Abstract
The widely expressed anion exchanger polypeptide AE2/SLC4A2 is acutely inhibited by acidic intracellular (pHi), by acidic extracellular pH (pHo), and by the calmodulin inhibitor, calmidazolium, whereas it is acutely activated by NH4+. The homologous erythroid/kidney AE1/SLC4A1 polypeptide is insensitive to these regulators. Each of these AE2 regulatory responses requires the presence of AE2's C-terminal transmembrane domain (TMD). We have now measured 36Cl− efflux from Xenopus oocytes expressing bi- or tripartite AE2–AE1 chimeras to define TMD subregions in which AE2-specific sequences contribute to acute regulation. The chimeric AE polypeptides were all functional at pHo 7.4, with the sole exception of AE2(1-920)/AE1(613-811)/AE2(1120-1237). Reciprocal exchanges of the large third extracellular loops were without effect. AE2 regulation by pHi, pHo and NH4+ was retained after substitution of C-terminal AE2 amino acids 1120–1237 (including the putative second re-entrant loop, two TM spans and the cytoplasmic tail) with the corresponding AE1 sequence. In contrast, the presence of this AE2 C-terminal sequence was both necessary and sufficient for inhibition by calmidazolium. All other tested TMD substitutions abolished AE2 pHi sensitivity, abolished or severely attenuated sensitivity to pHo and removed sensitivity to NH4+. Loss of AE2 pHi sensitivity was not rescued by co-expression of a complementary AE2 sequence within separate full-length chimeras or AE2 subdomains. Thus, normal regulation of AE2 by pH and other ligands requires AE2-specific sequence from most regions of the AE2 TMD, with the exceptions of the third extracellular loop and a short C-terminal sequence. We conclude that the individual TMD amino acid residues previously identified as influencing acute regulation of AE2 exert that influence within a regulatory structure requiring essential contributions from multiple regions of the AE2 TMD.
The plasmalemmal SLC4 AE anion exchangers mediate electroneutral Na+-independent Cl−–HCO3− exchange to regulate intracellular pH (pHi), intracellular [Cl−] and cell volume. Under usual physiological conditions, AE anion exchangers extrude HCO3− from cells, and load cells with acid and Cl−. These processes are harnessed by polarized epithelial cells for transepithelial transport of salt, water and acid–base equivalents. Electroneutral Na+-independent Cl−–HCO3− exchange is mediated by at least three homologous, differentially expressed SLC4 gene products: SLC4A1/AE1, SLC4A2/AE2 and SLC4A3/AE3 (Alper, 2002; Romero et al. 2004). In contrast to the restricted distribution of AE1 predominantly in erythrocytes and in renal collecting duct Type A intercalated cells, the nonerythroid anion exchangers AE2 and AE3 are widely expressed in epithelial and other cell types. The SLC4 AE anion exchangers exhibit distinct patterns of acute regulation. In contrast to AE1, AE2 and AE3 are regulated by acute changes in pHi (Stewart et al. 2001) and extracellular pH (pHo) (Stewart et al. 2002). AE2 is also stimulated by NH4+ and hypertonicity via mechanisms requiring intracellular Ca2+ ([Ca2+]i), and by calmidazolium in a calmodulin-independent manner (Chernova et al. 2003). The structural basis for these regulatory differences between the closely related AE1 and AE2 anion exchanger polypeptides remains incompletely understood.
The SLC4 AE gene products AE1–3 share, along with other SLC4 transporters, a tripartite domain structure comprising a cytoplasmic N-terminal domain of 400–700 amino acids (aa), a transmembrane domain (TMD) of ∼500 aa that traverses the lipid bilayer 12–14 times, and a short C-terminal tail proposed to bind carbonic anhydrase II (Vince & Reithmeier, 2000). The orthologous TMDs of AE1–3 are ∼65% identical in aa sequence, with shorter regions of greater sequence conservation. In contrast, the orthologous N-terminal cytoplasmic domains share only ∼35% sequence identity. The AE TMDs expressed in erythrocyte membranes, in Xenopus oocytes or in HEK-293 cells suffice to mediate anion exchange (Grinstein et al. 1978; Kopito et al. 1989; Lindsey et al. 1990; Zhang et al. 1996). However, removal of the AE2 N-terminal domain alters regulation of AE2-mediated Cl− transport by pH (Zhang et al. 1996; Stewart et al. 2001), NH4+, hypertonicity and calmidazolium (Chernova et al. 2003).
Whereas removal of most of the AE2 N-terminal cytoplasmic domain abolishes sensitivity to pHi, the sensitivity of the remaining AE2 TMD to changes in pHo is acid-shifted but not abolished. This indicates the presence within the AE2 TMD of amino acid residues that are required for normal regulation by pH. Our initial studies of the AE2 TMD have identified individual TMD His residues (Stewart et al. 2007b) and TMD charged residues which contribute to normal regulation of AE2 by pH (Stewart et al. 2007a). However, our inability to identify a single non-conserved amino acid residue responsible for pH sensitivity of AE2-mediated Cl− transport suggests that multiple TMD residues comprise a ‘pH sensor’ in the AE2 transmembrane domain.
In the present study, we sought to identify subregions of the AE2 TMD required for normal regulation by pHi and by pHo, as well as to identify subregions important for regulation by NH4+ and by calmidazolium. To do so, we constructed a series of bipartite and tripartite chimeric AE2–AE1 transporters (see Fig. 1A (Stewart et al. 2007a) for sequence alignment). The encoded chimeric polypeptides were expressed in Xenopus oocytes and assayed for regulation of AE-mediated 36Cl− transport by pHi, by pHo, by NH4+ and by the calmodulin inhibitor, calmidazolium. We show that replacement of AE2 TMD residues in the large third extracellular loop (EC3) and at the TMD C-terminus with corresponding residues of AE1 alters minimally or not at all the AE2 regulatory phenotype for pHi, pHo and NH4, whereas substitution of any other tested subregion of the AE2 TMD with corresponding AE1 sequence abrogates these responses. Thus, broad stretches of the AE2 TMD collaborate in this regulatory phenotype of AE2-mediated anion exchange. In contrast, the C-terminal 118 residues of AE2 are both necessary and sufficient to confer inhibition by calmidazolium upon the normally calmidazolium-insensitive AE1.
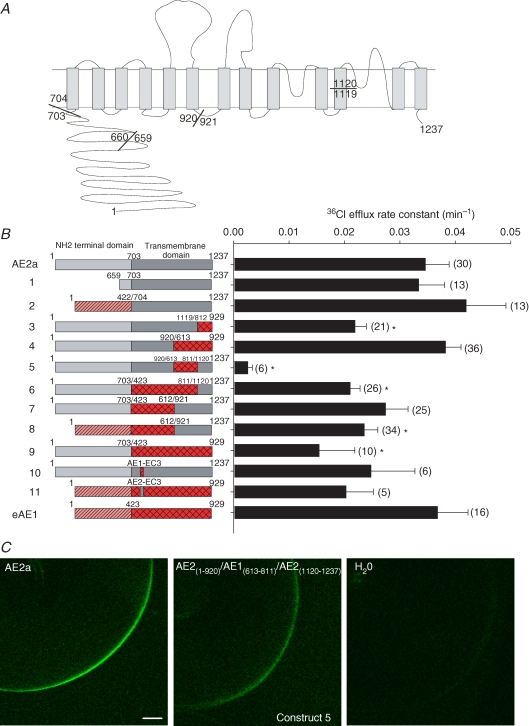
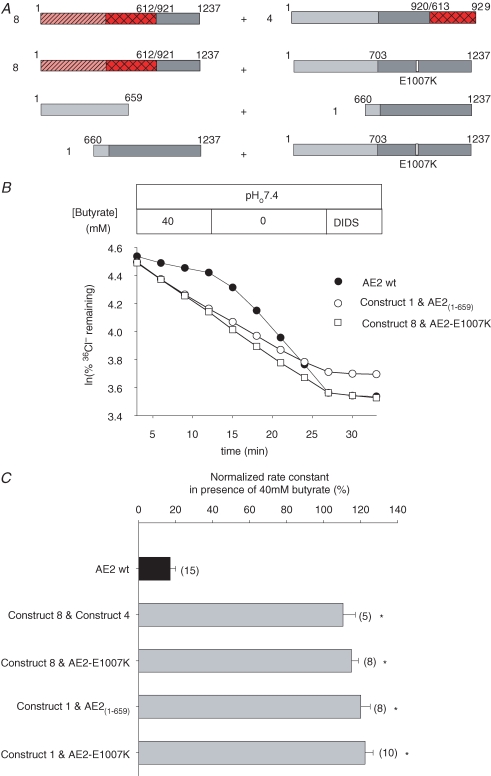
Figure 1. Chimeric and other mutant constructs studied.
A, schematic of the AE2 TMD structure modelled on the human AE1 TMD topography proposed by Zhu et al. (2003), with mouse AE2a aa numbering (GenBank J04036) at N- and C-termini, and at junctions utilized for construction of chimeric and truncated cDNAs. B, wildtype, truncations and chimeric AE-mediated 36Cl− efflux from (n) Xenopus oocytes presented as rate constants (bar graph at right) for each numbered construct (schematics at left). Domains from mouse AE2a are light grey (N-terminal cytoplasmic) or dark grey (TMD). Domains from mouse eAE1 are hatched (N-terminal cytoplasmic domain) or cross-hatched (TMD). Amino acid numbers indicated at domain and/or chimera junctions. *P < 0.05 compared with wildtype AE2a. C, representative confocal fluorescence images of Xenopus oocytes expressing C-terminal GFP fusion proteins of wildtype AE2a (left), or the minimally active construct 5 (centre), or previously injected with water (right). Bar, 100 μm.
Methods
Analytical grade reagents were purchased from Sigma, Fluka (St Louis, MO, USA) or Calbiochem (San Diego, CA, USA). Na36Cl was obtained from ICN (Irvine, CA, USA). Taq DNA polymerase was from Roche Biochemicals (Mannheim, Germany) and dNTPs were from Promega (Madison, WI, USA). Restriction enzymes and T4 DNA ligase were from New England BioLabs (Beverly, MA, USA).
Construction of chimeric AE cDNAs
Plasmid pΔX (Alper et al. 1989) or plasmid pXT7 encoding mouse AE2 (Accession: J04036), and plasmid pBL encoding mouse erythroid AE1 (Accession no. X02677) or plasmid pL2AΔ encoding mouse kidney AE1 (Brosius et al. 1989) served as cDNA templates for polymerase chain reaction (PCR) and RNA transcription. Most chimeric cDNAs were constructed by the four primer PCR method previously described (Zhang et al. 1996; Chernova et al. 1997; Stewart et al. 2001), using the oligonucleotides (Biosynthesis, Woodlands, TX, USA), restriction enzymes and backbone acceptor fragment vectors listed in Supplemental Table 1. Integrity of PCR fragments and ligation sites was confirmed by DNA sequencing of both strands.
cRNA expression in Xenopus oocytes
Ovarian segments were excised from female Xenopus laevis (Xenopus One, Madison, WI, USA) anaesthetized with 0.17% tricaine according to protocols approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center. Minced ovarian fragments were incubated in 2 mg ml−1 Type A collagenase (Roche) for 1 h at room temperature in ND-96, pH 7.4, containing (mm): 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 Hepes, 2.5 sodium pyruvate and 5 mg (100 ml)−1 gentamicin. Individual stage V–VI oocytes were manually defolliculated and injected on the same day with 50 nl cRNA or with H2O. Capped mAE2, mAE1 and chimera cRNAs were transcribed from XbaI-linearized pXT7 or from ClaI-linearized pΔX templates with the T7 MEGAscript kit (Ambion, Austin, TX, USA), purified with the RNAeasy kit (Qiagen, Valencia, CA, USA), and resuspended in diethylpyrocarbonate-treated water. Formaldehyde agarose gel electrophoresis confirmed cRNA integrity, and cRNA concentration was estimated by A260 (Nanodrop, Wilmington, DE, USA). The amount of injected cRNA (0.5–25 ng) was titrated for each chimeric construct to approximate the 36Cl− efflux at pHo 7.4 associated with injection of 10 ng wildtype AE2 cRNA. cRNA-injected oocytes were maintained at 19°C in ND-96 for 2–6 days in the continued presence of gentamicin and pyruvate until used for experiments.
36Cl− efflux assays in Xenopus oocytes
36Cl− efflux assays were performed as previously described (Stewart et al. 2004, 2007b). Briefly, oocytes injected with 50 nl of 260 mm Na36Cl (15 000–20 000 c.p.m.) were allowed to recover for ∼10 min in Cl−- free medium (mm: 96 sodium isethionate, 2 potassium gluconate, 1.8 calcium gluconate, 1 magnesium gluconate, 5 Hepes, pH 7.4). 36Cl− efflux was initiated by transferring individual oocytes to 6 ml borosilicate glass tubes containing 1 ml ND-96 (mm: 96 NaCl, 2KCl, 1.8 CaCl2, 1 MgCl2, 5 Hepes, pH 7.4). 0.95 ml of this efflux solution were removed at 3 min intervals and replaced by an equal volume of fresh ND-96. At the end of the assay, integrity of AE2-mediated Cl− transport and of the oocyte was confirmed by a final efflux period in the presence of the anion transport inhibitor 4,4′-di-isothiocyanatostilbene-2,2′-disulphonic acid (DIDS, 200 μm). Individual oocytes were lysed in 100 μl 1% sodium dodecyl sulphate (SDS) and lysates and efflux samples were subjected to scintillation counting for 3–4 min or until the magnitude of two s.d.s was < 5% of the sample mean.
36Cl− efflux activity of tested chimeric AE polypeptides was compared with that of wildtype AE2 at pHo 7.4 and to that of water-injected oocytes on each experimental day. Each cRNA construct was tested in oocytes harvested from at least two frogs. Experimental data were plotted as ln (% c.p.m. remaining in the oocyte) versus time. 36Cl− efflux rate constants were calculated from linear fits to data from at least three time points for each experimental condition. Injected cRNA quantities were titrated for wildtype AE2, AE1 and chimeric AE polypeptides to yield comparable 36Cl efflux rate constants at pHo 7.4. The amount of cRNA used for each construct is presented in Supplemental Table 3 together with complete 36Cl− efflux data for all experiments shown.
Measurements of pHo-dependent AE2-mediated 36Cl− efflux and of pHi-dependent AE2-mediated 36Cl− efflux were conducted as previously described and validated (Stewart et al. 2004). AE2-mediated Cl− transport sensitivity to pHi at constant pHo was tested by selective oocyte pHi reduction (∼0.5 pH units, time constant = 403 ± 55 s, n= 8) with bath addition of 40 mm sodium butyrate (Stewart et al. 2001), followed by intracellular alkalinization (∼0.5 pH units, time constant = 315 ± 15 s, n= 6) accompanying removal of butyrate from the bath.36Cl− efflux rate constants for pHi dependence of AE2 were normalized as the ratio of the efflux rate constant in the presence of 40 mm butyrate to that in the absence of butyrate at constant pHo 7.4. Butyrate is neither a substrate nor an inhibitor of AE2 (Stewart et al. 2001). AE2 sensitivity to pHo at near-constant pHi was measured by varying bath solution pH as previously described (Stewart et al. 2002).
Confocal laser immunofluorescence microscopy
The expression of AE chimeras at or near the oocyte surface was assessed in oocytes expressing AE chimera polypeptides fused at their carboxy-termini with Green Fluorescent Protein (GFP). At least eight oocytes expressing each AE–GFP fusion construct were fixed overnight in phosphate-buffered saline (PBS) containing 3% paraformaldehyde, washed three times in PBS, and stored at 4°C. Fixed, intact oocytes were imaged as whole-mount preparations with a Bio-Rad MRC1024 (Fig. 1C) or Zeiss LSM 510 laser scanning confocal microscope (Supplemental Fig. 1A) with a × 10 objective at constant pinhole, gain and filter settings. Representative images of whole-mount equatorial sections were compiled in Microsoft PowerPoint and oocytes exhibiting median levels of fluorescence intensity are shown in Fig. 1C and Supplemental Fig. 1A.
Surface polypeptide expression was correlated with 36Cl− efflux rate constant in oocytes previously injected with varying amounts of cRNA encoding wildtype AE2a–GFP. GFP fluorescence intensity at the oocyte surface (greyscale range 0–255 units) measured in four standardized regions of interest (width, 52 μm; height, 9 μm) in whole-mount confocal images (NIH ImageJ, http://rsb.info.nih.gov/nih-image/) was transferred into Microsoft Excel. After correction for background fluorescence intensity measured in oocytes previously injected with H2O, mean surface pixel intensity was calculated for individual oocytes. Functional activities of wildtype AE2a–GFP were plotted against oocyte polypeptide surface expression across a wide range of injected cRNA quantities. The data were well described by a least squares linear fit (r2= 0.98), with saturation between 5 and 40 ng injected cRNA (Supplemental Fig. 1B).
Statistical analysis
Data are reported as mean ±s.e.m. Values for individual AE chimeras and groups of chimeras were compared with those of wildtype AE2 by Dunnett's 2-way t test, with significance defined as P < 0.05.
Results
Most chimeric AE1–AE2 polypeptides constructed are functional anion transporters
The C-terminal TMDs of murine AE1 and AE2 share ∼67% sequence identity (Alper, 1991) and mediate comparable rates of Cl−–Cl− exchange in the absence of most of their preceding cytoplasmic N-terminal domains (Zhang et al. 1996; Stewart et al. 2001). To define which portions of the AE2 TMD contribute to acute regulation of AE2-mediated anion transport, we constructed chimeras in which subdomains of the AE2 TMD were substituted with the corresponding TMD regions from the relatively pH-insensitive AE1 anion exchanger. Selection of junctions for the AE regions to be swapped (Fig. 1A) was guided by conserved unique restriction enzyme sites and by recent (Fujinaga et al. 1999) and current topographical models of human AE1 (Zhu et al. 2003). The conserved BsrGI site (AE2 aa 1119; AE1 aa 811) was used (Supplemental Table 1) to swap the C-terminal portions of the TMDs. The intracellular loop between putative TMDs 6 and 7 (AE2 aa 920/921) was chosen based on previous subdomain co-expression and deletion experiments for human AE1 (Groves & Tanner, 1999). With one exception, the AE chimeras exhibited 36Cl− efflux activity at pHo 7.4 which was at least 50% those of wildtype AE2 and AE1. Only construct 5, AE2(1-920)–AE1(613-811)–AE2(1120-1237), was functionally impaired to the degree that regulation by pHi and pHo could not be accurately assessed (Fig. 1B). This loss of function correlated with reduced expression at or near the oocyte surface (26.5 ± 3.9 greyscale units (n= 8) for construct 5 versus 189 ± 12.2 greyscale units (n= 6) for wildtype or 14% of the wildtype level; Fig. 1C). Thus, reduced surface expression of construct 5 largely if not entirely accounted for its severely reduced transport activity.
Replacement of the AE2 EC3 sequence with that of AE1 does not alter AE2 regulation by pHi or pHo
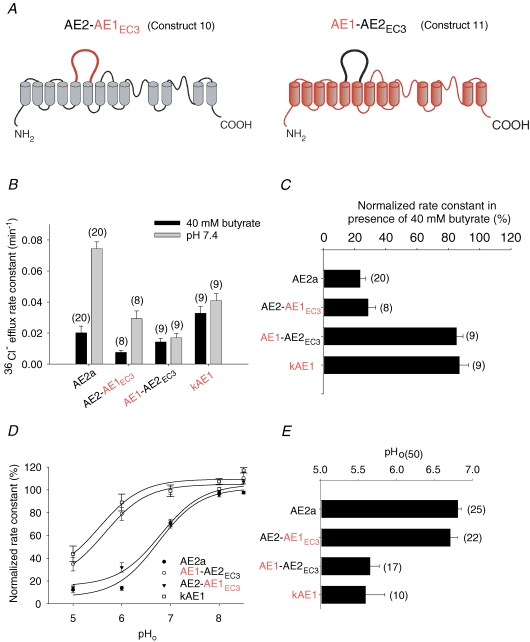
We assessed the contribution to regulation of AE2 by pH of the poorly conserved sequence in the large exofacial loop connecting transmembrane spans 5 and 6 (EC3) by studying chimeras in which the EC3 sequence of mouse AE1 was inserted into mouse AE2 and vice versa (Fig. 2A). The resulting construct 10 (AE2–AE1EC3) contains no N-glycans, whereas the complementary construct 11 (AE1–AE2EC3) is predicted to contain the three N-glycans of the AE2 EC3 sequence plus the single N-glycan of AE1 EC4. Swapping EC3 loops between AE1 and AE2 had little effect on DIDS-sensitive 36Cl− efflux at pHo 7.4 as compared with that of wildtype AE2 or AE1 (Basal 36Cl− efflux, Supplemental Table 3). As shown in Fig. 2B and summarized in Fig. 2C, chimera AE2–AE1EC3 retained the wildtype AE2a phenotype of pHi sensitivity, whereas chimera AE1–AE2EC3 shared in this assay the pHi insensitivity characteristic of wildtype AE1. Figure 2D compares the pHo dependence of normalized 36Cl− efflux activity for these EC3 chimeras to those of wildtype AE2 and AE1. As summarized in Fig. 2E, the pHo(50) values for wildtype AE2a and AE2–AE1EC3 were comparable, and the indistinguishable pHo(50) values for wildtype AE1 and AE1–AE2EC3 were acid-shifted to the same large extent (P > 0.05). Thus, the EC3 loop of AE2 is neither necessary for AE2 regulation by pH, nor sufficient to confer the AE2 pH-regulatory phenotype upon AE1. These data also demonstrate that N-glycosylation is not needed for functional expression of AE2 at the oocyte surface.
Figure 2. EC3 contribution to AE2 regulation by pHi and pHo.
A, schematic of constructs 10 (AE2–AE1EC3) and 11 (AE1–AE2EC3). B, regulation of 36Cl− efflux by pHi in (n) oocytes expressing the indicated AE polypeptides. C, normalized rate constants for the indicated AE polypeptides in (n) oocytes in the presence of butyrate (acidic pHi). Interchange of EC3 residues does not alter regulation by pHi. D, regulation of AE-mediated 36Cl− efflux at sequentially changing pHo values. E, pHo(50) values in (n) oocytes expressing the indicated AE polypeptides. Interchange of EC3 residues does not alter regulation by pHo.
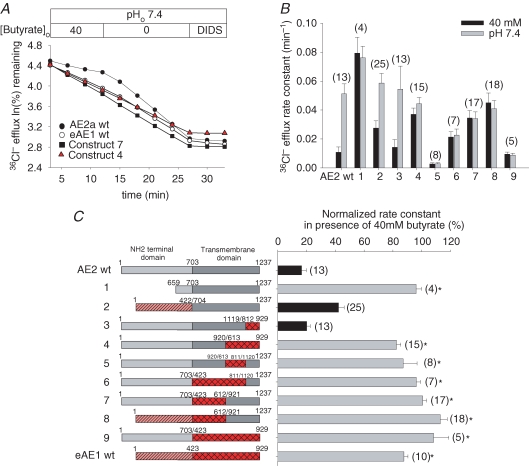
Replacement of any tested part of the AE2 TMD except C-terminal aa 1120–1237 with corresponding AE1 residues abolishes AE2 regulation by pHi
Figure 3A shows 36Cl− efflux time courses from representative oocytes expressing wildtype or mutant AE polypeptides. The summarized efflux rate constants in the presence (black bars) and absence (grey bars) of 40 mm butyrate are presented in Fig. 3B. Figure 3C presents normalized pHi sensitivity data for all tested chimeras. The cytoplasmic N-terminal domain of AE2 is required for wildtype regulation by pHi (construct 1), consistent with our previous demonstration that conserved residues within that domain mediate this pHi sensitivity (Stewart et al. 2002). The inhibition of wildtype AE2 by acidic pHi was preserved (black bars) upon substitution of AE2 C-terminal aa 1120–1237 with the corresponding AE1 residues (construct 3) encoding a putative second re-entrant loop, two final transmembrane spans and the short C-terminal cytoplasmic tail (Fig. 1A). AE2 pHi sensitivity was preserved in attenuated form upon replacement of its N-terminal cytoplasmic domain with the shorter corresponding domain from AE1 (construct 2). All other tested chimeras (constructs 4–9) shared with wildtype AE1 a lack of inhibition by acidic pHi (grey bars). These data show that non-conserved residues within the AE2 TMD separately encompassing aa 704–920 and aa 921–1119 are required for AE2 regulation by pHi. However, substitution of AE2 aa 921–1237 into AE1 does not suffice to confer pHi sensitivity on AE1. These data also confirm the ability of a substituted AE1 N-terminal cytoplasmic domain to sustain regulation by pHi when joined to the AE2 TMD (Stewart et al. 2001).
Figure 3. TMD contribution to AE2 regulation by pHi.
A, representative 36Cl− efflux traces from individual oocytes expressing the indicated AE polypeptides in the presence and subsequent absence of butyrate, followed by exposure to the inhibitor, DIDS. (See Methods for the effects of butyrate on pHi.) B, 36Cl− efflux rate constants for (n) oocytes expressing the indicated wildtype or mutant AE polypeptides in the presence (black bars) and subsequent absence of butyrate (grey bars). C, normalized inhibition by butyrate of 36Cl− efflux (right) of (n) wildtype, mutant and chimeric AE polypeptides (schematics at left). Efflux was not significantly different (black bars) or was significantly different (grey bars) from wildtype AE2 (*P < 0.05).
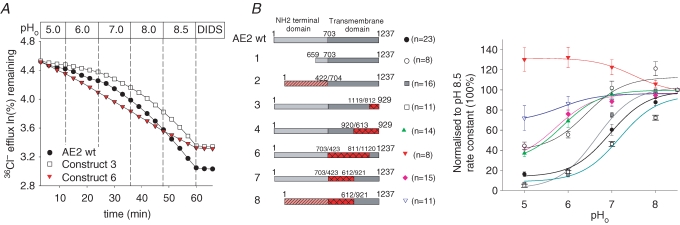
Replacement of any part of the AE2 TMD except aa 1120–1237 with corresponding AE1 residues severely acid-shifts or abolishes AE2 regulation by pHo
Since pHo regulates AE2 activity independently from pHi (Stewart et al. 2002), we assessed the response of the AE2–AE1 chimeras to varying pHo. Figure 4A shows that while replacement of AE2 N-terminal TMD residues 704–1119 with the corresponding AE1 residues 423–811 (construct 6) abolishes regulation of AE2-mediated Cl−–Cl− exchange by pHo, replacement of AE2 C-terminal TMD residues 1120–1237 with the corresponding AE1 TMD residues 812–929 (construct 3) moderately alkali-shifts pHo(50) (7.24 ± 0.11, n= 11) from the wildtype AE2 value (6.89 ± 0.06, n= 23, P < 0.05). All other AE chimeras tested displayed either a greatly acid-shifted pHo (constructs 3, 4 and 8) or, in the case of AE2(1-703)–AE1(423-811)–AE2(1120-1237) (construct 6), complete loss of inhibition by acidic pHo (Fig. 4B). These data show that replacement of either the N- or C-terminal half of the AE2 TMD with corresponding residues from AE1 severely attenuates regulation by pHo, and AE1 replacement of most of the AE2 TMD abolishes that regulation. However, near-normal or enhanced regulation by pHo results from replacement of AE2 C-terminal aa 1120–1237 with corresponding AE1 residues and maintains near-normal anion exchange regulation by pHo. Thus, those C-terminal AE2 residues within aa 1120–1237 that are not conserved in AE1 are not critical for regulation by either pHo or pHi.
Figure 4. TMD contribution to AE2 regulation by pHo.
A, representative 36Cl− efflux traces from individual oocytes expressing the indicated AE polypeptides during sequential pHo transitions from 5.0 to 8.5, followed by exposure to the inhibitor, DIDS. B, normalized pHo dependence of 36Cl− efflux (right) by (n) oocytes expressing the indicated wildtype and chimeric AE2 polypeptides (numbered schematics and symbols at left).
Co-expression of separate, complementary AE2 sequences does not restore pHi sensitivity to pHi-insensitive AE1–AE2 chimeras or AE2 truncation mutants
We have shown previously that the Cl−–HCO3− exchange-incompetent mouse AE1 mutant E699Q can restore Cl−–HCO3− exchange activity to kAE1 mutants with missense or truncation mutations of the C-terminal cytoplasmic tail (Dahl et al. 2003) We therefore tested the hypothesis that the corresponding transport-incompetent AE2 mutants E1007Q and E1007K might restore pHi sensitivity to pHi-insensitive chimeric AE polypeptides (see Figs 3C and 5A) or to pHi-insensitive AE2 cytoplasmic N-terminal deletion mutants (Stewart et al. 2001). We also tested possible rescue of pHi sensitivity by co-expressed pHi-insensitive chimeric polypeptides of complementary sequence (Fig. 5A, construct 8 and construct 4). In view of the role of the AE2 N-terminal cytoplasmic domain in regulation by pHi (Stewart et al. 2004), we further examined the hypothesis that co-expression of the N-terminal cytoplasmic and C-terminal transmembrane domains of AE2 as separate polypeptides might reconstitute pHi-sensitive anion transport. Figure 5A summarizes the tested combinations.
Figure 5. Loss of AE2 inhibition by acidic pHi is not rescued by complementary sequences co-expressed in trans.
A, schematics of co-expressed AE chimeras and mutants. B, representative 36Cl− efflux traces from individual oocytes expressing the indicated wildtype or mutant AE polypeptides in the presence and subsequent absence of butyrate, followed by DIDS inhibition. C, normalized rate constants in the presence of butyrate for the indicated wildtype and mutant AE polypeptides expressed in (n) oocytes. Grey bars indicate significant difference from wildtype AE2 (*P < 0.05).
Figure 5B shows that co-expression of the pHi-insensitive chimera, AE1(1-612)–AE2(921-1237) (construct 8) with the transport-inactive AE2 mutant E1007K did not restore regulation by pHi. AE2 E1007K was similarly unable to rescue pHi sensitivity of transport by the AE2 TMD (AE2(660-1237); construct 1) (Fig. 5C). Co-expression of the AE2 TMD (AE2(660-1237); construct 1) with its complementary N-terminal cytoplasmic domain (AE2(1-659) also failed to restore pHi sensitivity to AE2-mediated Cl−–Cl− exchange (Fig. 5B and C). In addition, rescue of pHi-sensitive 36Cl− efflux was unsuccessful with co-expression of complementary chimeras AE1(1-612)–AE2(921-1237) and AE2(1-920)–AE1(613-929) (constructs 8 and 4, Fig. 5C). The data provide no evidence under the conditions tested for the ability of residues on one AE monomer to confer pHi sensitivity on a pHi-insensitive second monomer within a heterodimer known to form in the context of AE1 (Dahl et al. 2003). Nor can the free AE2 N-terminal cytoplasmic domain rescue pHi sensitivity of co-expressed AE2 TMD. AE2 regulation by pHi may require intramolecular interactions.
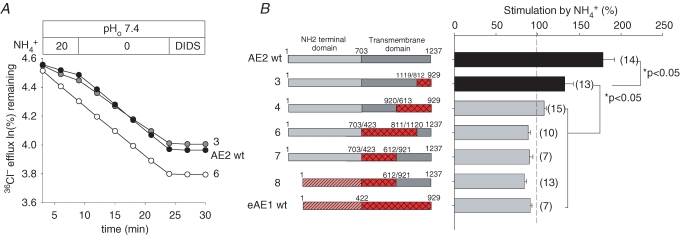
Partial replacement of the AE2 TMD by corresponding AE1 residues attenuates or abolishes stimulation by NH4+
AE2 is acutely stimulated by NH4+ despite the fact that it acidifies the oocyte to pHi values otherwise sufficient to inhibit activity completely (Humphreys et al. 1997; Stewart et al. 2001). This stimulation, an effect not shared by AE1, is inhibited by chelation of intracellular Ca2+ (Chernova et al. 2003; Kurschat et al. 2006). Our preliminary chimera studies showed a requirement for both the TMD and the N-terminal cytoplasmic domain of AE2 for NH4+-stimulated AE2 activity (Chernova et al. 2003). The AE TMD chimeras studied above have now allowed determination of those sections of AE2-specific TMD sequence required for NH4+ stimulation. Figure 6 shows that whereas wildtype AE2 and the chimera AE2(1-1119)–AE1(812-929) (construct 3) were stimulated by 20 mm NH4+, the chimeric AE constructs 4, 6, 7 and 8 resembled wildtype AE1 in their insensitivity to stimulation. Thus, although maximal NH4+ stimulation of AE2 appears to require the entire AE2 TMD, detectable stimulation is preserved when C-terminal AE2 aa 1120–1237 are substituted with the corresponding AE1 aa 812–929.
Figure 6. TMD contribution to AE2 stimulation by NH4+.
A, representative 36Cl− efflux traces from individual oocytes expressing the indicated wildtype or mutant AE polypeptides in the absence and subsequent presence of 20 mm NH4+, followed by DIDS inhibition. B, normalized stimulation by NH4+ in (n) oocytes (right) expressing the indicated wildtype and chimeric AE polypeptides (numbered schematics at left). Grey bars denote absence of stimulation.
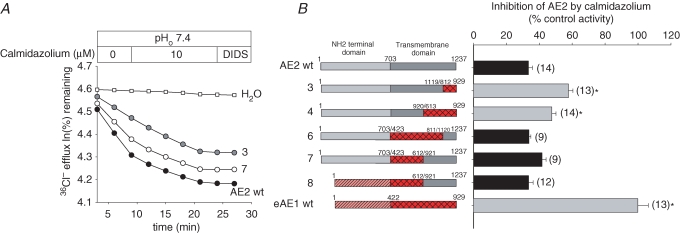
Partial replacement of the AE2 TMD by corresponding AE1 residues attenuates or abolishes inhibition by calmidazolium
The calmodulin inhibitor calmidazolium inhibits both basal and NH4+-stimulated AE2 activity, whereas AE1 is insensitive to the drug. However, other calmodulin antagonists and CaMKII inhibitors are not AE2 inhibitors. AE2 inhibition by calmidazolium does not require the presence of either the AE2 or the AE1 N-terminal cytoplasmic domains (Chernova et al. 2003). Figure 7 shows that replacement of the N-terminal half (construct 7) or three-quarters of the AE2 TMD (construct 6) with AE1 sequence, whether with or without AE1 substitution of the N-terminal cytoplasmic domain (construct 8), preserved the calmidazolium inhibition phenotype of wildtype AE2. This inhibition was attenuated but nonetheless sustained when AE1 sequence was substituted for the C-terminal half (construct 4) or quarter (construct 3) of the AE2 TMD. Although maximal inhibition of AE2 by calmidazolium may require other TMD residues, substantial inhibition is preserved when AE2 aa 1120–1237 (construct 3) or 921–1237 (construct 4) are substituted with the corresponding sections of the AE1 TMD. Thus, within the TMD, AE2 aa 1120–1237 are both necessary (construct 3) and sufficient (construct 6) for wildtype calmidazolium inhibition of AE2.
Figure 7. TMD contribution to AE2 inhibition by calmidazolium.
A, representative 36Cl− efflux traces from individual oocytes expressing the indicated wildtype or mutant AE polypeptides in the absence and subsequent presence of 10 μm calmidazolium, followed by DIDS inhibition. B, normalized inhibition by calmidazolium of (n) oocytes (right) expressing the indicated wildtype and chimeric AE polypeptides (numbered schematics at left). Grey bars indicate significant differences from wildtype AE2 (P < 0.05).
Discussion
The structural basis for the distinct, acute regulatory properties of the SLC4 AE anion exchangers remains incompletely understood. We have shown with chimeric AE2–AE1 polypeptides that regulation of AE2 by pHi and by pHo requires AE2-specific amino acid residues from nearly the entire TMD sequence, with the exceptions of the third extracellular loop (Fig. 2) and of the C-terminal aa 1120–1237 (Figs 3 and 4) encompassing the last two putative transmembrane spans and the short C-terminal cytoplasmic tail (construct 3). These data suggest that the TMD pH sensor is comprised of multiple amino acid residues within distinct TMD subdomains.
The entire AE2 TMD sequence was also required for full AE2 stimulation by NH4+ and for full inhibition by calmidazolium. However, substitution of AE2 aa 1120–1237 with the corresponding AE1 sequence (construct 3) preserved partial stimulation by NH4+ (Fig. 6) and partial inhibition by calmidazolium (Fig. 7). The presence of AE2 aa 1120–1237 as the only AE2 sequence in the TMD of the reciprocal chimeric construct 6 sufficed to confer inhibition by calmidazolium on a chimera with its remaining TMD derived entirely from the calmidazolium-insensitive AE1.
Thus, the AE2-specific sequences within the third extracellular loop and within AE2 aa 1120–1237 are not required for transport inhibition by acidic pH, but are involved in wildtype regulation by NH4+ and are required for maximal inhibition by calmidazolium.
Anion transport function of AE2–AE1 chimeras
All but one of the chimeric constructs exhibited DIDS-sensitive Cl−–Cl− exchange activity within 2-fold of the transport activities of wildtype AE2 and AE1 (Fig. 1B). Although the low activity of construct 5 (AE2(1-920)–AE1(613-811)–AE2(1120-1237)) precluded tests of inhibition by acidic pHi, pHo or calmidazolium, its activity remained DIDS sensitive. This low activity was explained in part by reduced surface expression (Fig. 1C). Thus (with the exception of construct 5), preservation of near-native polypeptide conformation tolerated considerable latitude in the placement of AE2–AE1 chimera junctions, suggesting only subtle differences in folding between the chimeric polypeptides and their wildtype parents. In contrast, TMD chimeras of AE2 with AE3 (which is processed to the HEK-293 cell surface 8-fold less efficiently than AE2) exhibit the AE3 TMD phenotype of low level surface expression (Fujinaga et al. 2003). Similarly, the mouse AE1 chimera in which substitution of putative TM spans 6–11 with the corresponding trout AE1 sequence led to loss of function probably due to absence from the oocyte surface (Borgese et al. 2004). Conformational studies utilizing introduced N-glycosylation sites engineered into human AE1 have suggested that both TM 12 and the N-terminal TMs 1–3 are both required for efficient membrane insertion of the first putative re-entrant loop of the AE1 TMD (Kanki et al. 2002). The interposed AE1 sequence in chimera 5 may thus alter interaction between these N-terminal and near C-terminal regions of the AE2 TMD, reducing anion transport to a greater degree than surface trafficking.
Structural subdomains of the AE2 TMD
The current study defines specific subdomains within the AE2 TMD that are required for normal regulation of anion transport. AE1 TMD subdomains are assembled during biosynthesis, and membrane insertion and trafficking as groups of transmembrane spans, based on the ability of co-expressed AE1 TMD subfragments to reconstitute 36Cl− influx (Groves & Tanner, 1999). The groups proposed were TM 1–5, TM 6–8, TM 9–12 and TM 13–14 (numbered according to a hydropathy-based topographical model of that time). TM 13–14 (hAE1 aa 825–911, roughly corresponding to AE2 aa 1120–1237 minus the putative re-entrant loop) was proposed to be placed on the periphery of the assembly of transmembrane spans, perhaps as a mobile element (Groves & Tanner, 1999). Choi et al. (2007) showed more recently that electrogenic Na+–HCO3− cotransport requires interaction between two halves (TM 1–5 and TM 6-end) of the NBCe1 TMD. The chimera containing TM 1–5 from the electroneutral NBCn1 and TM 6-end from the electrogenic NBCe1 mediated electroneutral Na+–HCO3− cotransport, implicating TM 1–5 of the TMD of NBCn1 as sufficient to confer electroneutrality. In contrast, both halves of the NBCe1 TMD were required for electrogenicity. The missense mutation experiments of Muller-Berger et al. (1995) led them to propose that TM 5, TM 8–10 and TM 13 contribute aa residues required for AE1-mediated Cl− transport. All of these data are consistent with the observed requirement for most of the AE2 TMD to preserve wildtype AE2 patterns of acute regulation, and for the unique regulatory properties of AE2 aa 1120–1237.
Lack of evident contribution from inter-monomeric interactions to AE2 regulation by pH
The AE polypeptides form dimers or higher-order oligomers in the plasma membrane (Casey & Reithmeier, 1991; Zolotarev et al. 1999; Taylor et al. 2001). However, a single functional AE1 monomer within a dimer suffices to mediate Cl−–HCO3− exchange in erythrocytes (Jennings & Gosselink, 1995) and Xenopus oocytes (Dahl et al. 2003). Functional evidence nevertheless suggests interaction between mutant and wildtype AE1 monomers within heteroligomers (Kuma et al. 2002; Cordat et al. 2006). We tested here the possibility that pH sensitivity can be conferred on a pH-insensitive but functional monomer by a co-expressed, Cl− transport-incompetent AE2 variant. However, loss of pH regulatory function was not successfully complemented in trans by complementary AE2 sequence in the context of either co-expressed chimeras or AE2 subdomains (Fig. 5). Thus, these results provide no evidence for an intermonomeric component of AE2 regulation by pH.
Lack of regulatory function of AE2 N-glycans
N-glycosylation is important for normal trafficking or function for some membrane proteins. Thus, engineered or enzymatic lack of N-glycosylation in the GAT1 GABA–Na+–Cl− cotransporter leads to reduced protein stability, surface delivery, Na+ affinity and slowed catalytic cycling (Cai et al. 2005). Mutation of N-glycosylation sites in the Fe2+–H+ cotransporter DMT1 prevents sorting to the apical membrane (Tabuchi et al. 2002). Moreover, disruption of N-glycosylation at N39 of the Cl−-dependent (Rizwan et al. 2007) organic anion exchanger hOAT1 abolishes transport activity without altering surface expression, but mutation of all N-glycosylation sites also impairs surface delivery (Tanaka et al. 2004). However, as AE2–AE1EC3 (construct 10) is devoid of N-glycans, whereas AE1–AE2EC3 has four N-glycans (three from the EC3 of AE2 and one from the EC4 of AE1), the presence or absence of N-glycans on AE2 is not important for surface expression, function or regulation by pH in the Xenopus oocyte system (Fig. 2). These results parallel the lack of effect of tunicamycin treatment on either surface expression or function of AE2 expressed in HEK-293 cells (Fujinaga et al. 2003). Similar conclusions were reached for AE1 (Parker & Tanner, 2004) and for NBCe1 (Choi et al. 2003) expressed in Xenopus oocytes. The N-glycan of kAE1 could also be transferred from EC4 to EC3 without apparent consequence to trafficking in polarized MDCK cells (Quilty et al. 2002).
AE2 TMD regions important for regulation by pH
Replacement of C-terminal AE2 aa 1120–1237 with corresponding AE1 sequence (as in construct 3) is the only AE1 substitution besides that of EC3 which did not abrogate regulation of AE2 by pHi. This distal region of AE2 includes the putative second re-entrant loop, the last two putative transmembrane spans that may include part of the anion selectivity filter (Zhu & Casey, 2004) and the C-terminal cytoplasmic tail encompassing a proposed binding site for carbonic anhydrase (Vince et al. 2000) (Figs 1 and 2). Replacement of this region by AE1 sequence slightly enhanced sensitivity to inhibition by extracellular protons (Fig. 4), whereas replacement of any other large section of AE2 TMD with the corresponding AE1 sequence abolished or greatly reduced sensitivity to inhibition by acidic pH on either side of the plasma membrane (Figs 3 and 4). Thus, AE2 TMD aa 703–1119 are required for regulation by both pHi and pHo. We previously identified several individual aa residues of the AE2 TMD whose mutation alters AE2 regulation by pH (Stewart et al. 2007a,b). Some of these mutations selectively altered regulation by pH changes on only one side of the plasma membrane, but none individually constituted the complete ‘pH sensor’. In addition, conversion of single amino acids within AE2 aa 1120–1237 to their AE1 counterparts led to enhanced proton sensitivity, consistent with the current data on the role of AE2 aa 1120–1237 in regulation by pH. These results further support the hypothesis that multiple amino acid residues located among several TM spans regulate AE2 activity in response to changing pH (Fig. 8 of Stewart et al. 2007a). They also provide further support for independent AE2 TMD sensors for pHi and for pHo, but more precise definition of the proposed separate sensors will require additional data. The minimal contribution of AE2 aa 1120–1237 to inhibition by acid pH does not arise from a greater degree of sequence identity with AE1 than is present in other tested TMD regions (Supplemental Table 2).
We have previously identified several conserved amino acids of the AE2 N-terminal cytoplasmic domain that, when mutated, dramatically alter regulation by pHo and by pHi (Stewart et al. 2004; Kurschat et al. 2006). We propose that such cytoplasmic domain residues interact with the TMD components of the pH sensor(s) to mediate inhibition of AE2 by protons. A similar interaction between a TMD cytoplasmic loop and C-terminal cytoplasmic tail residues has been proposed for the proton-mediated inhibition of connexin permeability (Duffy et al. 2002) additionally regulated by cytoplasmic CO2 and aminosulphonates (Tao & Harris, 2004).
AE2 TMD regions important for stimulation by NH4+
Stimulation of AE2 by NH4+ also required integrity of the entire TMD (Fig. 6). However, replacement of AE2 aa 1120–1237 with the corresponding AE1 sequence was not entirely without effect, as observed for AE2 regulation by pH. Replacement of these residues attenuated but did not abolish AE2 stimulation by NH4+. Additional removal of TMD residues N-terminal to aa 1119 did abolish the stimulatory effect of NH4+. When AE2 aa 1120–1237 were substituted into a chimera with the remainder of its TMD from AE1 (Fig. 6, construct 6), these AE2 residues were not sufficient together with the AE2 N-terminal cytoplasmic domain to confer stimulation by NH4+. AE2 aa 1120–1237 may contribute to the action of the highly conserved aa 340–348 of the N-terminal cytoplasmic domain to mediate stimulation by NH4+ (Chernova et al. 2003). AE2 stimulation by NH4+ is attenuated by chelation of intracellular Ca2+, but the mechanism by which NH4+ stimulates AE2 otherwise remains little understood.
AE2 TMD regions important for inhibition by calmidazolium
As observed for NH4+ stimulation, inhibition by calmidazolium was substantially attenuated but not abolished by substitution of AE2 aa 1120–1237 with corresponding AE1 sequence (Fig. 7, construct 3). However, attenuated inhibition by calmidazolium was preserved even when AE1 substitution extended to AE2 aa 921–1237, whereas stimulation by NH4+ was abolished. Another difference from the structural requirements of NH4+ stimulation was that AE2 aa 1120–1237 sufficed to confer calmidazolium inhibition onto construct 6, a chimera with all remaining TMD residues derived from AE1. Since the AE2 N-terminal cytoplasmic domain is not required for inhibition by calmidazolium (Chernova et al. 2003), non-conserved residues present within AE2 aa 1120–1237 are probably the sole determinants of calmidazolium inhibition. As other inhibitors of calmodulin or of CaM-kinase II fail to replicate calmidazolium's inhibition of AE2 in Xenopus oocytes (Chernova et al. 2003), calmodulin-independent mechanisms of calmidazolium must be envisioned. These include direct inhibition of calcineurin and of adenylyl cyclases II and IX, activation of phospholipase A2, activation of Cai2+ entry through store-operated Ca2+ channels, inhibition of L-type Ca2+ channels and inhibition of acid secretion. These diverse actions might reflect calmidazolium's high affinity binding to the plasma membrane inner leaflet. The consequent reduction of inner leaflet negative surface charge may in turn decrease electrostatic membrane association of polycationic signalling proteins such as Kras, Src, MARCKS and the cytoplasmic tails of receptor tyrosine kinases. In addition, the calmidazolium-induced decrease in transmembrane electric field will be experienced as depolarization by intramembrane voltage sensors (Sengupta et al. 2007). Future experiments will determine whether calmidazolium inhibition can be localized to the C-terminal cytoplasmic tail or to the immediately preceding transmembrane spans of AE2.
Conclusion
Previous work has identified individual amino acids located across the entire AE2 TMD (both non-conserved and conserved in AE1) that are required for normal AE2 regulation by pH. However, no single amino acid residue substitution abolished regulation by pH, consistent with contributions of multiple TMD subdomains to the AE2 ‘pH sensor’. We have now identified subdomains of the AE2 TMD that are required for isoform-specific sensitivity to four types of acute regulation to which the related AE1 polypeptide is unresponsive. Normal inhibition of AE2 by acidic intracellular or extracellular pH and normal stimulation of transporter activity by NH4+ each require interaction between two TMD regions encompassing AE2 amino acids 703–843 and 920–1119. AE2-specific residues within C-terminal AE2 aa 1120–1237 are not critical for regulation by acidic pH and are not sufficient to confer normal stimulation by NH4+. In contrast, AE2 aa 1120–1237 are required for wildtype AE2 inhibition by calmidazolium, and substitution into the AE1 TMD of AE2 aa 1120–1237 suffices to confer full sensitivity to inhibition by calmidazolium. Future experiments will define more precisely the contributions of individual amino acid residues from each TMD subdomain to the complex TMD pH ‘sensor(s)’ that acutely regulate(s) AE2-mediated anion transport.
Supplementary material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.136119/DC1
and
http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.136119
References
- Alper SL. The band 3-related anion exchanger (AE) gene family. Annu Rev Physiol. 1991;53:549–564. doi: 10.1146/annurev.ph.53.030191.003001. [DOI] [PubMed] [Google Scholar]
- Alper SL. Genetic diseases of acid-base transporters. Annu Rev Physiol. 2002;64:899–923. doi: 10.1146/annurev.physiol.64.092801.141759. [DOI] [PubMed] [Google Scholar]
- Alper SL, Natale J, Gluck S, Lodish HF, Brown D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci U S A. 1989;86:5429–5433. doi: 10.1073/pnas.86.14.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese F, Renard C, Gabillat N, Pellissier B, Guizouarn H. Molecular mapping of the conductance activity linked to tAE1 expressed in Xenopus oocyte. Biochim Biophys Acta. 2004;1664:80–87. doi: 10.1016/j.bbamem.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Brosius FC, 3rd, Alper SL, Garcia AM, Lodish HF. The major kidney band 3 gene transcript predicts an amino-terminal truncated band 3 polypeptide. J Biol Chem. 1989;264:7784–7787. [PubMed] [Google Scholar]
- Cai G, Salonikidis PS, Fei J, Schwarz W, Schulein R, Reutter W, Fan H. The role of N-glycosylation in the stability, trafficking and GABA-uptake of GABA-transporter 1. Terminal N-glycans facilitate efficient GABA-uptake activity of the GABA transporter. FEBS J. 2005;272:1625–1638. doi: 10.1111/j.1742-4658.2005.04595.x. [DOI] [PubMed] [Google Scholar]
- Casey JR, Reithmeier RA. Analysis of the oligomeric state of Band 3, the anion transport protein of the human erythrocyte membrane, by size exclusion high performance liquid chromatography. Oligomeric stability and origin of heterogeneity. J Biol Chem. 1991;266:15726–15737. [PubMed] [Google Scholar]
- Chernova MN, Jiang L, Crest M, Hand M, Vandorpe DH, Strange K, Alper SL. Electrogenic sulfate/chloride exchange in Xenopus oocytes mediated by murine AE1 E699Q. J Gen Physiol. 1997;109:345–360. doi: 10.1085/jgp.109.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernova MN, Stewart AK, Jiang L, Friedman DJ, Kunes YZ, Alper SL. Structure-function relationships of AE2 regulation by Cai2+-sensitive stimulators NH4+ and hypertonicity. Am J Physiol Cell Physiol. 2003;284:C1235–C1246. doi: 10.1152/ajpcell.00522.2002. [DOI] [PubMed] [Google Scholar]
- Choi I, Hu L, Rojas JD, Schmitt BM, Boron WF. Role of glycosylation in the renal electrogenic Na+-HCO3− cotransporter (NBCe1) Am J Physiol Renal Physiol. 2003;284:F1199–F1206. doi: 10.1152/ajprenal.00131.2002. [DOI] [PubMed] [Google Scholar]
- Choi I, Soo Yang H, Boron WF. The electrogenicity of the rat sodium–bicarbonate cotransporter NBCe1 requires interactions among transmembrane segments of the transporter. J Physiol. 2007;578:131–142. doi: 10.1113/jphysiol.2006.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordat E, Kittanakom S, Yenchitsomanus PT, Li J, Du K, Lukacs GL, Reithmeier RA. Dominant and recessive distal renal tubular acidosis mutations of kidney anion exchanger 1 induce distinct trafficking defects in MDCK cells. Traffic. 2006;7:117–128. doi: 10.1111/j.1600-0854.2005.00366.x. [DOI] [PubMed] [Google Scholar]
- Dahl NK, Jiang L, Chernova MN, Stuart-Tilley AK, Shmukler BE, Alper SL. Deficient HCO3− transport in an AE1 mutant with normal Cl− transport can be rescued by carbonic anhydrase II presented on an adjacent AE1 protomer. J Biol Chem. 2003;278:44949–44958. doi: 10.1074/jbc.M308660200. [DOI] [PubMed] [Google Scholar]
- Duffy HS, Sorgen PL, Girvin ME, O'Donnell P, Coombs W, Taffet SM, Delmar M, Spray DC. pH-dependent intramolecular binding and structure involving Cx43 cytoplasmic domains. J Biol Chem. 2002;277:36706–36714. doi: 10.1074/jbc.M207016200. [DOI] [PubMed] [Google Scholar]
- Fujinaga J, Loiselle FB, Casey JR. Transport activity of chimaeric AE2-AE3 chloride/bicarbonate anion exchange proteins. Biochem J. 2003;371:687–696. doi: 10.1042/BJ20030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga J, Tang XB, Casey JR. Topology of the membrane domain of human erythrocyte anion exchange protein, AE1. J Biol Chem. 1999;274:6626–6633. doi: 10.1074/jbc.274.10.6626. [DOI] [PubMed] [Google Scholar]
- Grinstein S, Ship S, Rothstein A. Anion transport in relation to proteolytic dissection of band 3 protein. Biochim Biophys Acta. 1978;507:294–304. doi: 10.1016/0005-2736(78)90424-8. [DOI] [PubMed] [Google Scholar]
- Groves JD, Tanner MJ. Structural model for the organization of the transmembrane spans of the human red-cell anion exchanger (band 3; AE1) Biochem J. 1999;344:699–711. [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Chernova MN, Jiang L, Zhang Y, Alper SL. NH4Cl activates AE2 anion exchanger in Xenopus oocytes at acidic pHi. Am J Physiol. 1997;272:C1232–1240. doi: 10.1152/ajpcell.1997.272.4.C1232. [DOI] [PubMed] [Google Scholar]
- Jennings ML, Gosselink PG. Anion exchange protein in Southeast Asian ovalocytes: heterodimer formation between normal and variant subunits. Biochemistry. 1995;34:3588–3595. doi: 10.1021/bi00011a013. [DOI] [PubMed] [Google Scholar]
- Kanki T, Sakaguchi M, Kitamura A, Sato T, Mihara K, Hamasaki N. The tenth membrane region of band 3 is initially exposed to the luminal side of the endoplasmic reticulum and then integrated into a partially folded band 3 intermediate. Biochemistry. 2002;41:13973–13981. doi: 10.1021/bi026619q. [DOI] [PubMed] [Google Scholar]
- Kopito RR, Lee BS, Simmons DM, Lindsey AE, Morgans CW, Schneider K. Regulation of intracellular pH by a neuronal homolog of the erythrocyte anion exchanger. Cell. 1989;59:927–937. doi: 10.1016/0092-8674(89)90615-6. [DOI] [PubMed] [Google Scholar]
- Kuma H, Abe Y, Askin D, Bruce LJ, Hamasaki T, Tanner MJ, Hamasaki N. Molecular basis and functional consequences of the dominant effects of the mutant band 3 on the structure of normal band 3 in Southeast Asian ovalocytosis. Biochemistry. 2002;41:3311–3320. doi: 10.1021/bi011678+. [DOI] [PubMed] [Google Scholar]
- Kurschat CE, Shmukler BE, Jiang L, Wilhelm S, Kim EH, Chernova MN, Kinne RK, Stewart AK, Alper SL. Alkaline-shifted pHo sensitivity of AE2c1-mediated anion exchange reveals novel regulatory determinants in the AE2 N-terminal cytoplasmic domain. J Biol Chem. 2006;281:1885–1896. doi: 10.1074/jbc.M509734200. [DOI] [PubMed] [Google Scholar]
- Lindsey AE, Schneider K, Simmons DM, Baron R, Lee BS, Kopito RR. Functional expression and subcellular localization of an anion exchanger cloned from choroid plexus. Proc Natl Acad Sci U S A. 1990;87:5278–5282. doi: 10.1073/pnas.87.14.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Berger S, Karbach D, Konig J, Lepke S, Wood PG, Appelhans H, Passow H. Inhibition of mouse erythroid band 3-mediated chloride transport by site-directed mutagenesis of histidine residues and its reversal by second site mutation of Lys 558, the locus of covalent H2DIDS binding. Biochemistry. 1995;34:9315–9324. doi: 10.1021/bi00029a006. [DOI] [PubMed] [Google Scholar]
- Parker MD, Tanner MJ. The disruption of the third extracellular loop of the red cell anion exchanger AE1 does not affect electroneutral Cl−/HCO3− exchange activity. Blood Cells Mol Dis. 2004;32:379–383. doi: 10.1016/j.bcmd.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Quilty JA, Li J, Reithmeier RA. Impaired trafficking of distal renal tubular acidosis mutants of the human kidney anion exchanger kAE1. Am J Physiol Renal Physiol. 2002;282:F810–F820. doi: 10.1152/ajprenal.00216.2001. [DOI] [PubMed] [Google Scholar]
- Rizwan AN, Krick W, Burckhardt G. The chloride dependence of the human organic anion transporter 1 (hOAT1) is blunted by mutation of a single amino acid. J Biol Chem. 2007;282:13402–13409. doi: 10.1074/jbc.M609849200. [DOI] [PubMed] [Google Scholar]
- Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO3− transporters. Pflugers Arch. 2004;447:495–509. doi: 10.1007/s00424-003-1180-2. [DOI] [PubMed] [Google Scholar]
- Sengupta P, Ruano MJ, Tebar F, Golebiewska U, Zaitseva I, Enrich C, McLaughlin S, Villalobo A. Membrane-permeable calmodulin inhibitors (e.g. W-7/W-13) bind to membranes, changing the electrostatic surface potential: dual effect of W-13 on epidermal growth factor receptor activation. J Biol Chem. 2007;282:8474–8486. doi: 10.1074/jbc.M607211200. [DOI] [PubMed] [Google Scholar]
- Stewart AK, Chernova MN, Kunes YZ, Alper SL. Regulation of AE2 anion exchanger by intracellular pH: critical regions of the NH2-terminal cytoplasmic domain. Am J Physiol Cell Physiol. 2001;281:C1344–C1354. doi: 10.1152/ajpcell.2001.281.4.C1344. [DOI] [PubMed] [Google Scholar]
- Stewart AK, Chernova MN, Shmukler BE, Wilhelm S, Alper SL. Regulation of AE2-mediated Cl− transport by intracellular or by extracellular pH requires highly conserved amino acid residues of the AE2 NH2-terminal cytoplasmic domain. J Gen Physiol. 2002;120:707–722. doi: 10.1085/jgp.20028641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AK, Kerr N, Chernova MN, Alper SL, Vaughan-Jones RD. Acute pH-dependent regulation of AE2-mediated anion exchange involves discrete local surfaces of the NH2-terminal cytoplasmic domain. J Biol Chem. 2004;279:52664–52676. doi: 10.1074/jbc.M408108200. [DOI] [PubMed] [Google Scholar]
- Stewart AK, Kurschat CE, Alper SL. Role of nonconserved charged residues of the AE2 transmembrane domain in regulation of anion exchange by pH. Pflugers Arch. 2007a;454:373–384. doi: 10.1007/s00424-007-0220-8. [DOI] [PubMed] [Google Scholar]
- Stewart AK, Kurschat CE, Burns D, Banger N, Vaughan-Jones RD, Alper SL. Transmembrane domain histidines contribute to regulation of AE2-mediated anion exchange by pH. Am J Physiol Cell Physiol. 2007b;292:C909–C918. doi: 10.1152/ajpcell.00265.2006. [DOI] [PubMed] [Google Scholar]
- Tabuchi M, Tanaka N, Nishida-Kitayama J, Ohno H, Kishi F. Alternative splicing regulates the subcellular localization of divalent metal transporter 1 isoforms. Mol Biol Cell. 2002;13:4371–4387. doi: 10.1091/mbc.E02-03-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Xu W, Zhou F, You G. Role of glycosylation in the organic anion transporter OAT1. J Biol Chem. 2004;279:14961–14966. doi: 10.1074/jbc.M400197200. [DOI] [PubMed] [Google Scholar]
- Tao L, Harris AL. Biochemical requirements for inhibition of Connexin26-containing channels by natural and synthetic taurine analogs. J Biol Chem. 2004;279:38544–38554. doi: 10.1074/jbc.M405654200. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Zhu Q, Casey JR. Cysteine-directed cross-linking localizes regions of the human erythrocyte anion-exchange protein (AE1) relative to the dimeric interface. Biochem J. 2001;359:661–668. doi: 10.1042/0264-6021:3590661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JW, Carlsson U, Reithmeier RA. Localization of the Cl−/HCO3− anion exchanger binding site to the amino-terminal region of carbonic anhydrase II. Biochemistry. 2000;39:13344–13349. doi: 10.1021/bi0015111. [DOI] [PubMed] [Google Scholar]
- Vince JW, Reithmeier RA. Identification of the carbonic anhydrase II binding site in the Cl−/HCO3− anion exchanger AE1. Biochemistry. 2000;39:5527–5533. doi: 10.1021/bi992564p. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chernova MN, Stuart-Tilley AK, Jiang L, Alper SL. The cytoplasmic and transmembrane domains of AE2 both contribute to regulation of anion exchange by pH. J Biol Chem. 1996;271:5741–5749. doi: 10.1074/jbc.271.10.5741. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Casey JR. The substrate anion selectivity filter in the human erythrocyte Cl−/HCO3− exchange protein, AE1. J Biol Chem. 2004;279:23565–23573. doi: 10.1074/jbc.M401380200. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Lee DW, Casey JR. Novel topology in C-terminal region of the human plasma membrane anion exchanger, AE1. J Biol Chem. 2003;278:3112–3120. doi: 10.1074/jbc.M207797200. [DOI] [PubMed] [Google Scholar]
- Zolotarev AS, Shmukler BE, Alper SL. AE2 anion exchanger polypeptide is a homooligomer in pig gastric membranes: a chemical cross-linking study. Biochemistry. 1999;38:8521–8531. doi: 10.1021/bi990337h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.