Abstract
Objectives
We examined the expression of Rho Kinase (ROK) isoforms in young and old rabbit’s detrusor smooth muscles (SM) during the progression of short term partial bladder outlet obstruction and correlated them with the time course of obstruction.
Methods
Detrusor samples were obtained from bladders after 1, 3, 7, and 14 days of obstruction and also sham operated control rabbits. Reverse transcriptase-polymerase chain reaction, sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting were used to determine the relative levels of ROK isoform expression at the mRNA and protein levels.
Results
Bladder weight for young rabbits increased between 1 and 7 days obstruction and came back toward control levels at 14 days obstruction. In old rabbits bladder weight increased after obstruction reaching a maximum at 3 days and remaining at this level throughout the 14 days. In young rabbits, the expression of ROKα increased in 1 to 7-day obstructed groups and decreased in the 14 day, while it increased progressively in the old rabbits, both at the mRNA and protein levels. There was a significant decrease in the expression of ROKβ in young obstructed rabbits, which gradually decreased during the course of 1–7 day obstruction period and increased after 14 days of obstruction. In old groups, there was a decrease in expression after 1 day of obstruction and values remained at a decreased level throughout the course of the study.
Conclusions
The young rabbit bladders are better able to adapt to bladder outlet obstruction and ROK isoforms respond in a similar way.
Keywords: Aging, Bladder, Obstruction, Contractility, Rho- kinase
INTRODUCTION
Partial bladder outlet obstruction (PBOO) is observed in children with posterior urethral valves or severe voiding dysfunction and in adults with benign prostatic hyperplasia or urethral stricture.1 Experimentally, many pathologic characteristics of human obstructed bladder disease can be reproduced in animal models of PBOO.2 Smooth muscle structure and function is significantly altered following PBOO. Insufficient adaptive capacity of the bladder in response to PBOO is one of the major reasons for decreased bladder contractile function 3, 4.
It is well established that SM tone is triggered by an increase in cytosolic Ca2+ concentration ([Ca2+]c). At increased concentration, Ca2+ binds to calmodulin (CaM), and Ca2+/CaM then activates myosin light chain kinase, which phosphorylates the regulatory myosin light chain. The result is a conformational change that allows actin to stimulate myosin ATPase activity, crossbridges to cycle, and actin to slide past myosin resulting in myocyte contraction.5–8 Conversely, a decrease in [Ca2+]i causes relaxation. Recently, secondary mechanisms were identified that can modulate SM contractility independently of Ca2+. Activation of excitatory G-protein coupled receptor can cause contraction of SM without necessarily changing [Ca2+]i, a process termed ‘Ca2+-sensitization.7 ROK, a serine/threonine protein with two isoforms, ROKα and ROKβ has been shown to play important roles in this process. ROK can regulate the phosphorylation of myosin light chain either through phosphorylation and subsequent inhibition of myosin phosphatase or direct phosphorylation of MLC on Ser 19 and Thr 18.3,5,7,9 It has been shown that ROK plays an important role in the regulation of urinary bladder SM contraction and tone.6,10,11, 12.
In our recent study on the response to short-term partial outlet obstruction in young and mature rabbits, the old rabbits were more severely affected by partial outlet obstruction than were the young rabbits 13. Although expression of ROK isoforms after obstruction has been reported in mature rabbits 10,12 there are no data in young rabbits. In this study, we focused on the expression of ROK isoforms in response to short term PBOO in young, as compared to old rabbits.
MATERIAL AND METHODS
Surgery protocol
A total of 20 young (3 moths old) and 20 mature male New Zealand white rabbits were divided into 4 sub-groups of 5 rabbits each. 4 rabbits in each group underwent partial outlet obstruction. The other rabbit in each group underwent sham operations. The rabbits in each group were evaluated after 1, 3, 7, and 14 days of obstruction. At the end of experiment period, bladders were weighed.
RNA Extraction and RT-PCR
Total RNA was extracted from the frozen bladder body SM tissues (100 mg) ground to a fine powder in a mortar cooled in liquid nitrogen using TRIzol reagent (Invitrogen, Carlsbad, CA). Of total RNA, 3.5µg was then amplified as previously described14 using SuperScript III One Step RT-PCR with Platinum Taq DNA Polymerase (Invitrogen, Carlsbad, CA). Forward and reverse oligonucleotide primers used, and predicted size of PCR products were as follows:5′-GTGATGGTTACTATGGGCGAGAAT-3′ (upstream) and 5′- GTTAAGAAGGCACAGATGAGAT-3′ (downstream) for ROKα (202 bp) and 5′- AAGTAGTTCTCGCATTGG-3′ (upstream) and 5′-TATCATCAGGGAAAGTAAGTG-3′ (downstream) for ROKβ (366 bp). In all reactions, α-actin was amplified as an internal control, since α-actin expression is not altered in bladder SM hypertrophy.13 PCR products were electrophoresed on a 2% agarose gel and visualized after ethidium bromide staining. Band intensities were quantitated by scanning the gel photographs using a densitometer (KODAK Image Station 440CF) and subsequent analyses using the KODAK 1D image analysis software (Scientific Image System, Rochester, NY).
Western blot analysis
Total protein extracts were prepared from the bladder SM tissues and quantified as previously described. 14 Equal amounts (20 µg) of total protein from control and obstructed rabbit bladders were loaded on 8% SDS-PAGE gels and transferred to Immobilon-P membranes. Membranes were incubated with anti-ROKα and anti-ROKβ antibody (Transduction Laboratories, Lexington, KY). It was then incubated with the secondary antibody anti-mouse IgG at 1:4,000 dilutions (Amersham Biosciences, Piscataway, NJ). Between incubations, membranes were washed thoroughly with PBS containing 0.05% Tween 20. Antibody reactivity was detected using an enhanced chemiluminescence kit (Amersham Biosciences Plus, GE Healthcare, UK) and quantitated by scanning densitometry . Amounts of protein loaded on gels for Western blotting were adjusted to the linear portion of the concentration vs. absorption curve.
Statistical analysis
All the data are expressed as the mean ± SEM with p< 0.05 considered statistically significant. Analysis of variance followed by the Bonferonni test for individual differences was used for comparative purposes.
RESULTS
The ratios of bladder weight to body weight are shown in table 1. The ratio in young rabbits increased 1 to 7 days obstruction and came back toward control levels at 14 days obstruction. In mature rabbits the ratio increased after obstruction reaching a maximum at 3 days and remaining at this level throughout the 14 days.
Table 1.
Comparison of Body Weight & Bladder Weight
| YOUNG | OLD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1 Day-Obst. | 3 Day-Obst. | 7 Day-Obst | 14 Day-Obst. | Control | 1 Day-Obst. | 3 Day-Obst. | 7 Day-Obst. | 14 Day-Obst. | |
| Body Weight (kg) | 1.78±0.01 | 1.75±0.02 | 1.81±0.09 | 1.86±0.05 | 2.23±0.10 | 3.76±0.07¥ | 3.47±0.05¥ | 3.61±0.09¥ | 3.31±0.03¥ | 3.66±0.09¥ |
| Bladder Weight (g) | 1.23±0.33 | 2.77±0.66* | 5.82±0.76* | 5.84±0.48* | 2.68±0.37* | 2.67±0.52¥ | 5.03±0.34* ¥ | 6.87±0.25* | 7.08±0.94* | 6.90±1.03* ¥ |
significantly different from young, p < 0.05. n= 4
significantly different from control
ROK α and ROK β expression
The expressions of ROKα in the young and old rabbit bladder SM, as determined by RT-PCR using total RNA and quantitative values of densitometric scanning of ethidium bromide stained bands are represented in Figure 1. In young rabbits, the expression of ROKα was increased after obstruction, reaching a maximum level in 7 day obstruction group and decreasing to the day 1 level in the 14 day obstructed group. In old rabbits, there was a significant increase in all the obstructed groups. Figure 2 demonstrates the expression of ROKα by Western blotting and quantitative data obtained from densitometric analysis. The amount of ROKα in young obstructed groups increased during the first 1 to 7 days after obstruction and by 7 days, the increase was two fold. After 7 days the level of ROKα decreased to the level of the day 1 obstructed group. In the older group, it increased progressively throughout the course of obstruction. The amount of ROKα in the 14 day obstructed group was higher in old rabbits than young groups. These results were consistent with RT-PCR experiments.
Figure 1.
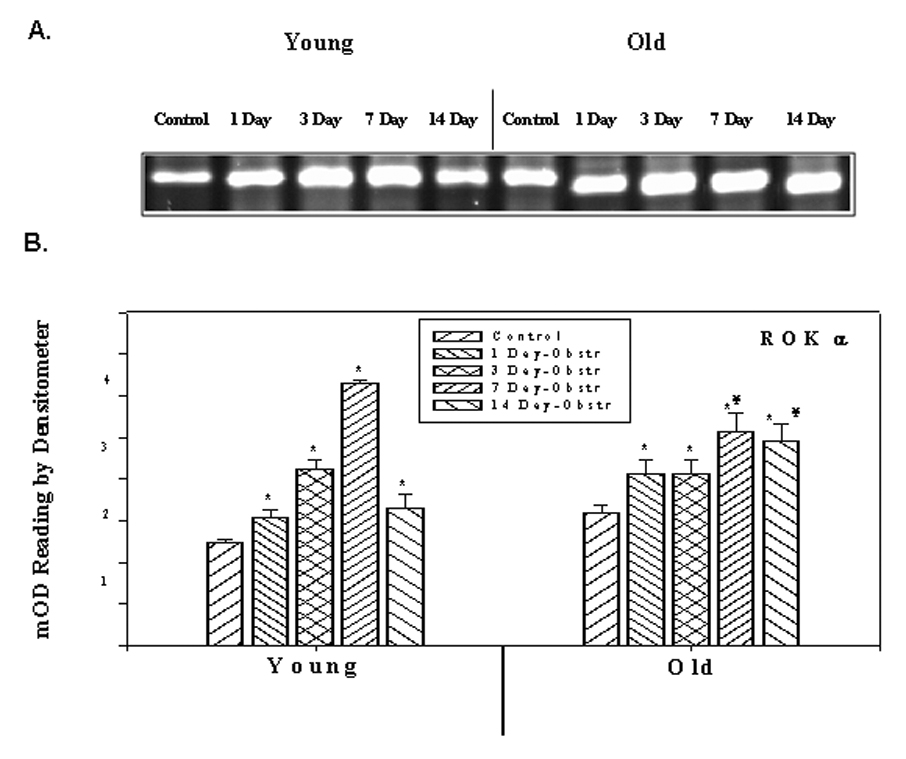
RT-PCR analysis of ROK α. A). ROK α mRNA expression in young and old rabbits’ control and obstructed bladders. B) Average expression in different samples of young and old rabbits. Each bar is the mean +/− SEM of 4 obstructed and 4 control rabbits. * = significantly different from control, ¥ = significantly different from young, p < 0.05.
Figure 2.
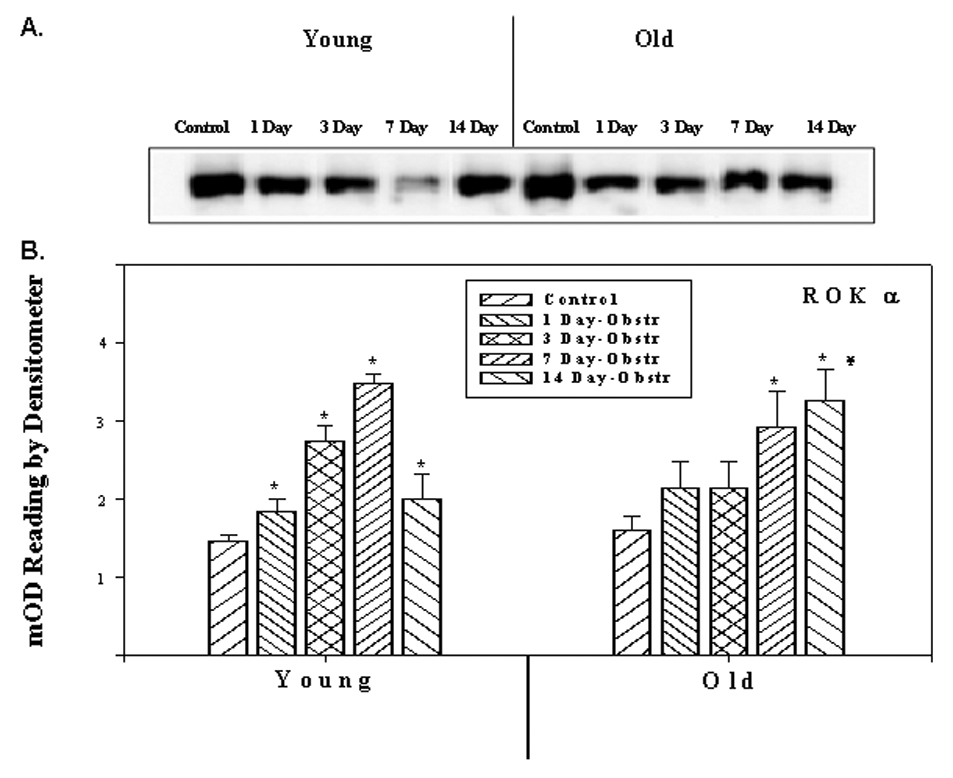
Western blot analyses showing the expression of ROK α at the protein level. A) Protein extracts from detrusor smooth muscles of young and old rabbit’s control and obstructed rabbit bladders probed with antibody against ROK α. B) Panel B depicts the relative expression of ROK α as obtained from scanning the bands. Each bar is the mean +/− SEM of 4 obstructed and 4 control rabbits. * = significantly different from control, ¥ = significantly different from young, p < 0.05.
Whereas there was an increase for ROKα, there was a significant decrease in the expression of ROKβ in both young and old obstructed groups. Figure 3 represents the expression of ROKβ at the mRNA level and quantitative data of densitometric scanning. The level gradually decreases during the course of the 1–7 day obstruction period in the young groups and then increases to the day 1 level. However, in old rabbits, there was a significant decrease in all the post-obstruction groups; the increased expression seen in the 14 day obstructed young group was not seen in the 14 day old group. These changes in the expression of ROKβ were also seen at the protein level (Figures 4).
Figure 3.
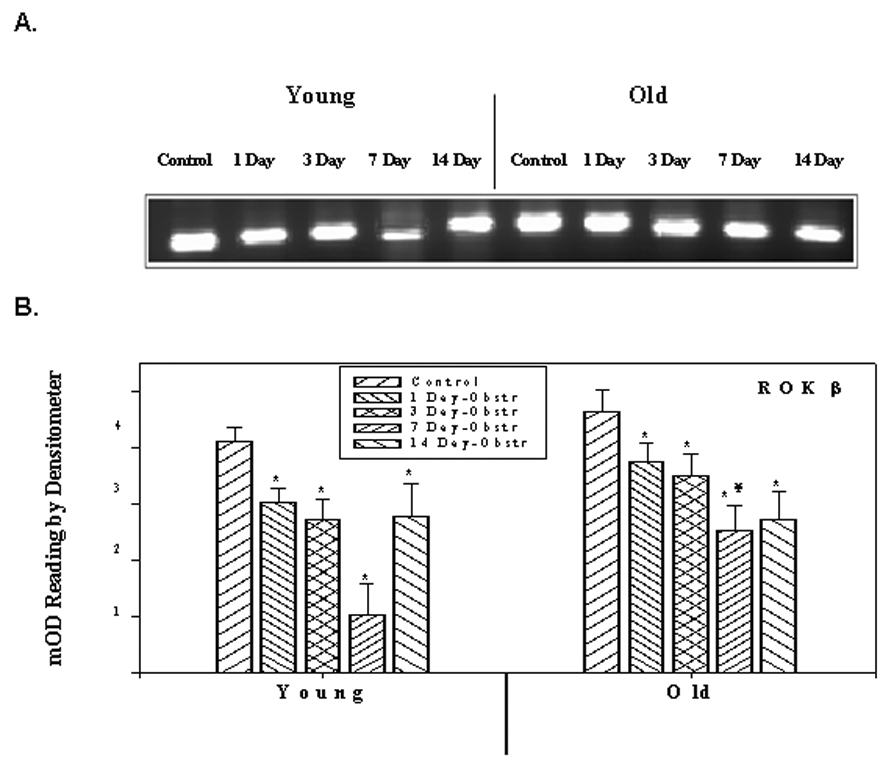
RT-PCR analysis of ROK β. A) ROK β mRNA expression in young and old rabbits’ control and obstructed bladders. B) Average expression in different samples of young and old rabbits. Each bar is the mean +/− SEM of 4 obstructed and 4 control rabbits. * = significantly different from control, ¥ = significantly different from young, p < 0.05.
Figure 4.
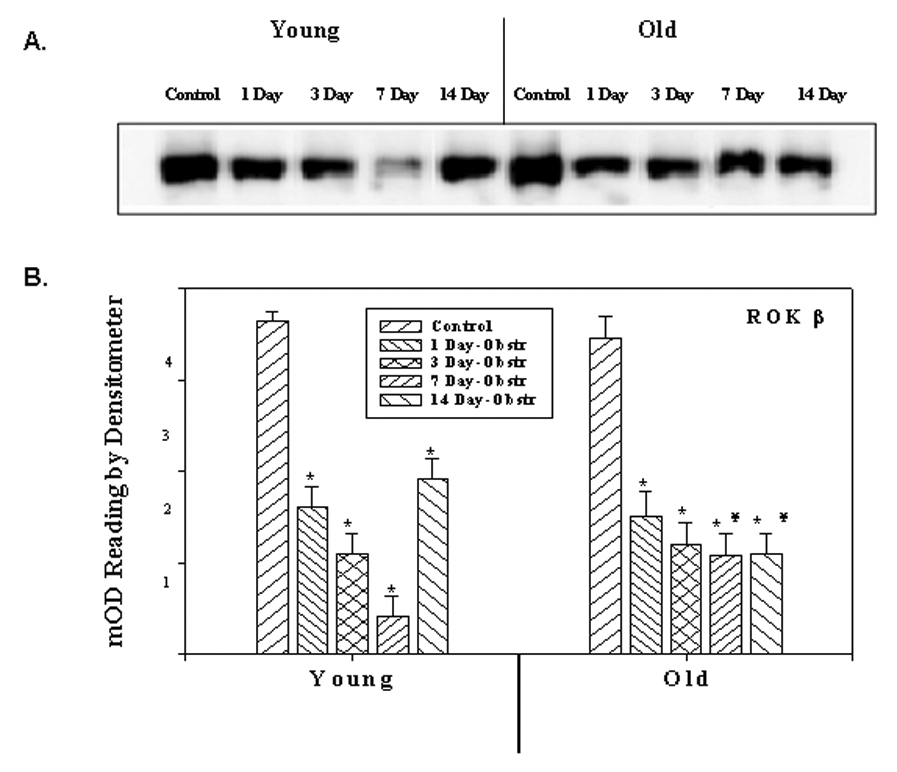
Western blot analysis showing the expression of ROK β at the protein level. A) Protein extracts from detrusor smooth muscles of young and old rabbit’s control and obstructed rabbit bladders probed with antibody against of control and obstructed rabbit bladders probed with antibody against ROK β. B) Panel B depicts the relative expression of ROK β as obtained from scanning the bands. Each bar is the mean +/− SEM of 4 obstructed and 4 control rabbits.* = significantly different from control, ¥ = significantly different from young, p < 0.05.
COMMENTS
In this study, we focused on the relationship between aging and ROK isoforms and compared changes of ROK expression at short-term PBOO between young and old rabbits. We clearly show incremental changes in ROKα and ROKβ with a different pattern of change after obstruction. Further, we show that young animals can recover from changes in ROK better than older animals. In addition to finding that ROKα was increased and ROKβ was decreased, we found that there were changes in both ROKα and ROKβ over time after obstruction. Although the exact differences between ROKα and ROKβ isoforms are not clearly defined, in our study we have found that they changed in opposite directions with increasing ROKα and decreasing ROKβ during the course of obstruction. Activation of the RhoA/ROK signaling cascade results in cytoskeletal reorganization and is dependent on the integration of multiple downstream signals.15 The activation of ROK inhibits the myosin phosphatase, leading to phosphorylation of the MLC, contraction happens as a result of MLC dephosphorylation. Therefore, regulation of ROK activity can be controlled either by altering expression level of ROK itself or by altering expression levels of RhoA.5 It is assumed that, the ROK mediated pathway may be partly responsible for the high degree of force maintenance in the obstructed rabbit bladder to expel urine. Essentially, inducing SM contraction fails due to obstruction; the bladder becomes Ca2+ sensitive and ROKα increases, presumable as a compensatory mechanism. In a previous paper on the expression of ROK isoforms in PBOO rabbits, Bing et al reported that in mature male rabbits, ROKα was not changed and ROKβ was overexpressed in the SM in response to mild partial bladder outlet obstruction for two weeks.10 Our study is different in time course of obstruction. In addition, there may be differences in the antibody used and the degree of obstruction created. The role for ROK in SM contraction was shown in other organs. Aikawa et al showed that ROK activity plays an important role in stretch induced activation of extracellular signal regulated protein kinases, expression of α-actin and an increase in protein synthesis in cardiac myocytes. 16 In endothelium, Y-27632, a specific inhibitor of ROK, selectively inhibits SM contraction and corrects blood pressure in several hypertensive rat models. 17 Rajasekaran et al. demonstrated that inhibition of ROK activity with Y-27632 suppressed the bladder hyperactivity noted in the spontaneously hypertensive rat. 18 However, the precise role of ROK in the bladder and the specific effect of ROK inhibitor on the bladder function are still being elucidated.
Our data suggest that an enhanced ROKα and reduced ROKβ mediated signal transduction pathway has a significant role in the adaptive changes of the young rabbits. There is no data about aging and ROK expression in the bladder tissue. Observations indicate that the structural proteins of cytoskeleton and its connection to plasma membrane and extracellular matrix are dynamically regulated and play essential role in contractile responses. 19 Greenland et al. has demonstrated that bladder contraction is accompanied by cyclical ischemia/reperfussion (I/R).20 As the bladder gets thicker the severity of I/R increases. Age-related intolerance to cyclical I/R may cause changes at ROK signaling. It was shown that Rho-Kinase activation is involved in the pathogenesis of myocardial I/R injury and pretreatment with ROK inhibitor before reperfusion suppresses the development of myocardial infarction in dogs in vivo. In another study, Rikitake et al reported that inhibition of ROK leads to increased cerebral blood flow and suggests that it can be a therapeutic target for ischemic stroke. 21 Therefore, the upregulation of ROKα and downregulation of ROKβ contributes to the faster adaptive changes of young rabbit bladder. In this regard, inhibition of ROKα and activation of ROKβ may be useful for treatment of bladder dysfunction due to obstruction.
CONCLUSIONS
The upregulation of ROKα and downregulation of ROKβ may play an important role in the adaptation of the smooth muscle to outlet obstruction. Further studies on the pharmacological effects of Rho-kinase may provide novel strategies for the treatment of PBOO.
Acknowledgments
This material is based upon work supported in part by the Office of Research and Development Medical Research Service, Department of Veteran’s Affairs; in part by NIH grant RO-1-DK 067114; and in part by a generous grant from the Astellas USA Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chatelain C, Denis L, Foo K, Khoury S. Benign Prostatic Hyperplasia. Paris: Health Publications Ltd; 2001. p. 535. [Google Scholar]
- 2.Levin RM, Haugaard N, O'Connor L, Buttyan R, Das A, Dixon JS, Gosling JA. Obstructive response of human bladder to BPH vs. rabbit bladder response to partial outlet obstruction: a direct comparison. Neurourol Urodyn. 2000;19:609–629. doi: 10.1002/1520-6777(2000)19:5<609::aid-nau7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004;84:935–986. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 4.Levin RM, Chicester P, Hass MA, Gosling JA, Buttyan RB. Obstructive bladder dysfunction: morphological, biochemical, and molecular changes. Eur Urol Suppl. 2002;1:14–20. [Google Scholar]
- 5.Guven A, Kalorin C, Onal B, Whitbeck C, Chichester P, Kogan BA, Levin RM, Mannikarottu A. Novel biomarkers of bladder decompensation after partial bladder obstruction. Neurourol Urodyn. 2007 doi: 10.1002/nau.20433. [DOI] [PubMed] [Google Scholar]
- 6.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522(Pt 2):177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi R, Nishimura J, Hirano K, Seki N, Naito S, Kanaide H. Ca2+ sensitization in contraction of human bladder smooth muscle. J Urol. 2004;172:748–752. doi: 10.1097/01.ju.0000130419.32165.6b. [DOI] [PubMed] [Google Scholar]
- 8.Horowitz A, Menice CB, Laporte R, Morgan KG. Mechanisms of smooth muscle contraction. Physiol Rev. 1996;76:967–1003. doi: 10.1152/physrev.1996.76.4.967. [DOI] [PubMed] [Google Scholar]
- 9.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 10.Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999;274:37385–37390. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- 11.Bing W, Chang S, Hypolite JA, DiSanto ME, Zderic SA, Rolf L, Wein AJ, Chacko S. Obstruction-induced changes in urinary bladder smooth muscle contractility: a role for Rho kinase. Am J Physiol Renal Physiol. 2003;285:F990–F997. doi: 10.1152/ajprenal.00378.2002. [DOI] [PubMed] [Google Scholar]
- 12.Wibberley A, Chen Z, Hu E, Hieble JP, Westfall TD. Expression and functional role of Rho-kinase in rat urinary bladder smooth muscle. Br J Pharmacol. 2003;138:757–766. doi: 10.1038/sj.bjp.0705109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guven A, Onal B, Kalorin C, Whitbeck C, Chichester P, Kogan B, Levin R, Mannikarottu A. Long term partial bladder outlet obstruction induced contractile dysfunction in male rabbits: a role for Rho-kinase. Neurourol Urodyn. 2007 doi: 10.1002/nau.20435. [DOI] [PubMed] [Google Scholar]
- 14.Mannikarottu AS, Hypolite JA, Zderic SA, Wein AJ, Chacko S, Disanto ME. Regional alterations in the expression of smooth muscle myosin isoforms in response to partial bladder outlet obstruction. J Urol. 2005;173:302–308. doi: 10.1097/01.ju.0000142100.06466.49. [DOI] [PubMed] [Google Scholar]
- 15.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 16.Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Zhu W, Kadowaki T, Yazaki Y. Rho family small G proteins play critical roles in mechanical stress-induced hypertrophic responses in cardiac myocytes. Circ Res. 1999;84:458–466. doi: 10.1161/01.res.84.4.458. [DOI] [PubMed] [Google Scholar]
- 17.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 18.Rajasekaran M, Wilkes N, Kuntz S, M EA. Rho-kinase inhibition suppresses bladder hyperactivity in spontaneously hypertensive rats. Neurourol Urodyn. 2005;24:295–300. doi: 10.1002/nau.20122. [DOI] [PubMed] [Google Scholar]
- 19.Sylvester JT. The tone of pulmonary smooth muscle: ROK and Rho music? Am J Physiol Lung Cell Mol Physiol. 2004;287:L624–L630. doi: 10.1152/ajplung.00215.2004. [DOI] [PubMed] [Google Scholar]
- 20.Greenland JE, Brading AF. The effect of bladder outflow obstruction on detrusor blood flow changes during the voiding cycle in conscious pigs. J Urol. 2001;165:245–248. doi: 10.1097/00005392-200101000-00072. [DOI] [PubMed] [Google Scholar]
- 21.Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, Moskowitz MA, Liao JK. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36:2251–2257. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]


