Abstract
In the present study, we investigated the effect of chronic ethanol (CE) administration on the polypeptide levels of the δ-subunit of GABAA receptors and [³H]muscimol binding to the immunoprecipitated δ-subunit-containing GABAA receptor assemblies in the rat brain. CE administration resulted a down-regulation of polypeptide levels of the δ-subunit of GABAA receptors in the rat cerebellum and hippocampus, whereas there were no changes in the δ-subunit polypeptide levels in the rat cerebral cortex. Further, CE administration caused a down-regulation of native δ-subunit-containing GABAA receptor assemblies in the rat cerebellum as determined by [³H]muscimol binding to the immunoprecipitated receptor assemblies. These results indicate that the δ-subunit-containing GABAA receptors may play a role in chronic ethanol-induced tolerance and dependence.
Keywords: Chronic ethanol, GABAA receptors, δ-Subunit, Polypeptide levels, Immunoprecipitation, Radioligand binding
1. Introduction
Extensive structural heterogeneity exists among various subtypes of GABAA receptor since native pentameric receptor assemblies are derived from several subunits such as α1–6, β1–3, γ1–3, δ, ε, π, and θ (see Mehta and Ticku, 1999a; Sieghart et al., 1999; Whiting, 1999). Most common subunits-combination for GABAA receptors in the brain is α1β2γ2 (Fritschy et al., 1992). GABAA receptors gene expression is affected by physiological and pathological processes as well as by the drugs that modulate GABAA receptors (Aguayo et al., 2002; Cagetti et al., 2003; Follesa et al., 2003; Sanna et al., 2003). Ethanol is known to modulate the activity of a variety of receptors and ion channels (Lovinger et al., 1989; Lovinger and White 1991; Lovinger, 1999; Lewohl et al., 1999; Mihic, 1999; Narahashi et al., 1999; Woodward, 1999; Walter and Messing, 1999; Weight et al., 1999; Lei et al., 2000; Aguayo et al., 2002; Carta et al., 2003; Davies et al., 2003; Roberto et al., 2003; Gehlert et al., 2007), and role of GABAA receptors in the behavioral effects of ethanol is well documented (see Ticku and Mehta, 1995).
Chronic ethanol (CE) as well as chronic intermittent ethanol (CIE) treatment is known to modulate the expression of the major subunits of GABAA receptors in the brain (Montpied et al., 1991; Mhatre and Ticku, 1992, 1994; Devaud et al., 1995, 1997; see Mehta and Ticku, 1999a; Cagetti et al., 2003; Marutha Ravindran and Ticku, 2006a, 2006b; Marutha Ravindran et al., 2007). Recently, it has been suggested that the GABAA receptor derived from δ-subunit is important site for the pharmacological actions of ethanol (Mihalek et al., 2001; Sundstrom-Poromaa et al., 2002; Cagetti et al., 2003; Wallner et al., 2003; Wei et al., 2004; Hanchar et al., 2004; Glykys et al., 2007). On the other hand, there are also reports contradicting the involvement of the δ-subunit-containing GABAA receptors in the pharmacology of clinically relevant low concentrations of ethanol (Borghese et al., 2006; Yamashita et al., 2006; Casagrande et al., 2007). Recently, we have reported that ethanol (up to 50 mM) does not affect [³H]muscimol binding to the immunoprecipitated δ-subunit-containing GABAA receptor assemblies in the rat cerebellum and hippocampus, thereby suggesting that the native δ-subunit-containing GABAA receptors do not play a major role in the pharmacology of clinically relevant low concentrations of ethanol (Mehta et al., 2007). In the present study, we investigated the effect of CE administration on the polypeptide levels of the δ-subunit of GABAA receptors in the rat cerebellum, hippocampus and cerebral cortex. Further, we investigated the effect of chronic administration of ethanol on the regulation of native δ-subunit-containing GABAA receptor assemblies in the rat cerebellum so as to examine whether δ-subunit-containing GABAA receptors are a potential target site for the chronic ethanol-induced phenomena.
2. Results
2.1. Effect of CE administration on the polypeptide levels of the δ-subunit of GABAA receptors in the rat brain
Western blot experiments revealed that chronic administration of ethanol causes down-regulation of the polypeptide levels of the δ-subunit of GABAA receptors in the rat cerebellum (Fig. 1A–a,c; ANOVA F2,6 = 43.59, p = 0.0003; Dunnett’s multiple comparison p < 0.01) and hippocampus (Fig. 1B–a,c; ANOVA F2,6 = 29.79, p = 0.0008; Dunnett’s multiple comparison p < 0.01). These changes in the polypeptide levels of δ-subunit reverted back to control levels in the ethanol-withdrawn group (48 h) as shown in Fig. 1A and Fig. 1B. However, chronic administration of ethanol did not result any change in the polypeptide levels of the δ-subunit of GABAA receptors in the rat cerebral cortex (Fig. 1C–a,c; ANOVA F2,6 = 1.104, p = 0.39; Dunnett’s multiple comparison p > 0.05). Notably, there was equal intensity of the protein actin band following probing with actin antibody by Western blotting (Fig. 1A–b, Fig. 1B–b and Fig. 1C–b).
Fig. 1.
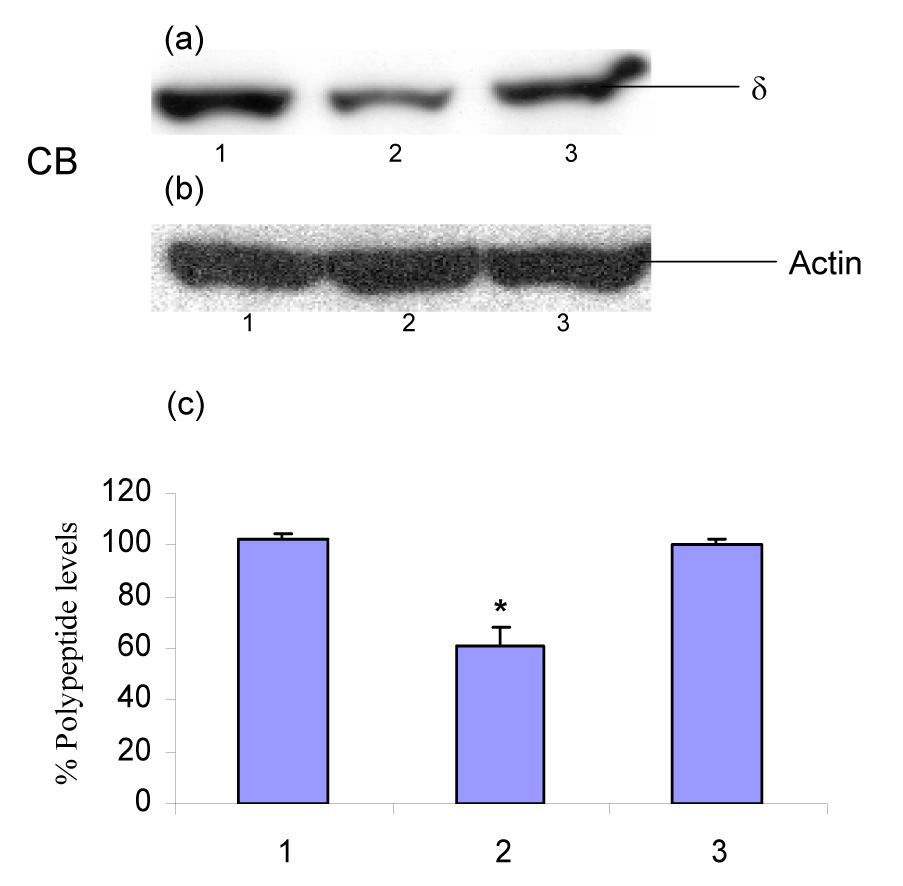
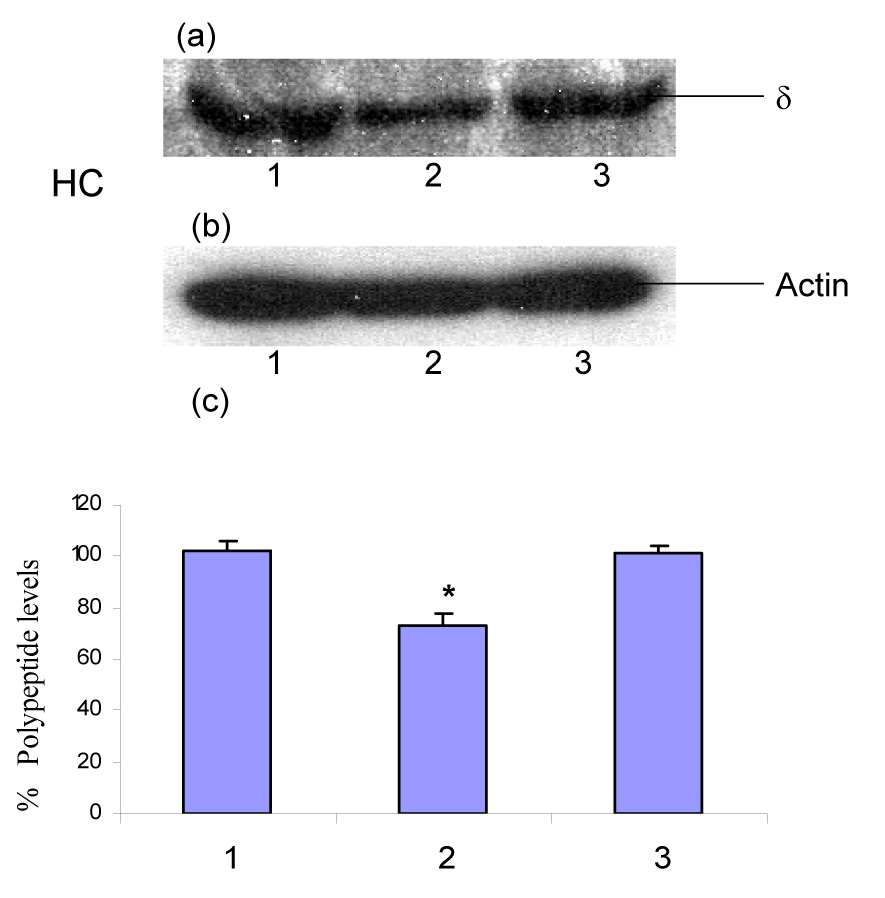

Effect of chronic administration of ethanol and its withdrawal on the polypeptide levels of the δ-subunit of GABAA receptors in the rat (A) cerebellum, i.e., CB, (B) hippocampus, i.e., HC, and (C) cerebral cortex, i.e., CC. Panel (a): Western blot for the GABAA receptors δ-subunit polypeptide; panel (b): reprobed with actin; and panel (c): percentage polypeptide levels of the δ-subunit of GABAA receptors. Molecular mass of the δ-subunit of GABAA receptors was found to be 54 kDa in Western blot analysis. Each value is % mean ± S.E.M derived from three different Western blots performed on different days using pooled tissue from 10 rats in each group. % Polypeptide levels were calculated by comparing the intensity of each band with the average control intensity (100%) from three different Western blots as detailed in materials and methods. Lane 1: proteins extracted from control tissues (saline-administered rats); lane 2: proteins extracted from tissues harvested from chronic ethanol-administered rats; lane 3: proteins extracted from tissues harvested from ethanol-withdrawn rats (48 h).
*p < 0.01 as compared to the control group by one-way analysis of variance (ANOVA) followed by Dunnett test.
2.2. Effect of CE administration on the GABAA receptor assemblies derived from δ-subunit in the rat cerebellum
Antiserum for the rat GABAA receptors δ-subunit immunoprecipitated 18.0 ± 1.1% of the GABAA receptor assemblies in the rat cerebellum as determined with [³H]muscimol (Table 1). Chronic administration of ethanol to the rats decreased the percentage immunoprecipitation of the binding activity for the GABAA receptor assemblies derived from δ-subunit in the rat cerebellum as determined with [³H]muscimol (11.1 ± 0.7% vs. 18.0 ± 1.1%, ANOVA F2,15 = 16.67, p = 0.0002, Dunnett’s multiple comparison p < 0.01, Table 1), and this effect reverted to control level following withdrawal of chronic ethanol treatment (48 h) as shown in Table 1.
Table 1.
Effect of chronic administration of ethanol on the quantitative immunoprecipitation of the δ-subunit-containing GABAA receptor assemblies in the rat cerebellum.
| Group | % Immunoprecipitation of the binding activity |
|---|---|
| [³H]Muscimol (36 nM) | |
| Control | 18.0 ± 1.1 |
| Chronic ethanol | 11.1 ± 0.7* |
| Ethanol withdrawn (48 h) | 17.6 ± 0.8 |
The values are mean ± S.E.M. of six individual experiments, each performed in triplicate using the pooled cerebellum from ten rats in each group. Immunoprecipitations were done using 30 µl of the antiserum specific for the rat δ-subunit of GABAA receptors. [³H]Muscimol (36 nM) binding (100%) to the solubilized receptors (pellet+supernatant) of the rat cerebellum was 0.28 ± 0.02 pmol/mg protein.
p < 0.01 as compared to the control group by one-way analysis of variance (ANOVA) followed by Dunnett test.
3. Discussion
Role of GABAA receptors in the pharmacology of ethanol has been investigated in detail (see Ticku and Mehta, 1995; Mehta and Ticku, 1999a; Sieghart et al., 1999; Whiting, 1999; Hanchar et al., 2004). The subunit composition and stoichiometry of native GABAA receptors are currently unknown, but the most abundant population of native GABAA receptors in the mammalian brain is believed to be the α1β2γ2 subunit combination (Benke et al., 1991; Fritschy et al., 1992; McKernan and Whiting 1996), and these subunits are present in abundant amount in almost every region of the brain (see Mehta and Ticku 1999a). Chronic administration of ethanol is reported to reduce the levels of α1-, α2-, and α5-subunits mRNA and polypeptide levels in cerebellum and cerebral cortex (Mhatre and Ticku 1992; Devaud et al., 1997; Marutha Ravindran et al., 2007). However, the α4-, γ1-, γ2S- (Devaud et al., 1995), α6- (Mhatre and Ticku 1992) and β2/3-subunits (Mhatre and Ticku 1994) mRNA levels increase in the ethanol-dependent animals. CE as well as CIE exposure of cultured cortical neurons of mice results in up-regulation of the β2-subunit, down-regulation of the α1-subunit and no change in the polypeptide levels of the γ2-subunit of the GABAA receptors (Marutha Ravindran and Ticku, 2006a, 2006b).
Our present study revealed that chronic administration of ethanol results in a down-regulation of the polypeptide levels of the δ-subunit of the GABAA receptors in the rat cerebellum and hippocampus. However, chronic administration of ethanol did not elicit any changes in the polypeptide levels of the δ-subunit of GABAA receptors in the rat cerebral cortex. Our results with CE paradigm are consistent with the report indicating that CIE treatment of rats elicits a decrease in the polypeptide levels of the δ-subunit of the GABAA receptors in hippocampus (Cagetti et al., 2003). On the other hand, there is also a report indicating that CE treatment of cultured cerebellar granule neurons causes a statistically insignificant reduction in the expression of δ-subunit (Follesa et al., 2005). However, these researchers also observed a statistically significant reduction in the expression of δ-subunit in the cultured cerebellar granule neurons at 6 h of ethanol-withdrawal following CE paradigm, which returned to control values at 12 h of ethanol-withdrawal (Follesa et al., 2005). Further, these researchers found that CE paradigm results in an increase in the expression of δ-subunit in cultured hippocampal neurons (Follesa et al., 2005). These differences may be due to different experimental models (intact rats versus cultured neurons). Notably, CE-induced changes in polypeptide levels of δ-subunit of GABAA receptors in our present study reverted back to normal levels in the rat cerebellum and hippocampus following 48 h of ethanol withdrawal.
Consistent with chronic ethanol-induced down-regulation of the polypeptide levels of the GABAA receptors δ-subunit in our study, we also observed a down-regulation of δ-subunit-containing GABAA receptor assemblies in the rat cerebellum as determined by immunoprecipitation followed by [³H]muscimol binding. It is possible that chronic ethanol-induced down-regulation of native δ-subunit-containing GABAA receptor assemblies may be due to altered receptor trafficking, as reported recently in the case of α1-subunit-containing GABAA receptors (Kumar et al., 2003). Down-regulation of the polypeptide levels of the GABAA receptors δ-subunit as well as δ-subunit-containing GABAA receptor assemblies following the chronic administration of ethanol may alter the GABAergic transmission and synaptic responses, thereby leading to tolerance and dependence. Thus our study suggests δ-subunit-containing GABAA receptors as an important potential target for the treatment of chronic ethanol-induced tolerance and dependence.
In summary, CE administration caused a down-regulation of the polypeptide levels of the GABAA receptors δ-subunit in the rat cerebellum and hippocampus. Further, chronic administration of ethanol resulted in a down-regulation of native δ-subunit-containing GABAA receptor assemblies in the rat cerebellum. These CE-induced changes in the δ-subunit-containing GABAA receptors may have important implications in chronic ethanol-induced dependence and tolerance.
4. Experimental procedures
Adult male Sprague-Dawley rats (Harlan, Indianapolis IN, U.S.A.) weighing 200–250 g were maintained at a constant room temperature (22°C) on a 12-h light/12-h dark cycle. All experiments were conducted in accordance with the Declaration of Helsinki and/or with the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the National Institutes of Health. Adequate measures were taken to minimize pain or discomfort to the animals. Food and water were available ad libitum. They were divided into three groups: saline control, ethanol-maintained (chronic ethanol-administered) and ethanol-withdrawn rats.
4.1. Chronic administration of ethanol to the rats
The animals were intoxicated by intragastric intubation method for 6 days as described earlier (Majchrowicz 1975; Mhatre et al., 1988; Mehta and Ticku 1999b; Marutha Ravindran et al., 2007). Briefly, at the beginning of the experiment, a priming dose of ethanol of 5 g/kg (20% v/v in normal saline) followed by 9 g/kg (20% v/v in normal saline) over 24 h period in three divided doses was administered orally to all animals for six days. The control rats received normal saline. Chronic ethanol-maintained rats were sacrificed 1 h after the last dose of ethanol and the ethanol-withdrawn rats were sacrificed 48 h after the last dose of ethanol. Different regions of the rat brain were dissected, and tissues were stored at −80°C until use.
4.2. Electrophoresis and immunoblotting
Tissues were homogenized in ice-cold lysis buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 1% Triton X 100, 10 mM NaF, 1 mg/ml bacitracin, 50 mM β-glycerophosphate, 1 mM phenyl methyl sulfonyl fluoride (PMSF), 1 mM N-ethylmaleimide, 1 mM Na3VO4, 10 µg/ml leupeptin). These samples were centrifuged (2300 g, 10 min, 4°C) so as to remove the supernatant containing protein (Bjornstrom et al., 2002; Marutha Ravindran and Ticku, 2006a, 2006b; Marutha Ravindran et al., 2007). Fifty micrograms of protein was boiled in a boiling water-bath for 5 min to denature the proteins, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli, 1970) and transferred to polyvinylidene difluoride membrane followed by treatment with 5% nonfat dry milk (Nestle, Ohio, USA) in Tris-buffered saline containing 0.1% Tween 20. Following this, the membrane was incubated with 1:100 dilution of the antiserum for the GABAA receptors δ-subunit since preliminary experiments revealed that 1:100 dilution of the δ-subunit antiserum yields the intensity of its protein band in a linear range. The membrane was washed several times and peroxidase-coupled secondary antibody (anti-mouse IgG/anti-rabbit IgG; New England Biolabs, USA) was added and incubated for 1 h. Following this, the membrane was washed and specific bands were visualized using super signal west pico chemiluminescent substrate kit (Pierce Biotechnology Inc, USA). Intensities of the bands on the membrane were scanned using a densitometer. Average intensity of the bands from three different Western blots of the control group was considered as 100% and the changes in the intensity of the band in the experimental group were determined as detailed earlier (Marutha Ravindran and Ticku, 2004, 2006a, 2006b; Marutha Ravindran et al., 2007). The data are expressed as % mean ± S.E.M. from three different Western blots performed on different days using pooled tissue from 10 rats in each group. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Dunnett test. Antiserum for the rat GABAA receptors δ-subunit (AA 1–11) was procured from Alpha Diagnostics (San Antonio, TX, USA). We have reported the characterization of this antiserum for the GABAA receptors δ-subunit recently (Mehta et al., 2007). Pre-incubation with the antigenic peptide (AA 1–11; 20 µg/ml) for the δ-subunit of GABAA receptors antibody blocked the immunoprecipitation in control experiments, and molecular mass of the δ-subunit of GABAA receptors was found to be 54 kDa in Western blot analysis (Mehta et al., 2007).
4.3. Immunoprecipitation and [³H]muscimol binding
The rat cerebellum membrane preparation, the GABAA receptors solubilization, immunoprecipitation and [³H]muscimol binding assays were performed as reported in the literature (Khan et al., 1993; Mehta and Ticku, 1999b, 2005). Briefly, the frozen tissue was thawed, homogenized in ice-cold 0.32 M sucrose solution (pH 7.4) and it was then centrifuged at 78,000 g for 30 min at 4°C. The pellet was suspended in 0.32 M sucrose solution (pH 7.4) and kept frozen overnight at −80°C. After thawing, Tris-HCl (50 mM, pH 7.4) was added to the tissue, and it was centrifuged at 30,000 g for 10 min at 4°C. The pellet was resuspended in Tris-HCl buffer (50 mM, pH 7.4), and centrifuged at 30,000 g for 10 min at 4°C. The last step was repeated four more times. The pellet was resuspended in Tris-HCl buffer, and kept frozen overnight at −80°C. After thawing, Tris-HCl buffer was added to the tissue, and the mixture was centrifuged at 78,000 g for 30 min at 4°C. The membranes were then suspended in Tris-HCl (50 mM, pH 7.4), distributed in aliquots, and kept frozen at −80°C until use. GABAA receptors were solubilized in modified radioimmune precipitation assay buffer (RIPA), i.e., solubilization buffer (pH 7.4) containing NaCl (0.137 M), sodium deoxycholate (1% w/v), Triton X-100 (1% v/v), sodium dodecyl sulfate, i.e, SDS (0.1% w/v), Tris (10 mM) and a cocktail of protease inhibitors containing EDTA (1 mM), EGTA (1 mM), benzamidine HCl (2 mM), trypsin inhibitor type 1-S (0.1 mg/ml), bacitracin (0.1 mg/ml) and phenylmethylsulfonyl fluoride (0.3 mM). After incubation for 1 h at 4°C, insoluble material was removed by centrifugation at 100,000 g for 1 h at 4°C. A sample of 400 µl (≈300 µg protein) of the solubilized receptors was incubated overnight at 4°C with 30 µl of the antiserum for the rat GABAA receptors δ-subunit since preliminary experiments revealed that 30 µl of the antiserum elicits maximal immunoprecipitation of the δ-subunit-containing GABAA receptor assemblies. The receptor-antibody complexes were recovered by incubation with protein A-agarose suspension (60 µl of 40% v/v) followed by centrifugation. Immunoprecipitation was quantified by determining the binding of [³H]muscimol (36 nM) to the immunoprecipitated pellet and supernatant. Non-specific radioligand binding was determined with GABA (100 µM). Protein was estimated using Micro BCA™ Protein Assay Kit (Pierce, Rockford IL U.S.A.). The data are expressed as mean ± S.E.M. of six individual experiments, each performed in triplicate using the pooled cerebellum from ten rats in each group. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Dunnett test.
Acknowledgements
This research work was supported by National Institute on Alcohol and Alcohol Abuse (NIAAA) grant AA10552.
Abbreviations
- CE
chronic ethanol
- CIE
chronic intermittent ethanol
- CB
cerebellum
- HC
hippocampus
- CC
cerebral cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aguayo LG, Peoples RW, Yeh HH, Yevenes GE. GABAA receptors as molecular sites of ethanol action. Direct or indirect actions? Curr. Top. Med. Chem. 2002;2:869–885. doi: 10.2174/1568026023393426. [DOI] [PubMed] [Google Scholar]
- Benke D, Mertens S, Trzeciak A, Gillessen D, Mohler H. GABAA receptors display association of γ2-subunit with α1- and β2/3-subunits. J. Biol. Chem. 1991;266:4478–4483. [PubMed] [Google Scholar]
- Bjornstrom K, Sjolander A, Schippert A, Eintrei C. A tyrosine kinase regulates propofol-induced modulation of the β-subunit of the GABAA receptor and release of intracellular calcium in cortical rat neurones. Acta Physiol. Scand. 2002;175:227–235. doi: 10.1046/j.1365-201X.2002.00991.x. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Storustovu SI, Ebert B, Herd MB, Belelli D, Lambert JJ, Marshall G, Wafford KA, Harris RA. The δ subunit of γ-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J. Pharmacol. Exp. Ther. 2006;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol. Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Carta M, Ariwodola OJ, Weiner JL, Valenzuela CF. Alcohol potently inhibits the kainate receptor-dependent excitatory drive of hippocampal interneurons. Proc. Natl. Acad. Sci. U.S.A. 2003;100:6813–6818. doi: 10.1073/pnas.1137276100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande S, Cupello A, Pellistri F, Robello M. Only high concentrations of ethanol affect GABAA receptors of rat cerebellum granule cells in culture. Neurosci. Lett. 2007;414:273–276. doi: 10.1016/j.neulet.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Davies AG, Pierce-Shimomura JT, Kim H, Van Hoven MK, Thiele TR, Bonci A, Bargmann CI, McIntire SL. A central role of the BK potassium channel in behavioral response to ethanol in C. elegans. Cell. 2003;115:655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Smith FD, Grayson DR, Morrow AL. Chronic ethanol consumption differentially alters the expression of γ-aminobutyric acidA receptor subunit mRNAs in rat cerebral cortex: competitive, quantitative reverse transcriptase-polymerase chain reaction analysis. Mol. Pharmacol. 1995;48:861–868. [PubMed] [Google Scholar]
- Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Bidirectional alterations of GABAA receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J. Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Follesa P, Mancuso L, Biggio F, Mostallino MC, Manca A, Mascia MP, Busonero F, Talani G, Sanna E, Biggio G. γ-Hydroxybutyric acid and diazepam antagonize a rapid increase in GABAA receptors α4 subunit mRNA abundance induced by ethanol withdrawal in cerebellar granule cells. Mol. Pharmacol. 2003;63:896–907. doi: 10.1124/mol.63.4.896. [DOI] [PubMed] [Google Scholar]
- Follesa P, Mostallino MC, Biggio F, Gorini G, Caria S, Busonero F, Murru L, Mura ML, Sanna E, Biggio G. Distinct patterns of expression and regulation of GABAA receptors containing the δ subunit in cerebellar granule and hippocampal neurons. J. Neurochem. 2005;94:659–671. doi: 10.1111/j.1471-4159.2005.03303.x. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Benke D, Mertens S, Oertel WH, Bachi T, Mohler H. Five subtypes of type A γ-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc. Natl. Acad. Sci. U.S.A. 1992;89:6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Lê AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J. Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat. Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Wallner M, Olsen RW. Alcohol effects on γ-aminobutyric acid type A receptors: are extrasynaptic receptors the answer? Life Sci. 2004;76:1–8. doi: 10.1016/j.lfs.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Fernando LP, Escriba P, Busquets X, Mallet J, Miralles CP, Filla M, De Blas AL. Antibodies to the human γ2 subunit of the γ-aminobutyric acidA/benzodiazepine receptor. J. Neurochem. 1993;60:961–971. doi: 10.1111/j.1471-4159.1993.tb03243.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kralic JE, O'Buckley TK, Grobin AC, Morrow AL. Chronic ethanol consumption enhances internalization of α1 subunit-containing GABAA receptors in cerebral cortex. J. Neurochem. 2003;86:700–708. doi: 10.1046/j.1471-4159.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lei Q, Jones MB, Talley EM, Schrier AD, McIntire WE, Garrison JC, Bayliss DA. Activation and inhibition of G protein-coupled inwardly rectifying potassium (kir3) channels by G protein beta gamma subunits. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9771–9776. doi: 10.1073/pnas.97.17.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewohl JM, Wilson WR, Mayfield RD, Brozowski SJ, Morrisett RA, Harris RA. G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat. Neurosci. 1999;2:1084–1090. doi: 10.1038/16012. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. 5-HT3 receptor and the neural actions of alcohols: an increasingly exciting topic. Neurochem. Int. 1999;35:125–130. doi: 10.1016/s0197-0186(99)00054-6. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G. Ethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol. Pharmacol. 1991;40:263–270. [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacology. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Marutha Ravindran CR, Ticku MK. Changes in methylation pattern of NMDA receptor NR2B gene in cortical neurons after chronic ethanol treatment in mice. Mol. Brain Res. 2004;121:19–27. doi: 10.1016/j.molbrainres.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Marutha Ravindran CR, Ticku MK. Tyrosine kinase phosphorylation of GABAA receptor subunits following chronic ethanol exposure of cultured cortical neurons of mice. Brain Res. 2006a;1086:35–41. doi: 10.1016/j.brainres.2006.02.106. [DOI] [PubMed] [Google Scholar]
- Marutha Ravindran CR, Ticku MK. Tyrosine kinase phosphorylation of GABAA receptor α1, β2, and γ2 subunits following chronic intermittent ethanol (CIE) exposure of cultured cortical neurons of mice. Neurochem. Res. 2006b;31:1111–1118. doi: 10.1007/s11064-006-9124-9. [DOI] [PubMed] [Google Scholar]
- Marutha Ravindran CR, Mehta AK, Ticku MK. Effect of chronic administration of ethanol on the regulation of tyrosine kinase phosphorylation of the GABAA receptor subunits in the rat brain. Neurochem. Res. 2007;32:1179–1187. doi: 10.1007/s11064-007-9288-y. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res. Rev. 1999a;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. Prevalence of the GABAA receptor assemblies containing α1-subunit in the rat cerebellum and cerebral cortex as determined by immunoprecipitation: lack of modulation by chronic ethanol administration. Mol. Brain Res. 1999b;67:194–199. doi: 10.1016/s0169-328x(99)00020-0. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. Effect of chronic administration of ethanol on GABAA receptor assemblies derived from α2-, α3-, β2- and γ2-subunits in the rat cerebral cortex. Brain Res. 2005;1031:134–137. doi: 10.1016/j.brainres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Marutha Ravindran CR, Ticku MK. Low concentrations of ethanol do not affect radioligand binding to the δ-subunit-containing GABAA receptors in the rat brain. Brain Res. 2007;1165:15–20. doi: 10.1016/j.brainres.2007.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhatre MC, Mehta AK, Ticku MK. Chronic ethanol administration increases the binding of the benzodiazepine inverse agonist and alcohol antagonist [³H]Ro 15-4513 in rat brain. Eur. J. Pharmacol. 1988;153:141–145. doi: 10.1016/0014-2999(88)90599-7. [DOI] [PubMed] [Google Scholar]
- Mhatre MC, Ticku MK. Chronic ethanol administration alters γ-aminobutyric acidA receptor gene expression. Mol. Pharmacol. 1992;42:415–422. [PubMed] [Google Scholar]
- Mhatre MC, Ticku MK. Chronic ethanol treatment upregulates the GABA receptor β subunit expression. Mol. Brain Res. 1994;23:246–252. doi: 10.1016/0169-328x(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Bowers BJ, Wehner JM, Kralic JE, VanDoren MJ, Morrow AL, Homanics GE. GABAA-receptor δ subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol. Clin. Exp. Res. 2001;25:1708–1718. [PubMed] [Google Scholar]
- Mihic SJ. Acute effects of ethanol on GABAA and glycine receptor function. Neurochem. Int. 1999;35:115–123. doi: 10.1016/s0197-0186(99)00053-4. [DOI] [PubMed] [Google Scholar]
- Montpied P, Morrow AL, Karanian JW, Ginns EI, Martin BM, Paul SM. Prolonged ethanol inhalation decreases γ-aminobutyric acidA receptor α subunit mRNAs in the rat cerebral cortex. Mol. Pharmacol. 1991;39:157–163. [PubMed] [Google Scholar]
- Narahashi T, Aistrup GL, Marszalec W, Nagata K. Neuronal nicotinic acetylcholine receptors: a new target site of ethanol. Neurochem. Int. 1999;35:131–141. doi: 10.1016/s0197-0186(99)00055-8. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc. Natl. Acad. Sci. U.S.A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Busonero F, Talani G, Tranquilli S, Mameli M, Spiga S, Follesa P, Biggio G. Changes in GABAA receptor gene expression associated with selective alterations in receptor function and pharmacology after ethanol withdrawal. J. Neurosci. 2003;23:11711–11724. doi: 10.1523/JNEUROSCI.23-37-11711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Hoger H, Adamiker D. Structure and subunit composition of GABA(A) receptors. Neurochem. Int. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat. Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticku MK, Mehta AK. Effects of alcohol on GABA-mediated neurotransmission. In: Kranzler HK, editor. Handbook of Experimental Pharmacology: The pharmacology of Alcohol Abuse. Vol. 114. Heidelberg: Springer-Verlag; 1995. pp. 103–119. [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc. Natl. Acad. Sci. U.S.A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter HJ, Messing RO. Regulation of neuronal voltage-gated calcium channels by ethanol. Neurochem. Int. 1999;35:95–101. doi: 10.1016/s0197-0186(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by δ subunit-containing GABAA receptors in hippocampal neurons. J. Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weight FF, Li C, Peoples RW. Alcohol action on membrane ion channels gated by extracellular ATP (P2X receptors) Neurochem. Int. 1999;35:143–152. doi: 10.1016/s0197-0186(99)00056-x. [DOI] [PubMed] [Google Scholar]
- Whiting PJ. The GABA-A receptor gene family: new targets for therapeutic intervention. Neurochem. Int. 1999;34:387–390. doi: 10.1016/s0197-0186(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Woodward JJ. Ionotropic glutamate receptors as sites of action for ethanol in the brain. Neurochem. Int. 1999;35:107–113. [PubMed] [Google Scholar]
- Yamashita M, Marszalec W, Yeh JZ, Narahashi T. Effects of ethanol on tonic GABA currents in cerebellar granule cells and mammalian cells recombinantly expressing GABAA receptors. J. Pharmacol. Exp. Ther. 2006;319:431–438. doi: 10.1124/jpet.106.106260. [DOI] [PubMed] [Google Scholar]


