Abstract
We have previously shown that direct vagus nerve stimulation (VNS) reduces the slope of action potential duration (APD) restitution while simultaneously protecting the heart against induction of ventricular fibrillation (VF) in the absence of any sympathetic activity or tone. In the current study we have examined the role of nitric oxide (NO) in the effect of VNS. Monophasic action potentials were recorded from a left ventricular epicardial site on innervated, isolated rabbit hearts (n = 7). Standard restitution, effective refractory period (ERP) and VF threshold (VFT) were measured at baseline and during VNS in the presence of the NO synthase inhibitor NG-nitro-l-arginine (l-NA, 200 μm) and during reversing NO blockade with l-arginine (l-Arg, 1 mm). Data represent the mean ± s.e.m. The restitution curve was shifted upwards and became less steep with VNS when compared to baseline. l-NA blocked the effect of VNS whereas l-Arg restored the effect of VNS. The maximum slope of restitution was reduced from 1.17 ± 0.14 to 0.60 ± 0.09 (50 ± 5%, P < 0.0001) during control, from 0.98 ± 0.14 to 0.93 ± 0.12 (2 ± 10%, P = NS) in the presence of l-NA and from 1.16 ± 0.17 to 0.50 ± 0.10 (41 ± 9%, P = 0.003) with l-Arg plus l-NA. ERP was increased by VNS in control from 119 ± 6 ms to 130 ± 6 ms (10 ± 5%, P = 0.045) and this increase was not affected by l-NA (120 ± 4 to 133 ± 4 ms, 11 ± 3%, P = 0.0019) or l-Arg with l-NA (114 ± 4 to 123 ± 4 ms, 8 ± 2%, P = 0.006). VFT was increased from 3.0 ± 0.3 to 5.8 ± 0.5 mA (98 ± 12%, P = 0.0017) in control, 3.4 ± 0.4 to 3.8 ± 0.5 mA (13 ± 12%, P = 0.6) during perfusion with l-NA and 2.5 ± 0.4 to 6.0 ± 0.7 mA (175 ± 50%, P = 0.0017) during perfusion with l-Arg plus l-NA. Direct VNS increased VFT and flattened the slope of APD restitution curve in this isolated rabbit heart preparation with intact autonomic nerves. These effects were blocked using l-NA and reversed by replenishing the substrate for NO production with l-Arg. This is the first study to demonstrate that NO plays an important role in the anti-fibrillatory effect of VNS on the rabbit ventricle, possibly via effects on APD restitution.
Clinical studies have shown that abnormal autonomic states with impaired heart rate variability and baroreflex sensitivity – both measures of vagal activity – are strong prognostic factors in patients with heart failure (Nolan et al. 1998) and with previous myocardial infarctions (La Rovere et al. 1998). There is strong evidence that the relationship between impaired cardiac autonomic control and mortality is the result of an increased susceptibility to lethal ventricular arrhythmias (Schwartz, 1998). Historical work by Einbrodt (1859) with the ‘inductorium’ (induction coil) suggested that stimulation of the vagus nerve may reduce the inducibility of ventricular fibrillation (VF). The mechanisms underlying both the vagal protective effect in VF and the propensity towards VF during reduced vagal activity are not understood. Nitric oxide (NO) has been shown to mediate central and peripheral vagal activity (Chowdhary & Townend, 1999). There is evidence that NO enhances the bradycardic effects of vagal activity on the sinus node (Paterson, 2001) and the slowing of atrioventricular conduction (Conlon & Kidd, 1999), and that it may have anti-arrhythmic actions at the ventricular level in the heart (Kumar et al. 2003). The possibility that NO may be involved in mediating the vagal protective effect in the left ventricle, especially with respect to initiation of VF has never been explored.
It is believed that the onset of VF is associated with break up of spiral waves or rotors into multiple wavelets and oscillations of electrical activity (reviewed by Weiss et al. 2002). The ‘restitution hypothesis’ states that oscillations are facilitated when the slope of the action potential duration (APD) restitution curve is greater than 1 (Cao et al. 1999). In addition, there is recent evidence that drugs that reduce the slope of the restitution curve prevent the induction of VF (Garfinkel et al. 2000), thus supporting the notion that electrical restitution may be a key determinant in the initiation of VF, although this view is contentiously debated.
We have recently shown in the innervated isolated heart preparation that vagus nerve stimulation (VNS) in the absence of any background sympathetic activity or tone decreased the slope of the APD restitution curve and increased the threshold for initiation of fibrillation in the left ventricle of the rabbit (Ng et al. 2007). In the current study, we used the same preparation to investigate the role of NO in mediating the effects of direct VNS on ventricular electrophysiology and VF initiation. The NO synthase (NOS) inhibitor NG-nitro-l-arginine (l-NA) was used to study the effects of blocking NO production during VNS on initiation of VF after which l-arginine (l-Arg) was used to replenish substrate for NO production during NOS inhibition. The effects on APD restitution were studied and correlated with the initiation of VF.
Methods
Isolated rabbit heart preparation with intact dual autonomic innervation
The isolated heart preparation with intact autonomic innervation has been previously described (Ng et al. 2001a). In brief, adult male New Zealand White rabbits (2.0 ± 0.1 kg, n = 7) were premedicated with a mixture (i.m.) of Domitor (0.2 mg kg−1, Pfizer, Sandwich, UK), Vetalar (10 mg kg−1, Fort Lodge, Southampton, UK) and Torbugesic (0.05 mg kg−1, Pharmacia, Corby, UK), and anaesthetized with propofol (1 mg kg−1, i.v., Fresenius, Warrington, UK). The depth of anaesthesia was assessed at regular intervals using corneal and pedal reflexes and supplementary doses of propofol (0.1 mg kg−1) were given if necessary. The rabbit was ventilated, after tracheotomy, at 60 breaths min−1 using a small-animal ventilator (Harvard Apparatus Ltd, Ednebridge, Kent, UK) with an O2–air mixture. The vagus nerves were isolated and cut at the neck level and the blood vessels leading to and from the rib cage were ligated and dissected. The rabbit was killed with an overdose of Sagatal (Rhône Mérieux, Harlow, UK; 60 mg, i.v.) together with 500 U heparin i.v. The anterior portion of the rib cage was removed and the descending aorta cannulated. The preparation extending from the neck to thorax was dissected as described before (Ng et al. 2001a). All procedures were undertaken in accordance with the Animals (Scientific Procedures) Act 1986 in the UK and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996).
Langendorff perfusion
The preparation was perfused via the descending aorta in a modified Langendorff perfusion system with Tyrode solution containing (mm): Na+ 138, K+ 4.0, Ca2+ 1.8, Mg2+ 1.0, HCO3− 24.0, H2PO4− 0.4, Cl− 121 and glucose 11. The pH of the solution was maintained at 7.4 by continuously bubbling with 95% O2–5% CO2 and the temperature was maintained at 37°C. A constant perfusion rate of 100 ml min−1 was maintained using a Gilson Minipuls 3 peristaltic pump (Anachem, Luton, UK). A 3F catheter (Portex, Kent, UK) was inserted at the left ventricular apex to drain Thebesian venous effluent. Intraventricular pressure was monitored with a fluid-filled latex balloon connected with a 3F cannula to a pressure transducer (Washington PT 400, Elcomatin Ltd) and inserted into the left ventricle via the left atrium. The volume of the balloon was adjusted to give zero end-diastolic pressure. Perfusion pressure was monitored with a second pressure transducer in series with the aortic cannula.
VNS
The cervical vagus nerves were separated from adjacent tissues and supported on a pair of platinum electrodes, connected to a constant-voltage square-pulse stimulator (SD9, Grass Instruments, Astro-Medical Inc., USA), which allowed stimulation over a range of frequencies (1–20 Hz) and strengths (1–20 V) at 2 ms pulse width. The stimulation strength, at a frequency of 5 Hz, which produced a heart rate 80% of the maximal response, was used throughout the study. A frequency at this stimulus strength that would produce a steady-state heart rate just less than 100 beats min−1 with VNS was chosen to ensure that sinus rhythm was maintained during vagal stimulation (i.e. no atrioventricular block or asystole) (Ng et al. 2001a). The stimulation strength used in this study was 8.2 ± 1.2 V at a frequency of 11.6 ± 2.2 Hz.
In a previous preliminary study, we found that there was no significant difference between right and left VNS on ventricular electrophysiology (Ng et al. 2001b). Data presented in the current study on VNS are with right vagus nerve alone. Tyrode solution was intermittently applied to the vagus nerve to maintain viability throughout the experiment. Viability of VNS was confirmed by the presence of similar magnitude of heart rate responses towards the end of the experiments. Stable nerve stimulation effects were ascertained by the on-line monitoring of maintained heart rate response in between pacing protocols and during the 5 s gap periods during VF/ERP testing (see below).
Cardiac electrical recording and pacing
A custom-made suction electrode was used to record monophasic action potentials (MAPs) from a single site from the epicardial surface of the left ventricular free wall using a DC-coupled high-input impedance differential amplifier. The stability of the MAPs remained constant as indicated by measurements of MAP amplitude and duration (see below) throughout the period of each protocol. A bipolar electrode was inserted into the right ventricular apex for pacing at twice the diastolic threshold with a constant current stimulator using a stimulus pulse width of 2 ms (DS7A, Digitimer, Welwyn Garden City, UK).
Electrical restitution
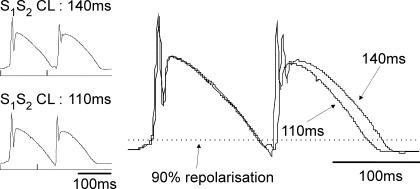
Standard restitution of MAP duration was obtained with right ventricular pacing using a 20 beat drive train (S1, 300 ms CL) followed by an extrastimulus (S2) (Ng et al. 2007). S1–S2 cycle lengths were repeated at progressively shorter intervals, by 10 ms from 300 ms to 200 ms and by 5 ms from 200 ms to ventricular effective refractory period (ERP). ERP was defined as the longest coupling interval that failed to capture the ventricles. Timings of the beginning of the S1 and S2 MAP signals were noted and the delays from the pacing stimuli measured. Timings and MAP durations was measured, using a custom-written program (Francis Burton, Glasgow University, UK) (Ng et al. 2007) from the beginning of the signal to 90% repolarization (MAPD90) with the amplitude of the signal measured from the peak of the dome to the isoelectric line. Restitution was examined by analysis of the relationship between S2 MAPD90 and preceding diastolic intervals (DI = interval between the S1 and S2 MAP signals minus S1 MAPD90; Ng et al. 2007). An exponential curve (MAPD90 = MAPD90max (1–e−DI/τ)) was fitted to the data using Microcal Origin (v6.1, Origin, San Diego, CA, USA) where MAPD90max was the maximum MAPD90 and τ was the time constant. The maximum slope of restitution curve was measured by analysing the first derivative of the fitted curve. An example of a standard restitution protocol where the monophasic action potentials exhibited a steep restitution curve that was greater than 1 is illustrated in Fig. 1, which shows a change in S2 MAPD that is greater than the change in diastolic interval.
Figure 1. Extrastimulus protocol to determine standard restitution.
Left panel shows the last S1 and S2 monophasic action potentials (MAPs) during the standard restitution protocol (see text) at S1–S2 intervals of 140 ms (top trace) and 110 ms (bottom trace). Right panel shows the same traces superposed and aligned at S1 to illustrate the difference in MAP durations with the different S1–S2 intervals.
VF threshold
VF threshold (VFT) was obtained at the end of a 20 beat drive train with an interstimulus cycle length of 300 ms, using a train of 30 stimuli (30 ms interval) spanning the refractory period. Using right ventricular pacing, the pacing current of each train was progressively increased in 0.5 mA steps, until sustained VF was produced. The current that produced this sustained period of VF we defined as the VFT. A 5 s rest period between pacing trains was maintained. A 2 ml bolus of Tyrode solution containing concentrated KCl (50 mm) was given via the side arm of the aortic cannula for conversion back to sinus rhythm. An equilibration period of 15 min was allowed when heart rate, ventricular pressure and ERP returned to baseline values. VFT varied less than 5% with repeated determinations of VFT in unstimulated conditions and subsequent conversions with KCl (data not shown). Between six and 10 determinations of VFT was carried out in each preparation with the experiment lasting no longer than 5 h, without any appreciable variation in VFT in unstimulated conditions.
Experimental protocol 1 – APD restitution, ERP and VFT
After an equilibration period of 30 min, standard restitution and ERP were studied. Heart rate, intraventricular pressure and perfusion pressure were monitored throughout. VFT was then determined using the VF induction protocol. A 2 ml bolus of Tyrode solution containing concentrated KCl (50 mm) was given via the side arm of the aortic cannula for conversion back to sinus rhythm. An equilibration period of 15 min was allowed when heart rate, ventricular pressure and ERP returned to baseline values. VFT varied less than 5% on average with repeated measurements after conversion with KCl (data not shown).
Similar measurements of electrical restitution, ERP and VFT were made during VNS when the new steady-state heart rate was reached during nerve stimulation. After VF induction, nerve stimulation was stopped and the hearts were cardioverted with concentrated KCl bolus.
Experimental protocol 2 – effect on inhibition of NOS
The involvement of NO in mediating the effects of VNS was investigated using the NOS inhibitor l-NA (200 μm, n = 7). The effects of VNS on heart rate, standard electrical restitution, ERP and VFT were measured during steady state after perfusion of l-NA for 10 min.
Experimental protocol 3 – reversing NOS inhibition with l-Arg
While still in the presence of l-NA, the effect of reversing NOS inhibition was studied using l-Arg (1 mm) to provide substrate for NO production. The effects of VNS on heart rate, standard electrical restitution, ERP and VFT were measured after 10 min perfusion with l-NA +l-Arg and compared with control values, as well as with values obtained during perfusion with l-NA alone (see below) and after washout with normal Tyrode solution. These agents mentioned above were perfused in the order described and were not randomized.
Experimental protocol 4 – effects of l-Arg alone
The effect of l-Arg alone was examined separately in five hearts during perfusion with Tyrode solution containing 1 mm l-Arg, and the effects of VNS on the electrical parameters were compared with control values (normal Tyrode solution).
Signal measurements and statistical analysis
All measured signals were recorded with a PowerLab 800/s system (ADInstruments Ltd) and digitized at 1 kHz using Chart software (ADInstruments Ltd), and the data were stored and displayed on a Power Macintosh G3 personal computer (Apple). All data are expressed as mean ± s.e.m. and analysed using (1) Student's t test, paired for comparisons between baseline values (before nerve stimulation) and during VNS and (2) repeated-measures ANOVA for comparing the change in parameters with VNS under the different protocols, using post hoc analysis with Tukey's test if a significant difference was identified. Two-tailed P value < 0.05 was considered significant.
Results
Effect of perfusion with l-NA and l-NA +l-Arg
Haemodynamic data and chronotropic effect of VNS
Perfusion with 200 μm l-NA led to a significant 12.7 ± 2.9% increase in coronary perfusion pressure that was reversed by perfusion of 1 mm l-Arg (Table 1). Left ventricular pressure was unaffected by the perfusion of any agent (Table 1). VNS significantly (P < 0.001) decreased heart rate under all conditions. Although baseline heart rate and the rates achieved during VNS were not significantly different between groups, the decrease in heart rate with VNS during l-NA perfusion was significantly less. Under control conditions, VNS decreased heart rate by 71.1 ± 7.2 beats min−1 but by 57.0 ± 6.9 beats min−1 in the presence of l-NA (P < 0.05). The chronotropic effect of VNS during perfusion with l-NA +l-Arg was significantly greater (64.1 ± 5.6 beats min−1) than with l-NA alone (P < 0.05).
Table 1.
Effect of perfusion with NG-nitro-l-arginine (l-NA) or l-NA plus l-arginine (l-Arg) on aortic perfusion pressure (AoP), left ventricular pressure (LVP), and heart rate (HR) at baseline and during vagus nerve stimulation (VNS)
| Control | l-NA | l-NA +l-Arg | Washout | |
|---|---|---|---|---|
| AoP (mmHg) | 40.1 ± 4.4 | 44.9 ± 4.4* | 41.1 ± 3.4 | 43.1 ± 3.3 |
| LVP (mmHg) | 51.6 ± 4.7 | 51.0 ± 4.4 | 50.4 ± 3.7 | 46.0 ± 3.8 |
| Baseline HR (beats min−1) | 158.7 ± 8.1 | 149.9 ± 6.6 | 149.3 ± 3.2 | 156.3 ± 5.3 |
| VNS HR (beats min−1) | 87.6 ± 2.6† | 92.9 ± 2.7† | 85.2 ± 4.7† | 90.7 ± 4.1† |
P < 0.05 versus control
P < 0.001 versus baseline, ANOVA (n = 7).
The effect of VNS on electrical restitution of MAP duration
We have previously shown that the threshold of ventricular pacing does not change during VNS (Ng et al. 2007). The threshold to capture the ventricles was tested in control, during l-NA perfusion and during perfusion with l-NA +l-Arg and did not significantly change during the experiment (control, 0.18 ± 0.03 mA; l-NA, 0.20 ± 0.05 mA; l-NA +l-Arg, 0.17 ± 0.02 mA).
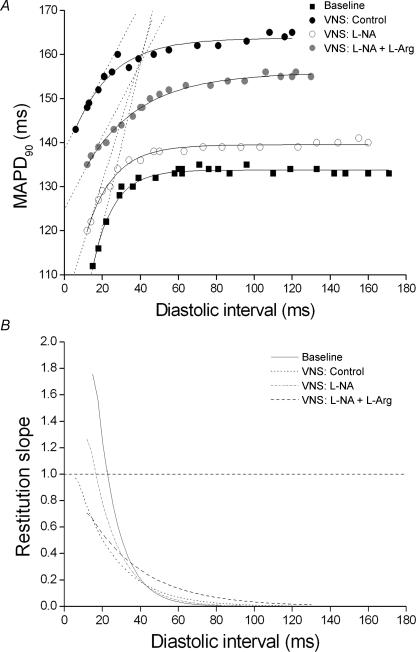
Figure 2A shows results from a typical experiment on the APD restitution curve where VNS increased steady-state MAP duration. The maximum slope of the restitution curve (dashed lines in Fig. 2A) became less steep with VNS when compared to baseline. Perfusion with l-NA reduced the effect of VNS which was restored by perfusion of l-Arg. Figure 2B shows the first derivative of the fitted restitution curves obtained in Fig. 2A. It shows that the maximum slope of restitution was 1.75 at baseline occurring at a diastolic interval of 15.0 ms. The maximum slope of electrical restitution was reduced to 0.96 at a diastolic interval of 6.0 ms during VNS. Perfusion with the NOS inhibitor l-NA reversed the slope of the restitution curve achieved during VNS and increased it back towards baseline (1.26 at a diastolic interval of 12.0 ms). Replenishing the substrate for NO production with l-Arg reversed the inhibition of l-NA and lowered the maximum slope of restitution curve back towards the control level of VNS (slope of 0.70 at a diastolic interval of 12.0 ms).
Figure 2. Standard restitution curve and slope of restitution.
A, plot of standard restitution curves (MAPD90versus preceding diastolic interval (DI)) in a typical experiment at baseline and during vagus nerve stimulation (VNS) under control conditions and VNS during perfusion with NG-nitro-l-arginine (l-NA) and l-NA together with l-arginine (l-NA +l-Arg). The curves were fitted to the exponential curve (MAPD90 = MAPD90max(1 − e−DI/τ); see text for definitions) (continuous lines) and the maximum slopes (dashed lines) during restitution were calculated. B, plot of first derivative of the fitted curves in A to calculate the slope of the restitution curves. A horizontal dashed line is plotted at slope = 1.
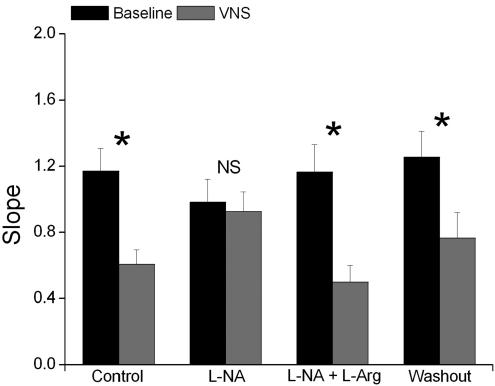
Results from the seven experiments are illustrated in Fig. 3 which shows mean maximum slope of restitution values obtained at baseline (i.e. without VNS) and during VNS in control, during perfusion with l-NA or l-NA +l-Arg and during washout. There was no significant effect on the maximum slope of restitution obtained at baseline throughout the experiment but there was a significant change in the effect obtained on the maximum slope of restitution during VNS. In control, VNS significantly decreased the slope of the restitution curve by 49.5 ± 4.7%, while simultaneously increasing steady-state MAPD90 from 128 ± 5.5 to 145.0 ± 4.9 ms (P < 0.05). Blocking NO production with l-NA completely abolished the reduction of the slope of the restitution curve (change of −1.8 ± 10.1%) and the increase in MAPD90 (138.9 ± 7.2 to 143.5 ± 5.6 ms, P > 0.05) obtained during VNS. The reduction in the slope of restitution curve (−54.9 ± 7.5%) and the increase in MAPD90 (134.5 ± 6.2 to 144.6 ± 7.2 ms, P < 0.05) during VNS was restored when NO substrate was replenished with l-Arg during l-NA inhibition. During washout, the effects of VNS on the slope of restitution curve (a decrease of 41.4 ± 9.4%) and on MAPD90 (143.5 ± 3.9 to 150.6 ± 5.7 ms) were comparable to those obtained during control.
Figure 3. Maximum slope of restitution.
Plot of the maximum slope of the restitution curve at baseline and during vagus nerve stimulation (VNS) during control and during perfusion with NG-nitro-l-arginine (l-NA) and l-NA together with l-arginine (l-NA +l-Arg) and during washout. *P < 0.001, NS P > 0.05 versus baseline (n = 7).
There was no significant effect of VNS on the diastolic interval where the maximum slope of restitution was achieved under control conditions (26.6 ± 8.6 to 27.0 ± 6.9 ms) or during perfusion with l-NA (25.0 ± 9.8 to 21.4 ± 6.8 ms), l-NA +l-Arg (16.5 ± 5.9 to 19.9 ± 5.9 ms) or at washout (17.8 ± 3.3 to 21.3 ± 4.3 ms).
The effect of VNS on ERP and VFT
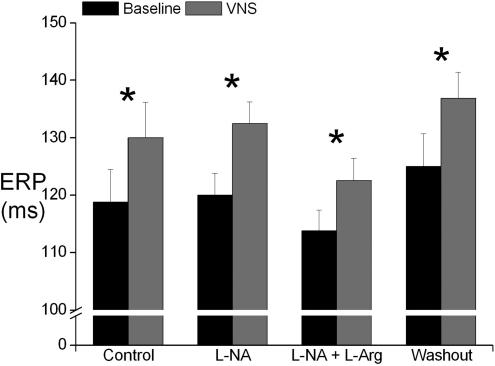
Figure 4 illustrates mean ERP values obtained at baseline (i.e. without VNS) and during VNS in control and during perfusion with l-NA, l-NA +l-Arg and washout. Although baseline ERP was higher during washout, there was no significant change between baseline ERP values or ERP values obtained during VNS throughout the experiment. In control, VNS significantly increased ERP from 118.8 ± 5.7 to 130.0 ± 6.2 ms (10.1 ± 4.6%, P < 0.05). Of more importance, the significant increase in ERP during VNS was not affected by blocking NO production (increase by 10.7 ± 2.5%) or by reversing NO blockade with l-Arg (increase of 7.8 ± 2.0%).
Figure 4. Ventricular effective refractory period.
Plot of ventricular effective refractory period (ERP) at baseline and during vagus nerve stimulation (VNS) during control and during perfusion with NG-nitro-l-arginine (l-NA) and l-NA together with l-arginine (l-NA +l-Arg) and during washout. *P < 0.05 versus baseline (n = 7).
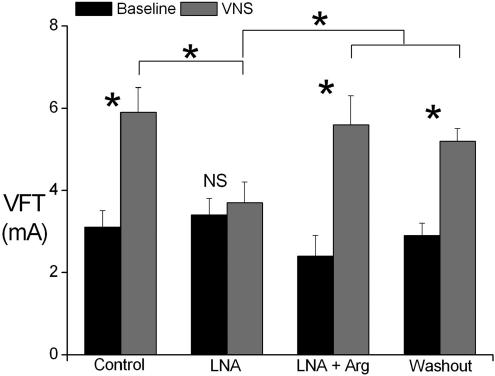
Significant differences, however, are seen in the effect of VNS on VFT (Fig. 5). VFT obtained at baseline was not significantly altered in the presence of l-NA or l-NA +l-Arg. The effect of VNS on the inducibility of VF was, however, affected by perfusion with l-NA. VNS increased VFT by 97.6 ± 11.9% during control and perfusion of l-NA completely abolished the increase in VFT during VNS (change of 13.0 ± 11.5%). In addition, VFT values obtained during VNS in the presence of l-NA were significantly (P < 0.05) lower when compared to the VFT values obtained during VNS under all other conditions. Perfusion with l-Arg while the hearts were perfused with l-NA restored the increase in VFT (175.4 ± 48.7%) seen during VNS.
Figure 5. Ventricular fibrillation threshold.
Plot of ventricular fibrillation threshold (VFT) at baseline and during vagus nerve stimulation (VNS) during control and during perfusion with NG-nitro-l-arginine (l-NA) and l-NA together with l-arginine (l-NA +l-Arg) and during washout. *P < 0.001, NS P > 0.05 versus baseline (n = 7).
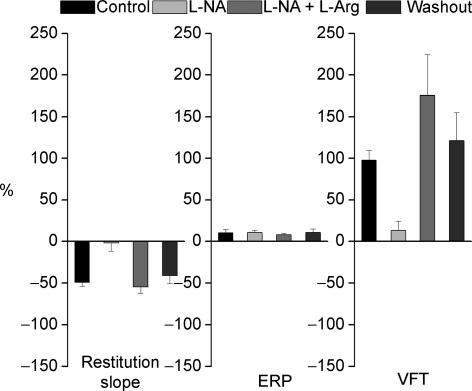
The percentage changes in the effect of VNS on the maximum slope of restitution, ERP and VFT are illustrated in Fig. 6.
Figure 6. Percentage change in electrophysiological parameters.
Plot of percentage change in maximum slope of standard restitution, ventricular effective refractory period (ERP) and ventricular fibrillation threshold (VFT) during vagus nerve stimulation (VNS) during control and during perfusion with NG-nitro-l-arginine (l-NA) and l-NA together with l-arginine (l-NA +l-Arg) and during washout (n = 7).
Control experiments with l-Arg
Aortic perfusion pressure (37.2 ± 4.1 to 40.5 ± 4.5 mmHg) and left ventricular pressure (47.7 ± 4.1 to 47.9 ± 4.6 mmHg) were unaltered by perfusion of l-Arg (n = 5). Mean heart rate at baseline, (control, 157.1 ± 7.3 beats min−1 and with l-Arg, 142.9 ± 4.3 beats min−1), and during VNS (control, 88.7 ± 1.7 beats min−1 and l-Arg, 75.4 ± 6.1 beats min−1) were not significantly different. Baseline values for the slope of the restitution curve (control, 1.168 ± 0.282; l-Arg, 1.411 ± 0.101), for ERP (control, 125.0 ± 11.5 ms; l-Arg, 118.3 ± 9.3 ms) and for VFT (control, 3.1 ± 0.5 mA; l-Arg, 2.8 ± 0.3 mA) were not significantly different in control and during l-Arg perfusion (n = 5). The significant (P < 0.05) effect of VNS on the slope of the restitution curve (control, 0.498 ± 0.194; l-Arg, 0.657 ± 0.120), ERP (control, 145.0 ± 2.9 ms; l-Arg, 145 ± 15.0 ms) and VFT (control, 5.9 ± 0.8 mA; l-Arg, 6.9 ± 1.0 mA) were unaffected by l-Arg perfusion.
Discussion
This is the first study to show that NO is involved in mediating the protective effects of direct VNS, in the absence of any background sympathetic activity or tone, against the induction of VF in the rabbit. The lack of any ongoing sympathetic tone or nerve activity is confirmed by the absence of any change in heart rate in this preparation during β-blockade that had also abolished the effects of strong sympathetic nerve stimulation (Ng et al. 2001a).
We have shown recently that the increased threshold to induce VF during VNS is associated with changes in electrical restitution with flattening of the APD restitution curve (Ng et al. 2007). The current study extends these findings and demonstrates that NO is involved in this mechanism. Thus the effects of the flattening of APD restitution curve and increase in VFT during VNS were blocked in the presence of the non-specific NOS inhibitor, l-NA and reversed by substrate replacement with l-Arg.
Vagus nerve activity, electrical restitution and VF
Vagus nervous system, ventricular arrhythmias and sudden death
There is strong evidence that the relationship between impaired cardiac autonomic control and mortality is the result of an increased susceptibility to lethal ventricular arrhythmias (Schwartz, 1998). It has been known for over 100 years since the work of Einbrodt and his induction coil that vagal stimulation increases the threshold for VF (Einbrodt, 1859). Work in anaesthetized animals confirmed this effect (Zuanetti et al. 1987) but debate existed as to whether it was a direct effect from VNS or whether it prevented the decrease in VFT induced by underlying sympathetic activity (Kolman et al. 1976). We have shown recently in the isolated innervated heart preparation in the absence of any other autonomic influence, that direct sympathetic stimulation increases whereas VNS decreases the susceptibility of the heart to VF (Ng et al. 2007). The benefit of this unanaesthetized and autonomically ‘clean’ preparation is that the confounding effects of haemodynamic reflexes and circulating humoral factors seen in vivo are avoided. Maybe of more importance, the interaction between the two arms of the autonomic nervous system can be strictly manipulated and precisely controlled. Together, these data therefore support the current study which demonstrates an uncontaminated and direct protective effect of VNS in the ventricle in the absence of sympathetic activity or tone.
APD restitution and VF
It is believed that the onset of VF is associated with the break up of spiral waves or rotors into multiple wavelets. This happens especially when the reentrant wavefronts undergo beat-to-beat oscillations. The ‘restitution hypothesis’ states that oscillations are facilitated when the slope of the APD restitution curve (gradient of APD versus previous diastolic interval) is greater than 1 (Gilmour & Chialvo, 1999). There is biological evidence that drugs that reduce the slope of the restitution curve prevent the induction of VF and convert existing VF into a periodic rhythm (Garfinkel et al. 2000). APD restitution is believed to be important in initiation of VF (Weiss et al. 2002), although other mechanisms may also be involved (Franz, 2003). One such mechanism is the effects on calcium cycling causing instability and alternans which some consider to play an important role in APD instability rather than APD restitution itself (Weiss et al. 2006).
The present study confirms the results from our previous study which demonstrated that the slope of the APD restitution curve correlated with VFT, with VNS reducing the slope and increasing VFT (Ng et al. 2007). The role of NO in mediating these changes has been explored in the current study and is discussed below.
NO in mediating VNS effects on cardiac electrophysiology
Compared to data on cardiac force generation (Han et al. 2002; Fischmeister et al. 2005), data on the effects of NO on cardiac electrophysiology are sparse. It has been shown that low levels of NO stimulate the hyperpolarization-induced pacemaker current (If) (Musialek et al. 1997) and increase heart rate by increasing firing of the sinus node. NO derived from neuronal NOS (nNOS) has been shown to modulate cardiac vagal control, facilitating cardiac vagal neurotransmission and enhancing the negative chronotropic and dromotropic action of vagus nerve activity at the atrial level (Conlon & Kidd, 1999). It has been shown that acetylcholine (ACh) release is increased by NO with enhanced muscarinic activation (Herring & Paterson, 2001). It would appear that the effects are both presynaptic and postsynaptic. Little is known about the effects of NO on ventricular electrophysiology (Fischmeister et al. 2005). Although there are some reports on the effects of NO on the ATP-sensitive K+ channel (Han et al. 2002), voltage-dependent fast Na+ current and Na+–K+ pump current (William et al. 2005), the electrophysiological relevance of these effects is unknown.
Role of NO in mediating the effect of VNS on APD restitution and VFT
VNS is known to prolong refractoriness (Martins & Zipes, 1980) and APD (Amlie & Refsum, 1981). Results from the current study are the first to support a role of NO in mediating the effects of VNS on ventricular electrophysiology. The increase in VFT and reduction of the slope of APD restitution curve with VNS were blocked in the presence of the non-specific NOS inhibitor, l-NA. Of note, the increase in ERP with VNS was not affected by NOS inhibition. This result suggests that NO is unlikely to be involved in this effect of VNS. Furthermore, these data indicate that the effect on ERP (considering the much smaller percentage change in comparison with restitution slope) is unlikely to be a main determinant in the protection from VF initiation by the vagus nerve. The significant inhibitory effects on the vagal actions on restitution slope and VFT with l-NA make it doubtful they are due to the changes in perfusion pressure or the chronotropic effect of VNS as the changes are disproportionately small. Furthermore, the vagal inhibitory effect occurred in the absence of any cardiac sympathetic activity or tone. These effects on VFT and APD restitution are likely to involve direct effects of vagal activity on ion channels and other cellular proteins in the ventricle. The direct effects of vagal activity on ventricular electrophysiology have not been examined in detail and need to be further explored.
Regarding possible cellular mechanisms, ACh mediates most actions from VNS and is a strong inhibitor of the L-type Ca2+ current (ICa,L) and contractile force in atrial cells (Wang et al. 1998). Apart from a reduction of ICa,L by ACh in ferret (Boyett et al. 1988), there are few studies to support a direct effect on unstimulated ICa,L in ventricular cells of most mammalian species (Bers, 2001). The ACh-activated K+ current, IK,Ach, is increased by ACh and vagal activity but is more abundant in atrial than ventricular cells (Hartzell, 1988). Modulation of these currents would not explain the results in the current study as the inhibition of ICa,L and activation of IK,Ach with vagal stimulation would both lead to opposite effects on ventricular electrical restitution. Other electrophysiological mechanisms that are beyond the scope of this study need to be explored.
In replenishing the substrate for NO synthesis with l-Arg, the reversal of the block on the effect of VNS on electrical restitution and VFT by NOS inhibition provides additional evidence that NO is involved in mediating these effects. Either l-NA or l-Arg alone did not alter the baseline parameters for restitution slope and VFT which argues against a direct effect of the agents on these parameters during VNS. Taken together, these data suggest that NO plays an important role in mediating the anti-fibrillatory effect of VNS on the ventricle possibly via the effects on APD restitution. While the indirect effects of vagal activity on adrenergically stimulated cAMP-dependent cellular events are well recognized, considering the absence of significant background sympathetic activity in this preparation, it is likely that the effects of NO on ion channels and Ca2+ cycling proteins are direct and cAMP-independent. These mechanisms will need to be explored.
In accord with this, others have shown in open-chest preparations in dogs with acute coronary artery occlusion, that intrapericardial perfusion with l-Arg to increase NOS activity, protected the myocardium against VF (Fei et al. 1997). This cardioprotective effect has also been shown in a recent study with intrapericardial administration of the NO donor nitroglycerin in closed-chest preparations in pigs (Kumar et al. 2003). In addition, ventricular myocytes isolated from mice lacking endothelial NOS have been shown to be more prone to arrhythmias as a result of triggered activity (Kubota et al. 2000). Conversely, treatment of human atrial strips with the NOS inhibitor NG-monomethyl l-arginine (l-NMMA) enhanced the arrhythmogenic effect of isoprenaline (Gauthier et al. 1998).
Limitations and further directions
In this study, we have demonstrated that NO plays an important role in mediating the protective effect from VF of vagal stimulation on the whole heart. To do this, we applied an electrical current from an external source to induce fibrillation as a surrogate marker to represent the susceptibility of hearts to VF. This method, however, may not be considered to be representative of what occurs naturally, and the link between VFT and electrical restitution may not be a causal one – currently no clear explanation is available from experimental evidence. In support of our study, we intentionally did not use any predisposing factors that may make it easier to induce VF, such as hypoxia or adrenergic activation, because it would make it more difficult to decipher the effects from direct VNS. In addition to this, the exogenous current that spans the refractory period during this protocol promotes electrical alternans development that deteriorates rapidly into a wavebreak and ultimately VF. This type of wavebreak is considered to depend on electrical restitution (Weiss et al. 2002). Hence, the effects of VNS and mediation by NO shown in the current study may be specific to this VF induction method.
The study was performed with MAPs measured from a single ventricular site in each preparation. The possibility of a heterogeneous response over the surface of the ventricular myocardium or transmurally from epicardium to endocardium needs to be explored to fully appreciate the mechanisms involved as dispersion of repolarization is known to be an important arrhythmogenic mechanism. Additionally, the limitation of using a single site with respect to the effect of left or right VNS and possible regional differences in innervation in the left ventricle warrants further investigation. This, however, should not detract from the results of the present study implicating a role for the involvement of NO in the peripheral effect of VNS in the ventricle in this species.
Although the underlying signalling pathways and effects on specific ion channels are unknown, data on APD restitution suggest this may be an important mechanism. Downstream pathways and potential effects on important ion channels involved in APD restitution will need to be investigated. The potential effects of VNS and the NO–cGMP pathway on this and other electrophysiological parameters such as conduction velocity, anisotropy and cardiac memory – all of which contribute to electrical restitution and arrhythmogenesis – need further investigation.
Conclusion
Direct VNS increased VFT and flattened the slope of APD restitution curve in the left ventricle of the isolated rabbit heart preparation with intact autonomic nerves and in the absence of any cardiac sympathetic nerve activity or tone. These effects were blocked by the NOS inhibitor l-NA and reversed by replenishing the substrate for NO production (l-Arg). These results suggest that NO plays an important role in the anti-fibrillatory effect of vagal stimulation on the ventricle, possibly via effects on APD restitution.
Acknowledgments
This study was supported by the British Heart Foundation (Project Grant PG/99008).
References
- Amlie JP, Refsum H. Vagus-induced changes in ventricular electrophysiology of the dog heart with and without beta-blockade. J Cardiovasc Pharmacol. 1981;3:1203–1210. doi: 10.1097/00005344-198111000-00006. [DOI] [PubMed] [Google Scholar]
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 1. Dordrecht: Kluwer Academic Publishers; 2001. [Google Scholar]
- Boyett MR, Kirby MS, Orchard CH, Roberts A. The negative inotropic effect of acetylcholine on ferret ventricular myocardium. J Physiol. 1988;404:613–635. doi: 10.1113/jphysiol.1988.sp017309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JM, Qu ZL, Kim YH, Wu TJ, Garfinkel A, Weiss JN, Karagueuzian HS, Chen PS. Spatiotemporal heterogeneity in the induction of ventricular fibrillation by rapid pacing: importance of cardiac restitution properties. Circ Res. 1999;84:1318–1331. doi: 10.1161/01.res.84.11.1318. [DOI] [PubMed] [Google Scholar]
- Chowdhary S, Townend JN. Role of nitric oxide in the regulation of cardiovascular autonomic control. Clin Sci (Lond) 1999;97:5–17. [PubMed] [Google Scholar]
- Conlon K, Kidd C. Neuronal nitric oxide facilitates vagal chrontropic and dromotropic actions on the heart. J Auton Nerv Syst. 1999;75:136–146. doi: 10.1016/s0165-1838(98)00185-4. [DOI] [PubMed] [Google Scholar]
- Einbrodt. Ueber Herzeizung und ihr Verhaeltnis zum Blutdruck. Akademie Wissenschaften (Vienna) Sitzungsberichte. 1859;38:345–359. [Google Scholar]
- Fei L, Baron AD, Henry DP, Zipes DP. Intrapericardial delivery of L-arginine reduces the increased severity of ventricular arrhythmias during sympathetic stimulation in dogs with acute coronary occlusion: nitric oxide modulates sympathetic effects on ventricular electrophysiological properties. Circulation. 1997;96:4044–4049. doi: 10.1161/01.cir.96.11.4044. [DOI] [PubMed] [Google Scholar]
- Fischmeister R, Castro L, Abi-Gerges A, Rochais F, Vandecasteele G. Species- and tissue-dependent effects of NO and cyclic GMP on cardiac ion channels. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:136–143. doi: 10.1016/j.cbpb.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Franz MR. The electrical restitution curve revisited: steep or flat slope – which is better? J Cardiovasc Electrophysiol. 2003;14:S140–S147. doi: 10.1046/j.1540.8167.90303.x. [DOI] [PubMed] [Google Scholar]
- Garfinkel A, Kim YH, Voroshilovsky O, Qu Z, Kil JR, Lee MH, Karagueuzian HS, Weiss JN, Chen PS. Preventing ventricular fibrillation by flattening cardiac restitution. Proc Natl Acad Sci U S A. 2000;97:6061–6066. doi: 10.1073/pnas.090492697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier C, Morteza E, Baron O, Balligand JLJ. Control of contractile and rhythmic properties of human atrial tissue by a NO pathway. Circulation. 1998;98:3838. [Google Scholar]
- Gilmour RF, Chialvo DR. Electrical restitution, critical mass, and the riddle of fibrillation. J Cardiovasc Electrophysiol. 1999;10:1087–1089. doi: 10.1111/j.1540-8167.1999.tb00281.x. [DOI] [PubMed] [Google Scholar]
- Han J, Kim N, Joo H, Kim E, Earm YE. ATP-sensitive K+ channel activation by nitric oxide and protein kinase G in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 2002;283:H1545–H1554. doi: 10.1152/ajpheart.01052.2001. [DOI] [PubMed] [Google Scholar]
- Hartzell HC. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophysics Mol Biol. 1988;52:165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Herring N, Paterson DJ. Nitric oxide-cGMP pathway facilitates acetylcholine release and bradycardia during vagal nerve stimulation in the guinea-pig in vitro. J Physiol. 2001;535:507–518. doi: 10.1111/j.1469-7793.2001.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolman BS, Verrier RL, Lown B. The effect of vagus nerve stimulation upon vulnerability of the canine ventricle: role of the sympathetic parasympathetic interactions. Circulation. 1976;52:578–585. doi: 10.1161/01.cir.52.4.578. [DOI] [PubMed] [Google Scholar]
- Kubota I, Han X, Opel DJ, Zhao YY, Baliga R, Huang P, Fishman MC, Shannon RP, Michel T, Kelly RA. Increased susceptibility to development of triggered activity in myocytes from mice with targeted disruption of endothelial nitric oxide synthase. J Mol Cell Cardiol. 2000;32:1239–1248. doi: 10.1006/jmcc.2000.1158. [DOI] [PubMed] [Google Scholar]
- Kumar K, Nguyen K, Waxman S, Nearing BD, Wellenius GA, Zhao SX, Verrier RL. Potent antifibrillatory effects of intrapericardial nitroglycerin in the ischemic porcine heart. J Am Coll Cardiol. 2003;41:1831–1837. doi: 10.1016/s0735-1097(03)00340-1. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- Martins JB, Zipes DP. Effects of sympathetic and vagal nerves on recovery properties of the endocardium and epicardium of the canine left ventricle. Circ Res. 1980;46:100–110. doi: 10.1161/01.res.46.1.100. [DOI] [PubMed] [Google Scholar]
- Musialek P, Lei M, Brown HF, Paterson DJ, Casadei B. Nitric oxide can increase heart rate by stimulating the hyperpolarization-activated inward current, If. Circ Res. 1997;81:60–68. doi: 10.1161/01.res.81.1.60. [DOI] [PubMed] [Google Scholar]
- Ng GA, Brack KE, Coote JH. Effects of direct sympathetic and vagal stimulation on the physiology of the whole heart – a novel model of isolated Langendorff perfused rabbit heart with intact dual autonomic innervation. Exp Physiol. 2001a;86:319–329. doi: 10.1113/eph8602146. [DOI] [PubMed] [Google Scholar]
- Ng GA, Brack KE, Coote JH. Differential effects of left and right vagus nerve stimulation on sinoatrial and atrioventricular nodes but not on ventricular electrophysiology – studies in the isolated rabbit heart with intact autonomic innervation. J Physiol. 2001b;531.P:182P. [Google Scholar]
- Ng GA, Brack KE, Patel VH, Coote JH. Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovasc Res. 2007;73:750–760. doi: 10.1016/j.cardiores.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen H, Baig W, Flapan AD, Cowley A, Prescott RJ, Neilson JM, Fox KA. Prospective study of heart rate variability and mortality in chronic heart failure – Results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-Heart) Circulation. 1998;98:1510–1516. doi: 10.1161/01.cir.98.15.1510. [DOI] [PubMed] [Google Scholar]
- Paterson DJ. Nitric oxide and the autonomic regulation of cardiac excitability. Exp Physiol. 2001;86:1–12. doi: 10.1113/eph8602169. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ. The autonomic nervous system and sudden death. Eur Heart J. 1998;19:F72–F80. [PubMed] [Google Scholar]
- Wang YG, Rechenmacher CE, Lipsius SL. Nitric oxide signaling mediates stimulation of L-type Ca2+ current elicited by withdrawal of acetylcholine in cat atrial myocytes. J Gen Physiol. 1998;111:113–125. doi: 10.1085/jgp.111.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JN, Chen PS, Qu Z, Karagueuzian HS, Lin SF, Garfinkel A. Electrical restitution and cardiac fibrillation. J Cardiovasc Electrophysiol. 2002;13:292–295. doi: 10.1046/j.1540-8167.2002.00292.x. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: the saga of cardiac alternans. Circ Res. 2006;98:1244–1253. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- William M, Vien J, Hamilton E, Garcia A, Bundgaard H, Clarke RJ, Rasmussen HH. The nitric oxide donor sodium nitroprusside stimulates the Na+-K+ pump in isolated rabbit cardiac myocytes. J Physiol. 2005;565:815–825. doi: 10.1113/jphysiol.2005.086447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuanetti G, Deferrari GM, Priori SG, Schwartz PJ. Protective effect of vagal stimulation on reperfusion arrhythmias in cats. Circ Res. 1987;61:429–435. doi: 10.1161/01.res.61.3.429. [DOI] [PubMed] [Google Scholar]