Abstract
Intestinal fluid secretion is driven by apical membrane, cystic fibrosis transmembrane conductance regulator (CFTR)-mediated efflux of Cl– that is concentrated in cells by basolateral Na+−K+−2Cl– cotransporters (NKCC1). An absolute requirement for Cl– efflux is the parallel activation of K+ channels which maintain a membrane potential that sustains apical anion secretion. Both cAMP and Ca2+ are intracellular signals for intestinal Cl– secretion. The K+ channel involved in cAMP-dependent secretion has been identified as the KCNQ1–KCNE3 complex, but the identity of the K+ channel driving Ca2+-activated Cl– secretion is controversial. We have now used a Kcnn4 null mouse to show that the intermediate conductance IK1 K+ channel is necessary and sufficient to support Ca2+-dependent Cl– secretion in large and small intestine. Ussing chambers were used to monitor transepithelial potential, resistance and equivalent short-circuit current in colon and jejunum from control and Kcnn4 null mice. Na+, K+ and water content of stools was also measured. Distal colon and small intestinal epithelia from Kcnn4 null mice had normal cAMP-dependent Cl– secretory responses. In contrast, they completely lacked Cl– secretion in response to Ca2+-mobilizing agonists. Ca2+-activated electrogenic K+ secretion was increased in colon epithelium of mice deficient in the IK1 channel. Na+ and water content of stools was diminished in IK1-null animals. The use of Kcnn4 null mice has allowed us to demonstrate that IK1 K+ channels are solely responsible for driving intestinal Ca2+-activated Cl– secretion. The absence of this channel leads to a marked reduction in water content in the stools, probably as a consequence of decreased electrolyte and water secretion.
Fluid secretion in the small and large intestine is a highly regulated process driven by electrogenic, active transport of Cl− ions (throughout this paper, we refer to a current consistent with anion secretion as Cl− secretion. We cannot discard the idea that a portion of this current is carried by HCO3− (see Discussion section)) across the epithelium (Barrett & Keely, 2000; Kunzelmann & Mall, 2002). The importance of this regulation is highlighted by the consequences of its disruption in pathophysiological states such as secretory diarrhoea and cystic fibrosis. Secretion is dependent upon the activation of apical CFTR Cl− channels. Such activation allows passive efflux of Cl− into the lumen of the intestine from those cells where it is found accumulated above electrochemical equilibrium. Intracellular accumulation is generated by uphill Cl− entry at the basolateral membrane via the Na+−K+−2Cl− cotransporter NKCC1. The ion gradients which drive Cl− influx through NKCC1 are maintained by the Na+−K+ pump, also present at the basolateral membrane. Basolateral membrane K+ channels are central in the anion secretion process, as they maintain a membrane potential favourable to continuous Cl− efflux and serve to recycle K+ taken up by NKCC1 and the Na+−K+ pump. Secretion of Cl− in intestinal epithelium is stimulated by various secretagogues, which differ in the intracellular mediator involved in their signalling. The primary intracellular mediators of intestinal Cl− secretion are cAMP (or cGMP) and intracellular Ca2+. In mammalian colon, K+ secretion occurs in addition to Cl− secretion, making an important contribution to K+ homeostasis (Kunzelmann & Mall, 2002).
Ca2+-dependent anion secretion in distal colon and in small intestinal epithelia requires the simultaneous activity of apical CFTR Cl− channels, activated by cAMP, and basolateral K+ channels activated by Ca2+ (Mall et al. 1998). Life-threatening rotaviral-induced secretory diarrhoea is known to be due to alterations in Ca2+ homeostasis (Ramig, 2004). On the other hand, if the magnitude of Ca2+-dependent Cl− secretion is sufficiently high to have an impact on the hydration of intestinal contents, it might determine the severity of the intestinal disease in cystic fibrosis (Bronsveld et al. 2001). The identification of the K+ channel involved in Ca2+-dependent intestinal Cl− secretion could provide a useful pharmacological target to modulate this type of secretion.
Three classes of Ca2+-activated K+ channels have been distinguished from their single channel conductance: the small-, intermediate- and large-conductance channels SK, IK1 and BK, respectively. SK channels are encoded by three genes, Kcnn1–3; IK1 and BK channels are the products of the Kcnn4 and Slo (Kcnma1) genes, respectively (Vergara et al. 1998). Ca2+-activated K+ channels of all three types have been identified in small intestine and colon (Hay-Schmidt et al. 2003; Joiner et al. 2003; Chen et al. 2004) and could participate in Ca2+-dependent anion secretion. There is evidence that IK1, encoded by the Kcnn4 gene, is the K+ channel involved in Ca2+-dependent intestinal Cl− secretion (Warth et al. 1999), but this has been strongly challenged in recent work (Halm et al. 2006). These investigations used mainly pharmacological tools to identify the conductance in question, thus alternative approaches are required to address this discrepancy.
In this report we tested the hypothesis that the intermediate conductance IK1 (Kcnn4) channel is important in Ca2+-dependent Cl− secretion in the small and large intestine, by investigating the effect of inactivating the Kcnn4 gene in the mouse. Our results strongly suggest that IK1 is necessary and sufficient for Ca2+-dependent Cl− secretion. Besides this, the genetic ablation of IK1 induces changes in the handling of Na+ and water leading to a marked increase in stool dehydration. Some of these results have been published previously in abstract form (Flores et al. 2005).
Methods
Animals
Male mice (in C57Bl/6J background) aged 2–5 months were used. They were bred at CECS mouse facility in Valdivia. The Kcnn4 null animal generation and their genotyping have been previously described (Begenisich et al. 2004).
Tissue isolation and Ussing chamber experiments
Animals were killed by cervical dislocation, a procedure performed according to international regulations for animal care and with the approval of the local bioethics committee. A segment of distal colon or jejunum was excised, rinsed with warm 0.9% NaCl, and cut open lengthwise through the mesenteric border. For colon, a partially stripped mucosal sheet was obtained by scraping the mucosa surface with a glass microscope slide (Catalán et al. 2004). The sheet obtained was mounted on a tissue-holding slider (aperture, 0.1 cm2) and put as a dividing membrane in a modified Ussing chamber (Physiologic Instruments Inc., San Diego, CA, USA). The transepithelial potential difference referred to the serosal compartment was measured continuously using an EVC4000 amplifier (World Precision Instruments, Sarasota, FL, USA). The current was clamped at zero and at 20 s intervals 1 s pulses to 10–20 μA were given. The voltage pulses were generated with pCLAMP 6 software (Axon Instruments, Union City, CA, USA) through a Labmaster interface, which also served to acquire the voltage. The difference in current and voltage was used to calculate the tissue resistance and equivalent short-circuit currents (Iscs) according to Ohm's law (Warth et al. 1999). A correction for the resistance of the fluid measured in the absence of tissue (60–64 Ω) was applied. The ΔIsc values were calculated as follows: amiloride-sensitive as Isc before minus Isc after drug addition; cAMP-induced as Isc after minus Isc before addition of forskolin/IBMX; chromanol 293B as Isc before minus Isc after addition of the drug in the continued presence of forskolin/IBMX, minus cAMP-induced; carbachol-induced as Isc after minus Isc before addition of the agonist in the continued presence of forskolin/IBMX and chromanol 293B. The bath solution was continuously gassed with CO2 5% and O2 95% and contained the following (mm): 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2 and 10 d-glucose. When using jejunum, d-glucose was replaced by d-mannitol in the apical bath solution, to avoid Na+-coupled glucose currents. Addition of drugs was by injection of small volumes from concentrated stocks in dimethylsulphoxide (DMSO). Addition of solvent only was without effect. Wash out of drugs was done by two washes of the appropriate compartment by complete fresh solution replacement. Most reagents were obtained from Sigma Chemical Company (USA). Mepyramine and chromanol 293B were purchased from Tocris Bioscience (USA).
Immunohistochemistry
Colonic tissue was removed and washed with warm saline before being fixed for 24 h at 4°C, and then for 24 h at room temperature, in PBS containing 2% picric acid and 2% paraformaldehyde. Immunohistochemistry was performed as previously described (Peña-Münzenmayer et al. 2005). Briefly, paraffin sections (4 μm thick) were incubated with anti-mouse BK antibody (APC-021, Alomone, Israel) diluted at 1: 50, 1: 100 or 1: 200 in PBS with 0.5% BSA, pH 7.4 at 22°C overnight. Bound antibodies were detected with the aid of the biotin–streptavidin–peroxidase technique. After peroxidase was developed, with diaminobenzidine and hydrogen peroxide, sections were counterstained with Harris haematoxylin.
RT-PCR reactions
Cells were isolated as previously described (Catalán et al. 2004). Total RNA from surface cells and crypts were isolated using Trizol reagent (Invitrogen) and then RT-PCR was performed. Three micrograms of total RNA were reverse transcribed with the SuperScript II system (Invitrogen), using the oligo (dT) primer and random hexamer primers. PCR amplification primers for KCNN1, KCNN2, KCNN3, KCNN4, KCNMA1, KCNMB1 and KCNMB4 are given in Supplementary Table 1. The reaction mixture contained aliquots of cDNA, 0.2 μm of each primer, 2.5 units Taq DNA polymerase (Promega), 2 mm dNTPs, and 1.5 mm MgCl2 in a total volume of 25 μl. Conditions were: initial denaturation at 95°C for 2 min, 30 cycles at 95°C for 30 s, annealing at 58°C for 30 s and extension at 74°C for 30 s, and final extension at 74°C lasting 5 min. cDNA samples from tissues with known expression of each of the transcripts were used as positive controls.
Water and electrolytes in stools
Freshly defecated faeces were collected, weighed immediately (WW) and then dried for 48 h, weighed again (DW), resuspended on 1 ml of deionized water and heated to 65°C for 30–60 min. Suspension were centrifuged at 12,000 g for 5 min and Na+ and K+ assays were performed on the supernatant using a Jenway PFP7 flame photometer (Essex, UK) under manufacturer's instructions. Calculation of water content was done as (WW − DW)/WW.
Data handling
Results are generally given as mean ±s.e.m. and the significance of differences tested by t test.
Results
Cl– secretion induced by the muscarinic agonist carbachol in large and small intestine shows an absolute dependence upon K+ channel IK1
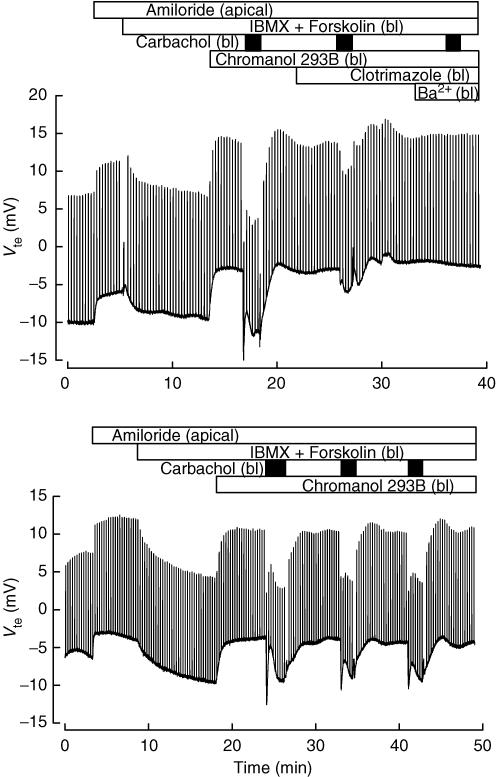
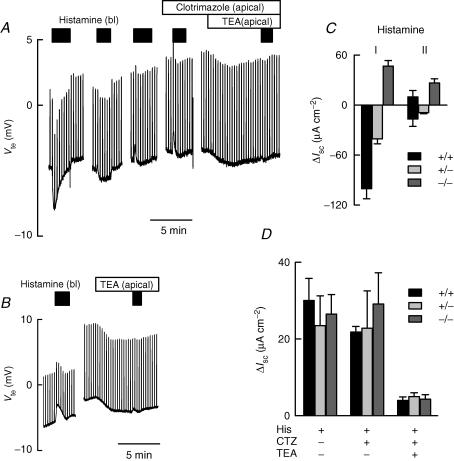
Muscarinic activation leads to Ca2+-dependent Cl− secretion in colonic epithelium from various species (Strabel & Diener, 1995; Mall et al. 1998; Warth et al. 1999; Carew & Thorn, 2000; Halm et al. 2006). Here we show the response to carbachol in the colon from our control animals. The upper panel of Fig. 1 shows a recording of transepithelial potential (Vte). A lumen-negative Vte corresponds to anion secretion or cation absorption. Apical amiloride (10 μm) was added to block Na+ current through the epithelial sodium channels (ENaC). Increasing intracellular cAMP by serosal addition of forskolin (1 μm) and isobutylmethylxanthine (IBMX, 100 μm) induced anion secretion, as revealed by the negative deflection in Vte. This secretion was abolished by serosal chromanol 293B (10 μm), a blocker of KCNQ1–KCNE3 K+ channels. Although chromanol 293B has been reported to inhibit recombinant CFTR expressed in Xenopus oocytes (Bachmann et al. 2001) and a mucosal effect of 293B, albeit with a threefold lower affinity, has been reported for rabbit colon mucosa (Lohrmann et al. 1995), in isolated rat colonic crypts 293B blocks K+ conductance but has no effect on cAMP-evoked depolarization induced by the opening of apical (CFTR) Cl− channels (Diener et al. 1996). In our hands mucosal chromanol 293B had no effect on cAMP-induced anion secretion in mouse colon (not shown), suggesting a lack of effect upon CFTR in our experimental protocol. Addition of serosal carbachol in the presence of 293B induced a robust anion secretion response. The effect of carbachol was reduced > 70% by the IK1 K+ channel inhibitor clotrimazole, and abolished in the combined presence of Ba2+ and clotrimazole in the serosal side. The partial effect of clotrimazole could be due to the rather low concentration used here to avoid undesired non-specific effects. Clotrimazole had no effect on cAMP-induced anion secretion (not shown). Separate control experiments, an example of which is shown in the lower panel of Fig. 1, demonstrated that the effect of successive additions of carbachol was comparable over time without any evidence for rundown. Carbachol did not always evoke anion secretion without pre-treatment with IBMX and forskolin (not shown). In tissues treated with indomethacin, to prevent spontaneous increase in cAMP via prostaglandin E2 release (Carew & Thorn, 2000), there was no Cl− secretory response at all (see Fig. 6, further described below). This suggests a lack of Ca2+-dependent apical chloride channel (CaCC) activity under the conditions of the experiment.
Figure 1. Effect of secretagogues on wild-type mouse colon mucosa.
The traces show continuous recordings of transepithelial potential (Vte) as function of time obtained in Ussing chamber experiments of mouse distal colon. The voltage deflections are caused by current pulse injection. Addition of 100 μm isobutylmethylxanthine (IBMX) plus 1 μm forskolin in both experiments, induces a negative change in Vte that was fully reversed by 10 μm chromanol 293B. Addition of 100 μm carbachol (black boxes) yielded fast negative changes in Vte that were maintained during repeated application (lower panel). Carbachol effect was almost completely blocked by the Kcnn4 inhibitor clotrimazole (CTZ, 3 μm) and totally blocked by further addition of 5 mm serosal Ba2+ (upper panel). In both experiments apical 10 μm amiloride was used to inhibit ENaC-mediated sodium currents. Apical and bl indicate additions to the mucosal and serosal compartment, respectively. Tissue resistances were, respectively, 86 and 83 Ω cm2 at the beginning and end of the experiment shown in the upper panel. The respective numbers for the lower panel experiment were 78 and 75 Ω cm2.
Figure 6. Carbachol-induced K+ secretion on mice colon.
After pre-incubation with indometacin (2 μm), colonic strips were challenged with carbachol. The inhibitory effect of indometacin on the cAMP-induced anionic secretion unmasked K+ secretion that was enhanced in the IK1 KO tissue (B) compared with that of the WT animal (A). The K+ secretion was not inhibited by clotrimazole but partially blocked by apical Ba2+ in both animals. C shows ΔIsc calculated for each condition. Values are means ±s.e.m., n = 4 (+/+) and n = 6 (−/−). *Significantly different from +/+ control; **significantly different from −/− control (t test).
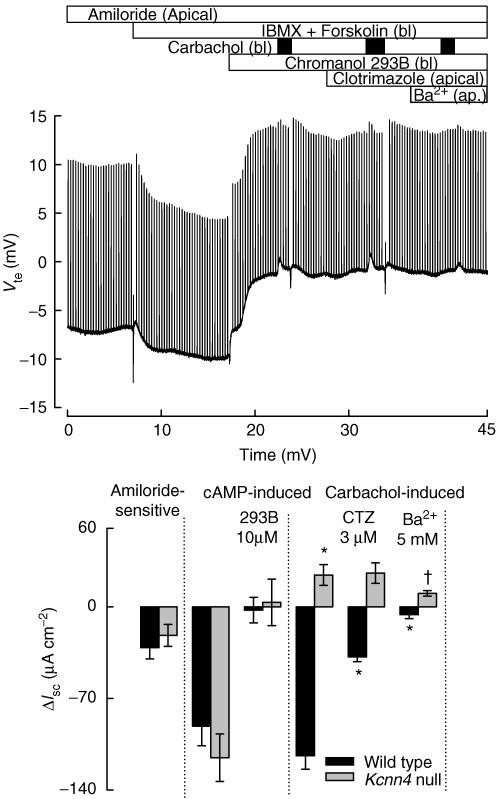
The upper panel in Fig. 2 shows a similar experiment to that in the upper panel of Fig. 1, but done on tissue from a Kcnn4 null mouse. After apical addition of amiloride to block ENaC, increasing cAMP induced the hyperpolarization expected during activation of cAMP-dependent Cl− secretion. As in wild-type (WT) colon, the effect was abolished by 293B. In the presence of 293B, addition of carbachol to the serosal side of the epithelium did not evoke the hyperpolarizing response observed in the WT epithelium. A rather small depolarizing response was induced by addition of the agonist. The IK1 channel inhibitor clotrimazole had no effect, but 5 mm apical Ba2+ (or 10 mm tetraethylammonium (TEA), not shown) abolished the modest depolarizing effect of carbachol in Kcnn4 null mice.
Figure 2. Effect of secretagogues on colon mucosa from Kcnn4 null mice.
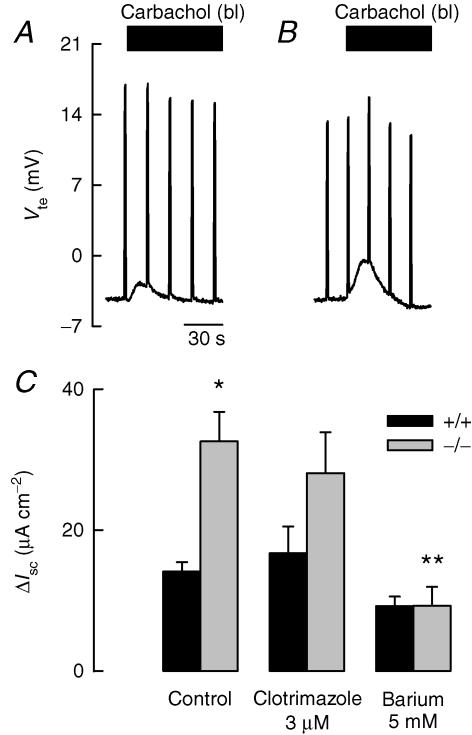
All details for the addition of agonists and blockers are as in Fig. 1. When colon strips of Kcnn4 null mice were challenged with 100 μm serosal carbachol there were positive instead of negative changes in Vte that were fully blocked by mucosal Ba2+ but not by clotrimazole. Tissue resistance at the beginning and end of the experiment were 89 and 76 Ω cm2. The lower panel summarizes differences in equivalent short-circuit currents (ΔIsc) obtained under different conditions with wild-type and Kcnn4 null mice colonic tissue. A P < 0.0005 level of significance for the difference with the effect of carbachol on WT tissue is indicated by *. † indicates P < 0.05 compared with carbachol effect on Kcnn4 null. Data are means ±s.e.m. of 5 and 4 separate experiments for WT and and KO, respectively.
The average change in equivalent short-circuit current (ΔIsc) across colon epithelium under the different treatments is summarized in the lower panel of Fig. 2. Both the Na+ absorption through ENaC channels and the 293B-inhibitable cAMP-dependent Cl− secretory currents were similar in colon from WT and IK1 knockout (KO) mice. The large anion secretory response seen in WT colon contrasted with the small, but significant, cation secretory current in the Kcnn4 null epithelium. Clotrimazole significantly inhibited carbachol-dependent anion secretion of WT epithelium but had no effect on the apparent cation secretion evoked in the colon from KO animals.
A further series of experiments was conducted to ascertain whether similar Ca2+-dependent anion secretion could be evoked in the small intestine. In Fig. 3A and B experiments with jejunal epithelium are illustrated. These follow a similar protocol as for colonic epithelium. Treatment with IBMX and forskolin evoked a hyperpolarizing response in jejunal epithelia from both control (A) and Kcnn4 null (B) animals. This response was abolished by the 293B K+ channel blocker in both types of tissue. The response to serosal carbachol addition in control jejunum was a transient hyperpolarization, which was greatly diminished by clotrimazole and abolished in the combined presence of serosal clotrimazole and Ba2+. Carbachol was without effect in jejunum from Kcnn4 null mouse. Figure 3C summarizes the calculated ΔIsc values for the different treatments in jejunal tissue from WT and KO animals.
Figure 3. Effect of secretagogues on jejunum epithelia from WT and Kcnn4 null mice.
All details for the addition of agonists and blockers are as in Fig. 1. When jejunum of WT mice (A) was challenged with 100 μm serosal carbachol, there were negative changes in Vte that were partially blocked by 3 μm clotrimazole and fully blocked by additional 5 mm Ba2+. B shows results for a jejunum from Kcnn4 null mouse, where carbachol addition did not produce the negative changes related with anionic secretion. C, summary of ΔIsc values for a series of experiments performed on jejunum epithelium from wild-type and Kcnn4 null mice. Data are means ±s.e.m. of 5 individual experiments for each animal. A P < 0.0005 level of significance for the difference with the effect of carbachol on WT tissue is indicated by *. None of the effects of carbachol on Kcnn4 KO tissue was significantly different from zero.
Na+-coupled d-glucose- and l-phenylalanine-stimulated currents are not affected in the jejunum of IK1 KO mice
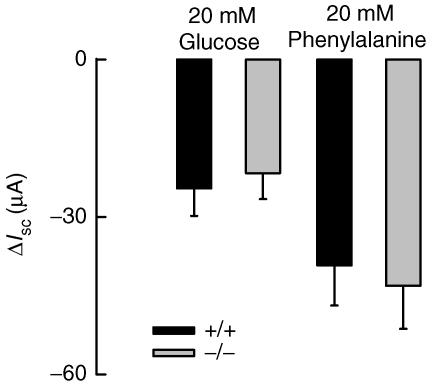
Na+-coupled absorption of sugars and amino acids in the small intestine is electrogenic and requires an active basolateral K+ conductance to avoid driving force collapse (Schultz, 1981). We tested therefore whether IK1 might contribute to this phenomenon by comparing the electrical effects of transported metabolites between WT and IK1 KO small intestine. Glucose and phenylalanine were increased from 0 to 20 mm in the apical side of jejunum strips placed in Ussing chambers and Vte monitored. Both metabolites induced negative changes on Vte consistent with Na+-coupled absorption. Calculation of the average change in equivalent short-circuit current in response to l-phenylalanine or d-glucose showed no significant differences between WT and KO tissues (Fig. 4). These results suggest that IK1 is not a key factor in the Na+-coupled absorption of sugars and amino acids in the small intestine.
Figure 4. Sodium-coupled sugar and amino acid transport on jejunum.
Jejunum strips from WT and IK1 KO mice, respectively, were challenged with 20 mm d-glucose or with 20 mml–phenylalanine. Tissue was previously incubated with 2 μm indomethacin to avoid cAMP-induced currents. ΔIsc values obtained with the indicated metabolite are shown. There were no differences between tissues. Data are means ±s.e.m. of 4 individual experiments each for WT and IK1 KO animals.
Histamine-evoked Cl– and K+ secretion in colonic epithelium
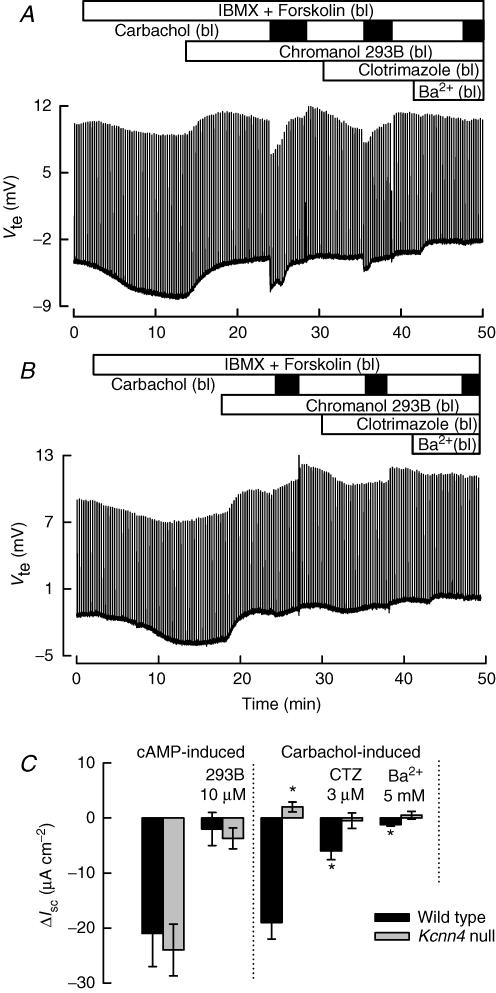
Histamine increases intracellular Ca2+ in intestinal crypts (Lindqvist et al. 2002) and induces Cl− secretion in rabbit (McCabe & Smith, 1984), human (Bronsveld et al. 2001) and rat (Schultheiss et al. 2006) large intestine. As expected, after activation of secretion by increasing cAMP and blockade of basolateral KCNQ1–KCNE3 with chromanol 293B, WT colon exhibited a robust, transient Cl− secretion (Fig. 5A), but only a cation secretory response in colon from IK1 KO animals (Fig. 5B). It is interesting to note that the Cl− secretory effect of histamine promptly desensitized, having disappeared at a second challenge, and becoming a cation secretory current from a third addition onwards. This effect is summarized by plotting the average responses to the first and second histamine challenges in Fig. 5C, which also reports results from experiments done with tissues from heterozygous animals (+/−). Notice that a second addition of histamine evoked either a small cation secretory response or a small anion secretion in WT colon. These two types of response are shown separately in Fig. 5C (black columns in histamine addition II). The idea that the cation secretory current corresponds to transient K+ secretion is supported by its blockade by apical TEA, but not clotrimazole (Fig. 5A). A similar result was obtained with the KO epithelia, where apical TEA largely eliminated the K+ secretory current (Fig. 5B). A summary for the results of the third to the fifth histamine application is shown in Fig. 5D.
Figure 5. Histamine effect on colonic ion secretion.
Tissues were initially treated with 100 μm IBMX plus 1 μm forskolin in all experiments, followed by serosal addition of 10 μm chromanol 293B as in Fig. 1. Serosal addition of histamine (150 μm) induced a transient Cl− current in the wild-type tissue. Further pulses of histamine on the same colonic preparation did not evoke a negative change in Vte. At a third pulse of histamine, a 10 mm TEA-sensitive K+ secretion is unmasked (A). Serosal histamine addition always evoked positive Vte change in the Kcnn4 null colon, which was also abolished by mucosal TEA (B). In C, a summary of evoked ΔIsc values in response to the first 2 histamine challenges on wild-type (+/+), heterozygous (+/−) and homozygous Kncc4 null (−/−) mice. Values are means ±s.e.m., n = 9 (+/+), n = 4 (+/−) and n = 7 (−/−). D shows ΔIsc for histamine-induced K+ secretion in tissues of the three different genotypes. The responses were measured successively from the third histamine application onwards, when the signal became stable. Values are means ±s.e.m., n = 6 (+/+), n = 3 (+/−) and n = 4 (−/−).
IK1 is not involved in K+ secretion in colonic epithelium stimulated by carbachol
Experiments in colon from Kcnn4 null mice suggested the presence of cation secretion (Fig. 2). To test whether this might correspond to Ca2+-dependent K+ secretion, experiments were carried out with tissues previously incubated with indomethacin (2 μm), to avoid basal release of prostaglandin and consequent cAMP increase. Carbachol evoked only cation secretory current in colon from WT (Fig. 6A) and KO (Fig. 6B) animals under these conditions. In both types of tissue the effect could be partially inhibited (Fig. 6C) by mucosal Ba2+ but not clotrimazole (added to both the mucosal and the serosal side), consistent with K+ secretion. These results suggest that Ca2+-dependent K+ secretion in the colon does not involve the IK1 channel. Moreover, K+ secretion appeared to be increased in the KO tissue, further supporting the idea that IK1 is not involved in this process. RT-PCR on isolated epithelial cells from the colon of both animals showed expression of various Ca2+-activated K+ channels. We confirmed the presence in both WT and IK-1 KO tissues of message for KCNN1 (SK1), KCNN3 (SK3) and KCNMA1 (BK, Slo, Maxi-K) and its β-subunits KCNMB1 and KCNMB4 (Fig. 7). KCNN4 was found in WT tissues only, as expected. KCNN2 transcript was not detected in either type of tissue (not shown).
Figure 7. Ca2+-activated K+ channel expression in colon tissue from WT and Kcnn4 null (KO) mice.
Agarose gel electrophoresis of RT-PCR products is shown for the indicated transcripts. Predicted product sizes were KCNN1 (241 bp), KCNN3 (275 bp), KCNN4 (957 bp), KCNMA1 (392 and 218 bp), KCNMB1 (246 bp) and KCNMB4 (285 bp). RT–, negative control omitting reverse transcriptase. C+ is a positive control from parotid gland cDNA, except for KCNN1 and KCNN3 which were done on brain cDNA. Arrows point to different number of base pairs as indicated on 100 or 1000 bp ladders.
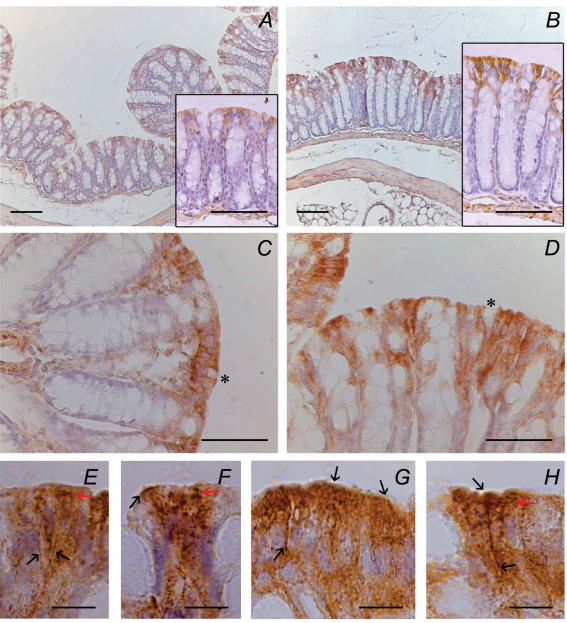
Immunohistochemical studies demonstrated that BK immunoreactivity was present in colonic epithelial tissue of both WT (Fig. 8A) and IK1 KO animals (Fig. 8B). BK-positive cells were encountered deeper into the crypts of KO colon (Fig. 8D) than in WT tissue (Fig. 8C), where the reaction was restricted to surface cells. This observation could explain the augmented K+ secretion in KO tissue when stimulated by carbachol. With the purpose of obtaining a stronger signal, anti-BK dilution was decreased to 1: 50 for WT (Fig. 8C), but kept at 1: 200 for the tissues from KO animals (Fig. 8D). A similar staining intensity was obtained despite the use of different antibody dilutions, which might suggest a higher expression level in tissues from KO animals. We failed to confirm this, however, in Western blots of protein extracts from total epithelium (not shown). Notice that the reaction was not restricted to any membrane domain, being present at apical (Fig. 8F, G and H) and basolateral (Fig. 8E, G and H) locations, in addition to strong cytoplasm staining.
Figure 8. Immunohistochemical localization of BK channels within mice colonic epithelium.
A and B, BK immunoreactivity using 1: 200 antibody dilution in WT (A) and IK1 KO (B) colon; insets show details of representative areas of each sample. C and D are higher magnification of WT and IK1 KO colon using 1: 50 and 1: 200 dilution, respectively. E–H, surface colonic epithelium displaying basolateral and apical immunoreactivity (black arrows); red arrows point out granular immunostaining concentrated in the upper portion of colonocytes. Asterisks mark absence of staining in goblet cells. Biotin–streptavidin–peroxidase-DAB technique. The images are representative of experiments with 3 different mice for each the WT and IK1 KO groups. Scale bars: A and B, 100 μm; C and D, 50 μm; E–H, 10 μm.
Kcnn4 null mice present decreased Na+ and water contents in the faeces
Animals were given free access to food and water. Analyses of serum demonstrated that Na+ and K+ concentrations did not differ between WT and IK1 KO animals (Table 1). In contrast, Na+ and K+ content was diminished in stools obtained from IK1 KO animals compared with WT. This phenomenon was paralleled by a marked decrease in water content in the faeces of IK1 KO animals, which meant that there was no significant change in cation concentration in stools water (Table 1).
Table 1.
Water and cation content of stools and serum electrolytes
| Wild-type | Kcnn4 null | ||
|---|---|---|---|
| Serum electrolytes | Na+ (mEq l−1) | 127 ± 12 (3) | 132 ± 6 (3) |
| K+ (mEq l−1) | 4.2 ± 0.4 (3) | 4 ± 0.2 (3) | |
| Faeces electrolytes | Na+ (mEq (g dry wt)−1) | 122 ± 14 (7) | 73 ± 7 (6)* |
| K+ (mEq (g dry wt)−1) | 87 ± 10 (7) | 58 ± 8 (6)* | |
| Na+ (mEq l−1) | 90 ± 10 (7) | 84 ± 6 (5) | |
| K+ (mEq l−1 l) | 58 ± 6 (7) | 67 ± 7 (6) | |
| Water content of stools (%) | 60 ± 3 (7) | 46 ± 2 (6)* |
Na+ and K+ concentrations in serum and faecal water in WT and IK1 KO mice. Na+ and K+ are also given as contents in dried stools. Data are means ±s.e.m. of the number of animals indicated in brackets.
indicates significant (P < 0.05) difference from WT figures.
Discussion
Cl− and fluid secretion in the intestinal epithelium depends crucially upon the activation of a basolateral membrane K+ conductance which both maintains a hyperpolarized membrane potential favourable to Cl− exit despite activation of CFTR, and recycles K+ taken up during Cl− accumulation. The K+ channel that supports cAMP-activated Cl− secretion is a complex formed by KCNQ1 and KCNE3 (Schroeder et al. 2000). Recent work with a KO mouse lacking KCNQ1 has confirmed this view (Vallon et al. 2005). Similarly, work with rat and rabbit colon suggests that, of the different Ca2+-activated K+ channels present in this epithelium, IK1 is a strong candidate to underlie the basolateral K+ conductance which supports Ca2+-dependent Cl− secretion (Warth et al. 1999). In addition there is immunocytochemical evidence for the presence of IK1 in the basolateral membrane of intestinal crypt epithelium (Furness et al. 2003; Joiner et al. 2003; Halm et al. 2006). There is, however, no agreement about the function of this channel as recent results obtained using guinea-pig colon suggest that the activity of basolateral IK1 is not necessary to support Ca2+-dependent Cl− secretion. These experiments relied mainly upon the use of pharmacological agents to dissect the different conductance pathways. It is clear, however, that these compounds can have non-specific effects that will obscure the interpretation of results. This makes it necessary to employ alternative approaches to help in understanding these complex epithelial transport mechanisms. We have now used an IK1 KO mouse to explore the role of this channel in intestinal Cl− secretion. Our data demonstrate that Ca2+-dependent Cl− secretion in mouse colon and small intestine is absolutely dependent upon the presence of the IK1 intermediate conductance, Ca2+-dependent K+ channel encoded by the Kcnn4 gene. Data presented here confirm and extend our results, communicated previously in abstract form, demonstrating lack of carbachol-induced anion secretion in IK1 KO mice (Flores et al. 2005). A very recent report also reaches a similar conclusion (Matos et al. 2007). The authors demonstrate the absence of carbachol-induced anion secretion in the colon of a different Kcnn4 null mouse, confirming our result in a different genetic background (129SV/C57Bl6 F2 generation and 129SV inbred). It is interesting to note that, by comparison with the mice used here (C57Bl/6J inbred) the response to carbachol is monophasic, lacking the fast component we describe here and that is also seen in rat and rabbit colon mucosa (Warth et al. 1999) and in mouse (strain not given) tissue (Carew & Thorn, 2000).
We attribute the anion secretory response observed in the present work to Cl− electrogenic flow. We cannot discard the idea, however, that part of this negative current in response to secretagogues is carried by HCO3−. Experiments with mouse colon have revealed that around 70% of total secretory current in the presence of HCO3− can be inhibited by blockade of basolateral NKCC1. In the absence of HCO3−, NKCC1 blockade achieved nearly full inhibition (Cuthbert et al. 1999). This could be interpreted to imply the presence of a HCO3− component to the anion secretory current. However, as HCO3−−Cl− exchange across the basolateral membrane has been shown to contribute to Cl− accumulation across this membrane (Grubb et al. 2000), an indirect effect of HCO3− removal on Cl− current could be taking place. Given the fact that CFTR has a low HCO3− permeability under physiological extracellular Cl− concentration (Shcheynikov et al. 2004), we believe that under our experimental conditions most anion secretory current will correspond to Cl− secretion.
The IK1-deficient mouse presents anomalies in osmotic responses in its T-lymphocytes and red blood cells, as expected from the proposed role of IK1 in these cells. Rather surprisingly, however, Kcnn4 KO mice have no apparent defect in Ca2+-dependent parotid gland fluid secretion nor cell volume regulation (Begenisich et al. 2004). Instead, the large conductance BK K+ channel, as demonstrated in a study using BK KO mice, is critical for the regulatory volume decrease of acinar cells and plays an important role in the sodium uptake and potassium secretion process in the ducts of these fluid-secreting salivary glands (Romanenko et al. 2006). In another report, KO animals for IK1 presented increased blood pressure due to impaired vascular dilatation induced by the endothelial-derived hyperpolarizing factor (Si et al. 2006). Our present examination of colonic and small intestinal epithelium shows normal Cl− secretory responses to increasing intracellular cAMP in the IK1 KO mice. These responses are probably supported by the KCNQ1–KCNE3 K+ channel (Vallon et al. 2005), as they were blocked by chromanol 293B, an inhibitor of this type of channel. The Cl− secretory response elicited by either the Ca2+ agonist carbachol or histamine in WT colon and small intestine was completely absent from the IK1-deficient animals, suggesting that this K+ channel is essential for Ca2+-dependent Cl− secretion. Despite the fact that several other Ca2+-activated K+ channels are known to be present in intestinal epithelium, none of them is capable of substituting for the basolateral IK1 channel. This might point to a failure to activate these channels at the intracellular Ca2+ concentrations reached or to their localization in different membrane domains or cell populations (Joiner et al. 2003). Indeed, the large conductance BK channel was found on the apical membrane where it supports K+ secretion (Sausbier et al. 2006). Our localization experiments found it to be on apical and basolateral locations of only surface cells. We hypothesize that in this cellular location BK cannot substitute for IK1 as the basolateral Ca2+-activated K+ channel needed for Ca2+-dependent Cl− secretion, which probably occurs in the crypts.
Ca2+-dependent intestinal Cl− secretion requires the simultaneous activation of CFTR by cAMP (Clarke et al. 1994; Strabel & Diener, 1995; Mall et al. 1998) and, as shown here, of IK1 Ca2+-dependent K+ channels. No CaCC channels appear to be expressed in adult mouse intestine to allow a CFTR-independent Cl− secretion. This appears at variance with what has been observed recently in rat distal colon, where fast transient muscarinic activation of an apical Cl− conductance takes place (Schultheiss et al. 2003, 2005). Nevertheless, in the IK1-null mouse intestine, or when CFTR activation is avoided by treating WT tissue with indomethacin, no Cl− secretion can be elicited by carbachol or histamine. What could be the physiological role of Ca2+-activated IK1 channels in the intestinal epithelium? They could provide an additional way to increase Cl− secretion which is rate-limited by the basolateral K+ conductance (Warth et al. 1999). The observation that the heterozygous IK1 colon has only about 40% of the histamine (Fig. 5C) or carbachol (not shown) Cl− secretory response of the WT, supports the view that the number or activity of basolateral K+ channels is rate-limiting to apical Cl− exit. IK1 channels apparently support CFTR-mediated secretion in the absence of cAMP-activated basolateral K+ conductance, providing an explanation for the lack of intestinal phenotype in KCNQ1 KO mice (Lee et al. 2000; Casimiro et al. 2001). This contrasts with the generalized intestinal obstruction observed in CFTR KO mice which severely limits their survival (Snouwaert et al. 1992; Ratcliff et al. 1993). The cAMP-induced Cl− secretion in jejunum from KCNQ1 KO mice is only reduced by about 50% (Vallon et al. 2005), suggesting that additional basolateral K+ channels must be present.
The physiological relevance of functional IK1 channels is underlined by the finding that its genetic ablation causes increased stool dehydration accompanied by decreased faecal Na+ and K+ content although not in concentration in faecal water. This might be caused by an increased absorption or a decreased secretion of water and electrolytes. Neither small intestinal Na+-coupled glucose and amino acid absorption nor ENaC-mediated colonic absorptive Na+ current are affected in the IK1 KO animals. Given the role of IK1 in Ca2+-dependent Cl− secretion in small and large intestine demonstrated here, we favour the hypothesis of a decreased secretion of water and electrolytes in Kcnn4 null animals. This would also be consistent with the decrease in faecal water content occurring without change in electrolyte concentration, suggestive of a deficit in an isotonic fluid secretion. The site of secretion for electrolytes and water has long been hypothesized to be in the crypt epithelium (Welsh et al. 1982). There is also evidence, however, that hypertonic water absorption occurs in distal colon crypts which dehydrate faeces from around 80% water content in the distal small intestine and proximal colon, to 60% water content in distal colon (Naftalin et al. 1999;.Thiagarajah et al. 2001). If the enhanced dehydration of faeces observed in the IK1 KO mouse is due to decreased secretion in the small intestine, this would provide drier initial luminal contents for the colon. On the other hand, it could occur in the distal colon, a tissue whose secretory capacity is affected by inactivation of Kcnn4, and which is also believed to be the sole segment capable of effectively dehydrating intestinal contents (Naftalin et al. 1999). In this case, we would conclude that distal colon crypts both absorb and secrete, thus providing fine control for faeces dehydration in distal colon.
In the IK1 KO colon, or when CFTR activation is avoided by treating the tissue with indomethacin, only cation secretion can be elicited by carbachol or histamine. This corresponds to Ca2+-activated electrogenic K+ secretion as it can be inhibited by K+ channel blockers added at the mucosal side of the epithelium (Binder & Sandle, 1994; Warth & Barhanin, 2003). Ca2+-dependent K+ secretion elicited by ATP in the colon depends absolutely upon the activity of apical membrane large conductance BK channels (Sausbier et al. 2006), and thus it was not expected to be affected in the IK1 KO mice as observed here. Surprisingly, it appears that IK1 ablation leads to an increased electrogenic K+ secretion in colon which correlates with a wider distribution of BK channels in the epithelium. A negative regulation of BK channels by IK1 has been demonstrated both in parotid glands and in a heterologous expression system (Thompson & Begenisich, 2006) and a direct interaction between the channels was proposed to take place to mediate this effect. In addition to its expression in crypt cells, IK1 has been reported at a similar location as BK in surface colonic epithelium (Joiner et al. 2003; Furness et al. 2003; Halm et al. 2006). An interaction between the channels at this location might possibly account for an increased BK activity in the absence of IK1. The mechanism for the effect of IK1 ablation on BK expression in colon remains to be elucidated.
Histamine can also promote Ca2+-dependent Cl− secretion in WT colon. Unlike what is seen with carbachol, the histamine effect on Cl− secretion rapidly desensitizes so that further challenges with the agonist lead to K+ secretion only. The fact that desensitization of the Cl−, but not the K+ secretory response to histamine, takes place suggests that these two processes occur in different cell populations within the epithelium. Cl− secretion occurs mainly in crypt cells (Welsh et al. 1982; Kunzelmann & Mall, 2002). Ca2+-dependent K+ secretion has an absolute dependence upon large conductance BK channels, which mediate K+ exit across apical epithelial membranes (Sausbier et al. 2006). Our present immunocytochemical localization study gives a mainly surface epithelial location for BK in mouse colon, supporting the idea that Ca2+-dependent K+ and Cl− secretion do indeed occur in different cell populations. This contrasts with a mainly crypt location also reported for mouse colon (Sausbier et al. 2006). This discrepancy might be due to the different genetic background of those animals (hybrid 129SV/C57Bl6, F2 generation) compared with ours (C57Bl/6J inbred). Other work shows BK channels localized to the apical membrane of surface cells of rabbit colon (Hay-Schmidt et al. 2003) and to the cytosol of surface and upper crypt cells in human colon (Mathialahan et al. 2005). Functional evidence indicates a surface, rather than crypt, cell location for aldosterone-regulated K+ secretion in rat distal colon (Grotjohann et al. 1998), whilst single channel recordings have given an apical membrane, surface cell location in rat (Butterfield et al. 1997) and human (Sandle et al. 2007) colon. Given the exceptionally high single channel conductance of BK, and the presence of β-subunits that very strongly modify its Ca2+ and voltage dependence (Orio et al. 2002), the presence of low numbers of BK channels in surface cells might suffice to account for electrogenic K+ secretion in colon. An alternative to the proposal that K+ and Cl− secretion are harboured in different cell populations would be that they are responsive to separate histamine receptors with differing desensitization properties. There is indeed evidence that histamine-induced K+ and Cl− secretion are controlled by different receptors in rat colon (Schultheiss et al. 2006). However, the specific H1 receptor antagonist mepyramine (Hill, 1990; Stack et al. 1995) abolished both K+ and Cl− secretion response in mouse colon (Supplementary Fig. 1). This would suggest that both these responses to histamine in mouse colon are mediated by H1 receptors. Differences in desensitization properties of histamine-dependent K+ and Cl− secretion could be due to differences in signalling compartmentalization associated with different membrane domains or a different cellular distribution of K+ and Cl− secretory functions.
In conclusion, inactivation of the Kcnn4 gene demonstrates that IK1 channels are responsible for the Ca2+-activated K+ conductance involved in Ca2+-dependent Cl− secretion of mouse intestinal epithelium, a process that appears to contribute to the hydration of faeces. Electrogenic K+ colonic secretion activated by the Ca2+-mobilizing agonists carbachol and histamine, is present in tissue from Kcnn4 KO mice. This observation suggests that IK1 is not part of this latter process. The key finding that IK1 is the only Ca2+-activated K+ channel driving Cl− secretion, suggests a unique role for IK1 channels in pathological states where Ca2+-dependent Cl− secretion is enhanced. This might arise in conditions such as rotaviral-induced diarrhoea where Ca2+ homeostasis in intestinal epithelium is known to be affected (Ramig, 2004). IK1 could serve as a useful target for new drugs for the treatment and/or prevention of virus-induced diarrhoea.
Acknowledgments
This work was supported by Fondecyt grant 1061069 and NIH grants DE09692 and DE08921. C.E.C.S. is supported by an Institute grant from the Millennium Science initiative and by Empresas CMPC. C.A.F. was supported by Conicyt.
Supplemental material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.134387/DC1
and
http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.
References
- Bachmann A, Quast U, Russ U. Chromanol 293B, a blocker of the slow delayed rectifier K+ current (IKs), inhibits the CFTR Cl− current. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:590–596. doi: 10.1007/s002100100410. [DOI] [PubMed] [Google Scholar]
- Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535–572. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- Begenisich T, Nakamoto T, Ovitt CE, Nehrke K, Brugnara C, Alper SL, Melvin JE. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J Biol Chem. 2004;279:47681–47687. doi: 10.1074/jbc.M409627200. [DOI] [PubMed] [Google Scholar]
- Binder HJ, Sandle GI. Electrolyte transport in the mammalian colon. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 2133–2171. [Google Scholar]
- Bronsveld I, Mekus F, Bijman J, Ballmann M, de Jonge HR, Laabs U, Halley DJ, Ellemunter H, Mastella G, Thomas S, Veeze HJ, Tummler B. Chloride conductance and genetic background modulate the cystic fibrosis phenotype of ΔF508 homozygous twins and siblings. J Clin Invest. 2001;108:1705–1715. doi: 10.1172/JCI12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield I, Warhurst G, Jones MN, Sandle GI. Characterization of apical potassium channels induced in rat distal colon during potassium adaptation. J Physiol. 1997;501:537–547. doi: 10.1111/j.1469-7793.1997.537bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew MA, Thorn P. Carbachol-stimulated chloride secretion in mouse colon: evidence of a role for autocrine prostaglandin E2 release. Exp Physiol. 2000;85:67–72. [PubMed] [Google Scholar]
- Casimiro MC, Knollmann BC, Ebert SN, Vary JC, Jr, Greene AE, Franz MR, Grinberg A, Huang SP, Pfeifer K. Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange–Nielsen Syndrome. Proc Natl Acad Sci U S A. 2001;98:2526–2531. doi: 10.1073/pnas.041398998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalán M, Niemeyer MI, Cid LP, Sepúlveda FV. Basolateral ClC-2 chloride channels in surface colon epithelium: regulation by a direct effect of intracellular chloride. Gastroenterology. 2004;126:1104–1114. doi: 10.1053/j.gastro.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Chen MX, Gorman SA, Benson B, Singh K, Hieble JP, Michel MC, Tate SN, Trezise DJ. Small and intermediate conductance Ca2+-activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:602–615. doi: 10.1007/s00210-004-0934-5. [DOI] [PubMed] [Google Scholar]
- Clarke LL, Grubb BR, Yankaskas JR, Cotton CU, McKenzie A, Boucher RC. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in Cftr (−/−) mice. Proc Natl Acad Sci U S A. 1994;91:479–483. doi: 10.1073/pnas.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert AW, Hickman ME, MacVinish LJ. Formal analysis of electrogenic sodium, potassium, chloride and bicarbonate transport in mouse colon epithelium. Br J Pharmacol. 1999;126:358–364. doi: 10.1038/sj.bjp.0702290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener M, Hug F, Strabel D, Scharrer E. Cyclic AMP-dependent regulation of K+ transport in the rat distal colon. Br J Pharmacol. 1996;118:1477–1487. doi: 10.1111/j.1476-5381.1996.tb15563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CA, Melvin JE, Sepúlveda FV. Cl− secretion induced by Ca2+ agonists is impaired in distal colon of a Kcnn4 null mouse. J Physiol. 2005;565P:C7. [Google Scholar]
- Furness JB, Robbins HL, Selmer IS, Hunne B, Chen MX, Hicks GA, Moore S, Neylon CB. Expression of intermediate conductance potassium channel immunoreactivity in neurons and epithelial cells of the rat gastrointestinal tract. Cell Tissue Res. 2003;314:179–189. doi: 10.1007/s00441-003-0808-z. [DOI] [PubMed] [Google Scholar]
- Grotjohann I, Gitter AH, Kockerling A, Bertog M, Schulzke JD, Fromm M. Localization of cAMP- and aldosterone-induced K+ secretion in rat distal colon by conductance scanning. J Physiol. 1998;507:561–570. doi: 10.1111/j.1469-7793.1998.561bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb BR, Lee E, Pace AJ, Koller BH, Boucher RC. Intestinal ion transport in NKCC1-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2000;279:G707–G718. doi: 10.1152/ajpgi.2000.279.4.G707. [DOI] [PubMed] [Google Scholar]
- Halm ST, Liao T, Halm DR. Distinct K+ conductive pathways are required for Cl− and K+ secretion across distal colonic epithelium. Am J Physiol Cell Physiol. 2006;291:C636–C648. doi: 10.1152/ajpcell.00557.2005. [DOI] [PubMed] [Google Scholar]
- Hay-Schmidt A, Grunnet M, Abrahamse SL, Knaus HG, Klaerke DA. Localization of Ca2+-activated big-conductance K+ channels in rabbit distal colon. Pflugers Arch. 2003;446:61–68. doi: 10.1007/s00424-002-0983-x. [DOI] [PubMed] [Google Scholar]
- Hill SJ. Distribution, properties, and functional characteristics of three classes of histamine receptor. Pharmacol Rev. 1990;42:45–83. [PubMed] [Google Scholar]
- Joiner WJ, Basavappa S, Vidyasagar S, Nehrke K, Krishnan S, Binder HJ, Boulpaep EL, Rajendran VM. Active K+ secretion through multiple KCa-type channels and regulation by IKCa channels in rat proximal colon. Am J Physiol Gastrointest Liver Physiol. 2003;285:G185–G196. doi: 10.1152/ajpgi.00337.2002. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- Lee MP, Ravenel JD, Hu RJ, Lustig LR, Tomaselli G, Berger RD, et al. Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. J Clin Invest. 2000;106:1447–1455. doi: 10.1172/JCI10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist S, Hernon J, Sharp P, Johns N, Addison S, Watson M, et al. The colon-selective spasmolytic otilonium bromide inhibits muscarinic M3 receptor-coupled calcium signals in isolated human colonic crypts. Br J Pharmacol. 2002;137:1134–1142. doi: 10.1038/sj.bjp.0704942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohrmann E, Burhoff I, Nitschke RB, Lang HJ, Mania D, Englert HC, et al. A new class of inhibitors of cAMP-mediated Cl− secretion in rabbit colon, acting by the reduction of cAMP-activated K+ conductance. Pflugers Arch. 1995;429:517–530. doi: 10.1007/BF00704157. [DOI] [PubMed] [Google Scholar]
- McCabe RD, Smith PL. Effects of histamine and histamine receptor antagonists on ion transport in rabbit descending colon. Am J Physiol Gastrointest Liver Physiol. 1984;247:G411–G418. doi: 10.1152/ajpgi.1984.247.4.G411. [DOI] [PubMed] [Google Scholar]
- Mall M, Bleich M, Schurlein M, Kuhr J, Seydewitz HH, Brandis M, Greger R, Kunzelmann K. Cholinergic ion secretion in human colon requires coactivation by cAMP. Am J Physiol Gastrointest Liver Physiol. 1998;275:G1274–G1281. doi: 10.1152/ajpgi.1998.275.6.G1274. [DOI] [PubMed] [Google Scholar]
- Mathialahan T, Maclennan KA, Sandle LN, Verbeke C, Sandle GI. Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol. 2005;206:46–51. doi: 10.1002/path.1750. [DOI] [PubMed] [Google Scholar]
- Matos JE, Sausbier M, Beranek G, Sausbier U, Ruth P, Leipziger J. Role of cholinergic-activated KCa1.1 (BK), KCa3.1 (SK4) and KV7.1 (KCNQ1) channels in mouse colonic Cl− secretion. Acta Physiol (Oxf) 2007;189:251–258. doi: 10.1111/j.1748-1716.2006.01646.x. [DOI] [PubMed] [Google Scholar]
- Naftalin RJ, Zammit PS, Pedley KC. Regional differences in rat large intestinal crypt function in relation to dehydrating capacity in vivo. J Physiol. 1999;514:201–210. doi: 10.1111/j.1469-7793.1999.201af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel β-subunits. News Physiol Sci. 2002;17:156–161. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- Peña-Münzenmayer G, Catalán M, Cornejo I, Figueroa CD, Melvin JE, Niemeyer MI, Cid LP, Sepúlveda FV. Basolateral localization of native ClC-2 chloride channels in absorptive intestinal epithelial cells and basolateral sorting encoded by a CBS-2 domain di-leucine motif. J Cell Sci. 2005;118:4243–4252. doi: 10.1242/jcs.02525. [DOI] [PubMed] [Google Scholar]
- Ramig RF. Pathogenesis of intestinal and systemic rotavirus infection. J Virol. 2004;78:10213–10220. doi: 10.1128/JVI.78.19.10213-10220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Evans MJ, Cuthbert AW, MacVinish LJ, Foster D, Anderson JR, Colledge WH. Production of a severe cystic fibrosis mutation in mice. Nat Genet. 1993;4:35–41. doi: 10.1038/ng0593-35. [DOI] [PubMed] [Google Scholar]
- Romanenko V, Nakamoto T, Srivastava A, Melvin JE, Begenisich T. Molecular identification and physiological roles of parotid acinar cell maxi-K channels. J Biol Chem. 2006;281:27964–27972. doi: 10.1074/jbc.M603871200. [DOI] [PubMed] [Google Scholar]
- Sandle G, Perry M, Mathialahan T, Linley J, Robinson P, Hunter M, Maclennan K. Altered cryptal expression of luminal potassium (BK) channels in ulcerative colitis. J Pathol. 2007;212:66–73. doi: 10.1002/path.2159. [DOI] [PubMed] [Google Scholar]
- Sausbier M, Matos JE, Sausbier U, Beranek G, Arntz C, Neuhuber W, Ruth P, Leipziger J. Distal colonic K+ secretion occurs via BK channels. J Am Soc Nephrol. 2006;17:1275–1282. doi: 10.1681/ASN.2005101111. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch TJ. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403:196–199. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- Schultheiss G, Hennig B, Schunack W, Prinz G, Diener M. Histamine-induced ion secretion across rat distal colon: involvement of histamine H1 and H2 receptors. Eur J Pharmacol. 2006;546:161–170. doi: 10.1016/j.ejphar.2006.07.047. [DOI] [PubMed] [Google Scholar]
- Schultheiss G, Ribeiro R, Schafer KH, Diener M. Activation of apical K+ conductances by muscarinic receptor stimulation in rat distal colon: fast and slow components. J Membr Biol. 2003;195:183–196. doi: 10.1007/s00232-003-0618-y. [DOI] [PubMed] [Google Scholar]
- Schultheiss G, Siefjediers A, Diener M. Muscarinic receptor stimulation activates a Ca2+-dependent Cl− conductance in rat distal colon. J Membr Biol. 2005;204:117–127. doi: 10.1007/s00232-005-0757-4. [DOI] [PubMed] [Google Scholar]
- Schultz SG. Homocellular regulatory mechanisms in sodium-transporting epithelia: avoidance of extinction by ‘flush-through’. Am J Physiol. 1981;83:497–512. doi: 10.1152/ajprenal.1981.241.6.F579. [DOI] [PubMed] [Google Scholar]
- Shcheynikov N, Kim KH, Kim KM, Dorwart MR, Ko SB, Goto H, Naruse S, Thomas PJ, Muallem S. Dynamic control of cystic fibrosis transmembrane conductance regulator Cl−/HCO3− selectivity by external Cl−. J Biol Chem. 2004;279:21857–21865. doi: 10.1074/jbc.M313323200. [DOI] [PubMed] [Google Scholar]
- Si H, Heyken WT, Wolfle SE, Tysiac M, Schubert R, Grgic I, et al. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res. 2006;99:537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- Snouwaert JB, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- Stack WA, Keely SJ, O'Donoghue DP, Baird AW. Immune regulation of human colonic electrolyte transport in vitro. Gut. 1995;36:395–400. doi: 10.1136/gut.36.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strabel D, Diener M. Evidence against direct activation of chloride secretion by carbachol in the rat distal colon. Eur J Pharmacol. 1995;274:181–191. doi: 10.1016/0014-2999(94)00728-p. [DOI] [PubMed] [Google Scholar]
- Thiagarajah JR, Jayaraman S, Naftalin RJ, Verkman AS. In vivo fluorescence measurement of Na+ concentration in the pericryptal space of mouse descending colon. Am J Physiol Cell Physiol. 2001;281:C1898–C1903. doi: 10.1152/ajpcell.2001.281.6.C1898. [DOI] [PubMed] [Google Scholar]
- Thompson J, Begenisich T. Membrane-delimited inhibition of maxi-K channel activity by the intermediate conductance Ca2+-activated K channel. J Gen Physiol. 2006;127:159–169. doi: 10.1085/jgp.200509457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Grahammer F, Volkl H, Sandu CD, Richter K, Rexhepaj R, et al. KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc Natl Acad Sci U S A. 2005;102:17864–17869. doi: 10.1073/pnas.0505860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- Warth R, Barhanin J. Function of K+ channels in the intestinal epithelium. J Membr Biol. 2003;193:67–78. doi: 10.1007/s00232-002-2001-9. [DOI] [PubMed] [Google Scholar]
- Warth R, Hamm K, Bleich M, Kunzelmann K, von Hahn T, Schreiber R, et al. Molecular and functional characterization of the small Ca2+-regulated K+ channel (rSK4) of colonic crypts. Pflugers Arch. 1999;438:437–444. doi: 10.1007/s004249900059. [DOI] [PubMed] [Google Scholar]
- Welsh MJ, Smith PL, Fromm M, Frizzell RA. Crypts are the site of intestinal fluid and electrolyte secretion. Science. 1982;218:1219–1221. doi: 10.1126/science.6293054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.