacy in the treatment of stress-related disease states.
4.6. Depression
The first suggestion that the brain RAS may be important in depression came with the observation that captopril induced an anti-depressant effect in hypertensive patients that also suffered from depression (Deicken, 1986; Germain and Chouinard, 1988, 1989; Zubenko and Nixon, 1984). There had been previous hints concerning this relationship from animal studies. Specifically, rats treated with antidepressants revealed decreased water intake induced by peripherally or centrally injected isoprenaline, either in the presence or absence of an α2-adrenoceptor antagonist (Goldstein et al., 1985; Przegalinski et al., 1988). Further testing indicated that each of the antidepressant drugs fluoxetine, desipramine, and tranylcypromine, reduced AngII-induced dipsogenicity in rats (Gard and Mycroft, 1991; Gard et al., 1994).
Captopril treatment has also been shown to protect animals against the forced swim induction method of learned helplessness/depression. This protocol requires the animal to swim within a small pool of water that has no escape. Eventually the animal stops swimming and becomes immobile. When placed in the pool the next day it assumes immobility significantly sooner than during the initial trial. On each subsequent test day the latency to evidence immobility decreases, i.e. learned helplessness. Pretreatment with captopril reduced immobility by mice equivalent to treatment with the antidepressants imipramine or mianserine (Giardina and Ebert, 1989). Learned helplessness induced by foot shock in rats could be prevented by pretreatment with captopril to the same degree as imipramine (Martin et al., 1990). Under both protocols the protective effects of captopril were reversed by naloxone, suggesting that the ACE inhibitor was exerting its antidepressant effects, at least in part, via opioid receptors. In addition, this effect is also dependent upon the brain RAS since pretreatment with losartan provided protection from immobility in the forced swim test (Gard, 2002; Gard et al., 1999). These results suggest that antidepressants exert their positive effects to some degree by inhibiting the brain RAS. The precise mechanism(s) of this inhibition remains to be determined.
5. Potential therapeutic applications
5.1. The angiotensin II/AT1 receptor subtype system
Treatment strategies for controlling hypertension include diuretics, central and peripheral sympathicolytics, calcium channel blockers, vasodilators, RAS inhibitors and receptor antagonists. As previously discussed it has been assumed that the AngII/AT1 receptor system is the primary regulator of blood pressure and body water balance. However, several years ago our laboratory reported that spontaneously hypertensive rats (SHRs) revealed increased pressor sensitivity to the icv infusion of AngII and AngIII as compared with normotensive Wistar–Kyoto and Sprague–Dawly rats (reviewed in Wright and Harding, 1992). These angiotensin-induced elevations in blood pressure persisted for a prolonged period of time in the SHR as compared with the normotensive strains. Subsequently we utilized icv delivery of the AP-N inhibitor, bestatin, to slow conversion of AngIII to AngIV resulting in a prolonged and heightened pressor response (Jensen et al., 1989); while the infusion of leucine aminopeptidase M that facilitated conversion of AngIII to AngIV significantly reduced blood pressure in SHRs (Wright et al., 1989). The more recent finding that inhibition of brain AP-A by the icv infusion of EC33 blocked blood pressure elevation due to the injection of AngII, further supports the notion that conversion of brain AngII to AngIII is necessary to the angiotensin-induced pressor response (Llorens-Cortes and Mendelsohn, 2002; Reaux et al., 1999; Zini et al., 1996). The selective AP-N inhibitor, PC18, administered icv prevented conversion of AngIII to AngIV and resulted in significant elevations in blood pressure. This response could be blocked by pretreatment with the AT1 receptor antagonist losartan. These findings offer a new level of insight into our understanding concerning the importance of AP-A mediated conversion of AngII to AngIII in the control of blood pressure, and represent an important therapeutic target for antihypertensive drug development.
5.2. The angiotensin IV/AT4 receptor subtype system
When our laboratory discovered the AT4 receptor in 1991 (Harding et al., 1992) we were attempting to purify the AT1 receptor subtype, and identify the binding site responsible for improvements in memory associated with icv infusion of AngIV (Braszko et al., 1988, 1991; Wright et al., 1993, 1995). Subsequent work by members of our laboratory indicated that spatial memory impairments induced by chemical disruption of the muscarinic cholinergic receptor system by scopolamine hydrobromide (Pederson et al., 1998, 2001), or perforant path knife cuts (Wright et al., 1999), could be over come with icv infusion of AngIV or AngIV analogs. These results were confirmed and extended by other laboratories (Holownia and Braszko, 2003; Lee et al., 2004; Tchekalarova et al., 2001b). We further showed that AngIV and its analogs enhanced long-term potentiation (LTP) in the CA1 region of rat hippocampal slices (Kramár et al., 2001) and in the dentate gyrus in vivo (Wayner et al., 2001). AngIV was shown to facilitate potassium-evoked acetylcholine release from rat hippocampal slices (Lee et al., 2001) suggesting that the brain cholinergic system may underlie, at least in part, the mechanism of AT4 receptor ligand memory enhancement. The potential use of angiotensin receptor agonists to treat dementias, including Alzheimer’s disease, has been suggested (Albiston et al., 2004; Gard and Rusted, 2004; Poljak et al., 2004; Savaskan, 2005), and represents a new therapeutic target.
Given the previous descriptions concerning the ability of AngIV and HGF to impact ischemia-induced injuries (Faure et al., 2006), seizures (Stragier et al., 2006), and renal blood flow (Handa, 2006), the AT4–c-Met receptor is also an exciting therapeutic target for the possible treatment of these disorders. Finally, emerging evidence suggests that the brain RAS is involved in the mediation of stress responses (Ruiz-Ortega et al., 2001) and depression (Gard, 2004). The intimate role played by angiotensin receptor subtypes in these disorders encourages the development of new treatment strategies.
6. Conclusion
This review considers available information regarding the synthesis and degradation of active angiotensins and the brain distributions of the three angiotensin receptor subtypes thus far characterized. The AT1 and AT2 receptor subtypes have been cloned and sequenced and are G-protein linked. There is debate concerning the AT4 subtype. Specifically, this subtype has been identified as insulin regulated aminopeptidase (IRAP); however recent findings challenge this assumption and indicate that the AT4 subtype is the c-Met receptor. This review provides relevant findings concerning the c-Met receptor where appropriate. Each of these subtypes is discussed and emphasis is placed on newly discovered functions and clinical applicability. The most promising targets include the development of an AP-A inhibitor to prevent conversion of AngII to AngIII to control hypertension, and the use of AngIV analogues to treat dementia, seizure and ischemia. The RAS continues to offer research challenges and the promise of an ever increasing constellation of important physiologies and behaviors. It is clear that the clinical importance of this system goes far beyond the treatment of dysfunctional blood pressure and body water balance.
Acknowledgments
The research from our laboratory presented in this review was supported by NIH grant RO1-HL64245-03, NSF grant IBN-0091337, the Edward E. and Lucille I. Lainge Endowment for Alzheimer’s Research, and funds provided for medical and biological research by the State of Washington to the Washington State University Program for Research on Alcohol and Drug Abuse. We thank Mrs. Ruth Day for the excellent secretarial assistance provided during the course of writing this manuscript.
Abbreviations
- ACD
acyl-coenzyme A dehydrogenase
- ACE
angiotensin converting enzyme
- ACE2
human angiotensin converting enzyme homologue
- Ach
acetylcholine
- ACTH
adrenocorticotrophin releasing hormone
- Ang
angiotensin
- Ang(1-9)
angiotensin I(1-9)
- Ang(1–7)
angiotensin II(1–7)
- Ang(2–7)
angiotensin II(2–7)
- Ang(3–7)
angiotensin II(3–7)
- AngI
angiotensin I
- AngII
angiotensin II
- AngIII
angiotensin III
- AngIV
angiotensin IV
- AP
area postrema
- AP-A
aminopeptidase A
- AP-N
aminopeptidase N
- Arg
arginine
- Asp
aspartate
- AT
angiotensin receptor subtype
- CA
Ammon’s horn
- Carb-P
carboxypeptidase P
- CRH
cortiocotrophin-releasing hormone
- CVOs
circumventricular organs
- DA
dopamine
- EC27
2-amino-pentane-1,5-dithiol
- EC33
3-amino-4-mercaptobutyl-sultonic acid
- ERK
extracellular signal-regulated kinase
- GLUT
glucose transporter molecules
- GST
glutathione-S-transferase
- GTPγS
guanosine triphosphate γ sulfate
- HEK
human embryonic kidney
- HGF
hepatocyte growth factor
- His
histidine
- icv
intracerebroventricular
- Ile
isoleucine
- IRAP
insulin-regulated aminopeptidase
- Leu
leucine
- L-NAME
nomega-nitro-1-arginine methylester
- LTD
long-term depression
- LTP
long-term potentiation
- LVV-H7
leucine-valine-valine-hemorphin-7
- Nle
norleucine
- NMDA
N-methyl-d-aspartate
- NO
nitric oxide
- NTS
nucleus of the solitary tract
- OVLT
organum vasculosum of the lamina terminalis
- PAI-1
plasminogen activator inhibitor-1
- PC18
2-amino-4-methylsulfonyl butane thiol
- Phe
phenylalanine
- PL
phospholipase
- PO
propyl oligopeptidase
- Pro
proline
- PVN
paraventricular nucleus
- RAS
renin–angiotensin system
- Sar
sarcosine
- Sar1,Ala8-AngII
saralasin
- Sar1,Ile8-AngII
sarile
- SFO
subfornical organ
- SH
sulfhyrdryl
- SHRs
spontaneously hypertensive rats
- SON
supraoptic nucleus
- Tyr
tyrosine
- Val
valine
- VDCCs
voltage dependent calcium channels
- VMN
ventral medial nucleus
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This article was published in an Elsevier journal. The attached copy is furnished to the author for non-commercial research and education use, including for instruction at the author’s institution, sharing with colleagues and providing to institution administration. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- Akimoto M, Baba A, Ikeda-Matsuo Y, Yamada MK, Itamura R, Nishiyama N, Ikegaya Y, Matsuki N. Hepatocyte growth factor as an enhancer of NMDA currents and synaptic plasticity in the hippocampus. Neuroscience. 2004;128:155–162. doi: 10.1016/j.neuroscience.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Albiston AL, McDowall SG, Matsacos D, Sim P, Clune E, Mustafa T, Lee J, Mendelsohn RA, Simpson RJ, Connolly LM, Chai SY. Evidence that the angiotensin IV (AT4) receptor is the enzyme insulin-regulated aminopeptidase. J Biol Chem. 2001;276:48623–48626. doi: 10.1074/jbc.C100512200. [DOI] [PubMed] [Google Scholar]
- Albiston AL, Mustafa T, McDowall SG, Mendelsohn FA, Lee J, Chai SY. AT(4) receptor is insulin-regulated membrane aminopeptidase: potential mechanisms of memory enhancement. Trends Endocrinol Metab. 2003;14:72–77. doi: 10.1016/s1043-2760(02)00037-1. [DOI] [PubMed] [Google Scholar]
- Albiston AL, Pederson ES, Burns P, Purcell B, Wright JW, Harding JW, Mendelsohn FA, Weisinger RS, Chai SY. Attenuation of scopolamine-induced learning deficits by LVV–hemorphin-7 in rats in the passive avoidance and water maze paradigms. Behav Brain Res. 2004;154:239–243. doi: 10.1016/j.bbr.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Allen AM, Oldfield BJ, Giles ME, Paxinos G, McKinley MJ, Mendelsohn FA. Localization of angiotensin receptors in the nervous system. In: Quirion R, Bjorklund A, Hodfelt T, editors. Handbook of Chemical Neuroanatomy. Elsevier; Amsterdam: 2000. pp. 79–124. [Google Scholar]
- Allen AM, Zhuo J, Mendelsohn FA. AT1-receptors in the central nervous system. J Renin Angiotensin Aldosterone Syst. 2001;2(Suppl 1):S95–S101. doi: 10.1177/14703203010020011701. [DOI] [PubMed] [Google Scholar]
- Arima S. Role of angiotennsin II and endogenous vasodilators in the control of grlomerular hemodynamics. Clin Exp Nephrol. 2003;7:172–178. doi: 10.1007/s10157-003-0249-8. [DOI] [PubMed] [Google Scholar]
- Armando I, Seltzer A, Bregonzio C, Saavedra JM. Stress and angiotensin II: novel therapeutic opportunities. Curr Drug Targets—CNS Neurol Dis. 2003;2:413–419. doi: 10.2174/1568007033482661. [DOI] [PubMed] [Google Scholar]
- Armstrong DL, Garcia EA, Ma T, Quinones B, Wayner MJ. Angiotensin II blockage of long-term potentiation at the perforant path-granule cell synapse in vitro. Peptides. 1996;17:689–693. doi: 10.1016/0196-9781(96)00030-7. [DOI] [PubMed] [Google Scholar]
- Avrith D, Fitzsimons J. Increased sodium appetite in the rat induced by intracranial administration components of the renin–angiotensin system. J Physiol. 1980;301:349–364. doi: 10.1113/jphysiol.1980.sp013210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banegas I, Prieto I, Vives F, Alba F, de Gasparo M, Segarra AB, Hermoso F, Duran R, Ramírez M. Brain aminopeptidases and hypertension. J Renin Angiotensin Aldosterone Syst. 2006;7:129–134. doi: 10.3317/jraas.2006.021. [DOI] [PubMed] [Google Scholar]
- Baranowska D, Braszko JJ, Wisniewski K. Effect of angiotensin II and vasopressin on acquisition and extinction of conditioned avoidance in rats. Psychopharmacology. 1983;81:247–251. doi: 10.1007/BF00427272. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Barnes NM, Costall B, Coughlan J, Horovitz ZP, Kelly ME, Naylor RJ, Tomkins DM. ACE inhibition and cognition. In: MacGregor GA, Sever PS, editors. Current Advances in ACE Inhibition. Churchill Livingston Press; New York: 1989. pp. 159–171. [Google Scholar]
- Barnes JM, Barnes NM, Costall B, Coughlan J, Kelly ME, Naylor RJ, Tomkins DM, Williams TJ. Angiotensin-converting enzyme inhibition, angiotensin, and cognition. J Cardiovasc Pharmacol. 1992;19(Suppl 6):563–571. doi: 10.1097/00005344-199219006-00011. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Champaneria S, Costall B, Kelly ME, Murphy DA, Naylor RJ. Cognitive enhancing actions of DuP 753 detected in a mouse habituation paradigm. Neuroreport. 1990;1:239–242. doi: 10.1097/00001756-199011000-00017. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Cheng CH, Costall B, Naylor RJ, Williams TJ, Wischik CM. Angiotensin converting enzyme density is increased in temporal cortex from patients with Alzheimer’s disease. Eur J Pharmacol. 1991a;20:289–292. doi: 10.1016/0014-2999(91)90584-d. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Costall B, Kelly ME, Murphy DA, Naylor RJ. Cognitive enhancing actions of PD123177 detected in a mouse habituation paradigm. Neuroreport. 1991b;2:351–353. doi: 10.1097/00001756-199106000-00013. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–417. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Belcheva I, Ternianov A, Georgiev V. Lateralized learning and memory effects of angiotensin II microinjected into the rat CA1 hippocampal area. Peptides. 2000;21:407–411. doi: 10.1016/s0196-9781(00)00163-7. [DOI] [PubMed] [Google Scholar]
- Bennett JP, Jr, Snyder SH. Angiotensin II binding to mammalian brain membranes. J Biol Chem. 1976;251:7423–7430. [PubMed] [Google Scholar]
- Bernier SG, Fournier A, Guillemette G. A specific binding site recognizing a fragment of angiotensin II in bovine adrenal cortex membranes. Eur J Pharmacol. 1994;271:55–63. doi: 10.1016/0014-2999(94)90264-x. [DOI] [PubMed] [Google Scholar]
- Bernier SG, Servant G, Boudreau M, Fournier A, Guillemette G. Characterization of a binding site for angiotensin IV on bovin endothelial cells. Eur J Pharmacol. 1995;291:191–200. doi: 10.1016/0922-4106(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Bernier SG, Bellemare JM, Escher E, Guillemette G. Characterization of AT4 receptor from bovine aortic endothel photosensitive analogues of angiotensin IV. Biochemistry. 1998;37:4280–4287. doi: 10.1021/bi972863j. [DOI] [PubMed] [Google Scholar]
- Bickerton RK, Buckley JP. Evidence for a central mechanism in angiotensin induced hypertension. Proc Soc Exp Biol Med. 1961;106:834–839. [Google Scholar]
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Blair-West JR, Coghlan JP, Denton DA, Funder JW, Scoggins BA, Wright RD. The effect of the heptapeptide (2–8) and hexapeptide (3–8) fragments of angiotensin II on aldosterone secretion. J Clin Endocrinol Metab. 1971;32:575–578. doi: 10.1210/jcem-32-4-575. [DOI] [PubMed] [Google Scholar]
- Blair-West JR, Carey KD, Denton DA, Madden LJ, Weisingeer RS, Shade RE. Possible contribution of brain angiotensin III to ingestive behaviors in baboons. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1633–R1636. doi: 10.1152/ajpregu.2001.281.5.R1633. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Garner-Medwin AR. Long-lastiing potentiation of synaptic transmission in the dentate area of the unanesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-term potentiation of synaptic transmission in the dentate area of the anesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth DA. Mechanism of action of norepinephrine in eliciting an eating response on injection into the rat hypothalamus. J Pharmacol Exp Ther. 1968;150:336–348. [PubMed] [Google Scholar]
- Bottari SP, Raylor V, King IN, Bogdal S, Whitebread S, DeGasparo M. Angiotensin II AT2 receptors do not interact with guanine nucleotide binding proteins. Eur J Pharmacol. 1991;207:157–163. doi: 10.1016/0922-4106(91)90091-u. [DOI] [PubMed] [Google Scholar]
- Braszko JJ. AT(2) but not AT(1) receptor antagonism abolishes angiotensin II increase of the acquisition of conditioned avoidance responses in rats. Behav Brain Res. 2002;131:79–86. doi: 10.1016/s0166-4328(01)00349-7. [DOI] [PubMed] [Google Scholar]
- Braszko JJ. D2 dopamine receptor blockade prevents cognitive effects of AngIV and des-Phe6 AngIV. Physiol Behav. 2006;88:152–159. doi: 10.1016/j.physbeh.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Braszko JJ, Wisniewski K, Kupryszewski G, Witczuk B. Psychotropic effects of angiotensin II and III in rats: locomotor and exploratory vs. cognitive behavior. Behav Brain Res. 1987;25:195–203. doi: 10.1016/0166-4328(87)90068-4. [DOI] [PubMed] [Google Scholar]
- Braszko JJ, Kupryszewski G, Witczuk B, Wisniewski K. Angiotensin II-(3–8)-hexapeptide affects motor activity, performance of passive avoidance and conditioned avoidance responses in rats. Neuroscience. 1988;27:777–783. doi: 10.1016/0306-4522(88)90182-0. [DOI] [PubMed] [Google Scholar]
- Braszko JJ, Wlasienko J, Koziolkiewicz W, Janecka A, Wisniewski K. The 3–7 fragment of angiotensin II is probably responsible for its psychoactive properties. Brain Res. 1991;542:49–54. doi: 10.1016/0006-8993(91)90996-9. [DOI] [PubMed] [Google Scholar]
- Braszko JJ, Kulakowska A, Winnicka MM. Effects of angiotensin II and its receptor antagonists on motor activity and anxiety in rats. J Physiol Pharmacol. 2003;54:271–281. [PubMed] [Google Scholar]
- Braszko JJ, Walesiuk A, Wielgat P. Cognitive effects attributed to angiotensin II may result from its conversion to angiotensin IV. J Renin Angiotensin Aldosterone Syst. 2006;7:168–174. doi: 10.3317/jraas.2006.027. [DOI] [PubMed] [Google Scholar]
- Braun-Menendez E, Fasciolo JC, Leloir LF, Munoz JM. The substance causing renal hypertension. J Physiol (Lond) 1940;98:283–298. doi: 10.1113/jphysiol.1940.sp003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregonzio C, Armando I, Ando H, Jezova M, Baiardi G, Saavedra JM. Anti-inflammatory effects of angiotensin II AT1 receptor antagonism prevent stress-induced gastric injury. Am J Physiol. 2003;285:G414–G423. doi: 10.1152/ajpgi.00058.2003. [DOI] [PubMed] [Google Scholar]
- Brix J, Haberl RL. The AT2-receptor mediates endothelium-dependent dilation of rat brain arerioles. FASEB J. 1992;6:A1264. [Google Scholar]
- Brosnihan KB, Li P, Ferrario CM. Angiotensin (1–7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension. 1996;27:523–528. doi: 10.1161/01.hyp.27.3.523. [DOI] [PubMed] [Google Scholar]
- Brown DC, Steward LJ, Ge J, Barnes NM. Ability of angiotensin II to modulate striatal dopamine release via the AT1 receptor in vitro and in vivo. Br J Pharmacol. 1996;118:414–420. doi: 10.1111/j.1476-5381.1996.tb15418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buggy J, Fisher AE. Anteroventral third ventricle site of action for angiotensin induced thirst. Pharmacol Biochem Behav. 1976;4:651–660. doi: 10.1016/0091-3057(76)90216-1. [DOI] [PubMed] [Google Scholar]
- Buggy J, Jonklaas J. Sodium appetite decreased by central angiotensin blockade. Physiol Behav. 1984;32:749–753. doi: 10.1016/0031-9384(84)90187-2. [DOI] [PubMed] [Google Scholar]
- Buggy J, Fisher AE, Hoffman WE, Johnson AK, Phillips MI. Ventricular obstruction: effect on drinking induced by intracranial injection of angiotensin. Science. 1975;190:72–74. doi: 10.1126/science.1166302. [DOI] [PubMed] [Google Scholar]
- Bumpus FM, Schwarz H, Page IH. Synthesis and pharmacology of the octapeptide angiotonin. Science. 1957;125:886–887. doi: 10.1126/science.125.3253.886. [DOI] [PubMed] [Google Scholar]
- Bumpus FM, Schwarz H, Page IH. Synthesis and properties of angiotonin. Circulation. 1958;17:664–667. doi: 10.1161/01.cir.17.4.664. [DOI] [PubMed] [Google Scholar]
- Burns KD, Inagami T, Harris RC. Cloning of a rabbit kidney cortex AT1 angiotensin II receptor that is present in proximal tubule epithelium. Am J Physiol (Renal Fluid Electrolyte Physiol 33) 1993;264:F645–F654. doi: 10.1152/ajprenal.1993.264.4.F645. [DOI] [PubMed] [Google Scholar]
- Campagnole-Santos MJ, Heeringer SB, Batista EN, Khosla MC, Santos RA. Differential baroreceptor reflex modulation by centrally infused angiotensin peptides. Am J Physiol. 1992;263:R89–R94. doi: 10.1152/ajpregu.1992.263.1.R89. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Lawrence AC, Towrie A, Kladis A, Valentijn AJ. Differential regulation of angiotensin peptide levels in plasma and kidney of the rat. Hypertension. 1991;18:763–773. doi: 10.1161/01.hyp.18.6.763. [DOI] [PubMed] [Google Scholar]
- Caputo R, Rowland N, Fregly M. Angiotensin-related intakes of water and NaCl in Fischer 344 and Sprague–Dawley rats. Am J Physiol. 1992;262:R382–R388. doi: 10.1152/ajpregu.1992.262.3.R382. [DOI] [PubMed] [Google Scholar]
- Carey RM, Saragy HM. Newly recognized components of the renin–angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003;24:261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- Caron AZ, Arguin G, Guillemette G. Angiotensin IV interacts with a juxtamembrane site on AT(4)/IRAP suggesting an allosteric mechanism of enzyme modulation. Regul Pept. 2003;113:9–15. doi: 10.1016/s0167-0115(02)00294-x. [DOI] [PubMed] [Google Scholar]
- Castren E, Saavedra JM. Repeated stress increases the density of angiotensin II binding sites in rat paraventricular nucleus and subfornical organ. Endocrinology. 1988;122:370–372. doi: 10.1210/endo-122-1-370. [DOI] [PubMed] [Google Scholar]
- Chai SY, Bastias MA, Clune EF, Matsacos DJ, Mustafa T, Lee JH, McDowall SG, Mendelsohn FA, Albiston AL, Paxinos G. Distribution of angiotensin IV binding sites (AT4 receptor) in the human forebrain, midbrain and pons as visualized by in vitro receptor autoradiography. J Chem Neuroanat. 2000;20:339–348. doi: 10.1016/s0891-0618(00)00112-5. [DOI] [PubMed] [Google Scholar]
- Chansel D, Czekalski S, Vandermeersch S, Ruffet E, Fournie-Zaluski M, Ardaillou R. Characteriation of angiotensin IV-degrading enzymes and receptors on rat mesangial cells. Am J Physiol. 1998;275:F535–F542. doi: 10.1152/ajprenal.1998.275.4.F535. [DOI] [PubMed] [Google Scholar]
- Chauvel EN, Llorens-Cortes C, Coric P, Wilk S, Roques BP, Fournie-Zaluski MC. Differential inhibition of aminopeptidase A and aminopeptidase N by new-amino thiols. J Med Chem. 1994;37:2950–2957. doi: 10.1021/jm00044a016. [DOI] [PubMed] [Google Scholar]
- Chen BZ, Mendelsohn FAO. Effect of acute and chronic administration of ceranapril on angiotensin converting enzyme in plasma, kidney, lung, brain-regions and cerebrospinal-fluid of rats. Neuropharmacology. 1992;31:929–935. doi: 10.1016/0028-3908(92)90132-9. [DOI] [PubMed] [Google Scholar]
- Chen JK, Zimpelmann J, Harris RC, Burns KD. Angiotensin IV induces tyrosine phosphorylation of focal adhesion kinase and paxillin in proximal tubule cells. Am J Physiol Renal Physiol. 2001;280:F980–F988. doi: 10.1152/ajprenal.2001.280.6.F980. [DOI] [PubMed] [Google Scholar]
- Chi NW, Lodish HF. Tankyrase is a Golgi-associated mitogen-activated protein kinase substrate that interacts with IRAP in GLUT4 vesicles. J Biol Chem. 2000;275:38437–38444. doi: 10.1074/jbc.M007635200. [DOI] [PubMed] [Google Scholar]
- Chiu AT, Herblin WF, McCall DE, Ardecky RJ, Carini DJ, Duncia JV, Pease LJ, Wong PC, Wexler RR, Johnson AL, Timmermans PB. Identification of angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989;165:196–203. doi: 10.1016/0006-291x(89)91054-1. [DOI] [PubMed] [Google Scholar]
- Chou SY, Porush JB, Faubert PF. Renal medullary circulation: hormonal control. Kidney Int. 1990;37:1–13. doi: 10.1038/ki.1990.1. [DOI] [PubMed] [Google Scholar]
- Coleman JK, Krebs LT, Hamilton TA, Ong B, Lawrence KA, Sardinia MF, Harding JW, Wright JW. Autoradiographic identification of kidney angiotensin IV binding sites and angiotensin IV-induced renal blood flow changes in rats. Peptides. 1998;19:269–277. doi: 10.1016/s0196-9781(97)00291-x. [DOI] [PubMed] [Google Scholar]
- Costall B, Coughlan J, Horovitz ZP, Kelly ME, Naylor RJ, Tomkins DM. The effects of ACE inhibitors captopril and SQ29852 in rodent tests of cognition. Pharmacol Biochem Behav. 1989;33:573–579. doi: 10.1016/0091-3057(89)90390-0. [DOI] [PubMed] [Google Scholar]
- Croog SH, Levine S, Testa MA, Brown B, Bulpitt CJ, Jinkins CD, Klerman GL, Williams GH. The effects of antihypertensive therapy on the quality of life. N Engl J Med. 1986;314:1657–1664. doi: 10.1056/NEJM198606263142602. [DOI] [PubMed] [Google Scholar]
- Culman J, Hohle S, Qadri F, Edling O, Blume A, Lebrun C, Unger T. Angiotensin as neuromodulator/neurotransmitter in central control of body fluid and electrolyte homoeostasis. Clin Exp Hypertens. 1995;17:281–293. doi: 10.3109/10641969509087071. [DOI] [PubMed] [Google Scholar]
- Culman J, Blume A, Gohlke P, Unger T. The renin–angiotensin system in the brain: possible therapeutic implications for AT1-receptor blockers. J Hum Hypertens. 2002;16:S64–S70. doi: 10.1038/sj.jhh.1001442. [DOI] [PubMed] [Google Scholar]
- Czekalski S, Chansel D, Vandermeersch S, Ronco P, Ardaillou R. Evidence for angiotnsin IV receptors in human collecting duct cells. Kidney Int. 1996;50:1125–1131. doi: 10.1038/ki.1996.419. [DOI] [PubMed] [Google Scholar]
- Dalmay F, Mazouz H, Allard J, Pesteil F, Achard JM, Fournier A. Non AT1-receptor-mediated protective effect of angiotensin against acute ischaemic stroke in the gerbil. J Renin Angiotensin Aldosterone Syst. 2001a;2:103–106. doi: 10.3317/jraas.2001.009. [DOI] [PubMed] [Google Scholar]
- Dalmay F, Pesteil F, Allard J, Nisse-Durgeat S, Fernandez L, Fournier A. Angiotensin IV decreases acute stroke mortality in the gerbil. Hypertension. 2001b;14:56A. [Google Scholar]
- Dampney RAL, Hirooka Y, Potts PD, Head GA. Functions of angiotensin peptides in the rostral ventrolateral medulla. Clin Exp Pharmacol Physiol. 1996;3(Suppl):S105–S111. [PubMed] [Google Scholar]
- Date I, Takagi N, Takagi K, Kago T, Matsumoto K, Nakamura T, Takeo S. Hepatocyte growth factor attenuates cerebral ischemia-induced learning dysfunction. Biochem Biophys Res Commun. 2004;319:1152–1158. doi: 10.1016/j.bbrc.2004.05.100. [DOI] [PubMed] [Google Scholar]
- Davis CJ, Kramar EA, De A, Meighan PC, Simasko SM, Wright JW, Harding JW. AT 4 receptor activation increases intracellular calcium influx and induces a non-N-methyl-d-aspartate dependent form of long-term potentiation. Neuroscience. 2006;137:1369–1379. doi: 10.1016/j.neuroscience.2005.10.051. [DOI] [PubMed] [Google Scholar]
- de Gasparo M, Siragy HM. The AT2 receptor: fact, fancy and fantasy. Regul Pept. 1999;81:11–24. doi: 10.1016/s0167-0115(99)00023-3. [DOI] [PubMed] [Google Scholar]
- de Gasparo M, Husain A, Alexander W, Catt KJ, Chiu AT, Drew M, Goodfriend T, Harding JW, Inagami T, Timmermans PB. Proposed update of angiotensin receptor nomenclature. Hypertension. 1995;25:924–939. doi: 10.1161/01.hyp.25.5.924. [DOI] [PubMed] [Google Scholar]
- de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International Union of Pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- Deicken RF. Captopril treatment of depression. Biol Psychiatry. 1986;12:1425–1428. doi: 10.1016/0006-3223(86)90334-3. [DOI] [PubMed] [Google Scholar]
- Denny JB, Polan-Curtain J, Wayner MJ, Armstrong DL. Angiotensin II blocks hippocampal long-term potentiation. Brain Res. 1991;567:321–324. doi: 10.1016/0006-8993(91)90812-a. [DOI] [PubMed] [Google Scholar]
- DeNoble VJ, DeNoble KF, Spencer KR, Chiu AT, Wong PC, Timmermans BM. Non-peptide angiotensin II receptor antagonist and angiotensin-converting enzyme inhibitor: effect on a renininduced deficit of a passive avoidance response in rats. Brain Res. 1991;561:230–235. doi: 10.1016/0006-8993(91)91599-v. [DOI] [PubMed] [Google Scholar]
- Dulin NO, Ernsberger P, Suciu DJ, Douglas JG. Rabbit renal epithelial angiotensin II receptors. Am J Physiol. 1994;267:F776–F782. doi: 10.1152/ajprenal.1994.267.5.F776. [DOI] [PubMed] [Google Scholar]
- Dulin N, Madhun ZT, Chang CH, Berti-Mattera L, Dickens D, Douglas JG. Angiotensin IV receptors and signaling in opossum kidney cells. Am J Physiol. 1995;269:F644–F652. doi: 10.1152/ajprenal.1995.269.5.F644. [DOI] [PubMed] [Google Scholar]
- Dzau VJ, Ingelfinger J, Pratt RE, Ellison KE. Identification of renin and angiotensinogen messenger RNA sequences in rat brain. Hypertension. 1986;8:544–548. doi: 10.1161/01.hyp.8.6.544. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Oto T. The hippocampus—what does it do? Behav Neural Biol. 1992;57:2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- Elliott DF, Peart WS. Amino-acid sequence in a hypertensin. Nature. 1956;177:527–528. doi: 10.1038/177527a0. [DOI] [PubMed] [Google Scholar]
- Epstein AN. Mineralocorticoids and cerebral angiotensin may act together to produce sodium appetite. Peptides. 1982;3:493–494. doi: 10.1016/0196-9781(82)90113-9. [DOI] [PubMed] [Google Scholar]
- Epstein AN, Fitzsimons JT, Rolls BJ. Drinking induced by injection of angiotensin into the brain of the rat. J Physiol (Lond) 1970;210:457–474. doi: 10.1113/jphysiol.1970.sp009220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdos EG. Angiotensin I converting enzyme and the changes in our concepts through the years. Hypertension. 1990;16:363–370. doi: 10.1161/01.hyp.16.4.363. [DOI] [PubMed] [Google Scholar]
- Evans RG, Correia AG, Weekes SR, Madden AC. Responses of regional kidney perfusion to vasoconstrictors in anaesthetized rabbits: dependence on agent and renal artery pressure. Clin Exp Pharmacol Physiol. 2000a;27:1007–1012. doi: 10.1046/j.1440-1681.2000.03377.x. [DOI] [PubMed] [Google Scholar]
- Evans RG, Madden AC, Denton KM. Diversity of responses of renal cortical and medullary blood flow to vasoconstrictors in conscious rabbits. Acta Physiol Scand. 2000b;169:297–308. doi: 10.1046/j.1365-201x.2000.00741.x. [DOI] [PubMed] [Google Scholar]
- Evans RG, Eppel GA, Anderson WP, Denton KM. Mechanisms underlying the differential control of blood flow in the renal medulla and cortex. J Hypertens. 2004;22:1439–1451. doi: 10.1097/01.hjh.0000133744.85490.9d. [DOI] [PubMed] [Google Scholar]
- Faure S, Chapot R, Tallet D, Javellaud J, Achard JM, Oudart N. Cerebroprotective effect of angiotensin IV in experimental ischemic stroke in the rat mediated by AT4 receptors. J Physiol Pharmacol. 2006;57:329–342. [PubMed] [Google Scholar]
- Feener EP, Northrup JM, Aiello LP, King GL. Angiotensin II induces plasminogen activator inhibitor-1 and -2 expression in vascular endothelial and smooth muscle cells. J Clin Invest. 1995;95:1353–1362. doi: 10.1172/JCI117786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez L, Spencer D, Kaczmar T. Angiotensin II decreases mortality rate in gerbils with unilateral carotid ligation. Stroke. 1986;17:82–85. doi: 10.1161/01.str.17.1.82. [DOI] [PubMed] [Google Scholar]
- Ferrario CM. Contribution of angiotensin-(1–7) to cardiovascular physiology and pathology. Curr Hypertens Rep. 2003;5:129–134. doi: 10.1007/s11906-003-0069-y. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Chappell MD. Novel angiotensin peptides. Cell Mol Life Sci. 2004;61:2720–2727. doi: 10.1007/s00018-004-4243-4. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Santos RA, Brosnihan KB, Block CH, Schiavone MT, Khosla MC, Greene LJ. A hypothesis regarding the function of angiotensin peptides in the brain. Clin Exp Hypertens A. 1988;10(Suppl 1):107–121. doi: 10.3109/10641968809075966. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin-(1–7) Hypertension. 1997;30:535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- Fink GD, Bruner CA. Hypertension during chronic peripheral and central infusion of angiotensin III. Am J Physiol. 1985;249:E201–E208. doi: 10.1152/ajpendo.1985.249.2.E201. [DOI] [PubMed] [Google Scholar]
- Fisher-Ferraro C, Nahmod VE, Goldstein DJ, Finkielman S. Angiotensin and renin in the rat and dog brain. J Exp Med. 1971;133:353–361. doi: 10.1084/jem.133.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald SM, Evans RG, Bergstrom G, Anderson WP. Renal hemodynamic responses to intrarenal infusion of ligands for the putative angiotensin IV receptor in anesthetized rats. J Cardiovasc Pharmacol. 1999;34:206–211. doi: 10.1097/00005344-199908000-00005. [DOI] [PubMed] [Google Scholar]
- Fitzsimons JT. The effect on drinking of peptide precursors and of shorter chain peptide fragments of angiotensin II injected into the rat’s diencephalons. J Physiol (Lond) 1971;214:295–303. doi: 10.1113/jphysiol.1971.sp009433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons JT. Angiotensin stimulation of the central nervous system. Rev Physiol Biochem Pharmacol. 1980;87:117–167. doi: 10.1007/BFb0030897. [DOI] [PubMed] [Google Scholar]
- Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- Fitts DA, Masson DB. Preoptic angiotensin and salt appetite. Behav Neurosci. 1990;104:643–650. doi: 10.1037//0735-7044.104.4.643. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE. Dose–response differences in the ability of ramipril to improve retention in diabetic mice. Eur J Pharmacol. 1993;240:311–314. doi: 10.1016/0014-2999(93)90916-6. [DOI] [PubMed] [Google Scholar]
- Ganong WF, Murakami K. The role of angiotensin II in the regulation of ACTH secretion. Ann N Y Acad Sci. 1987;512:176–186. doi: 10.1111/j.1749-6632.1987.tb24959.x. [DOI] [PubMed] [Google Scholar]
- Ganten D, Boucher R, Genest J. Renin activity in brain tissue of puppies and adult dogs. Brain Res. 1971a;33:557–559. doi: 10.1016/0006-8993(71)90137-5. [DOI] [PubMed] [Google Scholar]
- Ganten D, Marquez-Julio A, Granger P, Hayduk K, Karsunky KP, Boucher R, Genest J. Renin in dog brain. Am J Physiol. 1971b;221:1733–1737. doi: 10.1152/ajplegacy.1971.221.6.1733. [DOI] [PubMed] [Google Scholar]
- Ganten D, Hermann K, Bayer D, Unger T, Lang RE. Angiotensin synthesis in the brain and increased turnover in hypertensive rats. Science. 1983;221:869–871. doi: 10.1126/science.6879184. [DOI] [PubMed] [Google Scholar]
- Gard PR. The role of angiotensin II in cognition and behaviour. Eur J Pharmacol. 2002;438:1–14. doi: 10.1016/s0014-2999(02)01283-9. [DOI] [PubMed] [Google Scholar]
- Gard PR. Angioteinsin as a target for the treatment of Alzheimer’s disease, anxiety and depression. Expert Opin Ther Targets. 2004;8:7–14. doi: 10.1517/14728222.8.1.7. [DOI] [PubMed] [Google Scholar]
- Gard PR, Mycroft N. Reduction of angiotensin II-induced drinking in rats by 21 hour pretreatment with desipramine. J Pharm Pharmacol. 1991;43(Suppl 1) [Google Scholar]
- Gard PR, Rusted JM. Angiotensin and Alzheimer’s disease: therapeutic prospects. Expert Rev Neurother. 2004;4:87–96. doi: 10.1586/14737175.4.1.87. [DOI] [PubMed] [Google Scholar]
- Gard PR, Mandy A, Whiting JM, Nickels DP, Meakin AJ. Reduction of responses to angiotensin II by antidepressant drugs. Eur J Pharmacol. 1994;264:295–300. doi: 10.1016/0014-2999(94)00481-1. [DOI] [PubMed] [Google Scholar]
- Gard PR, Mandy A, Sutcliffe MA. Evidence of a possible role of altered angiotensin function in the treatment, but not aetiology, of depression. Biol Psychiatry. 1999;45:1030–1034. doi: 10.1016/s0006-3223(98)00101-2. [DOI] [PubMed] [Google Scholar]
- Garreau I, Chansel D, Vandermeersch S, Fruitier I, Piot JM, Ardaillou R. Hemorphins inhibit angiotensin IV binding and interact with aminopeptidase N. Peptides. 1998;19:1339–1348. doi: 10.1016/s0196-9781(98)00075-8. [DOI] [PubMed] [Google Scholar]
- Germain L, Chouinard G. Treatment of recurrent unipolar major depression with captopril. Biol Psychiatry. 1988;23:637–641. doi: 10.1016/0006-3223(88)90010-8. [DOI] [PubMed] [Google Scholar]
- Germain L, Chouinard G. Captopril treatment of major depression with serial measurements of blood cortisol concentrations. Biol Psychiatry. 1989;25:489–493. doi: 10.1016/0006-3223(89)90203-5. [DOI] [PubMed] [Google Scholar]
- Georgiev V, Kambourova T. Angiotensin II effects on the threshold of chemical convulsion seizures. Comp Rendus Acad Bulgare Sci. 1983;36:1599–1602. Abstract. [Google Scholar]
- Georgiev V, Lazarova M, Petkov VD, Kambourova T. Interactions between angiotensin II. GABA and diazepam in convulsive seizures. Neuropeptides. 1986;7:329–336. doi: 10.1016/0143-4179(86)90026-0. [DOI] [PubMed] [Google Scholar]
- Georgiev V, Yonkov DI, Kambourova TS. Interactions between angiotensin II and baclofen in shuttle-box and passive avoidance performance. Neuropeptides. 1988;12:155–158. doi: 10.1016/0143-4179(88)90047-9. [DOI] [PubMed] [Google Scholar]
- Georgiev V, Stancheva S, Kambourova T, Getova D. Effect of angiotensin II on the Vogel conflict paradigm and on the content of dopamine and noradrenaline in rat brain. Acta Physiol Pharmacol Bulgarica. 1990;16:32–37. Abstract. [PubMed] [Google Scholar]
- Gesualdo L, Ranieri E, Monno R, Rossiello MR, Colucci M, Semeraro N, Grandaliano G, Schena FP, Ursi M, Cerullo G. Angiotensin IV stimulates plasminogen activator inhibitor-1 expression in proximal tubular epithelial cells. Kidney Int. 1999;56:461–470. doi: 10.1046/j.1523-1755.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- Giardina WJ, Ebert DM. Positive effects of captopril in the behavioural despair swim test. Biol Psychiatry. 1989;25:697–702. doi: 10.1016/0006-3223(89)90240-0. [DOI] [PubMed] [Google Scholar]
- Glossmann H, Baukal A, Catt KJ. Angiotensin II receptors in bovine adrenal cortex. Modification of angiotensin II binding by guanyl nucleotides. J Biol Chem. 1974;249:664–666. [PubMed] [Google Scholar]
- Goldstein JM, Knobloch-Litwin LC, Malick JB. Behavioural evidence for β-adrenoceptor subsensitivity after subacute antidepressant/α2-adrenoceptor antagonist treatment. Naunyn-Schmiedeberg’s Arch Pharmacol. 1985;329:355–358. doi: 10.1007/BF00496367. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Gentil CG, Peres VL, Covian MR. Lever-pressing behavior caused by intraseptal angiotensin II in water satiated rats. Pharm Biochem Behav. 1973;1:357–359. doi: 10.1016/0091-3057(73)90129-9. [DOI] [PubMed] [Google Scholar]
- Guo DF, Inagami T. The genomic organization of the rat angiotensin II receptor AT1B. Biochim Biophys Acta. 1994;1218:91–94. doi: 10.1016/0167-4781(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Haberl RL, Anneser F, Villringer A, Einhaupl KM. Angiotensin II induces endothelium-dependent vasodilation of rat cerebral arterioles. Am J Physiol. 1990;258:H1840–H1846. doi: 10.1152/ajpheart.1990.258.6.H1840. [DOI] [PubMed] [Google Scholar]
- Haberl RL, Decker-Hermann PJ, Hermann K. Effect of renin on brain arterioles and cerebral blood flow in rabbits. J Cereb Blood Flow Metab. 1996;16:714–719. doi: 10.1097/00004647-199607000-00023. [DOI] [PubMed] [Google Scholar]
- Hall KL, Hanesworth JM, Ball AE, Felgenhaner GP, Hosick HL, Harding JW. Identification and chracterization of a novel angiotensin binding site in cultured vascular smooth muscle cells that is specific for the hexapeptide (3–8) fragment of angiotensin II, angiotensin IV. Regul Pept. 1993;44:225–232. doi: 10.1016/0167-0115(93)90246-5. [DOI] [PubMed] [Google Scholar]
- Hamilton TA, Handa RK, Harding JW, Wright JW. A role for the AT4/angiotensin IV system in mediating natriuresis in the rat. Peptides. 2001;22:935–944. doi: 10.1016/s0196-9781(01)00405-3. [DOI] [PubMed] [Google Scholar]
- Handa RK. Metabolism alters the selectivity of angiotensin-(1–7) receptor ligands for angiotensin receptors. J Am Soc Nephrol. 2000;11:1377–1386. doi: 10.1681/ASN.V1181377. [DOI] [PubMed] [Google Scholar]
- Handa RK. Characterization of signaling of the AT(4) receptor in human proximal tubule epithelial (HK-2) cells. J Am Soc Nephrol. 2001;12:440–449. doi: 10.1681/ASN.V123440. [DOI] [PubMed] [Google Scholar]
- Handa RK. Biphasic actions of angiotensin IV on renal blood flow in the rat. Regul Pept. 2006;136:23–29. doi: 10.1016/j.regpep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Handa RK, Krebs LT, Harding JW, Handa SE. Angiotensin IV AT4-receptor system in the rat kidney. Am J Physiol. 1998;274:F290–F299. doi: 10.1152/ajprenal.1998.274.2.F290. [DOI] [PubMed] [Google Scholar]
- Hanesworth JM, Sardinia JF, Krebs LT, Hall KL, Harding JW. Elucidation of a specific binding site for angiotensin II(3–8), angiotensin IV, in mammalian heart membranes. J Pharmcol Exp Ther. 1993;266:1036–1042. [PubMed] [Google Scholar]
- Harding JW, Cook VI, Miller-Wing AV, Hanesworth JM, Sardinia MF, Hall KL, Stobb JW, Swanson GN, Coleman JK, Wright JW, Harding EC. Identification of an AII (3–8) AIV binding site in guinea pig hippocampus. Brain Res. 1992;583:340–343. doi: 10.1016/s0006-8993(10)80047-2. [DOI] [PubMed] [Google Scholar]
- Harding JW, Stone LP, Wright JW. The distribution of angiotensin II binding sites in rodent brain. Brain Res. 1981;205:265–274. doi: 10.1016/0006-8993(81)90338-3. [DOI] [PubMed] [Google Scholar]
- Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, el-Dahr SS. Immunohistochemical localization of AngII AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol (Renal Fluid Electrolyte Physiol 41) 1997;273:F170–F177. doi: 10.1152/ajprenal.1997.273.1.F170. [DOI] [PubMed] [Google Scholar]
- Head GA. Role of AT1 receptors in the central control of sympathetic vasomotor function. Clin Exp Pharmacol Physiol. 1996;3(Suppl):S93–S98. [PubMed] [Google Scholar]
- Heinemann A, Sattler V, Jocic M, Wienen W, Holzer P. Effect of angiotensin II and telmisartan, an angiotensin 1 receptor antagonist, on rat gastric mucosal blood flow. Aliment Pharmacol Ther. 1999;13:347–355. doi: 10.1046/j.1365-2036.1999.00496.x. [DOI] [PubMed] [Google Scholar]
- Hellner K, Walther T, Schubert M, Albrecht D. Angiotensin-(1–7) enhances LTP in the hippocampus through the G-protein-coupled receptor. Mas Mol Cell Neurosci. 2005;29:427–435. doi: 10.1016/j.mcn.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Hermann K, Lang RE, Unger T, Bayer C, Ganten D. Combined high-performance liquid chromatograpy radioimmunoassay for the characterization and quantitative measurement of neuropeptides. J Chromatogr. 1984;312:273–284. doi: 10.1016/s0021-9673(01)92781-5. [DOI] [PubMed] [Google Scholar]
- Holownia A, Braszko JJ. Effect of angiotensin IVon the acquisition of the water maze task and ryanodine channel function. Pharmacol Biochem Behav. 2003;76:85–91. doi: 10.1016/s0091-3057(03)00190-4. [DOI] [PubMed] [Google Scholar]
- Iwai N, Inagami T. Identification of two subtypes in the rat type 1 angiotensin II receptor. FEBS Lett. 1992;298:257–260. doi: 10.1016/0014-5793(92)80071-n. [DOI] [PubMed] [Google Scholar]
- Iwai N, Yamano Y, Chak S, Konishi F, Bardham S, Tibbitts C, Sasaki K, Hasegawa M, Matsuda Y, Inagami T. Rat angiotensin II receptor: cDNA sequence and regulation of gene expression. Biochem Biophys Res Commun. 1991;177:299–304. doi: 10.1016/0006-291x(91)91982-i. [DOI] [PubMed] [Google Scholar]
- Iwai N, Liu H, Chen R. Important role of angiotensin II type 2 receptor in focal cerebral ischemia induced by middle cerebral artery occlusion: study using receptor gene deficient mice. J Hypertens. 2004;22:S9. [Google Scholar]
- Jaiswal N, Diz DI, Chappell MC, Khasla MC, Ferrario CM. Stimulation of endothelial cell prostaglandin production by angiotensin peptides. Characterization of receptors. Hypertension. 1992;21:900–905. doi: 10.1161/01.hyp.19.2_suppl.ii49. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Gessner GW, Ly CG. The angiotensin hexapeptide 3–8 fragment potently inhibits [125I] angiotensin II binding to non-AT1 or -AT2 recognition sites in bovine adrenal cortex. Eur J Pharmacol. 1992;219:319–322. doi: 10.1016/0014-2999(92)90312-r. [DOI] [PubMed] [Google Scholar]
- Jensen LL, Harding JW, Wright JW. Increased blood pressure induced by central application of aminopeptidase inhibitors is angiotensinergic-dependent in normotensive and hypertensive rat strains. Brain Res. 1989;490:48–55. doi: 10.1016/0006-8993(89)90429-0. [DOI] [PubMed] [Google Scholar]
- Jezova D, Ochedalski T, Kiss A, Aguilera GJ. Brain angiotensin II modulates sympathoadrenal and hypothalamic pituitary adrenocortical activation during stress. Neuroendocrinology. 1998;10:67–72. doi: 10.1046/j.1365-2826.1998.00182.x. [DOI] [PubMed] [Google Scholar]
- Jiang WG, Martin TA, Parr C, Davies G, Matsumoto K, Nakamura T. Hepatocyte growth factor, its receptor, and their potential value in cancer therapies. Crit Rev Oncol/Hematol. 2005;53:35–69. doi: 10.1016/j.critrevonc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Johnston CI. Biochemistry and pharmacology of the renin–angiotensin system. Drugs. 1990;39:21–31. doi: 10.2165/00003495-199000391-00005. [DOI] [PubMed] [Google Scholar]
- Kagiyama T, Kagiyama S, Phillips MI. Expression of angiotesnin type 1 and 2 receptos in brain after transient middle cerebral artery occlusion in rats. Regul Pept. 2003;110:241–247. doi: 10.1016/s0167-0115(02)00223-9. [DOI] [PubMed] [Google Scholar]
- Kakar SS, Sellers JC, Devor DC, Musgrove LC, Neill JD. Angiotensin II type-1 receptor subtype cDNAs: differential tissue expression and hormonal regulation. Biochem Biophys Res Commun. 1992;31:1090–1096. doi: 10.1016/s0006-291x(05)80302-x. [DOI] [PubMed] [Google Scholar]
- Kambayashi Y, Bardham S, Takahashi K, Tsuzuki S, Inui H, Hamakubo T, Inagami T. Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J Biol Chem. 1993;268:24543–24546. [PubMed] [Google Scholar]
- Kandror KV, Pilch PF. gp160, a tissue-specific marker for insulin-activated glucose transport. Proc Natl Acad Sci USA. 1994;91:8017–8021. doi: 10.1073/pnas.91.17.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri H, Asano T, Yamada T, Aoyama T, Fukushima Y, Kikuchi M, Kodama T, Oka Y. Acyl-coenzyme A dehydrogenases are localized on GLUT4-containing vesicles via association with insulin-regulated aminopeptidase in a manner dependent on its dileucine motif. Mol Endocrinol. 2002;16:1049–1059. doi: 10.1210/mend.16.5.0831. [DOI] [PubMed] [Google Scholar]
- Keller SR, Scott HM, Mastick CC, Aebersold R, Lienhard GE. Cloning and characterization of a novel insulin-regulated membrane aminopeptidase from Glut4 vescicles. J Biol Chem. 1995;270:23612–23618. doi: 10.1074/jbc.270.40.23612. [DOI] [PubMed] [Google Scholar]
- Kerins DM, Hao Q, Vaughan DE. Angiotensin induction of PAI-1 expression in endothelial cells is mediated by the hexapeptide angiotensin IV. J Clin Invest. 1995;96:2515–2520. doi: 10.1172/JCI118312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusha V, Georgiev V, Petkov VD, Markovska V, Svirskis SV, Mucenietze R, Ancans ZE. Comparative studies on the central effects of the angiotensin analogue/Sar1Ile8/ATII. Acta Physiol Pharmacol Bulgarica. 1986;12:22–29. Abstract. [PubMed] [Google Scholar]
- Köller M, Krause HP, Hoffmeister F, Ganten D. Endogenous brain angiotensin II disrupts passive avoidance behavior in rats. Neurosci Lett. 1979;14:71–75. doi: 10.1016/0304-3940(79)95346-1. [DOI] [PubMed] [Google Scholar]
- Konoshi H, Kuroda S, Inada Y, Fujisawa Y. Novel subtype of human angiotensin II type 1 receptor: cDNA cloning and expression. Biochem Biophys Res Commun. 1994;199:467–474. doi: 10.1006/bbrc.1994.1252. [DOI] [PubMed] [Google Scholar]
- Kramár EA, Harding JW, Wright JW. Angiotensin II-,and IV-induced changes in cerebral blood flow: roles of AT1, AT2, and AT4 receptor subtypes. Regul Pept. 1997;68:131–138. doi: 10.1016/s0167-0115(96)02116-7. [DOI] [PubMed] [Google Scholar]
- Kramár EA, Armstrong DL, Ikeda S, Wayner MJ, Harding JW, Wright JW. The effects of angiotensin IV on long-term potentiation within the CA1 region of the hippocampus in vitro. Brain Res. 2001;897:114–121. doi: 10.1016/s0006-8993(01)02100-x. [DOI] [PubMed] [Google Scholar]
- Kucharewicz I, Chabielska E, Pawlak D, Matys T, Rolkowski R, Buczko W. The antithrombotic effect of angiotensin (1–7) closely resembles that of losartan. J Renin Angiotensin Aldosterone Syst. 2000;1:268–272. doi: 10.3317/jraas.2000.041. [DOI] [PubMed] [Google Scholar]
- Kucharewicz I, Pawlak R, Matys T, Chabielska E, Buczko W. Angiotensin (1–7): an active member of the renin–angiotensin system. J Physiol Pharmacol. 2002;53:533–540. [PubMed] [Google Scholar]
- Kulakowska A, Karwowska W, Wisniewski K, Braszko JJ. Losartan influences behavioural effects of angiotensin II in rats. Pharmacol Res. 1996;34:109–115. doi: 10.1006/phrs.1996.0073. [DOI] [PubMed] [Google Scholar]
- Landas S, Phillips MI, Stamler JF, Raizada MK. Visualization of specific angiotensin II binding sites in the brain by fluorescent microscopy. Science. 1980;210:791–793. doi: 10.1126/science.6254147. [DOI] [PubMed] [Google Scholar]
- Lawrence AC, Evin G, Kladis A, Campbell DJ. An alternative strategy for the radioimmunoassay of angiotensin peptides using amino-terminal-directed antisera: measurement of eight angiotensin peptides in human plasma. J Hypertens. 1990;8:715–724. doi: 10.1097/00004872-199008000-00005. [DOI] [PubMed] [Google Scholar]
- Lee J, Chai SY, Mendelsohn FAO, Morris MJ, Allen AM. Potentiation of cholinergic transmission in the rat hippocampus by angiotensin IV and LVV-hemorphin-7. Neuropharmacology. 2001;40:618–623. doi: 10.1016/s0028-3908(00)00188-x. [DOI] [PubMed] [Google Scholar]
- Lee J, Mustafa T, McDowall SG, Mendelsohn FA, Brennan M, Lew RA, Albiston AL, Chai SY. Structure–activity study of LVV-hemorphin-7: angiotensin AT4 receptor ligand and inhibitor of insulin-regulated aminopeptidase. J Pharmacol Exp Ther. 2003;305:205–211. doi: 10.1124/jpet.102.045492. [DOI] [PubMed] [Google Scholar]
- Lee J, Albiston AL, Allen AM, Mendelsohn FAO, Ping SE, Barrett GL, Morris MJ, McDowall SG, Chai SY. Effect of icv injection of AT4 receptor ligands, Nle1-angiotensin IV and LVV-hemorphin 7, on spatial learning in rats. Neuroscience. 2004;124:341–349. doi: 10.1016/j.neuroscience.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Leong DS, Terron JA, Falcon-Neri A, Armando I, Ito T, Johren O, Tonelli LH, Hoe KL, Saavedra JM. Restraint stress modulates brain, pituitary and adrenal expression of angiotensin II AT(1A), AT(1B) and AT(2) receptors. Neuroendocrinology. 2002;75:227–240. doi: 10.1159/000054714. [DOI] [PubMed] [Google Scholar]
- Lew RA, Mustafa T, Ye S, McDowall SG, Chai SY, Albiston AL. Angiotensin AT4 ligands are potent, competitive inhibitors of insulin regulated aminopeptidase (IRAP) J Neurochem. 2003;86:344–350. doi: 10.1046/j.1471-4159.2003.01852.x. [DOI] [PubMed] [Google Scholar]
- Li J, Culman J, Hortnagl H, Zhao Y, Gerova N, Blume A, Zimmermann M, Seidel K, Dirnagl U, Unger T. Angiotensin AT2 receptor protects against cerebral ischemia-induced neuronal injury. FASEB J. 2005;19:617–619. doi: 10.1096/fj.04-2960fje. [DOI] [PubMed] [Google Scholar]
- Li P, Chappell MC, Ferrarion CM, Broshinan KB. Angiotensin (1–7) augments bradykinin-induced vasodilation by competing with ACE and releasing nitric oxide. Hypertension. 1997;29:394–400. doi: 10.1161/01.hyp.29.1.394. [DOI] [PubMed] [Google Scholar]
- Li XC, Campbell DJ, Ohishi M, Yuan S, Zhuo JL. AT1 receptor-activated signaling mediates angiotensin IV-induced renal cortical vaso-constriction in rats. Am J Physiol Renal Physiol. 2006;290:F1024–F1033. doi: 10.1152/ajprenal.00221.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YD, Block ER, Patel JM. Activation of multiple signaling modules is critical in angiotensin IV-induced lung endothelial cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L707–L716. doi: 10.1152/ajplung.00024.2002. [DOI] [PubMed] [Google Scholar]
- Liao AT, McMahon M, London C. Characterization, expression and function of c-Met in canine spontaneous cancers. Vet Comp Oncol. 2005;3:61–72. doi: 10.1111/j.1476-5810.2005.00067.x. [DOI] [PubMed] [Google Scholar]
- Linazasoro JM, Diaz CJ, Mendoza HC. The kidney and thirst regulation. Bull Inst Med Res. 1954;7:53–61. [PubMed] [Google Scholar]
- Lind RW. Sites of action of angiotensin in the brain. In: Harding JW, Wright JW, Speth RC, Barnes CD, editors. Angiotensin and Blood Pressure Regulation. Academic Press; San Diego: 1988. pp. 135–163. [Google Scholar]
- Liu Y. Hepatocyte growth factor and the kidney. Curr Opin Nephrol Hypertens. 2002;22:23–30. doi: 10.1097/00041552-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tolbert EM, Sun AM, Dworkin LD. Primary structure of rat HGF receptor and induced expression in glomerular mesangial cells. Am J Physiol. 1996;271:F679–F688. doi: 10.1152/ajprenal.1996.271.3.F679. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tolbert EM, Lin L, Thursby MA, Sun AM, Nakamura T, Dworkin LD. Up-regulation of hepatocyte growth factor receptor: an amplification and targeting mechanism for hepatocyte growth factor action in acute renal failure. Kidney Int. 1999;55:442–453. doi: 10.1046/j.1523-1755.1999.00267.x. [DOI] [PubMed] [Google Scholar]
- Llorens-Cortes C, Mendelsohn FAO. Organisation and functional role of the brain angiotensin system. J Renin Angiotensin Aldosterone Syst. 2002;3:S39–S48. doi: 10.3317/jraas.2002.029. [DOI] [PubMed] [Google Scholar]
- Loot AE, Roks AJ, Henning RH, Tio RA, Suurmeijer AM, Boomsma F, van Gilst WH. Angiotensin-(1–7) attenuates the development of heart failure after myocardial infarction in rats. Circulation. 2002;105:1548–1550. doi: 10.1161/01.cir.0000013847.07035.b9. [DOI] [PubMed] [Google Scholar]
- Lynch G, Larson J, Staubli U, Granger R. Variance of synaptic potentiation and different types of memory operations in hippocampus and related structures. In: Squire LR, Weinberger NM, Lynch G, Mc-Gaugh JL, editors. Memory: Organization and Locus of Change. Oxford University Press; New York: 1991. pp. 330–363. [Google Scholar]
- Lynch KR, Simnad VI, Ben-Ari ET, Garrison JC. Localization of angiotensinogen messenger RNA sequences in the rat brain. Hypertension. 1986;8:540–543. doi: 10.1161/01.hyp.8.6.540. [DOI] [PubMed] [Google Scholar]
- Ma PC, Maulik G, Ghristensen J, Salgia R. c-Met: structure, functions and potential for therapeutic inhibition. Cancer Metast Rev. 2003;22:309–325. doi: 10.1023/a:1023768811842. [DOI] [PubMed] [Google Scholar]
- Martin P, Massol J, Puech AJ. Captopril as an antidepressant? Effects on the learned helplessness paradigm in rats. Biol Psychiatry. 1990;27:968–974. doi: 10.1016/0006-3223(90)90034-y. [DOI] [PubMed] [Google Scholar]
- Martinez JL, Derrick BE. Long-term potentiation and learning. Ann Rev Psychiatry. 1996;47:173–203. doi: 10.1146/annurev.psych.47.1.173. [DOI] [PubMed] [Google Scholar]
- Matsoukas JM, Goghari MH, Scanlon MN, Franklin KJ, Moore GJ. Synthesis and biological activities of analogues of angiotensins II and III containing O-methyltyrosine and D-tryptophan. J Med Chem. 1985;28:780–783. doi: 10.1021/jm00383a015. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. Hepatocyte growth factor: renotropic role and potential therapeutics for renal diseases. Kidney Int. 2001;59:2023–2038. doi: 10.1046/j.1523-1755.2001.00717.x. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FAO, Chai SY. The brain renin–angiotensin system: location and physiological roles. Int J Biochem Cell Biol. 2003;35:901–918. doi: 10.1016/s1357-2725(02)00306-0. [DOI] [PubMed] [Google Scholar]
- Mehta JL, Li DY, Yang H, Raizada MK. Angiotensin II and IV stimulate expression and release of plasminogen activator inhibitor-1 in cultured human coronary artery endothelial cells. J Cardiovasc Pharmacol. 2002;39:789–794. doi: 10.1097/00005344-200206000-00003. [DOI] [PubMed] [Google Scholar]
- Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- Melo JC, Graeff FG. Effect of intracerebroventricular bradykinia and related peptides on rabbit operant behavior. J Pharmacol Exp Ther. 1975;193:1–10. [PubMed] [Google Scholar]
- Meng W, Busija DW. Comparative effects of angiotensin (1–7) and angiotensin II on piglet pial arterioles. Stroke. 1993;24:2041–2044. doi: 10.1161/01.str.24.12.2041. [DOI] [PubMed] [Google Scholar]
- Mentlein R, Roos T. Proteases involved in the metabolism of angiotensin II, bradykinin, calcitonin gene-related peptide (CGRP), and neuropeptide Y by vascular smooth muscle cells. Peptides. 1996;17:709–720. doi: 10.1016/0196-9781(96)00066-6. [DOI] [PubMed] [Google Scholar]
- Miller-Wing AV, Hanesworth JM, Sardinia MF, Wright JW, Speth RC, Grove KL, Harding JW. Central angiotensin IV receptors: distribution and specificity in guinea pig brain. J Pharmacol Exp Ther. 1993;266:1718–1726. [PubMed] [Google Scholar]
- Miyazawa T, Matsumoto K, Ohmichi H, Katoh H, Yamashima T, Hakamura T. Protection of hippocampal neurons from ischemia-induced delayed neuronal death by hepatocyte growth factor: a novel neurotrophic factor. J Cereb Blood Flow Metab. 1998;18:345–348. doi: 10.1097/00004647-199804000-00001. [DOI] [PubMed] [Google Scholar]
- Moe KE, Weiss ML, Epstein AN. Sodium appetite during captopril blockade of endogenous angiotensin II formation. Am J Physiol. 1984;247:R356–R365. doi: 10.1152/ajpregu.1984.247.2.R356. [DOI] [PubMed] [Google Scholar]
- Mǿeller I, Lew RA, Mendelsohn FA, Smith AI, Brennan ME, Tetaz TJ, Chai SY. The globin fragment LVV-hemorphin-7 is an endogenous ligand for the AT4 receptor in the brain. J Neurochem. 1997;68:2530–2537. doi: 10.1046/j.1471-4159.1997.68062530.x. [DOI] [PubMed] [Google Scholar]
- Mondadori C, Etienne P. Nootropic effets of ACE inhibitors in mice. Psychopharmacology. 1990;100:301–307. doi: 10.1007/BF02244597. [DOI] [PubMed] [Google Scholar]
- Morgan TM, Routtenberg A. Angiotensin injected into the neostriatum after learning disrupts retention performance. Science. 1977;196:87–89. doi: 10.1126/science.402696. [DOI] [PubMed] [Google Scholar]
- Morishita R, Nakamura S, Hayashi S, Taniyama Y, Moriguchi A, Nagano T, Taiji M, Noguchi H, Takeshita S, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension. 1999;33:1379–1384. doi: 10.1161/01.hyp.33.6.1379. [DOI] [PubMed] [Google Scholar]
- Morris RG. Development of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Mukoyama M, Nakajiima M, Horiuchi M, Sasamura H, Pratt RE, Dzau VJ. Expression cloning of type-2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J Biol Chem. 1993;268:24539–24542. [PubMed] [Google Scholar]
- Muratami H. Brain angioensin and circulatory control. Clin Exp Pharmacol Physiol. 1996;23:458–464. doi: 10.1111/j.1440-1681.1996.tb02761.x. [DOI] [PubMed] [Google Scholar]
- Murphy TJ, Alexander RW, Griendling KK, Runge MS, Berstein KE. Isolation of a cDNA encoding the vascular type-1 angiotensin receptor. Nature. 1991;351:233–236. doi: 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- Muthalif MM, Benter IF, Uddin MR, Harper JL, Malik KU. Signal transduction mechanisms involved in angiotensin (1–7)-stimulated arachidonic acid release and prostanoid synthesis in rabbit aortic smooth muscle cells. J Pharmacol Exp Ther. 1998;284:388–398. [PubMed] [Google Scholar]
- Nagayama T, Nagayama M, Kohara S, Kamiguchi H, Shibuya M, Katoh Y, Itoh J, Shinohara Y. Post-ischemic delayed expression of hepatocyte growth factor and c-Met in mouse brain following focal cerebral ischemia. Brain Res. 2004;999:155–166. doi: 10.1016/j.brainres.2003.11.052. [DOI] [PubMed] [Google Scholar]
- Nairn RC, Mason CM, Corcoran AC. The production of serous effusions in nephrectomized animals by the administratin of renal extracts and renin. J Pathol Bacteriol. 1956;71:155–163. doi: 10.1002/path.1700710121. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Nishizwa T, Hagiya M, Siki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Muzuno S, Matsumoto K, Sawa Y, Matsuda H, Nakamura T. Myocardial protection from ischemia/reperfusion injury by endogenous and exogenous HGF. J Clin Invest. 2000;106:1511–1519. doi: 10.1172/JCI10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani T, Kim T, Uchida J, Kumata N, Kawashima H, Sugimura K. Hepatocyte growth factor ameliorates renal hemodynamic disorder after ischemia/reperfusion. Int J Mol Med. 2002;10:217–219. [PubMed] [Google Scholar]
- Navar LG, Harrison-Bernard LM, Imig JD, Cervenka L, Mitchell KD. Renal responses to AT1 receptor blockade. Am J Hypertens. 2000;13:45S–54S. doi: 10.1016/s0895-7061(99)00248-4. [DOI] [PubMed] [Google Scholar]
- Naveri L, Stromberg C, Savedra JM. Angiotensin IV reverses the acute cerebral blood flow reduction after experimental subarachnoid hemorrhage in the rat. J Cereb Blood Flow Metab. 1994;14:1096–1099. doi: 10.1038/jcbfm.1994.143. [DOI] [PubMed] [Google Scholar]
- Neves LA, Averill DB, Ferrario CM, Chappell MC, Aschner JL, Walkup MP, Brosnihan KB. Characterization of angiotensin-(1–7) receptor subtype in mesenteric arteries. Peptides. 2003;24:455–462. doi: 10.1016/s0196-9781(03)00062-7. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Ito T, Hoe KL, Saavedra JM. Chronic peripheral administration of the angiotensin II AT(1) receptor antagonist candesartan blocks brain AT(1) receptors. Brain Res. 2000;871:29–38. doi: 10.1016/s0006-8993(00)02377-5. [DOI] [PubMed] [Google Scholar]
- Olson ML, Olson EA, Qualls JH, Stratton JJ, Harding HW, Wright JW. Norleucine1-Angiotensin IV alleviates mecamylamine-induced spatial memory deficits. Peptides. 2004;25:233–241. doi: 10.1016/j.peptides.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Osei SY, Ahima RS, Minkes RK, Weaver JP, Khosla MC, Kadowitz PJ. Differential responses to angiotensin (1–7) in the feline mesenteric and hindquarters vascular beds. Eur J Pharmacol. 1993;234:35–42. doi: 10.1016/0014-2999(93)90703-k. [DOI] [PubMed] [Google Scholar]
- Overmier JB, Murison R. Anxiety and helplessness in the face of stress predisposes, precipitates, and sustains gastric ulceration. Brain Res. 2000;110:161–174. doi: 10.1016/s0166-4328(99)00193-x. [DOI] [PubMed] [Google Scholar]
- Page IH, Helmer OM. A crystaline pressor substance (angiotonin) resulting from the action between renin and renin-activator. J Exp Med. 1940;71:29–42. doi: 10.1084/jem.71.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzak A, Persson AE. Angiotensin II–nitric oxide interaction in the kidney. Curr Opin Nephrol Hypertens. 2007;16:46–51. doi: 10.1097/MNH.0b013e328011a89b. [DOI] [PubMed] [Google Scholar]
- Paula RD, Lima CV, Khosla MC. Angiotensin (1–7) potenitates hypotensive effect of bradykinin in conscious rats. Hypertension. 1995;26:1154–1159. doi: 10.1161/01.hyp.26.6.1154. [DOI] [PubMed] [Google Scholar]
- Pederson ES, Harding JW, Wright JW. Attenuation of scopolamine-induced spatial learning impairments by an angioensin IV analog. Regul Pept. 1998;74:97–103. doi: 10.1016/s0167-0115(98)00028-7. [DOI] [PubMed] [Google Scholar]
- Pederson ES, Krishnan R, Harding JW, Wright JW. A role for the angiotensin AT4 receptor subtype in overcoming scopolamine-induced spatial memory deficits. Regul Pept. 2001;102:147–156. doi: 10.1016/s0167-0115(01)00312-3. [DOI] [PubMed] [Google Scholar]
- Peng J, Phillips MI. Opposite regulation of brain angiotensin type 1 and type 2 receptors in cold-induced hypertension. Regul Pept. 2001;97:91–102. doi: 10.1016/s0167-0115(00)00218-4. [DOI] [PubMed] [Google Scholar]
- Phillips MI. Functions of angiotensin in the central nervous system. Annu Rev Physiol. 1987;49:413–435. doi: 10.1146/annurev.ph.49.030187.002213. [DOI] [PubMed] [Google Scholar]
- Phillips MI, Sumners C. Angiotensin II in central nervous system physiology. Regul Pept. 1998;78:1–11. doi: 10.1016/s0167-0115(98)00122-0. [DOI] [PubMed] [Google Scholar]
- Phillips MI, Weyhenmeyer JA, Felix D, Ganten D. Evidence for an endogenous brain renin angiotensin system. Fed Probat. 1979;38:2260–2266. [PubMed] [Google Scholar]
- Poljak A, McLean CA, Sachdev P, Brodaty H, Smythe GA. Quantification of hemorphins in Alzheimer’s disease brains. J Neurosci Res. 2004;75:704–714. doi: 10.1002/jnr.20020. [DOI] [PubMed] [Google Scholar]
- Przegalinski E, Siwanowicz J, Baran L. Effect of repeated administration of antidepressant drugs on the isoprenaline-induced drinking in rats. Pol J Pharmacol. 1988;40:251–258. [PubMed] [Google Scholar]
- Rajapakse NW, Eppel GA, Widdop RE, Evans RG. AngII type 2 receptors and neural control of intrarenal blood flow. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1669–R1676. doi: 10.1152/ajpregu.00183.2006. [DOI] [PubMed] [Google Scholar]
- Ramírez M, Arechage G, García S, Sanchez B, De Gandarias JM. Mn2(+)-activated aspartate aminopeptidase activity, subcellular localization in young and adult rat brain. Brain Res. 1990;522:165–167. doi: 10.1016/0006-8993(90)91595-8. [DOI] [PubMed] [Google Scholar]
- Reaux A, deMote N, Zini S. PC18, a specific aminopeptidase N inhibitor, induces vasopressin release by increasing the half-life of grain angiotensin III. Neuroendocrinology. 1999;69:370–376. doi: 10.1159/000054439. [DOI] [PubMed] [Google Scholar]
- Reudelhuber TL. The renin–angiotensin system: peptides and enzymes beyond angiotensin II. Curr Opin Nephrol Hypertens. 2005;14:155–159. doi: 10.1097/00041552-200503000-00011. [DOI] [PubMed] [Google Scholar]
- Reynier-Rebuffel AM, Aubineau P, Issertial O, Seylaz J. Nonuniformity of CBF response to NE- or AngII-induced hypertension in rabbits. Am J Physiol. 1987;253:H47–H57. doi: 10.1152/ajpheart.1987.253.1.H47. [DOI] [PubMed] [Google Scholar]
- Rich DH, Moon BJ, Harbeson S. Inhibition of aminopeptidases by amastatin and bestatin derivatives, effect of inhibitor structure on slow-binding processes. J Med Chem. 1984;27:417–422. doi: 10.1021/jm00370a001. [DOI] [PubMed] [Google Scholar]
- Richter CP. Increased salt appetite in adrenalectomized rats. Am J Physiol. 1936;115:155–161. [Google Scholar]
- Roberts KA, Krebs LT, Kramár EA, Shaffer MJ, Harding JW, Wright JW. Autoradiographic identification of brain angiotensin IV binding sites and differential c-Fos expression following intracerebroventricular injection of angiotensin II and IV in rats. Brain Res. 1995;682:13–21. doi: 10.1016/0006-8993(95)00289-3. [DOI] [PubMed] [Google Scholar]
- Roks AJ, Henning RH. Angiotensin peptides: ready to re(de)fine the angiotensin system? J Hypertens. 2003;21:1269–1271. doi: 10.1097/01.hjh.0000059057.65882.b5. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Jones BP, Fallows DJ. A comparison of the motivational properties of thirst. Physiol Behav. 1972;9:777–782. doi: 10.1016/0031-9384(72)90051-0. [DOI] [PubMed] [Google Scholar]
- Rossi NF. Dopaminergic control of angiotensin II-induced vasopressin secretion in vitro. Am J Physiol. 1998;275:687–693. doi: 10.1152/ajpendo.1998.275.4.E687. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Suzuki Y, Mezzano S, Plaza JJ, Egido J. Role of the renin–angiotensin system in vascular diseases: expanding the field. Hypertension. 2001;38:1382–1387. doi: 10.1161/hy1201.100589. [DOI] [PubMed] [Google Scholar]
- Ryu J, Hah JS, Park JS, Lee W, Rampal AL, Jung CY. Protein kinase C-zeta phosphorylates insulin-responsive aminopeptidase in vitro at Ser-80 and Ser-91. Arch Biochem Biophys. 2002;403:71–82. doi: 10.1016/S0003-9861(02)00261-8. [DOI] [PubMed] [Google Scholar]
- Saavedra JM. Brain and pituitary angiotensin. Endocr Rev. 1992;13:329–380. doi: 10.1210/edrv-13-2-329. [DOI] [PubMed] [Google Scholar]
- Saavedra JM. Emerging features of brain angiotensin receptors. Regul Pept. 1999;85:31–45. doi: 10.1016/s0167-0115(99)00081-6. [DOI] [PubMed] [Google Scholar]
- Saavedra JM. Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell Mol Neurobiol. 2005;25:485–512. doi: 10.1007/s10571-005-4011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadamura H, Hein L, Krieger JE, Pratt RE, Kobilka BK, Dzau VJ. Cloning, characterization, and expression of two angiotensin receptor (AT-1) isoforms from the mouse genome. Biochem Biophys Res Commun. 1992;185:253–259. doi: 10.1016/s0006-291x(05)80983-0. [DOI] [PubMed] [Google Scholar]
- Sadowski J, Badzynska B. Specific features and roles of renal circulation: angiotensin II revisited. J Physiol Pharmacol. 2006;57:169–178. [PubMed] [Google Scholar]
- Sakai RR, Epstein AN. Dependence of adrenalectomy-induced sodium appetite on the action of angiotensin II in the brain of the rat. Behav Neurosci. 1990;104:167–176. doi: 10.1037/0735-7044.104.1.167. [DOI] [PubMed] [Google Scholar]
- Sandberg K, Ji H, Catt KJ. Regulation of angiotensin II receptors in rat brain during dietary sodium changes. Hypertension. 1994;23:I-137–I-141. doi: 10.1161/01.hyp.23.1_suppl.i137. [DOI] [PubMed] [Google Scholar]
- Santos RA, Campagnole-Santos MJ, Andrade SP. Angiotensin(1–7): an update. Regul Pept. 2000;91:45–62. doi: 10.1016/s0167-0115(00)00138-5. [DOI] [PubMed] [Google Scholar]
- Santos RA, Campagnole-Santos MJ, Baracho NC, Fontes MA, Silva LC, Neves LA, Oliveira DR, Caligiorne SM, Rodrigues AR, Gropen C, Jr, Carvalho WS, Simoes E, Silva AC, Khosla M. Characterization of a new angiotensin antagonist selective for angiotensin-(1–7): Evidence that the actions of angiotensin-(1–7) are mediated by specific angiotensin receptors. Brain Res Bull. 1994;35:293–298. doi: 10.1016/0361-9230(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Sardinia MF, Hanesworth JM, Krebs LT, Harding JW. AT4 receptor binding characteristics: D-amino acid- and glycine-substituted peptides. Peptides. 1993;14:949–954. doi: 10.1016/0196-9781(93)90071-n. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Yamano Y, Bardhan S, Iwai N, Murray JJ, Hasegawa M, Matsuda Y, Inagami T. Cloning and expression of a complementary DNA encoding a bovine adrenal angiotensin II type-1 receptor. Nature (London) 1991;351:230–233. doi: 10.1038/351230a0. [DOI] [PubMed] [Google Scholar]
- Savaskan E. The role of the brain renin–angiotensin system in neurodegenerative disorders. Curr Alzheimer Res. 2005;2:29–35. doi: 10.2174/1567205052772740. [DOI] [PubMed] [Google Scholar]
- Sayeski PP, Ali MS, Semeniuk DJ, Doan TN, Bernstein KE. Angiotensin II signal transduction pathways. Regul Pept. 1998;78:19–29. doi: 10.1016/s0167-0115(98)00137-2. [DOI] [PubMed] [Google Scholar]
- Schiavone MT, Santos RAS, Brosnihan KB, Khosla MC, Ferrario CM. Release of vasopressin from the rat hypothalamo-neurohypophysial system by angiotensin (1–7) heptapeptide. Proc Natl Acad Sci USA. 1988;85:4095–4098. doi: 10.1073/pnas.85.11.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura M, Sato N, Waguri S, Uchiyama Y, Hayashi T, Iida H, Nakamura T, Ogihara T, Kaneda Y, Morishita R. Gene transfer of hepatocyte growth factor gene improves learning and memory in the chronic stage of cerebral infarction. Hypertension. 2006;47:742–751. doi: 10.1161/01.HYP.0000208598.57687.3e. [DOI] [PubMed] [Google Scholar]
- Shinomiya N, Vande Woude GF. Suppression of Met expression: a possible cancer treatment. Clin Cancer Res. 2003;9:5085–5090. [PubMed] [Google Scholar]
- Silva-Barcellos NM, Frezard F, Caligiorne S, Santos RA. Long-lasting cardiovascular effects of liposome-entrapped angiotesnin (1–7) at the rostral ventrolateral medulla. Hypertension. 2001;38:1266–1271. doi: 10.1161/hy1201.096056. [DOI] [PubMed] [Google Scholar]
- Simonnet G, Giorguieff-Chesselet MF. Stimulating effect of angiotensin II on the spontaneous release of newly synthesized [3H]dopamine in rat striatal slices. Neurosci Lett. 1979;15:153–158. doi: 10.1016/0304-3940(79)96105-6. [DOI] [PubMed] [Google Scholar]
- Simpson JB, Routtenberg A. Subfornical organ: site of drinking elicitation by angiotensin II. Science. 1973;181:1172–1175. doi: 10.1126/science.181.4105.1172. [DOI] [PubMed] [Google Scholar]
- Sirrett NE, McLean AS, Bray JJ, Hubbard JL. Distribution of angiotensin II receptors in rat brain. Brain Res. 1977;122:299–312. doi: 10.1016/0006-8993(77)90296-7. [DOI] [PubMed] [Google Scholar]
- Skeggs LT, Kahn JR, Lentz KE, Shumway NP. The preparation, purification and amino acid sequence of polypeptide renin substrate. J Exp Med. 1957;106:439–453. doi: 10.1084/jem.106.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slinker BK, Wu Y, Brennan AJ, Campbell KB, Harding JW. Angiotensin IV has mixed effects on left ventricle systolic function and speeds relaxation. Cardiovasc Res. 1999;42:660–669. doi: 10.1016/s0008-6363(98)00344-7. [DOI] [PubMed] [Google Scholar]
- Song L, Wilk S, Healy DP. Aminopeptidase A antiserum inhibits intracerebroventricular angiotensin II-induced dipsogenic and pressor responses. Brain Res. 1997;744:1–6. doi: 10.1016/s0006-8993(96)00952-3. [DOI] [PubMed] [Google Scholar]
- Speth RC, Thompson SM, Johns SJ. Angiotensin II receptors: structural and functional considerations. In: Mukhopadhyay AK, Raizada MK, editors. Current Concepts: Tissue Rennin Angiotensin Systems as Local Regulators in Reproductive and Endocrine Organs. Plenum Press; New York: 1995. pp. 169–192. [Google Scholar]
- Speth RC, Brown TE, Barnes RD, Wright JW. Brain angiotensinergic activity: the state of our current knowledge. Proc West Pharmacol Soc. 2003;46:11–15. [PubMed] [Google Scholar]
- Stoker M, Gherardi E, Perryman M, Fray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- Stragier B, Sarre S, Vanderheyden P, Vauquelin G, Fkournie-Zalluski MC, Ebinger G, Michotte Y. Metabolism of angioensin II is required for its in vivo effect on dopamine release in the striatum of the rat. J Neurochem. 2004;90:1251–1257. doi: 10.1111/j.1471-4159.2004.02600.x. [DOI] [PubMed] [Google Scholar]
- Stragier B, Clinckers R, Meurs A, De Bundel D, Sarre S, Ebinger G, Michotte Y, Smolders I. Involvement of the somatostatin-2 receptor in the anti-confulsant effect of angiotensin IV against pilocarpine-induced limbic seizures in rats. J Neurochem. 2006;98:1100–1113. doi: 10.1111/j.1471-4159.2006.03942.x. [DOI] [PubMed] [Google Scholar]
- Stromberg C, Naveri L, Saavedra JM. Angiotensin AT2 receptors regulate cerebral blood flow in rats. Neuroreport. 1992;3:703–704. doi: 10.1097/00001756-199208000-00013. [DOI] [PubMed] [Google Scholar]
- Stubley-Weatherly LA, Harding JW, Wright JW. Effects of discrete kainic acid-induced hippocampal lesions on spatial and contextual learning and memory. Brain Res. 1996;716:29–38. doi: 10.1016/0006-8993(95)01589-2. [DOI] [PubMed] [Google Scholar]
- Summy-Long JY, Keil LC, Sells G, Kirby A, Ohee O, Severs WB. Cerebroventricular sites for enkephalin inhibition of the central actions of angiotensin. Am J Physiol. 1983;244:R522–R529. doi: 10.1152/ajpregu.1983.244.4.R522. [DOI] [PubMed] [Google Scholar]
- Svirskis SV, Mucenietze R, Klusha V, Ancans ZE, Georgiev V, Getova D. Angiotensin and its analogues: comparative studies of their central effects. central and peripheral peptidergic regulation. Latvian Acad Sci, Inst Org Synth, Riga. 1991:120–128. [Google Scholar]
- Swanson GN, Hanesworth JM, Sardinia MF, Coleman JK, Wright JW, Hall KL, Miller-Wing AV, Stobb JW, Cook VI, Harding EC, Harding JW. Discovery of a distinct binding site for angiotensin II (3–8), a putative angiotensin IV receptor. Regul Pept. 1992;40:409–419. doi: 10.1016/0167-0115(92)90527-2. [DOI] [PubMed] [Google Scholar]
- Tada T, Zhan H, Tanaka Y, Hongo K, Matsumoto K, Nakamura T. Intraventricular administration of hepatocyte growth factor treats mouse communicating hydrocephalus induced by transforming growth factor beta1. Neurobiol Dis. 2006;21:576–586. doi: 10.1016/j.nbd.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Tallant EA, Lu X, Weiss RB, Chappell MC, Ferrario CM. Bovine aortic endothelial cells contain an angiotensin-(1–7) receptor. Hypertension. 1997;29(Part 2):388–393. doi: 10.1161/01.hyp.29.1.388. [DOI] [PubMed] [Google Scholar]
- Tamake K, Saku YL, Ogata J. Effects of angiotensin and atrial natriuretic peptide on the cerebral circulation. J Cereb Blood Flow Metab. 1992;12:318–325. doi: 10.1038/jcbfm.1992.44. [DOI] [PubMed] [Google Scholar]
- Tchekalarova JD, Georgiev VP. Adenosine–angiotensin II interactions in pentylenetetrazol seizure threshold in mice. J Physiol (Paris) 1999;93:191–197. doi: 10.1016/s0928-4257(99)80151-x. [DOI] [PubMed] [Google Scholar]
- Tchekalarova JD, Georgiev VP. Angiotensin peptides modulatory system: how is it implicated in the control of seizure susceptibility? Life Sci. 2005;76:955–970. doi: 10.1016/j.lfs.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Tchekalarova JD, Georgiev VP. Ang II and Ang III modulate PTZ seizure threshold in non-stressed and stressed mice: possible involvement of noradrenergic mechanism. Neuropeptides. 2006;40:339–348. doi: 10.1016/j.npep.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Tchekalarova JD, Kambourova T, Georgiev VP. Angiotensin III and IV influence on pentylenetetrazol seizure susceptibility (threshold and kindling). Interaction with adenosine A1 receptors. Brain Res Bull. 2001a;56:87–91. doi: 10.1016/s0361-9230(01)00568-8. [DOI] [PubMed] [Google Scholar]
- Tchekalarova JE, Kambourova T, Georgiev VP. Interaction between angiotensin IV and adenosine A1 receptor related drugs in passive avoidance conditioning in rats. Behav Brain Res. 2001b;123:113–116. doi: 10.1016/s0166-4328(01)00198-x. [DOI] [PubMed] [Google Scholar]
- Tchekalarova JD, Pehlivanova D, Kambourova T, Matsoukas J, Georgiev V. The effects of sarmesin, an angiotensin II analogue on seizure susceptibility, nociception and memory retention. Regul Pept. 2003;111:191–197. doi: 10.1016/s0167-0115(02)00285-9. [DOI] [PubMed] [Google Scholar]
- Thewke EP, Seeds NW. The expression of mRNAs for hepatocyte growth factor/scatter factor, its receptor c-met, and one of its activators tissue-type plasminogen activator show a systematic relationship in the developing and adult cerebral cortex and hippocampus. Brain Res. 1999;821:356–367. doi: 10.1016/s0006-8993(99)01115-4. [DOI] [PubMed] [Google Scholar]
- Thomas WG, Mendelsohn FAO. Molecules in focus: angiotensin receptors: form and function and distribution. Int J Biochem Cell Biol. 2003;35:774–779. doi: 10.1016/s1357-2725(02)00263-7. [DOI] [PubMed] [Google Scholar]
- Tiegerstedt R, Bergman PG. Niere und kreislauf. Skand Arch Physiol. 1898;8:223–231. [Google Scholar]
- Tonnaer JA, Wiegant VM, DeJong W, DeWied D. Central effects of angiotensin on drinking and blood pressure; structure–activity relationships. Brain Res. 1982;236:417–428. doi: 10.1016/0006-8993(82)90725-9. [DOI] [PubMed] [Google Scholar]
- Tsujimoto M, Mizutani S, Adachi H, Kimura M, Nakazato H, Tomoda Y. Identification of human placental leucine aminopeptidase as oxytocinase. Arch Biochem Biophys. 1992;292:388–392. doi: 10.1016/0003-9861(92)90007-j. [DOI] [PubMed] [Google Scholar]
- Tsuzuki N, Miyazawa T, Matsumoto K, Hakamura T, Shima K. Hepatocyte growth factor reduces the infarct volume after transient focal cerebral ischemia in rats. Neurol Res. 2001;23:417–424. doi: 10.1179/016164101101198659. [DOI] [PubMed] [Google Scholar]
- Tuncel N, Erkasap N, Sahinturk V, Ak DD, Tuncel M. The protective effect of vasoactive intestinal peptide (VIP) on stress-induced gastric ulceration in rats. Ann N Y Acad Sci. 1998;865:309–322. doi: 10.1111/j.1749-6632.1998.tb11191.x. [DOI] [PubMed] [Google Scholar]
- Tuor UI, Kondysar MH, Harding RK. Effect of angiotensin II and peptide YY on cerebral and circumventricular blood flow. Peptides. 1988;9:141–149. doi: 10.1016/0196-9781(88)90020-4. [DOI] [PubMed] [Google Scholar]
- Ueda S, Masumori-Maemoto S, Ashino K, Nagohara T, Gotoh E, Umemura S, Ishii M. Angiotensin (1–7) attenuates vasoconstriction evoked by angiotensin II but not by noradrenaline in man. Hypertension. 2000;35:998–1001. doi: 10.1161/01.hyp.35.4.998. [DOI] [PubMed] [Google Scholar]
- Unger T. The role of the renin–angiotensin–aldosterone system in heart failure. J Renin Angiotensin Aldosterone Syst. 2004;5:S7–S10. doi: 10.3317/jraas.2004.024. [DOI] [PubMed] [Google Scholar]
- Unger T, Becker H, Petty M, Demmert G, Schneider B, Ganten D, Lang RE. Differential effects of central angiotensin II and substance P on sympathetic nerve activity in conscious rats. Implications for cardiovascular adaptation to behavioral responses. Circ Res. 1985;56:563–575. doi: 10.1161/01.res.56.4.563. [DOI] [PubMed] [Google Scholar]
- Unger T, Badoer E, Ganten D, Lang RE, Rettig R. Brain angiotensin: pathways and pharmacology. Circulation. 1988;77:140–154. [PubMed] [Google Scholar]
- Unger T, Horst PJ, Bauer M, Demmert G, Rettig R, Rohmeiss P. Natriuretic action of central angiotensin II in conscious rats. Brain Res. 1989;486:33–38. doi: 10.1016/0006-8993(89)91274-2. [DOI] [PubMed] [Google Scholar]
- Van Belle E, Witzenbichler B, Chen D, Silver M, Chang L, Schwall R, Isner JM. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: the case for paracrine amplification of angiogenesis. Circulation. 1998;97:381–390. doi: 10.1161/01.cir.97.4.381. [DOI] [PubMed] [Google Scholar]
- Van Houten M, Schiffrin EL, Mann JF, Posner BJ, Boucher R. Radioautographic localization of specific binding sites for blood–borne angiotensin II in the rat brain. Brain Res. 1980;186:480–485. doi: 10.1016/0006-8993(80)90995-6. [DOI] [PubMed] [Google Scholar]
- Van Rodijnen WF, Van Lambalgen TA, Van Wijhe MH, Tangelder GJ, Ter Wee PM. Renal microvascular actions of angiotensin II fragments. Am J Physiol. 2002;283:F86–F92. doi: 10.1152/ajprenal.00121.2001. [DOI] [PubMed] [Google Scholar]
- Vaughan DE, Lazos SA, Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin–angiotensin system and thrombosis. J Clin Invest. 1995;95:995–1001. doi: 10.1172/JCI117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauquelin G, Michotte Y, Smolders I, Sarre S, Ebinger G, Dupont A, Vanderheyden P. Cellular targets for angiotensin II fragments: pharmacological and molecular evidence. J Renin Angiotensin Aldosterone Syst. 2002;3:195–204. doi: 10.3317/jraas.2002.041. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O. Angiotensin IV in the central nervous system. Cell Tissue Res. 2003;311:1–9. doi: 10.1007/s00441-002-0655-3. [DOI] [PubMed] [Google Scholar]
- Walker LL, Rajaratne AA, Blair-West JR, Harris PJ. The effects of angiotensin II on blood perfusion in the rat renal papilla. J Physiol. 1999;519:273–278. doi: 10.1111/j.1469-7793.1999.0273o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters SB, D’Auria M, Martin SS, Nguyen C, Kozma LM, Luskey KL. The amino terminus of insulin-responsive aminopeptidase causes Glut4 translocation in 3T3-L1 adipocytes. J Biol Chem. 1997;272:23323–23327. doi: 10.1074/jbc.272.37.23323. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Ono T, Nolley D. Effects of angiotensin II on central neurons. Pharm Biochem Behav. 1973;1:679–691. doi: 10.1016/0091-3057(73)90032-4. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Armstrong DL, Polan-Curtain JL, Denny JB. Ethanol and diazepam inhibition of hippocampal LTP is mediated by angiotensin II and AT1 receptors. Peptides. 1993;14:441–444. doi: 10.1016/0196-9781(93)90129-5. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Polan-Curtain JL, Armstrong DL. Dose and time dependency of angiotensin II inhibition of hippocampal long-term potentiation. Peptides. 1995;16:1079–1082. doi: 10.1016/0196-9781(95)00089-3. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Armstrong DL, Phelix CF, Wright JW, Harding JW. Angiotensin IV enhances LTP in rat dentate gyrus in vivo. Peptides. 2001;22:1403–1414. doi: 10.1016/s0196-9781(01)00475-2. [DOI] [PubMed] [Google Scholar]
- Weiss MI, Moe KE, Epstein AN. Interference with central action of angiotensin II suppresses sodium appetite. Am J Physiol. 1986;250:R250–R259. doi: 10.1152/ajpregu.1986.250.2.R250. [DOI] [PubMed] [Google Scholar]
- Whitebread S, Mele M, Kamber B, deGasparo M. Preliminary biochemical characterization of two angiotensin II receptor subtypes. Biochem Biophys Res Comm. 1989;163:284–288. doi: 10.1016/0006-291x(89)92133-5. [DOI] [PubMed] [Google Scholar]
- Wilk S, Healy DP. Glutamyl aminopeptidase (aminopeptidase A), the BP-1/6C3 antigen. Adv Neuroimmunol. 1993;3:195–207. [Google Scholar]
- Wilson WL, Roques BP, Llorens-Cortes C, Speth RC, Harding JW, Wright JW. Roles of brain angiotensins II and III in thirst and sodium appetite. Brain Res. 2005;1060:108–117. doi: 10.1016/j.brainres.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Wong PC, Price WA, Chiu AT, Duncia JV, Carini DJ, Wexler RR, Johnson AL, Timmermans PB. Non-peptide angiotensin II receptor antagonists. IX. Antihypertensive activity in rats of DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther. 1990;252:726–732. [PubMed] [Google Scholar]
- Wright JW, Harding JW. Regulatory role of brain angiotensins in the control of physiological and behavioral responses. Brain Res Rev. 1992;17:227–262. doi: 10.1016/0165-0173(92)90018-h. [DOI] [PubMed] [Google Scholar]
- Wright JW, Harding JW. Brain angiotensin receptor subtypes in the control of physiological and behavioral responses. Neurosci Biobehav Rev. 1994;18:21–53. doi: 10.1016/0149-7634(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Wright JW, Harding JW. Important roles for angiotensin III and IV in the brain renin–angiotensin system. Brain Res Rev. 1997;25:96–124. doi: 10.1016/s0165-0173(97)00019-2. [DOI] [PubMed] [Google Scholar]
- Wright JW, Harding JW. The brain angiotensin system and extra-cellular matrix molecules in neural plasticity, learning, and memory. Prog Neurobiol. 2004;72:263–293. doi: 10.1016/j.pneurobio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Wright JW, Morseth SL, Abhold RH, Harding JW. Pressor action and dipsogenicity induced by angiotensin II and III in rats. Am J Physiol. 1985;249:R514–R521. doi: 10.1152/ajpregu.1985.249.5.R514. [DOI] [PubMed] [Google Scholar]
- Wright JW, Jensen LL, Roberts KA, Sardinia MF, Harding JW. Structure–function analyses of brain angiotensin control of pressor action in rats. Am J Physiol. 1989;257:R1551–R1557. doi: 10.1152/ajpregu.1989.257.6.R1551. [DOI] [PubMed] [Google Scholar]
- Wright JW, Miller-Wing AV, Shaffer MJ, Higginson C, Wright DE, Hanesworth JM, Harding JW. Angiotensin II(3–8) [ANG IV] hippocampal binding: potential role in the facilitation of memory. Brain Res Bull. 1993;32:497–502. doi: 10.1016/0361-9230(93)90297-o. [DOI] [PubMed] [Google Scholar]
- Wright JW, Krebs LT, Stobb JW, Harding JW. The angiotensin IV system: functional implications. Front Neuroendocrinol. 1995;16:23–52. doi: 10.1006/frne.1995.1002. [DOI] [PubMed] [Google Scholar]
- Wright JW, Stubley L, Pederson ES, Kramar EA, Hanesworth JM, Harding JW. Contributions of the brain angiotensin IV-AT4 receptor subtype system to spatial learning. J Neurosci. 1999;19:3952–3961. doi: 10.1523/JNEUROSCI.19-10-03952.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JW, Kramar EA, Meighan SE, Harding JW. Extracellular matrix molecules, long-term potentiation, memory consolidation and the brain angiotensin system. Peptides. 2002a;23:221–246. doi: 10.1016/s0196-9781(01)00599-x. [DOI] [PubMed] [Google Scholar]
- Wright JW, Tamura-Myers E, Wilson WL, Roques BP, Llorens-Cortes C, Speth RC, Harding JW. Conversion of brain angiotensin II to angiotensin III is critical for pressor response in rats. Am J Physiol Regul Integr Comp Physiol. 2002b;284:R725–R733. doi: 10.1152/ajpregu.00326.2002. [DOI] [PubMed] [Google Scholar]
- Yang G, Wan Y, Zhu Y. Angiotensin II—an important stress hormone. Biol Signals. 1996;5:1–8. doi: 10.1159/000109168. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Hanesworth JM, Sardinia M, Alt JA, Wright JW, Harding JW. Structural analysis of angiotensin IV receptor (AT4) from selected bovine tissues. J Pharmacol Exp Ther. 1999;289:1075–1083. [PubMed] [Google Scholar]
- Zhu B, Herbert J. Central antagonism of atrial natriuretic peptides on behavioral and hormonal responses to angiotensin II: mapping with c-Fos. Brain Res. 1996;734:55–60. [PubMed] [Google Scholar]
- Zini S, Fournie-Zaluski MC, Chauvel E, Roques BP, Corvol P, Llorens-Cortes C. Identification of metabolic pathways of brain angiotensin II and III using specific aminopeptidase inhibitors: predominant role of angiotensin III in the control of vasopressin release. Proc Natl Acad Sci USA. 1996;93:11968–11973. doi: 10.1073/pnas.93.21.11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubenko GS, Nixon RA. Mood elevating effect of captopril in depressed patients. Am J Psychiatry. 1984;141:110–111. doi: 10.1176/ajp.141.1.110. [DOI] [PubMed] [Google Scholar]


