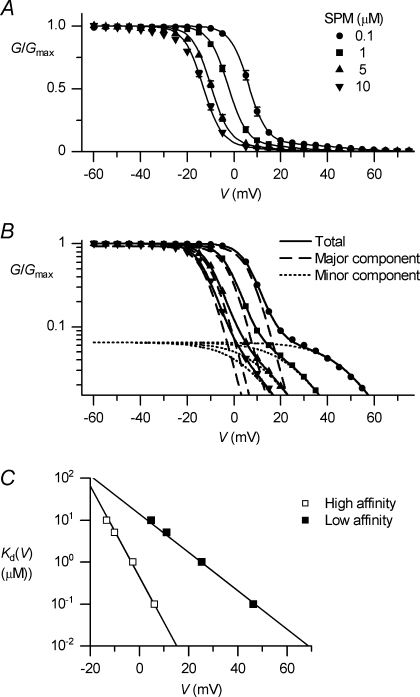
Abstract
The outward component of the strong inward rectifier K+ current (IKir) plays a pivotal role in polarizing the membranes of excitable and non-excitable cells and is regulated by voltage-dependent channel block by internal cations. Using the Kir2.1 channel, we previously showed that a small fraction of the conductance susceptible only to a low-affinity mode of block likely carries a large portion of the outward current. To further examine the relevance of the low-affinity block to outward IKir and to explore its molecular mechanism, we studied the block of the Kir2.1 and Kir2.2 channels by spermine, which is the principal Kir2 channel blocker. Current–voltage relations of outward Kir2.2 currents showed a peak, a plateau and two peaks in the presence of 10, 1 and 0.1 μm spermine, respectively, which was explained by the presence of two conductances that differ in their susceptibility to spermine block. When the current–voltage relations showed one peak, like those of native IKir, outward Kir2.2 currents were mediated mostly by the conductance susceptible to the low-affinity block. They also flowed in a narrower range than the corresponding Kir2.1 currents, because of 3- to 4-fold greater susceptibility to the low-affinity block than in Kir2.1. Reducing external [K+] shifted the voltage dependences of both the high- and low-affinity block of Kir2.1 in parallel with the shift in the reversal potential, confirming the importance of the low-affinity block in mediating outward IKir. When Kir2.1 mutants known to have reduced sensitivity to internal blockers were examined, the D172N mutation in the transmembrane pore region made almost all of the conductance susceptible only to low-affinity block, while the E224G mutation in the cytoplasmic pore region reduced the sensitivity to low-affinity block without markedly altering that to the high-affinity block or the high/low conductance ratio. The effects of these mutations support the hypothesis that Kir2 channels exist in two states having different susceptibilities to internal cationic blockers.
The outward component of the classical strong inward rectifier K+ current (IKir) is significantly smaller than the inward component and is negligible at depolarized voltages. This strong inward rectification is caused by a voltage-dependent block of the channels by intracellular cations, such as polyamines (spermine (SPM) and spermidine (SPD)) and Mg2+ (Lopatin et al. 1995; Stanfield et al. 2002), among which SPM is the principal determinant of the rectification (Fakler et al. 1995; Ishihara et al. 1996; Ishihara & Ehara, 2004). Despite its relatively small amplitude, as compared to the inward current, the current density of the outward IKir is large enough to bring the membrane potential to a negative value close to the K+ equilibrium potential (EK) in both excitable cells (e.g. cardiac myocytes and skeletal muscle fibres) and many types of non-excitable cells (e.g. smooth muscle cells, vascular endothelial cells and exocrine cells) (Quayle et al. 1997; Nilius & Droogmans, 2001; Hayashi et al. 2003). In cardiac myocytes, IKir (the cardiac current is known as IK1) is one of the major currents terminating the cardiac action potential (Matsuoka et al. 2003). However, the detailed mechanism underlying the outward IKir had not been well understood because the time-dependent gating of IKir, reflecting the block and unblock of the channel by SPM (Ishihara et al. 1996), seemed to be closed at voltages where the outward component of the cardiac IKir flows (Kurachi, 1985; Ishihara et al. 1989; Oliva et al. 1990).
IKir flows through tetrameric channels formed by subunits in the Kir2 family (Kir2.1–2.4). The polyamine block of Kir2 channels has been studied mainly using Kir2.1, which may be the predominant subunit mediating the cardiac IKir (Zaritsky et al. 2001; Miake et al. 2003). Studies have shown that block of the outward currents by internal particles involves both high- and low-affinity block (Yang et al. 1995a; Guo & Lu, 2000), but the molecular mechanisms for two types of block, and the physiological relevance of the low-affinity block, remained unclear. Yang et al. (1995a) showed that their dose–response data contained two distinct components corresponding to high and low affinity binding. Although they had considered the possibility that there are two different populations of channels with distinct affinities for the blockers, they later proposed that the high-affinity block may not abolish the current flow and that the binding of a second blocker to a channel may occur with lower affinity as a result of electrostatic repulsion and steric hindrance between the blockers (Yang et al. 1995b). Guo & Lu (2000) showed that the current–voltage (I–V) relations of the outward currents exhibit multiple phases, which they explained by assuming that, in the high-affinity blocking state, the polyamine traverses the pore to reduce the block at positive voltages. In our previous study (Ishihara & Ehara, 2004), we showed that the different shapes of the I–V relations of the outward currents obtained in the presence of various concentrations of polyamines are nicely explained by the sum of two conductances showing different susceptibilities to polyamine block. In that study, we demonstrated that the model of Guo & Lu (2000), in which two blocked states occur in a mutually exclusive manner (or that two types of blockers compete for block), does not explain the polyamine concentration dependence of the I–V relations. Moreover, internal Mg2+ at physiological intracellular concentrations (0.5–1 mm) appeared to block only the major conductance susceptible to high-affinity block, thereby inducing a time-dependent component in the outward currents that reflected the competition between Mg2+ and SPM for block of the channel (Yan & Ishihara, 2005). Thus, our current hypothesis is that Kir2 channels exist in two different states with differing susceptibilities to internal cationic blockers (Ishihara & Ehara, 2004).
Our earlier studies of Kir2.1 indicated that a large portion of the outward currents of the native IKir is likely carried by a small fraction of the conductance susceptible to the low-affinity mode of block (Ishihara & Ehara, 2004; Yan & Ishihara, 2005). To further examine the physiological relevance of the low-affinity block, here we first compared the SPM block of Kir2.2 with that of Kir2.1. The former shares 70% identity with the latter at the amino-acid level (Takahashi et al. 1994) and, together, Kir2.2 and Kir2.1 likely mediate the cardiac IKir (Yamashita et al. 1996; Zaritsky et al. 2001; Liu et al. 2001). We then studied the SPM block of Kir2.1 under the low external [K+] conditions. To explore the molecular mechanism of the two types of block, we also studied Kir2.1 mutants in which the negatively charged residues D172 and E224 situated on the wall of the pore (Nishida & Mackinnon, 2002; Kuo et al. 2003; Pegan et al. 2005) were replaced with corresponding uncharged residues in the weak inward rectifier Kir1.1. These residues are known to be involved in the high-affinity SPM block (Lu & Mackinnon, 1994; Stanfield et al. 1994; Yang et al. 1995a; Taglialatela et al. 1995), though the site to which polyamines bind to block the K+ permeation remains controversial (Guo & Lu, 2003; Guo et al. 2003; John et al. 2004; Kurata et al. 2004; Kurata et al. 2006). Our results indicate that E224 situated on the wall of the cytoplasmic pore plays a key role in facilitating the high-affinity block and its relief, but is not the binding site for block in the ‘high-affinity channel’, though it is a critical component of the binding site related to block of the ‘low-affinity channel.
Methods
Exogenous expression of Kir2 channels in 293T cells
293T cells (derived from the human embryonic kidney cell line 293 and expressing the SV40 large T antigen) were grown in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum, 0.15% sodium bicarbonate, 200 U ml−1 penicillin and 200 μg ml−1 streptomycin. Using Effectene transfection reagent (Qiagen Inc.), the cells were cotransfected with 1 μg of Kir2.2 (mouse; Takahashi et al. 1994), Kir2.1 (mouse; Kubo et al. 1993) or Kir2.1 mutant cDNA, all subcloned into the mammalian expression vector pCXN2 (Niwa et al. 1991), plus 0.1 μg pEGFP-N1 (Clontech) per 60 mm dish. Cells expressing exogenous genes were identified by visualizing the EGFP fluorescence using an inverted fluorescence microscope.
Current recordings from 293T cells expressing Kir2 channels
On the day of transfection, cells were seeded onto small pieces of collagen-coated cover glass (Asahi Techno Glass Corporation, Tokyo Japan). Within 24–56 h after transfection, a piece of cover glass was placed in a recording chamber (area: 4 mm × 23 mm; volume: ∼0.5 ml) mounted on the stage of an inverted fluorescence microscope (TMD300 or TE2000, Nikon, Tokyo, Japan), which was continuously perfused with bath solution at a rate of ∼3 ml min−1. Currents were recorded from inside-out patches with a patch-clamp amplifier (EPC-8, HEKA; or Axopatch 200B, Axon Instruments) using Silicone-coated and fire-polished patch electrodes made from borosilicate glass capillaries (1.65 mm o.d., 0.165 mm wall thickness; Hilgenberg GmbH, Malsfeld, Germany). The pipette (external) solution used to compare the SPM block of Kir2.1 and Kir2.2 (Fig. 1) and to examine the SPM block of Kir2.1 mutants (Figs 7, 8 and 9) contained (mM): 145 KCl, 1 CaCl2, and 5 Hepes (pH 7.4 adjusted with KOH). The pipette solutions used to study the external K+ dependence of the SPM block of Kir2.1 (Fig. 4) were devoid of CaCl2 and were buffered to pH 7.4 using 8 mm K2HPO4 and 2 mm KH2PO4: 150 mm K+, 50 mm K+ and 20 mm K+ solutions contained 132 mm, 32 mm and 2 mm KCl, respectively. The osmolarities of the 50 mm and 20 mm K+ solutions were not adjusted (see Discussion). The 5.4 mm K+ pipette solution (Fig. 6) contained (mm): 5.4 KCl, 139.6 NMDG-Cl, 1 CaCl2 and 5 Hepes (pH 7.4 adjusted with NMDG). The pipette resistance was typically 1.7–2.0 MΩ when filled with the pipette solution.
Figure 1.
SPM block of outward currents through Kir2.2 channels A, representative Kir2.2 currents obtained from an inside-out patch in the absence and presence of the indicated concentrations of SPM. a, currents elicited by test pulses to between −60 and +80 mV in 10 mV increments following a hyperpolarizing prepulse (−40 mV, 10 ms) to relieve the channels from block. The holding potential was 0 mV. b, enlarged outward currents at +20 mV and +70 mV. B, steady-state I–V relations constructed from the data in A. Currents were measured ∼200 ms after the pulse onset; except in the presence of 0.1 μm SPM they were measured ∼600 ms after onset. C, comparison of I–V relations of outward Kir2.2 (a) and Kir2.1 (b) currents in the presence of the indicated concentrations of SPM. Current amplitudes were normalized with respect to the inward-current amplitude at −40 mV in each relation. Shown are mean values for Kir2.2 obtained with 5–6 patches and for Kir2.1 obtained with 8–15 patches. Error bars are not shown when smaller than the symbol.
Figure 7.
SPM block of Kir2.1(D172N) channels A, representative currents recorded from an inside-out patch in the absence and presence of the indicated concentrations of SPM. Currents were elicited by test pulses to between −60 and +80 mV in 10 mV increments following a hyperpolarizing prepulse to −40 mV. The holding potential was 0 mV. B, steady-state I–V relations constructed from the currents in A. The right panel shows the reconstructed I–V relations for wild-type Kir2.1 in the presence of 0.1, 1 and 10 μm SPM (see the legend to Fig. 3 for details of the calculation). Red lines depict the components mediated by the ‘low-affinity conductance’. C, G–V relations in the presence of the indicated concentrations of SPM. Mean values from 3 to 4 patches are shown. Error bars are not shown when smaller than the symbol. Continuous lines are the Boltzmann relations fitted using a RT/z1F value of 8.4 mV. Reconstructed G/Gmax values for the wild-type Kir2.1 with only low-affinity block (φ= 0) were superimposed for comparison (red dotted lines); see the legend to Fig. 3 for details of the calculation. D, dissociation constants for the SPM block of Kir2.1(D172N) inferred from the half-blocking voltages of fitted Boltzmann relations (symbols). Fitting to eqn (2) using a RT/z1F value of 8.4 mV (continuous line) gave a Kd(0) value of 33 μm. Kd(V) values for the SPM block of the wild-type Kir2.1 (black dotted line, high; red dotted line, low) are also shown for comparison.
Figure 8.
SPM block of Kir2.1(E224G) channels A, representative currents recorded from an inside-out patch in the absence and presence of the indicated concentrations of SPM. a, currents elicited by test pulses to between −60 and +80 mV in 10 mV increments from a holding potential of −40 mV b, outward currents at +20, +40 and +60 mV in the presence of 1 μm (left) or 10 μm (right) SPM shown on a longer time scale. B, steady-state I–V relations constructed from the currents in A. Currents were measured at the end of 15, 5, 2 and 1 s test pulses for 1, 10, 100 and 500 μm SPM, respectively, and were normalized to the inward-current amplitude at −60 mV in each SPM concentration. C, G–V relations in the presence of the indicated concentrations of SPM before (a) and after (b) correcting for the instantaneous rectification observed in SPM-free solution. Open circles shown in a denote the instantaneous inward rectification in the SPM-free solution. Mean values from 3 to 4 patches are shown. Error bars are shown in a when larger than the symbol. Continuous lines shown in b are fits to eqn (1); dashed and dotted lines denote the major and minor Boltzmann components, respectively. Fitted RT/z1F and RT/z2F values were 7.9 mV and 18 mV, respectively. D, dissociation constants for the SPM block of Kir2.1(E224G) inferred from the half-activation voltages of the fitted Boltzmann components (open circles, high affinity; filled circles, low affinity). Continuous lines are the fits to eqn (2) using the RT/ziF values of 7.9 mV (high) and 18 mV (low). Fittings gave Kd(0) values of 1.7 μm and 14.9 mm for the high-affinity and low-affinity block, respectively. Kd(V) values for the SPM block of the wild-type Kir2.1 (black dotted line, high; red dotted line, low) are also shown for comparison.
Figure 9.
SPM block of Kir2.1(D172N & E224G) channels A, representative currents recorded from an inside-out patch in the presence of the indicated concentrations of SPM. Currents were elicited by test pulses to between −60 and +80 mV in 10 mV increments following a hyperpolarizing prepulse to −40 mV. The holding potential was 0 mV. Note that the current scales vary for the different SPM concentrations. B, steady-state I–V relations constructed from the currents in A. Current amplitudes were normalized to the inward-current amplitude at −60 mV at each SPM concentration. C, G–V relations in the presence of the indicated concentrations of SPM before (a) and after (b) correcting for the instantaneous rectification observed in SPM-free solution. Open circles shown in a denote the instantaneous inward rectification in the SPM-free solution. Mean values from 3 to 4 patches are shown. Error bars are shown in a when larger than the symbol. In b, reconstructed G/Gmax values for the Kir2.1(E224G) with only the low-affinity block (φ= 0) were superimposed for comparison (green line, 100 μm; blue line, 500 μm).
Figure 4.
SPM block of the outward Kir2.1 currents at low external [K+] A, representative currents recorded from inside-out patches in the absence and presence of the indicated concentrations of SPM in 150 mm (a), 50 mm (b) and 20 mm (c) external [K+]. The holding potential was set close to Vrev, and short (3 or 20 ms) hyperpolarizing prepulses (∼40 mV more negative than Vrev) were given before applying test pulses. Test pulses were from ∼50 mV more negative than Vrev to ∼80 mV more positive than Vrev in 10 mV increments. Currents in the same [K+] were obtained from one patch. B, enlarged outward currents at ∼20 mV and ∼70 mV more positive than Vrev in 150 mm (a), 50 mm (b) and 20 mm (c) external [K+]. C, steady-state I–V relations constructed from the data in A: upper panels, I–Vs for the outward currents in the presence of 0.1, 1 or 5 μm SPM; lower panels, I–Vs for the inward and outward currents in the presence of 0.1 or 10 μm SPM.
Figure 6.
SPM block of the outward Kir2.1 currents at 5.4 mm external [K+] A, representative currents from an inside-out patch with 1, 5 or 10 μm SPM. The holding potential was set at −70 mV (near Vrev), and a 5 ms hyperpolarizing prepulse to −110 mV (∼40 mV more negative than Vrev) was given before applying test pulses from −130 mV to 20 mV in 10 mV increments. Lower panels show enlarged outward currents at −50 mV and 0 mV (∼20 mV and ∼70 mV more positive than Vrev, respectively). NMDG was included in the 5.4 mm K+ pipette solution to correct the osmolarity. B, normalized I–V relations for the outward currents. Currents obtained from the same patch with different concentrations of SPM were normalized to the largest amplitude of the outward currents observed at ∼−50 mV in the presence of 5 μm SPM. Mean values from 4 patches (0.1, 1 and 5 μm SPM) and 3 patches (10 μm SPM) are shown. Error bars are not shown when smaller than the symbol.
The control bath (internal) solution contained (mm): 120 KCl, 4 K2EDTA, 7.2 K2HPO4 and 2.8 KH2PO4 (pH 7.2 adjusted with ∼4 mm KOH). The free Mg2+ and Ca2+ concentrations in this solution were calculated to be at submicromolar levels (Fabiato & Fabiato, 1979), assuming that the amounts of Ca2+ and Mg2+ contained in the solution were ∼10 μm each. Bath solutions containing various concentrations of SPM were made by adding the appropriate amount of spermine-4HCl (Nacalai Tesque, Kyoto, Japan; as a 10 mm stock solution in distilled water; stored in small aliquots at −20°C) to the control bath solution. Bath solution containing SPM was applied after the native inward rectification had been removed to as great an extent as possible in the control bath solution. All experiments were conducted at room temperature (24–25°C).
Data analysis
Steady-state current amplitudes were obtained using appropriate pulse duration. We did not subtract any background currents because of the difficulty in defining those currents. 293T cells show voltage-gated K+ currents activated at depolarized voltages under whole-cell conditions (Yu & Kerchner, 1998). These endogenous currents were usually negligibly small in our inside-out patch experiments using pipettes of ∼2 MΩ, especially when expression levels of exogenous Kir2 channels were high. Because Kir2 channels show an apparently ohmic open channel conductance (as can be seen from the instantaneous current amplitudes at various test potentials under the SPM free condition in Figs 1A and 4A), we analysed the normalized chord conductance (G/Gmax) to estimate the unblocked fraction of Kir2 channels at different voltages, as before (Ishihara & Ehara, 2004). Unless otherwise noted, the maximum value of the chord conductance (Gmax) obtained at the same SPM concentration was used for normalization to minimize the influence of current rundown. This analytical procedure was chosen because outward Kir2 currents exhibit slow gating, even in the nominal absence of SPM (e.g. Fig. 1A), which may cause a marginal overestimation of the block in this procedure, but may give rise to a significant underestimation of the block if the percentage block is calculated from isochronal current amplitudes in the presence and absence of SPM.
The voltage dependence of G/Gmax (G–V relation) was fitted to the sum of two Boltzmann relations:
 |
(1) |
where Vh1 and Vh2 are the half-activation voltages, z1 and z2 are the parameters for the voltage dependence of blocking, φ is the fractional amplitude of the first Boltzmann component, and R, T and F are the usual thermodynamic constants. In the experiments conducted under the symmetrical [K+] condition, G/Gmax approached a constant value at positive voltages where the Kir2 conductance became very small, presumably reflecting a non-specific leak conductance (GL). This value was less than 1% of Gmax in the analysed experiments. In the experiments conducted in low external [K+], the reversal potential of currents (Vrev) did not notably change when outward Kir2.1 currents were reduced by increasing the internal SPM concentration, and I–V relations crossed at the zero current potential (Fig. 4C), indicating that the contribution of the leak current was also insignificant. Still, to minimize interference by GL, eqn (1) was fitted to G–V relations using G/Gmax values larger than 3%. Fitting of the data to theoretical equations was done using Origin (version 6, OriginLab Corp., Northampton, MA, USA).
The junction potentials between the pipette and bath solutions were negligibly small (< 0.4 mV), except for those in the experiments shown in Fig. 6, which were ∼12 mV. These junction potentials were not corrected for.
All statistical values are given as means ±s.e.m.
Results
SPM-induced inward rectification of Kir2.2
We first examined the SPM-mediated block of Kir2.2 currents in symmetrical 150 mm[K+]. When SPM was added to the bath (internal) solution to final concentrations ranging from 0.1 μm to 10 μm, it blocked the outward component of Kir2.2 currents in a concentration-dependent manner without significantly affecting the inward component (Fig. 1A and B). The shapes of the I–V relations for the outward currents were distinct at different SPM concentrations: a peak was observed with 5 or 10 μm SPM, a plateau with 1 μm and two peaks with 0.1 μm (Fig. 1Ca). These findings were similar to those obtained with Kir2.1 (Fig. 1Cb in this study; Ishihara & Ehara, 2004). However, the outward-to-inward ratios of Kir2.2 current amplitudes were smaller than those obtained with Kir2.1; the ratio was roughly 2-fold smaller for the largest outward current observed at each SPM concentration (Fig. 1C). In the presence of 5 or 10 μm SPM, the outward components of Kir2.1 currents were similar to those of IKir in native cardiac ventricular myocytes in that they were observed at voltages ranging to at least Vrev+∼50 mV and showed a clear peak at Vrev+∼20 mV (e.g. Ishihara et al. 2002). For Kir2.2, the outward currents flowed in a narrower voltage range, and the peak amplitude was observed at more negative voltages.
We then analysed the G–V relations to estimate the unblocked fraction of Kir2.2 channels at different membrane potentials (Fig. 2A). The conductance values became smaller as the membrane potential become more positive, indicating the voltage-dependent block of the channels by SPM. Since the normalized conductance values for the outward currents were small, the relations were plotted on a semilogarithmic scale in Fig. 2B. As with Kir2.1 (Ishihara & Ehara, 2004), the G–V relations obtained in the presence of various concentrations of SPM could be approximated by the sum of two Boltzmann relations (eqn (1)). Increasing the SPM concentration shifted the half-activation voltages of the two Boltzmann components (dashed lines and dotted lines in Fig. 2B) in the negative direction without changing their fractional amplitudes. This finding strongly suggests that there are two conductances susceptible to different modes of SPM block. Indeed, the apparent dissociation constants for the two blocking modes, inferred from the relationship between the SPM concentration and the half-activation voltages of the Boltzmann components, declined exponentially with increasing membrane potential (Fig. 2C), and the relations could be expressed by the equation (Woodhull, 1973):
| (2) |
where Kd(0) is the dissociation constant at 0 membrane potential, using the ziF/RT value determined from the steepness of the voltage dependence of the fitted Boltzmann components (Fig. 2C, lines). The voltage dependence of the high-affinity block was steeper (zi= 6.4 with RT/F = 25.7 mV) than that of the low-affinity block (zi= 2.7 with RT/F = 25.7 mV). The Kd(0) values obtained from the fittings were 0.44 μm and 14 μm for the high-affinity and low-affinity block, respectively. The fractional conductance susceptible to low-affinity block was 0.065, which was smaller than the value of 0.1 we obtained for Kir2.1 under the same experimental conditions (Ishihara & Ehara, 2004).
Figure 2.
Voltage dependence of the SPM block of Kir2.2 channels A, G–V relations in the presence of the indicated concentrations of SPM. Mean values from 5 to 6 patches are shown. Error bars are not shown when smaller than the symbol. Continuous lines are fits to eqn (1). B, fitting of G–V relations with the sum of two Boltzmann relations shown on a semilogarithmic scale. Symbols are the mean values shown in A. Continuous lines are fits to eqn (1); dashed and dotted lines show the major and minor Boltzmann components, respectively. RT/z1F and RT/z2F values were 4 mV and 9.5 mV, respectively, and the fractional amplitude of the major Boltzmann component (φ) was 0.935. C, dissociation constants for the high-affinity (open squares) and low-affinity (filled squares) block inferred from the half-blocking voltages of the major and minor Boltzmann components, respectively. SPM concentrations were plotted against the half-activation voltages. Straight lines are fits with eqn (2) using the RT/z1F and RT/z2F values of the Boltzmann components.
In Fig. 3, we compared the outward components of the Kir2.1 and Kir2.2 currents based on the two-mode model of the SPM block. The characteristic shapes of the I–V relations and the quantiative difference between Kir2.1 and Kir2.2 currents were well reconstituted (Fig. 3B). The inward rectification of the Kir2.2 current caused by the SPM-mediated block was stronger than that of the Kir2.1 current because the dissociation constants for both the high-affinity and low-affinity block of Kir2.2 were smaller than those for Kir2.1 at membrane potentials > 0 mV, where currents flow outward (Fig. 3A). In addition, the fractional conductance susceptible to low-affinity block of Kir2.2 was smaller than that of Kir2.1 (0.065 versus 0.1). With 5–10 μm SPM (grey area in Fig. 3A), only one peak appeared in the I–V relations of the outward currents, which is consistent with observations of IKir in native cells and reflects the fact that the conductance susceptible to low-affinity block mediates a large portion of the outward currents (Fig. 3C and D). The 3- to 4-fold higher sensitivity to the low-affinity SPM block (Fig. 3A) caused the outward Kir2.2 currents to flow in a narrower voltage range than the corresponding Kir2.1 currents (Fig. 3D).
Figure 3.
External [K+] dependence of low-affinity SPM block
It is well known that the G–V relation of the whole-cell IKir shifts along the voltage axis in parallel with the shift in EK when the extracellular [K+] is altered at a constant intracellular [K+] (e.g. Hagiwara & Takahashi, 1974). We would thus expect that low-affinity block of Kir2 channels mediates the outward currents even under low external [K+] conditions. Our next aim was to carefully test that idea for Kir2.1 using inside-out patches. When [K+] in the pipette (external) solution was reduced to 50 mm or 20 mm, the characteristic shapes of the I–V relations of the outward currents, which showed a peak, a plateau (or a slightly positive slope region) and two peaks at 5 μm, 1 μm and 0.1 μm SPM, respectively, were largely unaffected (Fig. 4). The negative shift of the I–V relation of the inward currents seen with Kir2.1 in the presence of relatively high concentrations of SPM (Xie et al. 2002) was also seen at lower external [K+] (Fig. 4C, lower panels). This effect was not observed for Kir2.2 with 10 μm SPM, the highest concentration we used (Fig. 1B).
As was shown previously (Lopatin & Nichols, 1996), the open channel conductance of Kir2.1 was apparently ohmic, even with a [K+] gradient across the membrane, as can be seen from the instantaneous currents at various test potentials in the absence of SPM (Fig. 4A). We therefore simply compared G–V relations obtained at different external [K+] (Fig. 5A). The G–V relations could be fitted by eqn (1) using the same RT/z1F and RT/z2F values (4.7 mV and 9 mV, respectively) for all external [K+] (Fig. 5B). The voltage dependences of the dissociation constants for the high- and low-affinity blocks, inferred from the half-activation voltages of the Boltzmann components (Fig. 5C, symbols), were well approximated by eqn (2) using the aforementioned RT/z1F and RT/z2F values (Fig. 5C, lines), and were almost superimposable when plotted against the difference between the membrane potential and Vrev at each external [K+] (Fig. 5C). When the Kd(0) values obtained from those fittings were plotted against the external [K+] (Fig. 5D), they deviated slightly from the theoretical relation as [K+] was reduced, which may reflect the higher activity of K+ in the low [K+] pipette solutions (see Discussion). The fittings in Fig. 5B also showed that the fractional conductance susceptible to low-affinity block became slightly larger at lower external [K+]: the values were 0.075, 0.08 and 0.09 with 150 mm, 50 mm and 20 mm external K+, respectively.
Figure 5.
Voltage dependence of the SPM block of Kir2.1 channels at low external [K+] A, G–V relations in the presence of the indicated concentrations of SPM. Experiments in which current rundown was negligible were selected for analysis, and Gmax at 0.1 μm SPM was used to calculate G/Gmax values. Mean values were obtained from 5, 6–8 and 2 patches for 150 mm, 50 mm and 20 mm[K+], respectively. For 150 mm and 50 mm[K+], error bars are not shown when smaller than the symbol. Statistical errors were not calculated for 20 mm[K+]. Vertical lines denote mean values for Vrev (2.0 ± 0.4, –24.9 ± 0.5 and −46.9 mV at 150, 50 and 20 mm K+, respectively). Continuous lines are fits to eqn (1): the reductions in the conductances for the inward currents related to the negative shift in the inward I–V relation (Fig. 4C, lower panels) were ignored in the fittings, as before (Ishihara & Ehara, 2004). B, fitting of G–V relations using the sum of two Boltzmann relations shown on a semilogarithmic scale. Symbols are the mean values in A. Continuous lines are fits to eqn (1); dotted lines depict minor Boltzmann components. C, dissociation constants for the high-affinity (open symbols) and low-affinity (filled symbols) block inferred from the fitted Boltzmann components plotted against V – Vrev. Straight lines are the fits to eqn (2) using the RT/z1F and RT/z2F values from the fitted Boltzmann relations. D, relations between the fitted Kd(0) values and the external [K+] shown on a log–log scale. Straight lines are the theoretical relation: Kd(0) =Kd(0)*exp(ziln([K+]o/150)), where Kd(0)* is the Kd(0) value at 150 mm[K+]o, and zi values are 5.5 (high) and 2.9 (low).
Figure 6 shows outward Kir2.1 currents recorded at 5.4 mm external [K+], the physiological extracellular [K+] typically used in whole-cell current recordings. In this set of experiments, ∼140 mm NMDG was added to the pipette solution to compensate for the reduced osmolarity and to obtain a high seal resistance. In the presence of external NMDG, inward currents during hyperpolarizing steps showed a significant relaxation, which hampered the analysis of G–V relations (see Discussion). Nevertheless, it could be clearly seen that the shape of the I–V relation of the outward currents at each SPM concentration (Fig. 6B) was similar to that observed at higher external [K+] (Fig. 4C), which confirms that the conductance susceptible to low-affinity block mediates the outward currents, even under the physiologically low external [K+] conditions.
Block of Kir2.1(D172N) is similar to low-affinity block of the wild-type channel
It has been shown that neutralization of the negatively charged residues situated on the wall of the transmembrane pore (D172) and the cytoplasmic pore (e.g. E224) of Kir2.1 reduces the channel's sensitivity to high-affinity block by internal cations, which suggests that these sites are involved in the high-affinity binding related to block (see Introduction for references). In Fig. 7, we show that the susceptibility of Kir2.1(D172N) to the SPM block was comparable to that of the wild-type Kir2.1 to low-affinity SPM block. Figure 7A shows Kir2.1(D172N) currents recorded in the absence and presence of SPM. In the control (SPM-free) solution, the instantaneous amplitudes of the outward currents showed a nearly ohmic I–V relation, and the outward currents showed a slow decay, which were similar to wild-type Kir2.1 currents (Fig. 7A, cf. Fig. 4A), the inward rectification of Kir2.1(D172N) currents caused by 0.1–10 μm SPM was weaker than the rectification of the wild-type currents (Fig. 7B left, cf. Fig. 4C). Moreover, in contrast to the wild-type currents, the I–V relations of the outward currents obtained with 0.1 μm, 1 μm and 10 μm SPM all showed a single peak. Interestingly, the voltage at which the outward component of Kir2.1(D172N) currents showed the largest amplitude at each SPM concentration was comparable to that at which the outward currents mediated by the low-affinity conductance of the wild-type channel appeared to have the largest amplitude (Fig. 7B, right panel, red lines). Accordingly, in the presence of 10 μm SPM, the shape of the I–V relation of the outward Kir2.1(D172N) currents was very similar to that of the corresponding wild-type currents, which are mediated mostly by the conductance susceptible to low-affinity block. The G–V relations of Kir2.1(D172N) did not show two phases like those of the wild-type, but seemed to be approximated by a single-Boltzmann relation (Fig. 7C). The steepness of the voltage dependence and the half-blocking voltages of the fitted Boltzmann relations were similar to those for the low-affinity block of the wild-type channel (Fig. 7C and D).
Low-affinity block is markedly weakened in Kir2.1(E224G)
By contrast, E224G mutation of Kir2.1 greatly reduced the channel's sensitivity to low-affinity block without markedly altering its susceptibility to high-affinity block or the fractional conductance susceptible only to the low-affinity block. Consistent with previously reported findings (Yang et al. 1995a; Xie et al. 2003), the E224G mutation induced a weak instantaneous inward rectification in control (SPM-free) solution (Fig. 8Aa). Moreover, it dramatically slowed the time courses of the block and unblock by SPM – i.e. the decay phase of the outward currents and the activation phase of the inward currents (Fig. 8Aa) (Yang et al. 1995a; Taglialatela et al. 1995; Kubo & Murata, 2001; Panama & Lopatin, 2006). We therefore used long pulses to obtain the steady-state amplitudes of the outward currents (Fig. 8Ab). The steady-state I–V relations showed unique shapes at different SPM concentrations (Fig. 8B). Even though 1 μm SPM largely blocked the outward currents in the voltage range close to Vrev, a nearly ohmic I–V relation was observed at positive voltages, suggesting that the currents around Vrev and at the positive voltages had different susceptibilities to SPM block. When the SPM concentration was increased (10–500 μm), the inward and outward currents in the positive voltage range were both blocked in a concentration-dependent manner.
The G–V relations of Kir2.1(E224G) plotted on a semilogarithmic scale (Fig. 8Ca) clearly showed two phases, like those of the wild-type channel (Fig. 5B). Because the instantaneous inward rectification of Kir2.1(E224G) currents in the control (SPM-free) solution (Fig. 8Ca, open circles) may be a property of the unblocked channel (Yang et al. 1995a), we further normalized these G–V relations with the instantaneous G–V relation acquired in SPM-free solution and then fitted them with the sum of two Boltzmann relations (eqn (1)) (Fig. 8Cb). The fractional conductance susceptible to low-affinity block was 0.15, which was slightly larger than that of the wild-type channel. The E224G mutation caused the voltage dependence of the high-affinity block to become slightly shallower, but the Kd(V) values for the high-affinity block were similar to those of the wild-type channel (Fig. 8D). By contrast, the sensitivity of low-affinity block was significantly reduced; the Kd(V) value at +60 mV was estimated to be increased by more than 104-fold, as compared to the wild-type.
When we tested the effects of a D172N and E224G double mutation, we found that the susceptibility of the channel to the SPM block was dramatically reduced (Fig. 9A and B), such that SPM concentrations of 1 μm and below did not noticeably affect the currents (Fig. 9Ca). The degree of the instantaneous rectification of Kir2.1(D172N and E224G) observed in the control (SPM-free) solution (Fig. 9Ca, open circles) was nearly identical to that of Kir2.1(E224G) (Fig. 8Ca). The G–V relations of Kir2.1(D172N and E224G) corrected for the instantaneous inward rectification were similar to those for the low-affinity component of the Kir2.1(E224G) (Fig. 9Cb). This suggests that addition of the D172N mutation made almost all of the Kir2.1(E224G) channels susceptible only to the low-affinity block, as it did in the wild-type channel.
Discussion
We studied the mechanism underlying the outward currents carried by Kir2.1 and Kir2.2 channels, which play important roles in setting the membrane potentials of both excitable and non-excitable cells. The results obtained under the symmetrical [K+] condition strongly suggest that, as with Kir2.1 currents (Ishihara & Ehara, 2004), a large portion of the outward Kir2.2 currents are mediated by the conductance susceptible only to low-affinity mode of block by internal cations; this was also confirmed for Kir2.1 under the low external [K+] conditions. When the negatively charged residues on the wall of the pore of Kir2.1, which are conserved among the members of the Kir2 family, were replaced with their uncharged counterparts in the weak inward rectifier Kir1.1, the D172N mutation within the transmembrane pore made virtually all of the Kir2.1 conductance susceptible only to low-affinity block, whereas the E224G mutation in the cytoplasmic pore reduced the susceptibility to low-affinity block without significantly altering that to the high-affinity block or the fractional conductance susceptible to low-affinity block. The effects of these mutations are consistent with the hypothesis that Kir2 channels exist in two states showing different sensitivities to internal blockers.
Comparison of Kir2.1 and Kir2.2
We previously showed that the characteristic shapes of the I–V relations of the outward component of Kir2.1 currents observed in the presence of different concentrations of internal SPM/SPD are explained by the presence of two (large and small) conductances, which are susceptible to the high-affinity and low-affinity modes of polyamine block, respectively (Ishihara & Ehara, 2004). In the present study, the same finding was more clearly obtained with Kir2.2 and SPM because the SPM in the concentration range between 0.1 and 10 μm did not diminish the maximum conductance (Fig. 1B), enabling us to determine the fractional size of the two conductances in a straightforward manner. In the case of Kir2.1, there was a ‘shallow block’, which reduced the maximum conductance in a concentration-dependent manner by negatively shifting the I–V relation of the inward currents (Figs 5A and 4C in this study; Lopatin et al. 1995; Xie et al. 2002; Panama & Lopatin, 2006). Xie et al. (2002) showed that this shallow component of block is likely to be caused by a charge screening effect of SPM due to binding to the negatively charged residues (E224 and E299) within the cytoplasmic pore. This shallow block was also observed under the low external [K+] condition, suggesting it depends on the difference between the membrane potential and EK (Fig. 4C). We ignored this shallow component in fitting the G–V relations of Kir2.1 with eqn (1) (Fig. 5 in this study; Ishihara & Ehara, 2004) by tentatively assuming that its effect on the inward current conductance is additive with the high- and low-affinity block we analysed. Since E224 and E299 are conserved in Kir2.2, the difference in the behaviour of Kir2.1 and Kir2.2 may reflect a slight structural difference in the cytoplasmic pore.
The SPM-induced inward rectification of Kir2.2 currents was stronger than that of Kir2.1 currents (Fig. 1C) because the fractional conductance susceptible to low-affinity block is smaller in Kir2.2 than in Kir2.1 (0.065 versus 0.1), and the susceptibility to block is greater in Kir2.2 (Fig. 3A). This is consistent with earlier observations that in mammalian cells exogenously expressing Kir2.1 or Kir2.2, whole-cell Kir2.2 currents showed stronger inward rectification than Kir2.1 currents (Dhamoon et al. 2004; Panama & Lopatin, 2006). The outward-to-inward ratios of the Kir2.2 current amplitudes were about 2-fold smaller than those for Kir2.1 at the largest outward current observed at each SPM concentration (Fig. 1C). This difference is substantial for cells such as cardiac ventricular myocytes, in which the density of the outward IKir is significantly larger than those of other current systems (see Matsuoka et al. 2003).
The findings discussed above may be of particular importance in terms of cardiac electrophysiology because the outward IKir, which mediates membrane repolarization in the working cardiac muscle, is thought to be carried mainly by channels composed of Kir2.1 and Kir2.2 subunits, though the precise contributions of the respective subunits are still not clear and may differ among different human races (Kaibara et al. 2002). Although Kir2.3 also has been shown to be expressed in the cardiac myocytes (Liu et al. 2001), it likely does not significantly contribute to the cardiac IKir, as the cardiac IKir does not exhibit the sensitivity to external pH characteristic of the Kir2.3 channel (Yan et al. 2005). The results shown in the present study indicate that the characteristic I–V relation of the cardiac IKir is not reconstituted with the Kir2.2 homotetramer; the outward currents flowed in narrower voltage range than the native cardiac IKir (Fig. 1Ca). This finding may be indicative of the crucial role played by Kir2.1 in mediating the outward component of the cardiac IKir at relatively positive voltages, where a change in IKir could easily affect cardiac repolarization, thereby increasing the risk of arrhythmia. Indeed, mutations of Kir2.1(KCNJ2) have been implicated in disorders of cardiac repolarization that predispose patients to arrhythmias (Plaster et al. 2001; Priori et al. 2005).
Low external K+ experiments and SPM block in asymmetrical K+
We attempted to verify whether the parameters of the SPM block of Kir2.1 obtained in symmetrical [K+] could be adopted under conditions in which the external [K+] was at a physiological level. For this purpose, we used low [K+] pipette solutions without compensating for the reduced osmolarity so as to eliminate previously observed relaxation of inward currents (Shieh, 2000), which we found to be mainly caused by NMDG included in the pipette solution to compensate for the osmolarity. Internal NMDG also blocks Kir2.1 in a voltage-dependent manner, and the same step was taken to examine the internal [K+] dependence of the Kir2.1 conductance (Lopatin & Nichols, 1996). Elimination of Hepes and Ca2+, which also have been shown to cause relaxation of inward Kir2.1 currents (Guo & Lu, 2002), did not clearly reduce that observed with NMDG (see Fig. 6A). Even with the low-K+ pipette solutions devoid of NMDG, Hepes and Ca2+, we sometimes still saw some relaxation and thought it may be caused by depletion of K+ at the external rim of a patch membrane, which occurred especially when a large patch of membrane was drawn deeply into the pipette. Those data were discarded from the analysis of the G–V relations (Fig. 5A). The Vrev values of currents thus obtained at 50 mm and 20 mm[K+] were more positive than the predicted EK by ∼2.5 and 5 mV, respectively (see the legend to Fig. 5 for the mean Vrev values). This was not due to the contribution of leak currents (see Method), but is explained by the increased activity of K+ ion in the hypotonic low-K+ solutions: the activity coefficients for K+ in the 50 mm and 20 mm K+ solutions were calculated to be higher than in the 150 mm K+ solution by a factor of ∼1.2, which seems reasonable for a mixture of KCl and K-phosphate solutions (Robinson & Stokes, 1959; Akiyama & Fozzard, 1975). Therefore, it is likely that both the high- and low-affinity block depended very much on the difference between the membrane potential and EK, when the external [K+] was altered at a constant internal [K+]. We find it noteworthy that the fractional conductance susceptible to low-affinity SPM block measured in 150 mm external [K+] using Hepes- and Ca2+-free pipette solution as a control for the low external [K+] data was smaller than that obtained in the presence of external Hepes and Ca2+ (0.075 versus 0.1). The difference may be caused in part by an apparently time-independent block of the inward currents by Hepes and Ca2+ in the external solution.
The mechanisms of the two modes of block
Given that the crystal structures of Kir channels (Nishida & MacKinnon, 2002; Kuo et al. 2003; Pegan et al. 2005) show that there are not two pathways for K+ through a single Kir2 channel (with different affinities for SPM block, one conducting ∼9 times more K+ than the other), we speculate that Kir2 channels can exist in two states that are susceptible to the high-affinity and low-affinity modes of SPM block, respectively, and that there is a higher probability (∼90%) that the channel will be in the ‘high-affinity state’ (Ishihara & Ehara, 2004) (Fig. 10). We showed that the D172N mutation in the transmembrane pore of Kir2.1, which is known to reduce its affinity for block by internal cationic particles, made almost all of the Kir2.1 channels susceptible only to low-affinity block (Fig. 7), suggesting that the block of the Kir2.1(D172N) channel occurred in the same manner as that of the wild-type channel in the ‘low-affinity state’. This is consistent with the observations that the D172N mutation eliminates the time-dependent gating of the wild-type channel (Wible et al. 1994; Stanfield et al. 1994) and that relief of high-affinity SPM block is time dependent, while relief of low-affinity SPM block is virtually instantaneous, in the wild-type channel (Ishihara & Ehara, 2004). When we assume that a channel in the ‘high-affinity state’ possesses both high- and low-affinity binding sites related to block (to which SPM binds either mutually exclusively or non-exclusively) and their affinities for block are as shown in Fig. 3A, the blocked fractions of currents are negligibly different from those of a channel possessing only the high-affinity site for block (calculations not shown). Thus, our finding fits with the idea that a channel in the ‘high-affinity state’ is also susceptible to low-affinity block and that the D172N mutation eliminated the binding site for high-affinity block, rendering the channel blockable only at the low-affinity site. Indeed, recent evidence suggests that the interaction of the trailing amine group of SPM with a negative charge provided by D172 stabilizes the blockade of K+ permeation by its head amine at a deeper site, probably at the selective filter (John et al. 2004; Kurata et al. 2004; Kurata et al. 2006). A different and more imaginative possibility is that a polyamine binding to D172 in a non-blocking configuration is crucial for stabilizing the conformational state susceptible to high-affinity block. Recently, Xie et al. (2005) proposed that such interaction of a polyamine with D172 may allosterically strengthen the interaction of the channel with PIP2 (which stabilizes the open configuration of the channel) without blocking the channel. In that case, however, the channel bound at D172 with a polyamine in a non-blocking configuration may not be the ‘high-affinity state’ of the channel, because our analyses of G–V relations showed that the ratio of low/high affinity states is almost constant at different SPM concentrations (e.g. Fig. 2), and the voltage-dependent increase in the ‘high-affinity state’ is inconsistent with the G–V relations obtained from the experiments.
Figure 10.
Two-mode model for the mechanism of the high-affinity and low-affinity polyamine block of Kir2 channels We propose that Kir2 channels exist in two conformational states with differing susceptibilities to internal cationic blockers. In the ‘high-affinity state’, the block of K+ permeation by polyamines occurs within the transmembrane pore. Negatively charged D172 within the transmembrane pore may form the polyamine binding site for the block. An intermediate binding step of polyamines to negatively charged E224 and E299 within the cytoplasmic pore may facilitate this block and its relief (Kubo & Murata, 2001; Xie et al. 2003) and may also cause the ‘instantaneous shallow block’ preceding the time-dependent (steady state) steep block described by Lopatin et al. (1995). In the ‘low-affinity state’, blockade of K+ permeation occurs within the cytoplasmic pore, where E224 and E299 together form the polyamine binding site. Polyamines may not access the transmembrane pore due to steric hindrance. The block of Kir2.1(E224G) channel in the ‘high-affinity state’ occurs extremely slowly, and that in the ‘low-affinity state’ is greatly attenuated, due to reduced electrostatic interaction at position 224. The ratio of the low/high affinity state in the presence of internal SPM (1/9) differs from that in the presence of SPD (1/3), and may be regulated by the binding of polyamines to an internal regulatory site (Ishihara & Ehara, 2004).
Previous studies have shown that E224G mutation within the cytoplasmic pore of Kir2.1 reduces the affinity for the high-affinity block mainly by reducing the on-rate of the block, and this effect has been attributed to a reduction in the electrostatic interaction between the negatively charged E224 and cationic blockers (Yang et al. 1995a; Taglialatela et al. 1995). A similar effect was elicited by neutralizing the negatively charged E299 (Kubo & Murata, 2001; Xie et al. 2003), which is situated near E224 and together with E224 forms a ring of negative charges within the cytoplasmic pore (Kuo et al. 2003). In the present study, the steady-state I–V relations obtained using long pulses revealed that the affinity for the high-affinity block is not significantly affected by the E224G mutation (Fig. 8D), indicating that E224 plays a role in facilitating the high-affinity block and its relief (Kubo & Murata, 2001; Xie et al. 2003), but does not contribute to the binding sites for the high-affinity block (cf. Guo & Lu, 2003; Guo et al. 2003). E224 (and E299) may lower the energy barrier for polyamines to pass through the narrow part of the cytoplasmic pore and to access the high-affinity binding site in the transmembrane pore. Furthermore, the intermediate binding step to E224 and E299 may also exist (Kubo & Murata, 2001; Xie et al. 2003). The ‘instantaneous shallow block’ preceding the time-dependent (steady state) steep component of the polyamine block described by Lopatin et al. (1995) may be related to this intermediate binding step, because it has been shown that neutralization of E224 and E299 eliminates this component of the shallow block (Xie et al. 2003).
A more important observation was that E224G mutation dramatically reduced the affinity for low-affinity block without significantly affecting the fractional conductance susceptible to low-affinity block (Fig. 8C), which is in good agreement with the data reported by Panama & Lopatin (2006). This observation indicates that the marked attenuation of the low-affinity block was not accompanied by an increase in the fraction of the high-affinity block. This supports our hypothesis that the conductance susceptible to low-affinity block is not susceptible to high-affinity block. The great reduction in the affinity for the low-affinity bock caused by the E224G mutation indicates that E224 significantly contributes to forming the binding site for the low-affinity block. This finding also reveals that the site for the polyamine block of Kir2.1(D172N) is at the cytoplasmic pore.
The mechanisms of the high-affinity and low-affinity block that we propose based on our previous and present studies are summarized in Fig. 10. Our results suggest that in the ‘low-affinity state’ polyamines cannot access the high-affinity blocking site in the transmembrane pore, probably due to some kind of steric hindrance. The steric hindrance may be induced by a conformational change in the narrow part of the cytoplasmic pore (Pegan et al. 2005). It is unlikely to be caused by polyamines binding within the cytoplasmic pore, because the proportion of the ‘low-affinity channel’ seems to be almost constant at different SPM concentrations. Another important possibility we considered for the mechanism of the two modes of block, which originates from the proposal of Yang et al. (1995b), is that the high-affinity block does not completely block the current flow but reduces the single channel conductance by the amount corresponding to the fractional conductance susceptible to the high-affinity block, and that the channel being blocked at the high-affinity site becomes susceptible to the low-affinity block. The model is,
where O is the open state that is susceptible only to the high-affinity block, BH is the state being blocked by a polyamine molecule at the high-affinity site showing a subconductance level and BHL is the state that is completely blocked by a second polyamine molecule at the low-affinity site. In this ‘two-blocker model’, the G/Gmax values determined by the SPM block are given by the following equation:
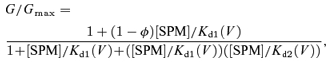 |
(3) |
and the values calculated using the Kd1(V) and Kd2(V) values shown in Fig. 3A well reproduce the G–V relations obtained from our experiments. The outward currents carried by the ‘low-affinity conductance’ (red lines in Fig. 7B) are mediated by the channels in BH state. However, if we assume that only the channels in the BH state can be blocked at the low-affinity site as in this model (the high-affinity block within the transmembrane pore may induce a conformational change of the cytoplasmic pore), the effect of D172N mutation may be difficult to explain; the absence of the four negative charges at the pore-facing position of the wide central cavity (Lu et al. 1999; Kuo et al. 2005) alone may not cause a conformational change of the cytoplasmic pore. Furthermore, voltage-dependent distributions of the two types of non-conductive states we previously determined from the tail-current analysis fit the two-mode model (Ishihara & Ehara, 2004), but not the two-blocker model (see online supplemental material, Fig. 1S). Thus, the idea depicted in Fig. 10 is more likely. Both models predict that the high-affinity and low-affinity block occur in different conformational states of the channel. Further study will be required to clarify the details of the molecular mechanism underlying the two-modes of block of Kir2 channels.
Acknowledgments
We would like to thank Dr L. Y. Jan, Dr Y. Kurachi and Dr Y. Kubo for providing the Kir2.1, Kir2.2 and mutant Kir2.1 clones, respectively. This study was supported by Scientific Research Grants from the Ministry of Education, Culture, Sports, and Technology of Japan and by grants from The Ichiro Kanehara Foundation and Mitsui Life Social Welfare Foundation.
Supplementary material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.136028/DC1
and
http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.136028
References
- Akiyama T, Fozzard HA. Influence of potassium ions and osmolality on the resting membrane potential of rabbit ventricular papillary muscle with estimation of the activity and the activity coefficient of internal potassium. Circ Res. 1975;37:621–629. doi: 10.1161/01.res.37.5.621. [DOI] [PubMed] [Google Scholar]
- Dhamoon AS, Pandit SV, Sarmast F, Parisian KR, Guha P, Li Y, Bagwe S, Taffet SM, Anumonwo JM. Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current. Circ Res. 2004;94:1332–1339. doi: 10.1161/01.RES.0000128408.66946.67. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- Fakler B, Brandle U, Glowatzki E, Weidemann S, Zenner HP, Ruppersberg JP. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 1995;80:149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- Guo D, Lu Z. Mechanism of IRK1 channel block by intracellular polyamines. J Gen Physiol. 2000;115:799–814. doi: 10.1085/jgp.115.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Lu Z. IRK1 inward rectifier K+ channels exhibit no intrinsic rectification. J Gen Physiol. 2002;120:539–551. doi: 10.1085/jgp.20028623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Lu Z. Interaction mechanisms between polyamines and IRK1 inward rectifier K+ channels. J Gen Physiol. 2003;122:485–500. doi: 10.1085/jgp.200308890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Ramu Y, Klem AM, Lu Z. Mechanism of rectification in inward-rectifier K+ channels. J Gen Physiol. 2003;121:261–275. doi: 10.1085/jgp.200208771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18:61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Komazaki S, Ishikawa T. An inwardly rectifying K+ channel in bovine parotid acinar cells: possible involvement of Kir2.1. J Physiol. 2003;547:255–269. doi: 10.1113/jphysiol.2002.035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Ehara T. Two modes of polyamine block regulating the cardiac inward rectifier K+ current IK1 as revealed by a study of the Kir2.1 channel expressed in a human cell line. J Physiol. 2004;556:61–78. doi: 10.1113/jphysiol.2003.055434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Hiraoka M, Ochi R. The tetravalent organic cation spermine causes the gating of the IRK1 channel expressed in murine fibroblast cells. J Physiol. 1996;491:367–381. doi: 10.1113/jphysiol.1996.sp021222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Mitsuiye T, Noma A, Takano M. The Mg2+ block and intrinsic gating underlying inward rectification of the K+ current in guinea-pig cardiac myocytes. J Physiol. 1989;419:297–320. doi: 10.1113/jphysiol.1989.sp017874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Yan D-H, Yamamoto S, Ehara T. Inward rectifier K+ current under physiological cytoplasmic conditions in guinea-pig cardiac ventricular cells. J Physiol. 2002;540:831–841. doi: 10.1113/jphysiol.2001.013470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John SA, Xie LH, Weiss JN. Mechanism of inward rectification in Kir channels. J Gen Physiol. 2004;123:623–625. doi: 10.1085/jgp.200409017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibara M, Ishihara K, Doi Y, Hayashi H, Ehara T, Taniyama K. Identification of human Kir2.2 (KCNJ12) gene encoding functional inward rectifier potassium channel in both mammalian cells and Xenopus oocytes. FEBS Lett. 2002;531:250–254. doi: 10.1016/s0014-5793(02)03512-3. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Murata Y. Control of rectification and permeation by two distinct sites after the second transmembrane region in Kir2.1 K+ channel. J Physiol. 2001;531:645–660. doi: 10.1111/j.1469-7793.2001.0645h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo A, Domene C, Johnson LN, Doyle DA, Venien-Bryan C. Two different conformational states of the KirBac3.1 potassium channel revealed by electron crystallography. Structure. 2005;13:1463–1472. doi: 10.1016/j.str.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–1926. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- Kurachi Y. Voltage-dependent activation of the inward-rectifier potassium channel in the ventricular cell membrane of guinea-pig heart. J Physiol. 1985;366:365–385. doi: 10.1113/jphysiol.1985.sp015803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata HT, Marton LJ, Nichols CG. The polyamine binding site in inward rectifier K+ channels. J Gen Physiol. 2006;127:467–480. doi: 10.1085/jgp.200509467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata HT, Phillips LR, Rose T, Loussouarn G, Herlitze S, Fritzenschaft H, Enkvetchakul D, Nichols CG, Baukrowitz T. Molecular basis of inward rectification: polyamine interaction sites located by combined channel and ligand mutagenesis. J Gen Physiol. 2004;124:541–554. doi: 10.1085/jgp.200409159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GX, Derst C, Schlichthorl G, Heinen S, Seebohm G, Bruggemann A, Kummer W, Veh RW, Daut J, Preisig-Muller R. Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes. J Physiol. 2001;532:115–126. doi: 10.1111/j.1469-7793.2001.0115g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin AN, Makhina EN, Nichols CG. The mechanism of inward rectification of potassium channels: ‘long-pore plugging’ by cytoplasmic polyamines. J Gen Physiol. 1995;106:923–955. doi: 10.1085/jgp.106.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin AN, Nichols CG. [K+] dependence of open-channel conductance in cloned inward rectifier potassium channels (IRK1, Kir2.1) Biophys J. 1996;71:682–694. doi: 10.1016/S0006-3495(96)79268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Mackinnon R. Electrostatic tuning of Mg2+ affinity in an inward-rectifier K+ channel. Nature. 1994;371:243–246. doi: 10.1038/371243a0. [DOI] [PubMed] [Google Scholar]
- Lu T, Nguyen B, Zhang X, Yang J. Architecture of a K+ channel inner pore revealed by stoichiometric covalent modification. Neuron. 1999;22:571–580. doi: 10.1016/s0896-6273(00)80711-4. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Sarai N, Kuratomi S, Ono K, Noma A. Role of individual ionic current systems in ventricular cells hypothesized by a model study. Jpn J Physiol. 2003;53:105–123. doi: 10.2170/jjphysiol.53.105. [DOI] [PubMed] [Google Scholar]
- Miake J, Marban E, Nuss HB. Functional role of inward rectifier current in heart probed by Kir2.1 overexpression and dominant-negative suppression. J Clin Invest. 2003;111:1529–1536. doi: 10.1172/JCI17959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- Nishida M, MacKinnon R. Structural basis of inward rectification: cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 Å resolution. Cell. 2002;111:957–965. doi: 10.1016/s0092-8674(02)01227-8. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Oliva C, Cohen IS, Pennefather P. The mechanism of rectification of iK1 in canine Purkinje myocytes. J Gen Physiol. 1990;96:299–318. doi: 10.1085/jgp.96.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panama BK, Lopatin AN. Differential polyamine sensitivity in inwardly rectifying Kir2 potassium channels. J Physiol. 2006;571:287–302. doi: 10.1113/jphysiol.2005.097741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegan S, Arrabit C, Zhou W, Kwiatkowski W, Collins A, Slesinger PA, Choe S. Cytoplasmic domain structures of Kir2.1 and Kir3.1 show sites for modulating gating and rectification. Nat Neurosci. 2005;8:279–287. doi: 10.1038/nn1411. [DOI] [PubMed] [Google Scholar]
- Plaster NM, Tawil R, Tristani-Firouzi M, Canun S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL, Jr, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptacek LJ. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell. 2001;105:511–519. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- Priori SG, Pandit SV, Rivolta I, Berenfeld O, Ronchetti E, Dhamoon A, Napolitano C, Anumonwo J, di Barletta MR, Gudapakkam S, Bosi G, Stramba-Badiale M, Jalife J. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res. 2005;96:800–807. doi: 10.1161/01.RES.0000162101.76263.8c. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Robinson RA, Stokes RH. Electrolyte Solutions. London: Butterworths; 1959. [Google Scholar]
- Shieh RC. Mechanisms for the time-dependent decay of inward currents through cloned Kir2.1 channels expressed in Xenopus oocytes. J Physiol. 2000;526:241–252. doi: 10.1111/j.1469-7793.2000.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield PR, Davies NW, Shelton PA, Sutcliffe MJ, Khan IA, Brammar WJ, Conley EC. A single aspartate residue is involved in both intrinsic gating and blockage by Mg2+ of the inward rectifier, IRK1. J Physiol. 1994;478:1–6. doi: 10.1113/jphysiol.1994.sp020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield PR, Nakajima S, Nakajima Y. Constitutively active and G-protein coupled inward rectifier K+ channels: Kir2.0 and Kir3.0. Rev Physiol Biochem Pharmacol. 2002;145:47–179. doi: 10.1007/BFb0116431. [DOI] [PubMed] [Google Scholar]
- Taglialatela M, Ficker E, Wible BA, Brown AM. C-terminus determinants for Mg2+ and polyamine block of the inward rectifier K+ channel IRK1. EMBO J. 1995;14:5532–5541. doi: 10.1002/j.1460-2075.1995.tb00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Morishige K, Jahangir A, Yamada M, Findley I, Koyama H, Kurachi Y. Molecular cloning and functional expression of cDNA encoding a second class of inward rectifier potassium channels in the mouse brain. J Biol Chem. 1994;269:23274–23279. [PubMed] [Google Scholar]
- Wible BA, Taglialatela M, Ficker E, Brown AM. Gating of inwardly rectifying K+ channels localized to a single negatively charged residue. Nature. 1994;371:246–249. doi: 10.1038/371246a0. [DOI] [PubMed] [Google Scholar]
- Woodhull AM. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie LH, John SA, Ribalet B, Weiss JN. Long polyamines act as cofactors in PIP2 activation of inward rectifier potassium (Kir2.1) channels. J Gen Physiol. 2005;126:541–549. doi: 10.1085/jgp.200509380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie LH, John SA, Weiss JN. Spermine block of the strong inward rectifier potassium channel Kir2.1: dual roles of surface charge screening and pore block. J Gen Physiol. 2002;120:53–66. doi: 10.1085/jgp.20028576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie LH, John SA, Weiss JN. Inward rectification by polyamines in mouse Kir2.1 channels: synergy between blocking components. J Physiol. 2003;550:67–82. doi: 10.1113/jphysiol.2003.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Horio Y, Yamada M, Takahashi N, Kondo C, Kurachi Y. Competition between Mg2+ and spermine for a cloned IRK2 channel expressed in a human cell line. J Physiol. 1996;493:143–156. doi: 10.1113/jphysiol.1996.sp021370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D-H, Ishihara K. Two Kir2.1 channel populations with different sensitivities to Mg2+ and polyamine block: a model for the cardiac strong inward rectifier K+ channel. J Physiol. 2005;563:725–744. doi: 10.1113/jphysiol.2004.079186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D-H, Nishimura K, Yoshida K, Nakahira K, Ehara T, Igarashi K, Ishihara K. Different intracellular polyamine concentrations underlie the difference in the inward rectifier K+ currents in atria and ventricles of the guinea-pig heart. J Physiol. 2005;563:713–724. doi: 10.1113/jphysiol.2004.077677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Jan YN, Jan LY. Control of rectification and permeation by residues in two distinct domains in an inward rectifier K+ channel. Neuron. 1995a;14:1047–1054. doi: 10.1016/0896-6273(95)90343-7. [DOI] [PubMed] [Google Scholar]
- Yang J, Jan YN, Jan LY. Determination of the subunit stoichiometry of an inwardly rectifying potassium channel. Neuron. 1995b;15:1441–1447. doi: 10.1016/0896-6273(95)90021-7. [DOI] [PubMed] [Google Scholar]
- Yu SP, Kerchner GA. Endogenous voltage-gated potassium channels in human embryonic kidney (HEK293) cells. J Neurosci Res. 1998;52:612–617. doi: 10.1002/(SICI)1097-4547(19980601)52:5<612::AID-JNR13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Zaritsky JJ, Redell JB, Tempel BL, Schwarz TL. The consequences of disrupting cardiac inwardly rectifying K+ current (IK1) as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes. J Physiol. 2001;533:697–710. doi: 10.1111/j.1469-7793.2001.t01-1-00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.