Abstract
Selective serotonin reuptake inhibitors (SSRI’s) are utilized in the treatment of depression in pregrant and lactating women. SSRI’s may be passed to the fetus through the placenta and the neonate through breastfeeding, potentially exposing them to SSRIs during peri and postnatal development. However, the long-term effects of this SSRI exposure are still largely unknown. The simplicity and genetic amenability of model organisms is a significant advantage to work with humans. This review will assess the current research done in animals that sheds light on the role of serotonin during development and the possible effects of SSRIs. Experimental studies in rodents show that administration of SSRIs during a key developmental window creates changes in brain circuitry and maladaptive behaviors that persist into adulthood. Similar changes result from the inhibition of serotonin transporter or monoamine oxidase, implicating these two regulators of serotonin signaling in developmental changes. Understanding the role of serotonin in brain development is critical to identifying the possible effects of SSRI exposure.
Keywords: serotonin, neurotransmitter, CNS development
Introduction
Serotonin signaling is involved in the modulation of the majority of neuronal circuits in the brain and likely plays a key role in the regulation of behavior and development. Levels of serotonin in the brain are largely regulated via the reuptake of serotonin through the serotonin transporter (SERT) and the degradation of serotonin by monoamine oxidase (MAO). Human polymorphisms in SERT and MAO are associated with depression and anxiety (Du et al., 2004, Caspi et al., 2003). Drugs targeting these two proteins have proven effective in the treatment of depression and other psychiatric illnesses. Selective serotonin reuptake inhibitors (SSRIs) are especially popular due to their limited side effects as compared to earlier drugs such as tricyclic antidepressants and MAO inhibitors (Drugs, 2000). However, the findings of maladaptive behaviors caused by polymorphisms in SERT and MAO suggest that altered levels of serotonin early in development may have unexpected effects on the developing brain. This is supported by recent discoveries in animal models.
Animal studies to date show the role of serotonin in early development and adulthood is not equivalent. While SSRI treatment alleviates symptoms of depression and anxiety in adult mouse models of these diseases, SSRI treatment in newborn mice causes an increase in these maladaptive behaviors that persists into adulthood. Furthermore, mice lacking SERT suffer from increased anxiety and depressive symptoms (Lira et al., 2003, Holmes et al., 2003b). This is thought to occur because the brains of these animals are exposed to high levels of extracellular serotonin throughout development. These results suggest the existence of a critical period where SSRI treatment may cause long-lasting changes that differ from that of treatment in adulthood. This is of special concern because SSRIs, which are the drug of choice for the treatment of depression in women during pregnancy and breastfeeding, can to cross the placental barrier and may be excreted in breast milk (Hendrick et al., 2003, Weissman et al., 2004). This highlights the need to explore and understand the possible effects of SSRI’s on the neonate.
This review will cover developmentally relevant aspects of serotonergic neuron structure and signaling. Most of what is known about serotonin signaling has been discovered through the use of model organisms (box reference). In this review, we will present a summary of the evidence from animal studies that indicate the importance of the serotonergic system in modifying the circuitry of the developing brain and the eventual behavior of the adult animal. We will begin with a brief introduction to the serotonergic system. This will be followed by a discussion of the developmental effects of serotonin degradation by MAO, reuptake of serotonin by SERT, and other studies that alter serotonin levels. Because MAO and SERT play a central role in regulating extracellular serotonin levels, emphasis will be placed on the developmental and behavioral effects of altering these two molecules.
Box + table 1: Animal models
Box + table 1.
Effects of altering serotonin signaling molecules–
| Animal | Human | ||
|---|---|---|---|
| Serotonin signaling molecule | Structural changes in KO mice | Behavioral changes in KO mice | Clinical significance |
| TPH 2 | no TPH2 KO | - | Possible polymorphism linked with depression |
| VMAT 2 | no structural abnormalities | lethal during early postnatal development | - |
| SERT | abnormal axonal arborization: retinal and thalamic neurons | increased anxiety, depression, decreased agression | Polymorphism (short variant) linked to increased anxiety SSRIs used for treatment of depression, anxiety, OCD, etc |
| MAO A | abnormal axonal arborization: retinal and thalamic neurons | increased aggression, anxiety | Polymorphism associated with increased aggression and depression |
| MAOA inhibitors used for treatment of depression | |||
| 5HT1A | increased anxiety | - | |
| 5HT1B | influences axonal arborization | increased aggression | - |
Animal models play a crucial role in our understanding of serotonin signaling on a genetic as well as molecular level. The scale of the system can range from 300,000 sertonergic neurons in a human to a mere 100 in a fruit fly, and yet the molecules, signaling cascades, and regulatory pathways are well conserved (Murphy et al., 2004, Hen, 1993). This conservation provides two major advantages for work with humans: simplicity and genetic amenability. The mouse and rat are the vertebrate workhorses of many studies because they can provide data ranging from molecular details to behavioral phenotypes. However, Drosophila’s simplicity and ease of genetic manipulation provide an excellent model for understanding the fundamentals of the serotonergic system. For example, the study of SERT and serotonin autoregulation in Drosophila identified a number of genes involved in regulating SERT expression, including robo2 and eagle (Couch et al., 2004, Hendricks et al., 2003). Lastly, behavioral and electrophysiological analysis of aplysia, a marine mollusk, has contributed greatly to our understanding of serotonin’s role in neural plasticity and long-term synaptic facilitation (Glanzman, 1994).
These studies have revealed connections important for understanding human disease. For instance, fibroblast growth factor (FGF) was initially identified as a key regulator of the onset of SERT expression in the grasshopper (Condron and Zinn, 1997). Subsequent gene expression studies showed it to be significantly decreased in patients with major depression (Evans et al., 2004).
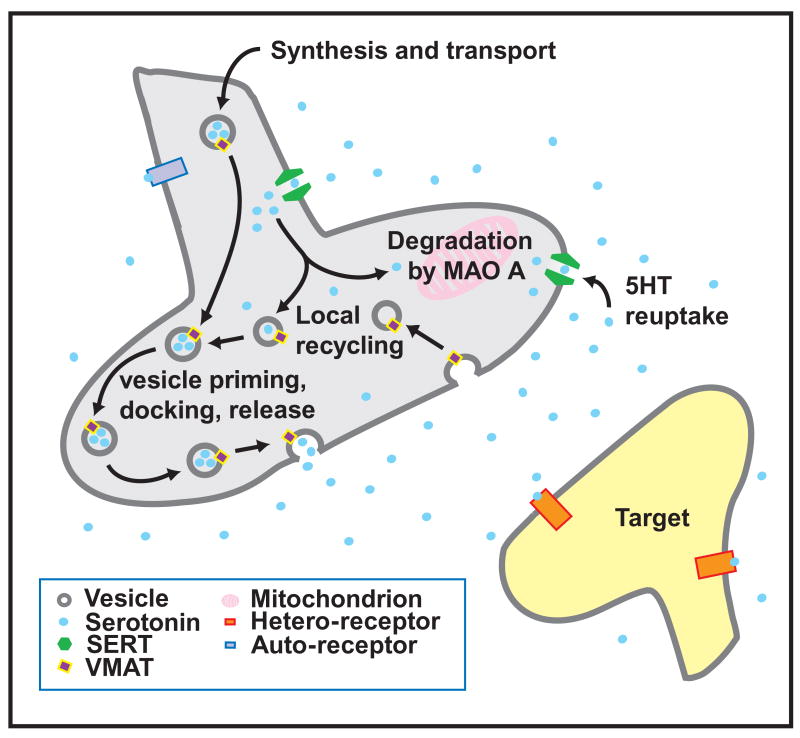
Box + Figure 1: Serotonin as a neurotransmitter
Serotonin synthesis is a two-step enzymatic process beginning with the rate-limiting enzyme, tryptophan hydroxylase (Tph), which converts the amino acid tryptophan into the intermediate 5-hydroxytryptophan. Aromatic amino acid decarboxylase (AADC) then converts the 5-hydroxytryptophan intermediate into serotonin. Tph polymorphisms have been proposed to be associated with depression and panic disorder (Zhou et al., 2005, 2005, Zill 2004, Maron 2006), however, see (Hahn and Blakely, 2002).
Once serotonin is synthesized it is packaged into vesicles for neurotransmission by the vesicular monoamine transporter (VMAT), which transports monoamines (serotonin, dopamine, histamine, and noradrenaline) from the cytoplasm into vesicles using a proton gradient.
Following release into the extracellular space, serotonin can interact with one of more than 14 receptor types. While one class of receptors acts through a serotonin gated ion channel, the remainder of serotonin receptors act through G protein coupled signaling. These receptors are expressed on a variety of different targets in the developing and mature organism, which allows serotonin to have its multitudinous effects (For a more detailed review of receptors, see (Barnes and Sharp, 1999)). Serotonin signaling through these receptors is dependent on extracellular serotonin levels.
Serotonin is removed from the extracellular space via SERT and then repackaged into secretory vesicles or degraded by MAO. Therefore the function of SERT and MAO is central to regulation of serotonin levels both inside and outside of the serotonergic neuron.
Serotonergic neurons
Serotonin is an ancient molecule, whose function has expanded to play a variety of roles in developing as well as adult humans. Serotonin is secreted hormonally by a number of peripheral structures including the pineal gland, parafollicular cells of the thyroid, enterochromaffun cells of the gut, and neuroepithelial bodies of the lung. Hormonally-released serotonin freely crosses the placenta and the immature blood-brain barrier, providing the fetus with a paracrine source of serotonin long before it gains the ability to synthesize the neurotransmitter (Vitalis and Parnavelas, 2003). In the central nervous system, serotonin is synthesized and released by serotonergic neurons located in the raphe nuclei within the brainstem. This review will focus on the role of serotonin in the developing central nervous system.
Serotonergic neurons develop very early, sending projections throughout the brain, and therefore are in a prime position to modulate the maturation of other neuronal circuits. Indeed, serotonergic fibers have been shown to make early, functional synaptic contacts with Cajal-Retzius cells (Janusonis et al., 2004), which are believed to play an essential role in the establishment of cortical layers (Ogawa et al., 1995) and radial columns (Luhmann et al., 2003).
Later in development, serotonin is transiently taken up by certain glutamatergic neurons found in the thalamus, limbic cortex, hypothalamus, retinal and superior olivary nuclei (Lebrand et al., 1996, Beltz et al., 2001). These so-called transient serotonergic neurons are involved in sensory processing. Lacking both Tph and AADC, the two enzymes necessary for serotonin synthesis, they take up extracellular serotonin via membranous serotonin transporter (Xu et al., 2004). Although SERT expression is seen only during a window of activity-dependent patterning, these neurons continue to express serotonin receptor 1B (5-HT-1b), thus retaining the ability to respond to the neurotransmitter. Because of their reliance on extracellular serotonin, serotonergic transients are likely to be more vulnerable to changes in extracellular serotonin levels during activity-dependent patterning. As such, aberrant patterning of areas of the cortex innervated by these neurons may contribute to the developmental effect of SSRIs. The mechanism by which serotonin effects the development of these regions is still controversial (Gaspar et al., 2003, Xu et al., 2004, Persico et al., 2000).
Out of the multitude of targets innervated by the transients, the barrel field area of the primary somatosensory cortex is the most investigated in rodent models. Here, inputs from each whisker of the snout normally map onto a precise cluster of projections corresponding to it (D’Amato et al., 1987, Rebsam et al., 2002). Due to the precise stereotyped distribution of neurons, subtle patterning defects can be examined. Therefore, analysis of the barrel area map is a powerful tool for understanding the structural consequences of altering serotonin levels during development.
Serotonin degradation by MAO
The significance of MAO in regulating serotonin levels is highlighted by the use of MAO inhibitors for the treatment of clinical depression. By preventing serotonin breakdown, MAO inhibition leads to significantly increased serotonin levels. Some MAO polymorphisms, which reduce serotonin levels, are associated with increased propensity towards violence (Meyer-Lindenberg et al., 2006) and major depression (Du et al., 2004) in humans. A critical first step towards understanding the effect of elevated serotonin during development was the creation of the MAO knockout mouse (For an in-depth review of this model see (Gaspar et al., 2003). In MAO KO mice, the cortical barrels are disrupted, leaving a jumble of overlapping fields along with decreased axonal branching (Rebsam et al., 2002). Additional patterning defects are seen in the segregation of retinal ganglion cell columns (Upton et al., 1999). Inhibition of serotonin synthesis during the perinatal period is sufficient to rescue the patterning defects. These studies implicate serotonin as a key regulator of activity-dependent patterning in serotonergic transient-rich areas such as the barrel cortex and superior colliculus. Furthermore, they suggest the existence of a developmental window characterized by increased sensitivity to changes in serotonin levels.
Analysis of MAO/5-HT1B double knockout mice helped to further refine the mechanism by which increased serotonin levels cause somatosensory patterning defects. Despite significantly elevated serotonin levels, double KO mice show a rescue of normal barrel morphology and retinal ganglion cell columns (Salichon et al., 2001). Therefore, it is likely that excess activation of the 1b receptor, which inhibits glutamate release, hampers activity-dependent axonal competition and pruning; Transient SERT expression may allow some of the glutamatergic neurons to escape serotonin-mediated inhibition and through doing so, gain a competitive advantage over their neighbors. When serotonin levels are artificially raised through MAO inhibition, this mechanism no longer functions appropriately, reducing pruning and leading to aberrant pattern formation. Since SSRI administration also leads to elevated serotonin levels, it has the potential to cause similar defects when administered during this critical developmental window.
Serotonin uptake by SERT
The importance of SERT during development in both circuitry formation and behavior is highlighted by a number of animal models. Two SERT knockout (KO) mice strains have been independently generated (Lira et al., 2003, Bengel et al., 1998). Complete loss of SERT results in four to six-fold increases in extracellular serotonin concentration (Mathews et al., 2004, Montanez et al., 2003) and 50% decreases in serotonin tissue concentration (Kim et al., 2005). SERT KO mice exhibit a number of defects including: alterations in sensory patterning, compensatory changes in receptor expression (Li, 2006), sleep pattern abnormalities, and long-term behavioral deficits, which depend on the genetic background of the mice.
Working through different mechanisms, both SERT and MAO knockouts cause increased serotonin levels during development. It is therefore not surprising that SERT KO mice show defects in barrel cortex and ganglion cell column patterning, similar to those seen in MAO knockouts. Furthermore, as would be predicted from MAO/5-HT1B studies, SERT/5-HT1B double knockout results in a rescue of the patterning defect (Salichon et al., 2001). Thus, both mouse models converge on the same pathway, through which serotonin may exert a developmental effect on cortical morphology. It is yet unclear if there is any link between these patterning defects and maladaptive behavioral responses seen in these mice.
Although precise modeling of human behavior in mice is difficult, a number of paradigms combining behavioral observation and/or biological monitoring allow correlations to aggression, anxiety, and/or depression. Tests in which performance is affected by the administration of SSRIs or anxiolytics tend to be the preferred means of measuring depressive or anxiery-like traits in rodents. SERT KO mice tend to exhibit significantly decreased levels of aggression when challenged with an intruder (Holmes et al., 2003a). Some mice show increased levels of anxiety-like behaviors (Holmes et al., 2003c), while others exhibit a depression-like phenotype, characterized by decreased attempts to escape unpleasant stimuli (Lira et al., 2003) and increased stress-sensitivity. Minor stressors cause exaggerated adrenocorticotropic and catecholamine release. The same mice also show a reduced dexamethasone-induced suppression of corticosterone secretion, pointing to a possible dysregulation of the hypothalamic-pituitary-adrenal axis (Prathiba et al., 1998, Li et al., 1999, Murphy et al., 2001, Tjurmina et al., 2002). The importance of genetic background in modulating the SERT KO phenotype suggests the existence of a number of modifier loci involved.
At least some of the behavioral phenotypes seen in SERT KO mice may be due to the excessive activation of the 5HT-1A receptor. Studies have shown that blockade of the 5HT-1A receptor with a drug, WAY 100635, early in development rescues some of the behavioral abnormalities found in SERT KO mice, such as altered sleep patterns and depression (Alexandre et al., 2006). Interestingly, 5-HT1a KO mice exhibit increased anxiety-like behavior (Groenink et al., 2003), possibly explaining the tendency of SERT KO mice to exhibit either primarily anxiety-like or depressive-like traits.
A critical observation is that SERT KO mice exhibit decreased performance on many of the same tasks normally improved by SERT inhibition in adulthood through SSRI administration or siRNA infusion in normal mice (Lucki et al., 2001, Thakker et al., 2005). This discrepancy is most likely to be a consequence of altered serotonin levels during critical a developmental window, with permanent consequences seen in the adult. As with MAO studies, this points to the different roles serotonin plays during development versus in the adult. The importance SERT in regulating serotonin levels during development is further highlighted by human genetic studies demonstrating that a relatively common polymorphism in the human SERT promoter, the short allelic form, results in reduced expression of SERT and an increased susceptibility to elevated anxiety, neuroticism, and depressive symptoms (Caspi et al., 2003, Lasky-Su et al., 2005). These defects are similar, although less extreme, to those seen in SERT KO mice.
Pharmacological manipulation of SERT function during the mouse postnatal period produces behaviors similar to those seen in SERT KO mice. This phenotype, described as neonatal antidepressant exposure syndrome (NADES) persists into adulthood (Maciag et al., 2006). Mice exposed to therapeutic doses of fluoxetine during the early postnatal period exhibit increased anxiety-like behavior and an impairment of shock avoidance (Ansorge et al., 2004). This behavioral phenotype has been replicated in similarly treated rats (Maciag et al., 2006, Hansen et al., 1997, Hansen and Mikkelsen, Mirmiran et al., 1981). Additional defects are seen in sexual competence/activity, alterations in locomotor activity, and REM sleep regulation. Furthermore, early SSRI treatment causes permanent reduction in TPH in the dorsal raphe and SERT expression in the cortex. None of these SSRI induced alterations are observed in adults undergoing similar type and duration of treatment, again reinforcing the idea of a critical serotonin-susceptibility period.
Other manipulations
The majority of the studies presented here focus on the developmental effect of elevated serotonin levels. It is, however important to point out that defects can also arise from depletion of serotonin levels. Depletion of extracellular serotonin in rodents was accomplished in a number of ways including: administration of serotonergic neurotoxins or synthesis inhibitors and preventing the packaging of serotonin into vesicles via a VMAT knockout (Bennett-Clarke et al., 1994, Wang et al., 1997, Persico et al., 2000, Persico et al.). Regardless of the methodology employed, decreased serotonin levels caused delayed barrel pattern maturation and decreased barrel size, although the effect is difficult to separate from a variable degree of growth impairment. The large number of systems influenced by serotonin often makes analyzing phenotypes resulting from whole-organism alterations in serotonin levels difficult, especially in more complex model systems.
A recent study injected serotonin-specific toxin directly into the medial forebrain bundle of newborn mice (Boylan et al., 2006) to avoid analyzing the exponentially more complex phenotype produced by global inhibition of serotonin signaling. This lesion produced numerous defects including: sex and region-specific increases in cerebral cortex width, impaired social learning and impulse control, increased repetitive behavior and aggression, hyper-responsiveness to environmental changes, and impaired fine motor performance. Due to behavioral and structural similarities, these mice have been proposed as a model for autism without mental retardation. The mechanism linking serotonin levels during development to autism is still unclear but this study further emphasizes the importance of tight control of extracellular serotonin during this critical period (Boylan et al., 2006, Whitaker-Azmitia, 2005, Kahne et al., 2002). Deviations in either direction from optimal serotonin levels during key developmental windows can produce dramatic and long-lasting alterations in the organism.
Conclusions
In this review, we have presented the major steps of serotonin signaling: serotonin synthesis and packaging, reuptake, and degradation. We have stressed the effects that each of these processes has on the regulation of serotonin levels and the developing brain. Animal studies have shown the importance of serotonin signaling in modulating neuronal circuitry, 5HT receptor level, and behavior. Regardless of the method employed, increased extracellular serotonin levels during the perinatal period can cause subtle changes in brain circuitry and maladaptive behaviors, such as increased anxiety, aggression, or depression, that are maintained into adulthood. These effects are thought occur through excessive activation of certain serotonin receptors as evidenced by the rescue of these phenotypes by pharmacological or genetic means.
Serotonin signaling is highly conserved, and therefore many of these animal model finding are relevant to humans. This is highlighted by the discovery of human polymorphisms in SERT and MAO that are associated with maladaptive behaviors that are very similar to the behaviors seen in knockout mice lacking these serotonin signaling genes. Altogether these findings stress the importance of regulating levels of extracellular serotonin during critical developmental windows. SSRI’s blockage during this time could have subtle effects on the developing brain that may not become apparent until adulthood. Very little is known about the timing or number of such critical periods in human development. It is clear that more studies in both animals and humans will need to be done to fully understand the effects of SSRIs on the developing brain.
While much of our current knowledge of serotonin signaling comes from mouse models, more basic animal systems may be necessary to tease out some of the fundamental rules governing serotonin signaling. For example, the fruit fly with its conserved serotonin signaling pathway, provides a more simple model to study the serotonergic system at the single neuron level. Studies done in the fruit fly, where excess serotonin has been shown to cause compensatory changes in the structure of the serotonergic neurons, have provided evidence for an autoregulatory role of serotonin. Further work in this simple model may yield more insight into the serotonergic signaling and its role in the developing brain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexandre C, Popa D, Fabre V, Bouali S, Venault P, Lesch KP, Hamon M, Adrien J. Early life blockade of 5-hydroxytryptamine 1A receptors normalizes sleep and depression-like behavior in adult knock-out mice lacking the serotonin transporter. J Neurosci. 2006;26:5554–64. doi: 10.1523/JNEUROSCI.5156-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–81. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Beltz BS, Benton JL, Sullivan JM. Transient uptake of serotonin by newborn olfactory projection neurons. Proc Natl Acad Sci U S A. 2001;98:12730–5. doi: 10.1073/pnas.231471298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–55. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Hankin MH, Leslie MJ, Chiaia NL, Rhoades RW. Patterning of the neocortical projections from the raphe nuclei in perinatal rats: investigation of potential organizational mechanisms. J Comp Neurol. 1994;348:277–90. doi: 10.1002/cne.903480209. [DOI] [PubMed] [Google Scholar]
- Boylan CB, Blue ME, Hohmann CF. Modeling early cortical serotonergic deficits in autism. Behav Brain Res. 2006 doi: 10.1016/j.bbr.2006.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Mcclay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Condron BG, Zinn K. Regulated neurite tension as a mechanism for determination of neuronal arbor geometries in vivo. Curr Biol. 1997;7:813–6. doi: 10.1016/s0960-9822(06)00343-5. [DOI] [PubMed] [Google Scholar]
- Couch JA, Chen J, Rieff HI, Uri EM, Condron BG. robo2 and robo3 interact with eagle to regulate serotonergic neuron differentiation. Development. 2004;131:997–1006. doi: 10.1242/dev.00962. [DOI] [PubMed] [Google Scholar]
- D’amato RJ, Blue ME, Largent BL, Lynch DR, Ledbetter DJ, Molliver ME, Snyder SH. Ontogeny of the serotonergic projection to rat neocortex: transient expression of a dense innervation to primary sensory areas. Proc Natl Acad Sci U S A. 1987;84:4322–6. doi: 10.1073/pnas.84.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugs CO. Use of psychoactive medication during pregnancy and possible effects on the fetus and newborn. Committee on Drugs. American Academy of Pediatrics. Pediatrics. 2000;105:880–7. doi: 10.1542/peds.105.4.880. [DOI] [PubMed] [Google Scholar]
- Du L, Bakish D, Ravindran A, Hrdina PD. MAO-A gene polymorphisms are associated with major depression and sleep disturbance in males. Neuroreport. 2004;15:2097–101. doi: 10.1097/00001756-200409150-00020. [DOI] [PubMed] [Google Scholar]
- Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, Lopez JF, Thompson RC, Meng F, Stead JD, Walsh DM, Myers RM, Bunney WE, Watson SJ, Jones EG, Akil H. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci U S A. 2004;101:15506–11. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–12. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Glanzman DL. Postsynaptic regulation of the development and long-term plasticity of Aplysia sensorimotor synapses in cell culture. J Neurobiol. 1994;25:666–93. doi: 10.1002/neu.480250608. [DOI] [PubMed] [Google Scholar]
- Groenink L, Van Bogaert MJ, Van Der Gugten J, Oosting RS, Olivier B. 5-HT1A receptor and 5-HT1B receptor knockout mice in stress and anxiety paradigms. Behav Pharmacol. 2003;14:369–83. doi: 10.1097/01.fbp.0000087737.21047.75. [DOI] [PubMed] [Google Scholar]
- Hahn MK, Blakely RD. Monoamine transporter gene structure and polymorphisms in relation to psychiatric and other complex disorders. Pharmacogenomics J. 2002;2:217–35. doi: 10.1038/sj.tpj.6500106. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Mikkelsen JD. Long-term effects on serotonin transporter mRNA expression of chronic neonatal exposure to a serotonin reuptake inhibitor. Eur J Pharmacol. 1998;352:307–15. doi: 10.1016/s0014-2999(98)00349-5. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Sanchez C, Meier E. Neonatal administration of the selective serotonin reuptake inhibitor Lu 10-134-C increases forced swimming-induced immobility in adult rats: a putative animal model of depression? J Pharmacol Exp Ther. 1997;283:1333–41. [PubMed] [Google Scholar]
- Hen R. Structural and functional conservation of serotonin receptors throughout evolution. Exs. 1993;63:266–78. doi: 10.1007/978-3-0348-7265-2_14. [DOI] [PubMed] [Google Scholar]
- Hendrick V, Stowe ZN, Altshuler LL, Hwang S, Lee E, Haynes D. Placental passage of antidepressant medications. Am J Psychiatry. 2003;160:993–6. doi: 10.1176/appi.ajp.160.5.993. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–47. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003a;2:365–80. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry. 2003b;54:953–9. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003c;28:2077–88. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- Janusonis S, Gluncic V, Rakic P. Early serotonergic projections to Cajal-Retzius cells: relevance for cortical development. J Neurosci. 2004;24:1652–9. doi: 10.1523/JNEUROSCI.4651-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahne D, Tudorica A, Borella A, Shapiro L, Johnstone F, Huang W, Whitaker-Azmitia PM. Behavioral and magnetic resonance spectroscopic studies in the rat hyperserotonemic model of autism. Physiol Behav. 2002;75:403–10. doi: 10.1016/s0031-9384(01)00673-4. [DOI] [PubMed] [Google Scholar]
- Kim DK, Tolliver TJ, Huang SJ, Martin BJ, Andrews AM, Wichems C, Holmes A, Lesch KP, Murphy DL. Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology. 2005;49:798–810. doi: 10.1016/j.neuropharm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Lasky-Su JA, Faraone SV, Glatt SJ, Tsuang MT. Meta-analysis of the association between two polymorphisms in the serotonin transporter gene and affective disorders. Am J Med Genet B Neuropsychiatr Genet. 2005;133:110–5. doi: 10.1002/ajmg.b.30104. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–35. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Li Q. Cellular and molecular alterations in mice with deficient and reduced serotonin transporters. Mol Neurobiol. 2006;34:51–66. doi: 10.1385/mn:34:1:51. [DOI] [PubMed] [Google Scholar]
- Li XM, Perry KW, Wong DT. Difference in the in vivo influence of serotonin1A autoreceptors on serotonin release in prefrontal cortex and dorsal hippocampus of the same rat treated with fluoxetine. Chin J Physiol. 1999;42:53–9. [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, Kung HF, Hofer MA, Hen R, Gingrich JA. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–71. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–22. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Hanganu I, Kilb W. Cellular physiology of the neonatal rat cerebral cortex. Brain Res Bull. 2003;60:345–53. doi: 10.1016/s0361-9230(03)00059-5. [DOI] [PubMed] [Google Scholar]
- Maciag D, Simpson KL, Coppinger D, Lu Y, Wang Y, Lin RC, Paul IA. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology. 2006;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–81. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, A RH, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A. 2006;103:6269–74. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmiran M, Van De Poll NE, Corner MA, Van Oyen HG, Bour HL. Suppression of active sleep by chronic treatment with chlorimipramine during early postnatal development: effects upon adult sleep and behavior in the rat. Brain Res. 1981;204:129–46. doi: 10.1016/0006-8993(81)90657-0. [DOI] [PubMed] [Google Scholar]
- Montanez S, Owens WA, Gould GG, Murphy DL, Daws LC. Exaggerated effect of fluvoxamine in heterozygote serotonin transporter knockout mice. J Neurochem. 2003;86:210–9. doi: 10.1046/j.1471-4159.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Mol Interv. 2004;4:109–23. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Li Q, Engel S, Wichems C, Andrews A, Lesch KP, Uhl G. Genetic perspectives on the serotonin transporter. Brain Res Bull. 2001;56:487–94. doi: 10.1016/s0361-9230(01)00622-0. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- Persico AM, Altamura C, Calia E, Puglisi-Allegra S, Ventura R, Lucchese F, Keller F. Serotonin depletion and barrel cortex development: impact of growth impairment vs. serotonin effects on thalamocortical endings. Cereb Cortex. 2000;10:181–91. doi: 10.1093/cercor/10.2.181. [DOI] [PubMed] [Google Scholar]
- Persico AM, Mengual E, Moessner R, Hall FS, Revay RS, Sora I, Arellano J, Defelipe J, Gimenez-Amaya JM, Conciatori M, Marino R, Baldi A, Cabib S, Pascucci T, Uhl GR, Murphy DL, Lesch KP, Keller F. Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J Neurosci. 2001;21:6862–73. doi: 10.1523/JNEUROSCI.21-17-06862.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prathiba J, Kumar KB, Karanth KS. Hyperactivity of hypothalamic pituitary axis in neonatal clomipramine model of depression. J Neural Transm. 1998;105:1335–9. doi: 10.1007/s007020050135. [DOI] [PubMed] [Google Scholar]
- Rebsam A, Seif I, Gaspar P. Refinement of thalamocortical arbors and emergence of barrel domains in the primary somatosensory cortex: a study of normal and monoamine oxidase a knock-out mice. J Neurosci. 2002;22:8541–52. doi: 10.1523/JNEUROSCI.22-19-08541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, De Maeyer E, Murphy DL, Mossner R, Lesch KP, Hen R, Seif I. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci. 2001;21:884–96. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker DR, Natt F, Husken D, Van Der Putten H, Maier R, Hoyer D, Cryan JF. siRNA-mediated knockdown of the serotonin transporter in the adult mouse brain. Mol Psychiatry. 2005;10:782–9. 714. doi: 10.1038/sj.mp.4001687. [DOI] [PubMed] [Google Scholar]
- Tjurmina OA, Armando I, Saavedra JM, Goldstein DS, Murphy DL. Exaggerated adrenomedullary response to immobilization in mice with targeted disruption of the serotonin transporter gene. Endocrinology. 2002;143:4520–6. doi: 10.1210/en.2002-220416. [DOI] [PubMed] [Google Scholar]
- Upton AL, Salichon N, Lebrand C, Ravary A, Blakely R, Seif I, Gaspar P. Excess of serotonin (5-HT) alters the segregation of ispilateral and contralateral retinal projections in monoamine oxidase A knock-out mice: possible role of 5-HT uptake in retinal ganglion cells during development. J Neurosci. 1999;19:7007–24. doi: 10.1523/JNEUROSCI.19-16-07007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitalis T, Parnavelas JG. The role of serotonin in early cortical development. Dev Neurosci. 2003;25:245–56. doi: 10.1159/000072272. [DOI] [PubMed] [Google Scholar]
- Wang YM, Gainetdinov RR, Fumagalli F, Xu F, Jones SR, Bock CB, Miller GW, Wightman RM, Caron MG. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron. 1997;19:1285–96. doi: 10.1016/s0896-6273(00)80419-5. [DOI] [PubMed] [Google Scholar]
- Weissman AM, Levy BT, Hartz AJ, Bentler S, Donohue M, Ellingrod VL, Wisner KL. Pooled analysis of antidepressant levels in lactating mothers, breast milk, and nursing infants. Am J Psychiatry. 2004;161:1066–78. doi: 10.1176/appi.ajp.161.6.1066. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? Int J Dev Neurosci. 2005;23:75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Xu Y, Sari Y, Zhou FC. Selective serotonin reuptake inhibitor disrupts organization of thalamocortical somatosensory barrels during development. Brain Res Dev Brain Res. 2004;150:151–61. doi: 10.1016/j.devbrainres.2003.02.001. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Salas R, Zhang L, De Biasi M, Dani JA. Corelease of dopamine and serotonin from striatal dopamine terminals. Neuron. 2005;46:65–74. doi: 10.1016/j.neuron.2005.02.010. [DOI] [PubMed] [Google Scholar]