Abstract
Fmoc-pSer-Ψ[(Z)CH=C]-Pro-(2)-N-(3)-ethylaminoindole 1, showed moderate inhibition towards the mitotic regulator, Pin1 (IC50 = 28.3μM). To improve the cell permeability, the charged phosphate was masked as the bis-pivaloyloxymethyl (POM) phosphate in Fmoc-(bisPOM)-pSer-Ψ[(Z)CH=C]-Pro-(2)-N-(3)-ethylaminoindole 2. Antiproliferative activity towards A2780 ovarian cancer cells of 1 (IC50 = 46.2 μM) was improved significantly in 2 (IC50 = 26.9 μM), comparable to the IC50 of 1 towards Pin1 enzymatic activity.
Cis-trans isomerization of proline-containing peptides has been implicated in a number of biologically important processes.1, 2 Peptidyl-prolyl isomerase (PPIase) enzymes catalyze the cis-trans isomerization of Xaa-Pro amide bonds in proteins.3 Pin1, a member of the PPIase family,4 is unique because it isomerizes prolyl residues preceded by phosphorylated Ser or Thr with selectivity up to 1300-fold greater (kcat/Km) than the unphosphorylated peptides.5, 6
The phosphorylation-dependent PPIase, Pin1, has been found to regulate mitosis through a simple conformational change,6 the cis-trans isomerization of phospho-Ser/Thr-Pro amide bonds in a variety of key cell cycle regulatory phosphoproteins, including the Cdc25 phosphatase, the p53 oncogene, and the c-Myc oncogene.6–8 Pin1 is essential for regulation of mitosis at G2 to M transition.4 Cells depleted of Pin1 are characterized by premature entry into mitosis, followed by mitotic arrest, nuclear fragmentation, and apoptosis, while overexpression of Pin1 inhibits the G2 to M transition.4, 7, 9 Therefore, Pin1 acts as a negative regulator for mitotic activity in G2, preventing lethal premature entry into mitosis. Pin1 is present at higher concentrations during mitosis,10 making it a potential target in the continuously dividing cells of cancer. The central role Pin1 plays in the cell cycle makes it an interesting target for inhibition, both for potential anticancer activity and for elucidation of the mechanism of mitosis.
Alkenes as cis- and trans-amide isosteres have been shown to be effective inhibitors of PPIases.1, 11 We have shown previously that Pin1 binds a cis isotere more tightly than a trans isostere.12 The cis alkene isostere was about 20-fold more potent than the trans alkene isostere in both the protease-coupled enzyme assay and the antiproliferative activity with A2780 cancer cells.12 Based on these results, only the cis isostere was incorporated into inhibitors 1 and 2 (Figure 1). The design of these two inhibitors was based on the selectivity of Pin1 for aromatic groups at the substrate termini.6, 13, 14
Figure 1.
Phosphorylated Pin1 inhibitors without (1) and with (2) bis- POM prodrug masking group.
One common problem for phosphorylated compounds is that they are generally not effective at penetrating cell membranes because of the negative charge on the phosphate group. A general strategy for circumventing this problem involves masking the phosphate in a form that neutralizes its negative charge and enhances its cell permeability.15–22 Upon cell entry, the mask is removed enzymatically and the inhibitors are converted to their biologically active forms.16, 17 Among the various approaches developed for reversibly masking phosphate compounds, the bisPOM (bis-pivaloyloxymethyl) strategy appears to be quite useful.16, 19, 22, 23 BisPOM derivatives are generally quite stable in buffer and plasma, and they are readily transformed to free phosphate derivatives inside various cell types.16
We now describe the design and synthesis of two Pin1 inhibitors containing the pSer-Ψ[(Z)CH=C]-Pro isostere, 1 and 2 (Figure 1). Inhibition of Pin1 and antiproliferative activity of human ovarian cancer cells in vitro is also reported. These inhibitors are a successful example of bisPOM prodrug approach applied to the cell cycle regulator Pin1, and they provide evidence to establish Pin1 as an anticancer drug target.
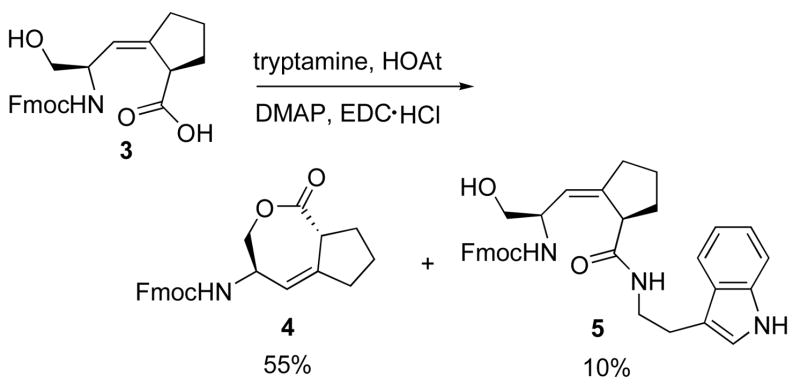
The intermediate Ser-Ψ[(Z)CH=C]-Pro isostere 6 for the synthesis of 1 and 2 was prepared previously through a stereoselective Still-Wittig rearrangement.24 Initially, the yield for the coupling reaction between Fmoc-Ser-Ψ[(Z)CH=C]-Pro-OH 3 (with unprotected alcohol side-chain) and tryptamine to give the desired product 5 was less than 10%. The 7-membered ring ε-lactone 4 was formed in greater than 50% yield (Scheme 1). Several different coupling reagents: DCC, HOBT/HBTU, HOAT/HATU were used for this coupling reaction, and lactone 4 was always the major product.
Scheme 1.
Lactonization of intermediate 3.
To circumvent this problem, TBS was introduced to protect the free hydroxyl group of 3. However, for the typical TBS protection reaction with imidazole as the base, the lactone 4 was still formed as the major product. The yield for the TBS protected product Fmoc-Ser(TBS)-Ψ[(Z)CH=C]-Pro-OH was less than 20%, which was not acceptable in the first step of the synthesis. If the phosphorylation step could be performed before the coupling step with tryptamine, the protected phosphate would prevent lactonization and eliminate one step for the deprotection. For this purpose, tert-butyl diisopropylphosphoramidite was used for the phosphorylation of 3.25 Unfortunately, the formation of the desired product Fmoc-Ser(PO(O-tBu)2)-Ψ[(Z)CH=C]-Pro-OH was still unfavorable in this phosphorylation reaction, while the formation of lactone 5 dominated. In summary, for the reaction of Fmoc-Ser-Ψ[(Z)CH=C]-Pro-OH 3, the use of any base led to the formation of 7-membered ring lactone 4 as the major product.
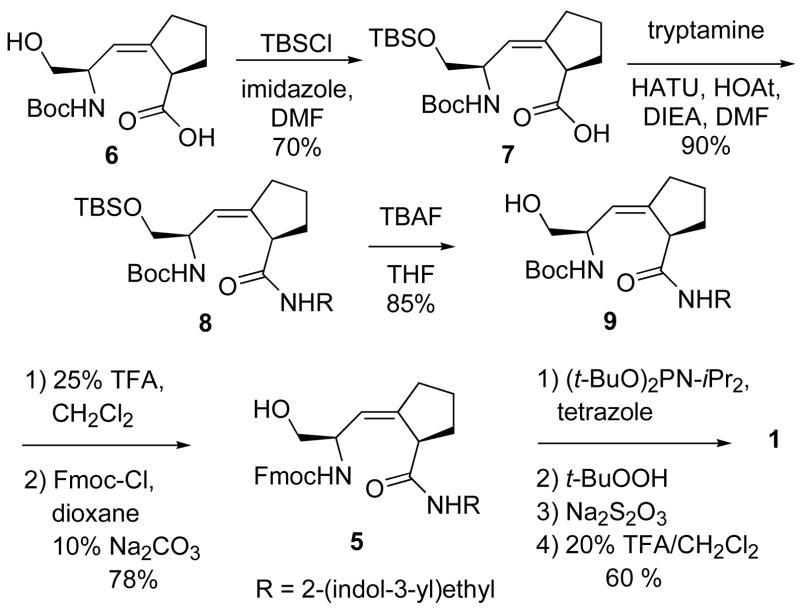
Successful synthesis of 5 was accomplished using a different starting material, Boc-Ser-Ψ[(Z)CH=C]-Pro-OH 6, for the coupling reaction.24 Quite different from reactions of Fmoc-protected 3, the side-chain protection step with TBSCl and imidazole of Boc-protected 6, proceeded in 70% yield (Scheme 2). The coupling of 7 with tryptamine gave 8 in 90% yield. After deprotection of the TBS group, Boc was changed to Fmoc at the N-terminus. The total yield for the conversion from 6 to 5 was 42%, which was much higher than the yield for the original synthetic route from 3 (5.5%). tert-Butyl diisopropylphosphoramidite was used again to phosphorylate 5.25,12 After removal of the tert-butyl groups with 20% TFA and HPLC purification, compound 1 was obtained as white solid in an overall yield of 30% from 6.
Scheme 2.
Synthesis of inhibitor 1.
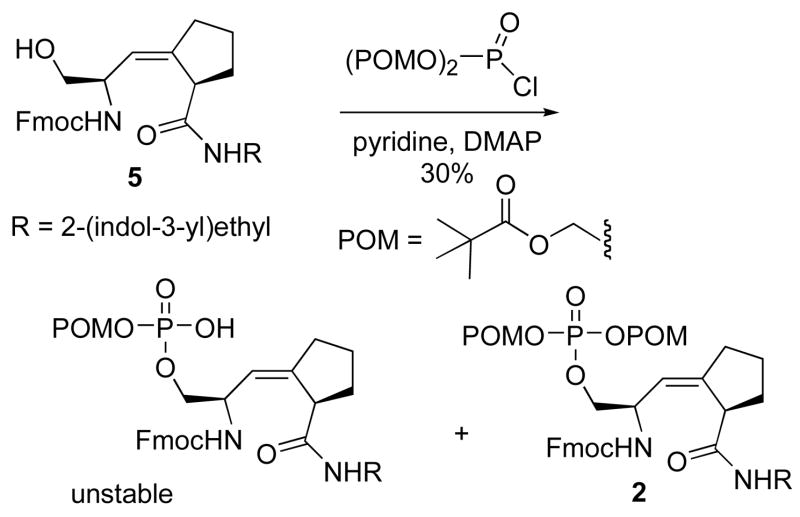
To synthesize compound 2, the prodrug form of 1, bisPOM was used as the masking group on the free phosphate of 1. Commonly, three general methods for the introduction of bisPOM have been described.20, 26, 27 In the first approach, the hydroxyl group is converted to the phosphate, followed by alkylation with pivaloyloxymethyliodide to give the desired products.20 In the second approach, bisPOM-phosphodiester is directly coupled with the hydroxyl compound.26 In the third approach, one-pot reaction between bisPOM-phosphoryl chloride and the hydroxyl compounds affords the desired products in high yield.27
Since the first approach involved 4–5 steps for the construction of bisPOM-protected phosphate and the overall yield is commonly low (<10%), the second and third approaches were tried. First, bisPOM phosphoric acid was used for the direct coupling with 6. 31P-NMR was used to monitor the reaction progress. Even after reaction for two days, there was no 31P NMR peak for the desired product (−3.8 to −4.0 ppm predicted from the calculation by ACD/XNMR Predictor™, experimental value −3.93 ppm).
The third approach was used because only one step was necessary for the construction of bisPOM phosphate, and the reported yield is generally higher than the other two methods. The bisPOM phosphoryl chloride was prepared by the reported method,27 however, no desired product was obtained in the phosphorylation step initially. 31P-NMR was used to follow the formation of bisPOM phosphoryl chloride and desired product 2 (Supporting Information Figure 4). For the formation of bisPOM phosphoryl chloride, slow addition of bisPOM phosphate to the solution of oxalyl chloride in DMF was critical to the success of the phosphorylation reaction.
Synthesis of 2 via nucleophilic addition of 5 to bisPOM phosphoryl chloride using triethylamine was problematic, affording only 10–20 % yield of 2 under the optimum conditions. Different bases were used to effect this phosphorylation (Supporting Information Table 1). Among them, a large excess of pyridine gave the highest yield of 2 (30 %). LC-MS analysis of the crude product showed that the mono-POM phosphate product was also formed, but it decomposed on standing. A steric effect may prevent the bulky bisPOM phosphoryl chloride from approaching the hindered hydroxyl group of 5, leading to the poor yield.
Inhibition of Pin1 by 1 was measured in the proteasecoupled PPIase assay in vitro as described.12 The enzymatic IC50 value obtained was 28.3 ± 2.1 (Table 1). In order to test whether the bisPOM strategy would improve the cell permeability of the inhibitors, 1 and 2 were tested for their antiproliferative activities towards A2780 ovarian cancer cells.28, 29 The IC50 value of 1 was 46.2 ± 3.0, a loss of about two-fold in activity. The IC50 value of the bisPOM prodrug 2 was 26.9 ± 1.5, within error the same as the IC50 value for the naked phosphoinhibitor 1 in the enzyme assay (Table 1). DMSO was included at the same concentration in all samples and controls. The IC50 values of 1 and 2 towards A2780 ovarian cancer cells suggest that the introduction of the bisPOM protecting group on the phosphate of 1 helps entry into the cell by neutralizing the negative charges on the phosphate, and improving the hydrophobicity of the inhibitor.
Table 1.
Inhibition of Pin1 PPIase enzymatic activity and antiproliferative activity towards A2780 ovarian cancer cells for 1 and 2.
| Compound | Inhibition of Pin1 PPIase activity IC50, μM | Inhibition of A2780 proliferation IC50, μMa |
|---|---|---|
| 1 | 28.3 (± 2.1) | 46.2 (± 3.0) |
| 2 | ND | 26.9 (± 1.5) |
Values are the means of three experiments, the error at 95 % confidence level is given in parentheses. (ND = not determined)
The IC50 value of 2 for the inhibition of A2780 cancer cell proliferation is comparable to the IC50 of 1 in the protease-coupled Pin1 enzyme assay, which suggests that the inhibition of the proliferation activity of A2780 ovarian cancer cell by these compounds is the result of Pin1 inhibition. The bisPOM masking group improves penetration of the hydrophobic cell membrane by inhibitor 2. These inhibitors provide additional evidence to establish Pin1 as a potential anticancer drug target.12, 13, 30, 31
Supplementary Material
Supporting Information
Experimental procedures and NMR spectra for compounds 1–10, HPLC chromatograms for compounds 1 and 2, phosphorylation conditions, enzyme assay conditions, and IC50 plots are included.
Scheme 3.
Synthesis of prodrug 2.
Acknowledgments
We thank Ms. Margaret Brodie and Professor David Kingston (Virginia Tech) for the A2780 cell-based assay results, and the NIH for Grant R01CA110940.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang XJ, Etzkorn FA. Biopolymers: Peptide Science. 2006;84:125. doi: 10.1002/bip.20240. [DOI] [PubMed] [Google Scholar]
- 2.Wiederrecht G, Etzkorn FA. In: Perspectives in Drug Discovery and Design. Sigal NH, Wyvratt MJ, editors. Vol. 2. ESCOM Science Publishers, B.V.; Leiden, The Netherlands: 1994. p. 57. [Google Scholar]
- 3.Fischer G. Angew Chem Int Ed Engl. 1994;33:1415. [Google Scholar]
- 4.Lu KP, Hanes SD, Hunter T. Nature. 1996;380:544. doi: 10.1038/380544a0. [DOI] [PubMed] [Google Scholar]
- 5.Ranganathan R, Lu KP, Hunter T, Noel JP. Cell. 1997;89:875. doi: 10.1016/s0092-8674(00)80273-1. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe MB, Schutkowski M, Shen M, Zhou XZ, Stukenberg PT, Rahfeld JU, Xu J, Kuang J, Kirschner MW, Fischer G, Cantley LC, Lu KP. Science. 1997;278:1957. doi: 10.1126/science.278.5345.1957. [DOI] [PubMed] [Google Scholar]
- 7.Shen M, Stukenberg PT, Kirschner MW, Lu KP. Genes Dev. 1998;12:706. doi: 10.1101/gad.12.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeh E, Cunningham M, Arnold H, Chasse D, Monteith T, Ivaldi G, Hahn WC, Stukenberg PT, Shenolikar S, Uchida T, Counter CM, Nevins JR, Means AR, Sears R. Nat Cell Biol. 2004;6:308. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- 9.Rippmann JF, Hobbie S, Daiber C, Guilliard B, Bauer M, Birk J, Nar H, Garin-Chesa P, Rettig WJ, Schnapp A. Cell Growth Differ. 2000;11:409. [PubMed] [Google Scholar]
- 10.Bao L, Kimzey A, Sauter G, Sowadski JM, Lu KP, Wang DG. Am J Pathol. 2004;164:1727. doi: 10.1016/S0002-9440(10)63731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart SA, Etzkorn FA. J Org Chem. 1999;64:2998. doi: 10.1021/jo990409a. [DOI] [PubMed] [Google Scholar]
- 12.Wang XJ, Xu B, Mullins AB, Neiler FK, Etzkorn FA. J Am Chem Soc. 2004;126:15533. doi: 10.1021/ja046396m. [DOI] [PubMed] [Google Scholar]
- 13.Wildemann D, Erdmann F, Alvarez BH, Stoller G, Zhou XZ, Fanghanel J, Schutkowski M, Lu KP, Fischer G. J Med Chem. 2006;49:2147. doi: 10.1021/jm060036n. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Daum S, Wildemann D, Zhou XZ, Verdecia MA, Bowman ME, Lucke C, Hunter T, Lu KP, Fischer G, Noel JP. ACS Chem Biol. 2007;2:320. doi: 10.1021/cb7000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefebvre I, Perigaud C, Pompon A, Aubertin AM, Girardet JL, Kirn A, Gosselin G, Imbach JL. J Med Chem. 1995;38:3941. doi: 10.1021/jm00020a007. [DOI] [PubMed] [Google Scholar]
- 16.Farquhar D, Chen R, Khan S. J Med Chem. 1995;38:488. doi: 10.1021/jm00003a012. [DOI] [PubMed] [Google Scholar]
- 17.Hernick M, Borch RF. J Med Chem. 2003;46:148. doi: 10.1021/jm0203229. [DOI] [PubMed] [Google Scholar]
- 18.Hernick M, Flader C, Borch RF. J Med Chem. 2002;45:3540. doi: 10.1021/jm020191b. [DOI] [PubMed] [Google Scholar]
- 19.Rose JD, Parker WB, Someya H, Shaddix SC, Montgomery JA, Secrist JA., 3rd J Med Chem. 2002;45:4505. doi: 10.1021/jm020107s. [DOI] [PubMed] [Google Scholar]
- 20.Rutschow S, Thiem J, Kranz C, Marquardt T. Bioorg Med Chem. 2002;10:4043. doi: 10.1016/s0968-0896(02)00269-9. [DOI] [PubMed] [Google Scholar]
- 21.Cebrat M, Kim CM, Thompson PR, Daugherty M, Cole PA. Bioorg Med Chem. 2003;11:3307. doi: 10.1016/s0968-0896(03)00265-7. [DOI] [PubMed] [Google Scholar]
- 22.Hwang Y, Ganguly S, Ho AK, Klein DC, Cole PA. Bioorg Med Chem. 2007;15:2147. doi: 10.1016/j.bmc.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farquhar D, Khan S, Srivastva DN, Saunders PP. J Med Chem. 1994;37:3902. doi: 10.1021/jm00049a009. [DOI] [PubMed] [Google Scholar]
- 24.Wang XJ, Hart SA, Xu B, Mason MD, Goodell JR, Etzkorn FA. J Org Chem. 2003;68:2343. doi: 10.1021/jo026663b. [DOI] [PubMed] [Google Scholar]
- 25.Perich JW, Johns RB. Synthesis. 1989:701. [Google Scholar]
- 26.Kemp BE, Perich JW. Peptide and Protein Phosphorylation. CRC Press; 1990. p. 289. [Google Scholar]
- 27.Hwang Y, Cole PA. Org Lett. 2004;6:1555. doi: 10.1021/ol049714v. [DOI] [PubMed] [Google Scholar]
- 28.Abdel-Kader M, Berger JM, Slebodnick C, Hoch J, Malone S, Wisse JH, Werkhoven MC, Mamber S, Kingston DG. J Nat Prod. 2002;65:11. doi: 10.1021/np0103261. [DOI] [PubMed] [Google Scholar]
- 29.Kapustin G, Fejér G, Gronlund JL, Seto E, McCafferty DG, Etzkorn FA. Org Lett. 2003;5:3053. doi: 10.1021/ol035056n. [DOI] [PubMed] [Google Scholar]
- 30.Lu KP. Cancer Cell. 2003;4:175. doi: 10.1016/s1535-6108(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 31.Uchida T, Takamiya M, Takahashi M, Miyashita H, Ikeda H, Terada T, Matsuo Y, Shirouzu M, Yokoyama S, Fujimori F, Hunter T. Chem Biol. 2003;10:15. doi: 10.1016/s1074-5521(02)00310-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Experimental procedures and NMR spectra for compounds 1–10, HPLC chromatograms for compounds 1 and 2, phosphorylation conditions, enzyme assay conditions, and IC50 plots are included.