Abstract
An F1 mutagenesis strategy was developed to identify conditional mutations affecting extracellular matrix (ECM) patterning. Tubulogenesis requires coordinated movement of epithelial cells and deposition of a multilayered ECM. In the Drosophila ovary, an epithelium of follicle cells creates the eggshells, including the paired tubular dorsal appendages (DAs) that act as breathing tubes for the embryo. A P-element mutagenesis strategy allowed for conditional overexpression of hundreds of genes in follicle cells. Conditional phenotypes were scored at the level of individual mutant (F1) female flies. ECM pattern regulators were readily identified including MAPK signaling gene ets domain lacking (fused DAs), Wnt pathway genes frizzled 3 and osa (long DAs), Hh pathway gene debra (branched DAs), and transcription factor genes sima/HIF-1α, ush, lilli, Tfb1, broad, and foxo. In moving cells the [Ca2+]/calcineurin pathway can regulate adhesion to ECM while adherens junctions link cells together. Accordingly, thin eggshell and DA phenotypes were identified for the calcineurin regulator calreticulin and the adherens junction component arc. Finally a tubulogenesis defect phenotype was identified for the gene pterodactyl, homologous to the mammalian serine/threonine receptor-associated protein (STRAP) that integrates the TGF-β and PI3K/AKT signaling pathways. Because phenotypes can be scored in each mutant fly before and after gene induction, this F1 conditional mutagenesis strategy should allow for increased scale in screens for mutations affecting repeated (reiterated) events in adult animals, including gametogenesis, movement, behavior, and learning.
ONE of the most basic and astonishing features of human development is the transformation of flat sheets of epithelial cells into tubes of various sizes, cell number, and branch structure, often in the absence of cell division or cell death. In response to growth factors and/or inductive interactions with other cells, the epithelial sheet undergoes oriented growth, cell movement, changes in the number of cell/cell contacts (cell intercalation), and changes in contacts with the extracellular matrix (ECM). The resulting tubulogenesis and branching morphogenesis creates the substructure of numerous tissues and organs, including the neural tube, kidney, lung, breast, and circulatory system. In adults the growth of new blood vessels (angiogenesis) is critical to wound healing as well as to tumor progression. Tumor metastasis and tubulogenesis share the common basic features of cell growth, cell movement, and altered contacts with ECM. Consequently the same growth factor and signaling pathways important in tubulogenesis are also implicated in tumor progression (van de Wetering et al. 2002; Reya and Clevers 2005; Glesne et al. 2006). As such the genes and pathways controlling tubulogenesis are of intense interest as possible targets for disease interventions in vivo and for controlling tissue and organ culture in vitro (Meyer et al. 2004; Soriano et al. 2004).
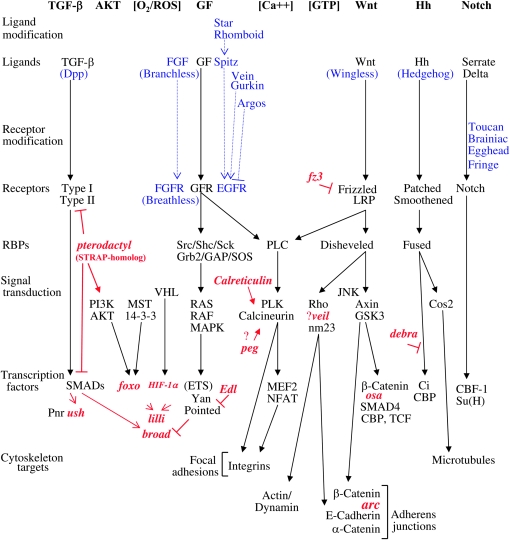
Mammalian in vitro systems implicate several specific growth factors (GF) and signaling pathways in tubulogenesis (summarized in Figure 1) (Han et al. 2004). For example, when hepatocyte growth factor is applied to primary cultured hepatocytes it binds the Met receptor tyrosine kinase and activates a signal cascade including MAPK components and ETS-family transcription factors, and ultimately results in branching morphogenesis (Rosario and Birchmeier 2003). Similarly, angiogenesis can be induced in cultured cells by vascular endothelial growth factor acting through the PI3K/AKT pathway and transcription factor ETS-1 (Lavenburg et al. 2003).
Figure 1.—
Outline of signaling pathways affecting tubulogenesis. Canonical pathway components are indicated in black. Details of Drosophila homologs are indicated in blue. Genes identified in this study are indicated in red. The ETS domain is a DNA-binding domain that specifically interacts with sequences containing the common core trinucleotide GGA and is involved in protein–protein interactions with cofactors that help determine its biological activity. RBPs, receptor binding proteins; ROS, reactive oxygen species.
Drosophila is an excellent model for the study of genes affecting tubulogenesis (Cabernard et al. 2004; Jung et al. 2005). The tracheae are the respiratory organ of Drosophila and genes have been identified that are required for each step of tracheole branching morphogenesis (Samakovlis et al. 1996; Petit et al. 2002; Neumann and Affolter 2006). These studies identify several signaling pathways conserved in humans, including the GF, TGF-β, Wnt, and Hh pathways. The pattern of tubules of the Drosophila wing vasculature (see Figure 3I) arises from another well-studied sequential gene action (De Celis 2003; Crozatier et al. 2004). The Hh and TGF-β signaling pathways set up positional information in the wing primordium and define the geometry of vein placement. Homeodomain transcription factors are involved in specifying cells competent for expression of Rhomboid, while the Notch signaling pathway is involved in further refining the boundaries of Rhomboid expression. The resulting pattern of Rhomboid expression predicts the ultimate vein pattern. Rhomboid is a potent stimulator of signaling through the EGFR pathway, which in turn promotes vein development. Additional patterning steps include intervein differentiation and crossvein development. Intervein structure has been demonstrated to be dependent upon the integrins, a family of transmembrane receptor proteins that link the ECM to the cytoskeleton, and that are also involved in tracheole morphogenesis (Araujo et al. 2003; Levi et al. 2006).
Figure 3.—
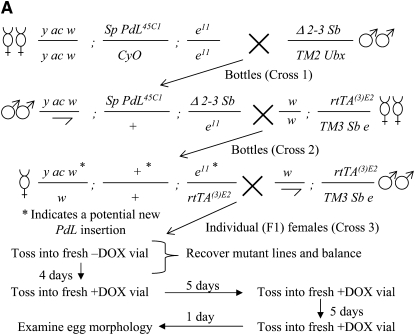
Identification of conditional, dominant mutations affecting the eggshell. (A) Scheme to identify conditional eggshell mutants. Crosses 1 and 2 were done en masse in bottles. F1 females bearing new PdL insertions obtained from the same bottle in cross 2 could contain unique events or the same event and were named to reflect that information. The mini-white+ gene in the rtTA(3)E2 insertion yields only an orange eye color, and so new white+ PdL insertions could be identified in this background by a more red or wild-type eye color. (B–H) Eggshell phenotypes. The indicated PdL mutant strains were crossed to rtTA(3)E2 driver strain, and female progeny were cultured in the presence and absence of DOX. The −DOX control eggs (B and F) are from strain edl4M127 (as in C), however they are characteristic of the normal morphology of control eggs for the other two lines shown (D and E) as well as most other PdL conditional eggshell mutations. (I) Recessive wing phenotype of pterodactyl2-4 insertion and excision derivatives. Representative wings are presented from wild-type flies and from flies homozygous for the starting pterodactyl2-4 insertion and homozygous for the indicated excision (exc) derivatives. The class of pterodactyl wing phenotype (mild, intermediate, severe) is indicated.
The construction of the eggshell (chorion) by the Drosophila ovarian follicle cells is emerging as a powerful model system in which to study tubulogenesis and ECM patterning (Tzolovsky et al. 1999; Berg 2005; Papadia et al. 2005; Kleve et al. 2006). The follicle cell epithelium surrounds the developing oocyte (see Figure 2, C and D) and in the absence of cell division synthesizes a multilayer ECM with a number of specialized features (see Figure 3, B and F). A subset of the dorsal/anterior columnar follicle cells detach from the underlying oocyte and embark on a dramatic anterior migration to synthesize the dorsal appendages (DAs). The DAs are the pair of large and elaborate gas-exchange tubes that project out from the anterior of the eggshell. Another subset of follicle cells synthesizes the tiny, tubular micropyle through which some intrepid sperm might pass. Formation of these structures requires coincident signaling through the EGFR and TGF-β pathways (Chen and Schüpbach 2006) (summarized in Figure 1). Gurken, a TGF-α homolog, is produced by the oocyte nucleus and signals the overlying follicle cells through the EGFR, RAS/RAF/MAPK signal transducers and ETS-domain transcription factor Pointed. Coincidently, TGF-β signals must be received from the anterior squamous follicle cells. At least two distinct groups of the columnar follicle cells then reorganize to create the DAs; “roof” cells express the transcription factor Broad and constrict their cytoskeleton on the apical side, while “floor” cells express Rhomboid (Dorman et al. 2004). Notch signaling acts to create the boundary between the roof and floor cell types (Ward et al. 2006). The coordinated anterior migration of the roof and floor cells extends the DAs and this involves additional signaling through the Jun-kinase (JNK) pathway.
Figure 2.—
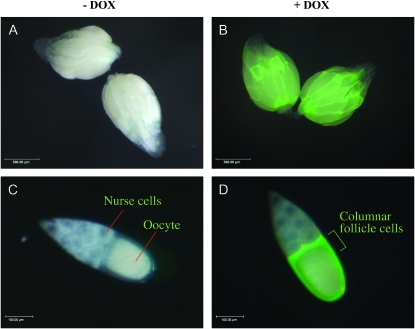
DOX-dependent gene overexpression in ovarian follicle cells. Ovaries and stage-10 egg chambers were photographed under a visible light source and fluorescent light source and merged pictures are shown. The scale is indicated in the lower left corner. (A) Ovaries dissected from female containing the GFP-reporter insertion and rtTA(3)E2 insertion cultured in absence of DOX. (B) Ovaries dissected from female containing the GFP reporter and rtTA(3)E2 cultured in the presence of DOX. (C) Stage-10 egg chamber dissected from female containing the GFP-reporter and rtTA(3)E2 cultured in the absence of DOX. (D) Stage-10 egg chamber dissected from female containing the GFP-reporter and rtTA(3)E2 cultured in the presence of DOX. Results are typical of multiple flies and experiments.
The migrating cells that generate Drosophila tracheoles and micropyles coordinate their movement through reciprocal signaling events sometimes called “social interactions” (Ghabrial and Krasnow 2006; Montell 2006). During tracheole branch formation the epithelial cells compete for the lead-cell position in a process involving GF and Notch pathway signals. The dynamic nature of tubulogenesis suggests that conditional mutations might be particularly useful for future genetic analyses, in that they might allow for more fine-scale dissection of individual steps.
A number of important questions remain to be answered for each of the tubulogenesis model systems, including how signals through the multiple pathways are integrated and how specific pathways activate specific tubulogenesis events at the level of the individual cell. A remarkably small number of cell-intrinsic surface-remodeling events can account for the movements required for tubulogenesis and cell intercalation (Lecuit and Pilot 2003; Pilot and Lecuit 2005; Neumann and Affolter 2006). “Focal adhesions” are specific contacts between the epithelial cell and the ECM involving integrins and the actin cytoskeleton. Cell movement over an ECM involves membrane protrusions and formation of new focal adhesions at the leading edge coordinated with disassembly of adhesions at the rear. Within the moving cell there are directional changes in actin/myosin polymerization and endocytic shuttling of membrane components to the leading edge. Cell intercalation additionally requires changes in the number and location of “adherens junctions,” which are specialized connections between epithelial cells involving catenins, cadherins, and the actin cytoskeleton (Neumann and Affolter 2006). GTPases such as Rho and nucleoside kinases including nm23 (awd) act downstream of Wnt and other pathways to directly regulate cytoskeleton organization and membrane dynamics in the moving cell (Palacios et al. 2002; Dammai et al. 2003).
Transposable elements with outwardly directed promoters are powerful tools for creating mutations by overexpression and/or misexpression of gene(s) near the insertion site and have been a rich source for mutations affecting development (Rorth et al. 1998; Peña-Rangel et al. 2002; Tseng and Hariharan 2002). A Drosophila P-type transposable element called PdL contains a doxycycline (DOX)-inducible promoter directed outward from its 3′ end (Landis et al. 2001). New PdL insertions cause the DOX-dependent overexpression of genes downstream of the insertion site, often hundreds of base pairs away. It is estimated that up to one-third of PdL insertions cause the overexpression of a downstream gene. The gene overexpression often, but not always, results in a conditional mutant phenotype. For example, 7% of PdL insertions are conditional larval lethal. Because PdL mutations are both dominant and conditional they lend themselves to efficient strategies for functional gene discovery. In these studies the conditional and dominant nature of the PdL mutations was used to allow identification of interesting phenotypes at the level of the individual F1 fly, thereby facilitating the screening of larger numbers of events.
MATERIALS AND METHODS
General:
Drosophila stocks and culture conditions are as previously described (Landis et al. 2001, 2003). All experiments were performed using the rtTA(3)E2 driver strain.
Isolation of genomic DNA from PdL lines:
DNA was isolated from PdL lines (50 female flies each) as previously described (Landis et al. 2001). It was further treated with RNaseA for 15 min at 37°, followed by phenol/chloroform extraction. The DNA was then precipitated, washed in 70% ethanol, dissolved in 50 μl of distilled water, and stored at −20°.
Inverse PCR amplification of PdL flanking sequences:
DNA equivalent to four flies was restriction digested with Taq1 at 65° for 5 hr. The DNA was extracted with phenol/chloroform, precipitated with ethanol, dissolved in 100 μl of distilled water, and 10 μl of this was treated with T4 DNA ligase overnight at 16°. PCR amplification was performed using primers Pry1 and IR as described (Landis et al. 2001). PCR protocol was as follows: step 1, 94° for 5 min; step 2, 96° for 30 sec; step 3, 51° for 1 min; step 4, 72° for 2 min; step 5, steps 2–4, repeat 39 times; and step 6, 72° for 10 min. PCR product was subcloned into the PCR2.1–TOPO cloning vector (Invitrogen, San Diego). Sequencing was carried out at the University of Southern California Microchemical Core Facility.
DNA sequence analyses:
PdL flanking DNA sequences were used to query GenBank databases using BLASTN program with default settings as provided at the NCBI web site (http://www.ncbi.nlm.nih.gov/).
Northern analyses:
Total RNA was isolated from adult female Drosophila using the TRIZOL reagent (Life Technologies) using standard protocol. RNA was fractionated on 1% formaldehyde gels and transferred to Gene Screen membranes (NEN, Boston).
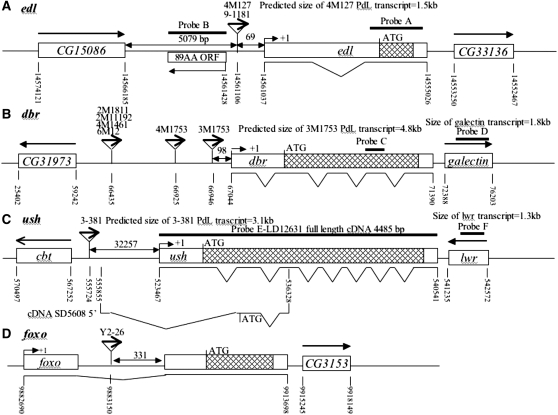
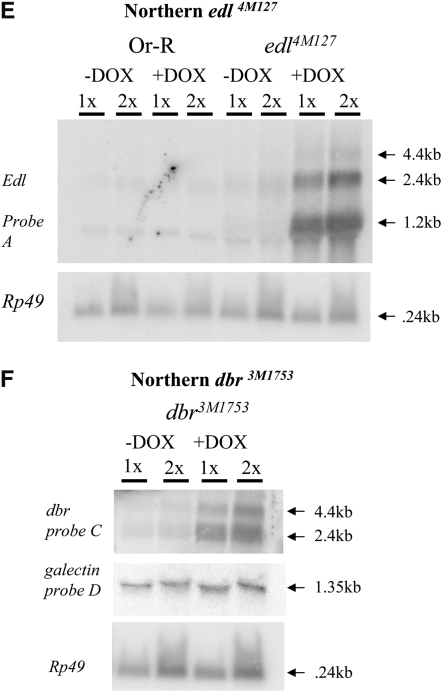
The DNA probe A for the edl gene (Figure 4A) was generated by PCR amplification from Drosophila genomic DNA using primers “EDL38381F” (cctagtccttagttctgctc) and “EDL39059R” (ccgttcgcaacgtttgagtt). The DNA probe B for the 89-aa ORF region (Figure 4A) was generated by PCR amplification from Drosophila genomic DNA using primers “89ORF44621F” (ctgttggctcaataagcagg) and “89ORF45410R” (tactgtaggtcagccatgtg).
Figure 4.—
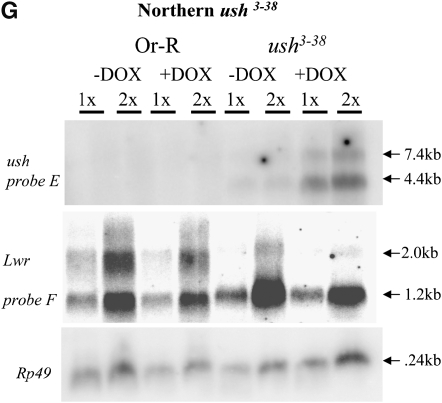
Molecular characterization of selected mutations. (A–D) Intron and exon boundaries of the mutated genes are indicated, with numbering according to DNA sequences obtained from the NCBI web site (http://www.ncbi.nlm.nih.gov/). Locations for transcriptional initiation are indicated by arrows. PdL inserts are indicated by triangles and each is oriented 5′ to 3′, as indicated by internal arrows. DNA fragments used as probes in Northern analyses are indicated at the top of each diagram. (A) edl (genomic scaffold sequence AE003798.3). (B) dbr (genomic scaffold sequence AE003590.3). (C) ush (genomic scaffold sequence AE003589.3; SD5608 cDNA sequence is from accession no. AI541608 in the NCBI database. (D) foxo. (E–G) Northern analysis of selected lines. Oregon R wild-type strain and the indicated PdL mutant strains were crossed to rtTA(3)E2 driver strain. Total RNA was isolated from adult progeny cultured 1 week in the presence and absence of DOX, transferred to Northerns blots, and hybridized with the gene-specific probes indicated. Ribosomal protein 49 gene Rp49 was used as control for loading. Two amounts of RNA were loaded for each sample (once and twice, as indicated), and signals were visualized by phosphoimager. (E) edl4M127 mutant strains and controls. (F) dbr3M1753 mutant strain and controls. (G) ush3-38 mutant strains and controls. Galectin probe D downstream of gene dbr and lwr probe F downstream of gene ush did not yield any altered signal in the presence and absence of DOX, indicating that transcription did not go beyond genes dbr and ush. The predicted sizes of the edl4M127, dbr3M1753, and ush3-38 PdL transcripts (A–C) matched the measured sizes of the corresponding +DOX transcripts (E–G).
The DNA probe C for the debra gene (Figure 4B) was generated by PCR amplification from Drosophila genomic DNA using primers “dbrF” (gttccagcaacccaaatcccacaccg) and “dbrR” (cctgctttaaatccacgattgagcag). The DNA probe D for the galacten gene was generated by PCR amplification from Drosophila genomic DNA using primers “galectinF” (caatgactctcgagatgtgg) and “galectinR” (acggattctgatagactcgc).
The DNA probe E for the ush gene (Figure 4C) was obtained from a full-length cDNA LD12631 (Stapleton et al. 2002). The DNA probe F for the lwr gene was generated by PCR amplification from Drosophila genomic DNA using primers “lwrF” (tgctcgactgcacatttgc) and “lwrR” (cagtagagaagcgagcaag).
The loading control was ribosomal protein gene Rp49 (O'Connell and Rosbash 1984). DNA probes were 32P-labeled using the Prime-It II DNA labeling kit (Stratagene, La Jolla, CA). Hybridization signals were scanned using the Phosphoimager (Molecular Dynamics, Sunnyvale, CA) and visualized using the Imagequant program. Transcript size was determined by comparison with 1 kb RNA ladder (GIBCO-BRL, Grand Island, NY) according to the manufacturer's instructions.
Electron microscopy:
Scanning electron microscopy (SEM) was carried out at the University of Southern California Center for Electron Microscopy and Microanalysis, using a Cambridge 360 SEM. Samples were prepared using standard methods. Briefly, young F1 female flies were cultured on food in the presence and absence of DOX for 10 days. The females were mated and fed yeast paste prepared with and without added DOX for the last 2 days to boost egg production. The females were allowed to lay eggs directly onto the SEM grid, and these were then sputter-coated with platinum and examined in the SEM.
Expression of GFP in follicle cells:
A transgenic reporter strain was generated in which expression of eGFP is driven by the “tet-on” promoter in USC 1.0 vector (Allikian et al. 2002). The creation and characterization of the construct and strains are described in detail elsewhere (N. Hoe and J. Tower, unpublished results). Males from the eGFP strain were crossed with rtTA(3)E2 females and progenies (F1) were collected that contained both constructs. F1 female flies were cultured on food in the presence and absence of DOX for 10 days before dissection and microscopy. The females were mated and fed yeast paste prepared with and without added DOX for 2 days before dissection to boost egg production. Ovaries and stage-10 egg chambers were dissected in 1× PBS buffer at room temperature and GFP images were generated using the Leica MZ FLIII fluorescence stereomicroscope and SPOT image capture system and software according to the manufacturer's instructions.
Mobilization of P-element insertion in the pterodactyl mutant line:
Females homozygous for the PeterodactlylPdL2-4 insertion, genotype y-ac-w;PdL2-4 were crossed to males from a line containing a source of P-element transposase (Robertson et al. 1988), genotype Sp/Cyo;delta2-3 Sb/TM2 Ubx. The first generation male flies containing both PdL and the delta2-3 transposase were collected and crossed to second chromosome balancer females, genotype y-ac-w;Sp/CyO. From the offspring, twelve single males with PdL excision (loss of white+ marker) were identified, each from a different vial and therefore representing independent events. These 12 excision chromosomes were made homozygous by crossing to second chromosome balancer stock.
RESULTS AND DISCUSSION
In the rtTA(3)E2 transgenic strain, the artificial transcription factor rtTA is expressed in all somatic cells using the powerful Actin 5C gene promoter (Bieschke et al. 1998). The rtTA protein will bind to its target site (tetO) and activate transcription only in the presence of DOX. To confirm efficient induction of target gene transcription in the ovary, a tetO-GFP reporter construct was assayed in adult females that had been cultured in the presence and absence of DOX in the food for 10 days. Fluorescence microscopy of dissected ovaries confirmed DOX-dependent expression of GFP in the follicle cells at all stages of oogenesis (Figure 2). Particularly abundant expression was present at stage 10 of oogenesis in the columnar follicle cell epithelium surrounding the oocyte (Figure 2D). GFP could also be readily detected in the squamous follicle cells stretched over the nurse cells and in the migrating border follicle cells. As expected, little to no GFP expression could be detected in the nurse cells or oocyte. Transgenic constructs are not efficiently expressed in Drosophila germ-line cells unless they contain specific germ-line transcription and RNA processing elements (Rorth 1998). As a consequence, the tetO promoters are not efficiently expressed in the germ-line cells, and any PdL mutant phenotypes should result from gene overexpression in the somatic follicle cells.
A PdL insertion on the second chromosome was mobilized to ∼3000 new chromosomal insertion sites by crossing to a strain that expresses the P-element transposase (Figure 3A). The strains and crossing strategy are designed to minimize time and effort and allow for simultaneous mutagenesis of each of the five Drosophila chromosomes (X, Y, 2, 3, and 4) using only two crosses performed en masse. Progeny are generated in cross 2 that contain a new PdL insertion as well as the rtTA(3)E2 transactivator, and these flies are referred to as F1. A single Drosophila female is capable of laying hundreds of eggs throughout her lifetime. Each of 3000 F1 females bearing a new PdL insertion was cultured individually in the absence of DOX for several days so that normal, viable eggs were laid, and the event could be recovered later if desired. The female was then serially transferred to culture vials containing DOX over several days, and the last two vials were inspected for the presence of eggs with abnormal shells using the dissecting microscope. DOX treatment and any subsequent gene overexpression was for a minimum of 10 days before scoring of eggshells (Figure 3A), and therefore on the basis of typical times for progression through stages of oogenesis, the follicle cells have potentially been overexpressing the gene from the time of stem-cell division through completion of eggshell synthesis (Margolis and Spradling 1995). Of 3000 females scored, 190 failed to lay eggs or produced eggs that were abnormal in the presence of DOX. Stable PdL insert lines were successfully established from the early-laid, normal eggs for 170 of the females. Conditional eggshell phenotypes were confirmed for 140 strains by testing cohorts of females from each strain in parallel, in the presence and absence of DOX. The gene downstream of the PdL insertion site was identified for patterning-defect lines by inverse PCR and sequencing (Table 1). For selected genes DOX-dependent overexpression was confirmed by Northern blot (Figure 4). It was previously observed that PdL-directed transcription and overexpression does not extend beyond the terminator of the first gene downstream (3′) of the PdL insertion site (i.e., no detectable “readthrough”) (Landis et al. 2001, 2003), and that result was confirmed here for genes dbr and ush using Northern analysis and downstream probes.
TABLE 1.
New PdL mutations with pattern defects
| Gene | ID number | Alleles | Functions | Egg phenotype |
|---|---|---|---|---|
| bad egg (beg) | CG7842 | 18 | Fatty acid synthesis | Thin shell |
| cracked (ckd) | CG14959 | 12 | ? | Thin shell, short DA |
| debra (dbr) | CG11371 | 9 | MVB, Hh signaling | Thin shell, branched DAs and micropyle |
| ets domain lacking (edl) | CG15085 | 2 | GF signaling | Fused DAs |
| foxo | CG3143 | 1 | Txn factor, AKT pway | Thin shell, short DAs |
| ushaped (ush) | CG2762 | 1 | Txn factor | Thin shell, short DAs |
| Tfb1 | CG8151 | 1 | Txn factor | Thin shell, long DAs |
| arc (a) | CG13505 | 1 | Adherens junction | DAs folded |
| lilliputian (lilli) | CG8817 | 1 | Txn factor | Thin shell, long DAs |
| calreticulin (Crc) | CG9429 | 1 | ER, protein folding | Thin shell, short DAs |
| peg | CG8583 | 1 | SRP, protein folding | Thin shell, short DAs |
| pterodactyl (pter) | CG3957 | 1 | TGF-β signaling | Long DAs |
| veil | CG4827 | 1 | 5′-nucleotidase | Thin shell, short DAs |
| frizzled 3 (fz3) | CG16785 | 1 | Wnt signaling | Long DAs |
| osa | CG7467 | 1 | Txn factor, Wnt signaling | Abnormal DAs |
| broad (br) | CG11491 | 1 | Txn factor | Thin shell |
| similar (sima) | CG7951 | 1 | Txn factor, HIF-1α homolog | Abnormal DAs |
| Spargel | CG9809 | 1 | PEP-dependent sugar phosphotransferase system, PPAR-γ C1A homolog | Long DAs |
| eIF3-S10 | CG9805 | 1 | Translation initiation | Short DAs |
| — | CG3563 | 1 | ? | Long DAs |
| — | CG13012 | 1 | ? | Long DAs |
| — | CG2127 | 1 | ? | Long DAs |
| — | CG2909 | 1 | ? | Long DAs |
| — | CG31246 | 1 | ? | Thin shell, long DAs |
| l(3)neo26 | CG6874 | 1 | ? | Long DAs |
| — | CG33188 | 1 | ? | Long DAs |
| — | CG15160 | 1 | ? | Long DAs |
Numerous mutations were recovered that altered ECM patterning, some with quite specific effects on tubulogenesis (Figure 3, Table 1). A PdL insertion was mapped 69 bp upstream of the edl gene and DOX-dependent overexpression of the gene was confirmed by Northern blot (Figure 4, A and E). Probe A located in the edl coding region hybridized to RNA species of approximate sizes 1.2 kb, 2.4 kb, and 4.4 kb, while probe B located upstream of the PdL insertion did not hybridize to any detectable RNA species in the presence or absence of DOX (data not shown). Overexpression of edl caused a dramatic fusion of the DAs (Figure 3C). The edl gene encodes a negative transcription factor that antagonizes Pointed, and overexpression of edl has previously been shown to yield fused DAs (Yamada et al. 2003). At least nine genes are known to give rise to the same phenotype when they are mutated to loss-of-function (Berg 2005). The products of the toucan, egghead, brainiac, and fringe genes all modify the Notch receptor, while the products of the Spitz, argos, and vein genes each encode EGFR ligands. Finally, pointed encodes an ETS-domain transcription factor activated by EGFR signaling (summarized in Figure 1). The data are consistent with a model in which Notch signaling and EGFR signaling through Pointed cooperate to specify the midline branch that results in two distinct DAs (Ward et al. 2006).
Overexpression of debra (dbr) yielded a novel phenotype characterized by multibranched DAs and multibranched micropyle (Figure 3D). PdL was mapped 98 bp upstream of the debra gene and overexpression was confirmed by Northern blot (Figure 4, B and F). Debra is a component of the multivesicular body (MVB), a type of late endosome in which regions of the limiting endosomal membrane invaginate to form internal vesicles. Membrane proteins are targeted to the vesicles by ubiquitination and are sequestered from the cytoplasm. The MVB functions in downregulation of membrane receptor signaling. Debra has been shown to mediate the ubiquitination and lysosomal destruction of the Hh pathway target transcription factor Ci during wing development (Dai et al. 2003). The phenotype observed here in the ovary suggests that Hh pathway signaling and Ci factor activity might favor branching.
The TGF-β and AKT pathways are critically implicated in tubulogenesis, but it is not known how these signals are integrated at the level of the individual epithelial cell. In mammals a WD-40 repeat-containing protein called serine/threonine receptor-associated protein (STRAP) has been identified that bridges these pathways (Datta et al. 1998; Datta and Moses 2000; Seong et al. 2005). STRAP interacts physically with both types I and II receptors and SMAD7 and inhibits TGF-β signaling, while in turn it physically interacts with PI3K and stimulates the AKT pathway (summarized in Figure 1). A PdL insertion was mapped at 3 bp upstream of the Drosophila homolog of STRAP, here designated pterodactyl (pter). Overexpression of pterodactyl in the follicle cells caused overly long DAs (Figure 3G), while a homozygous pterodactyl insertion (pter2-4) showed a loss-of-function phenotype of mild wing-vein defects (Figure 3I). The fact that pterodactyl overexpression and loss-of-function both affect branching morphogenesis supports the conclusion that the mutagenesis strategy is identifying true tubulogenesis regulators and not merely creating neomorphic effects via unrelated genes. The pter2-4 PdL insertion was mobilized using P-element transposase, and twelve lines were generated where the mini-white+ marker gene in PdL had been lost, indicating partial or complete loss of the pter2-4 insertion. Each excision derivative was homozygous viable and had wing vasculature defects that were more severe than the starting pter2-4 insertion. Specifically there was increased presence of ectopic wing vasculature. Relative to the mild phenotype of the starting pter2-4 mutation, seven of the new excision mutations (lines 1, 2, 6, 8, 9, 10, and 12) had an intermediate phenotype, while five lines (3, 4, 5, 7, and 11) had a severe phenotype (Figure 3I). Mutations from both the intermediate and severe class had the same wing phenotype when heterozygous to the starting pter2-4 mutation. The data support the conclusion that the starting pter2-4 mutation is indeed caused by an insertion of the PdL element marked with mini-white+. Moreover the lack of excision events with wild-type wing phenotype suggests that the starting pter2-4 insertion might have a complex structure that precludes reversion to wild type, such as an associated deletion, and it may be useful to further characterize this mutation in the future.
Overexpression of several transcription factor genes resulted in thin eggshells, including ush, foxo, lilli, and Tfb1 (Table 1; Figure 3, E and H). Ush is a zinc-finger transcription factor that acts downstream of TGF-β signaling during embryogenesis and cooperates with another transcription factor, Pnr, to regulate Wnt expression (Fossett et al. 2001). Overexpression of ush caused thin eggshells and small, short DAs, while positioning of the DAs appeared relatively normal (Figure 3E). The presence of an upstream promoter and alternative transcript for the ush gene is suggested by the location of the PdL insertion and the location of the 5′ end of the SD5608 cDNA sequence found in the GenBank database (Figure 4). The ush coding region probe (probe E) hybridized to both a 4.4-kb and a 7.5-kb transcript induced by DOX, suggesting that the large intervening sequence (30 kb) was spliced out and that the transcript from the upstream promoter is being overexpressed along with possible alternative splicing. The transcription factor Foxo acts downstream of insulin-like signaling, AKT/PI3K signaling, and JNK signaling pathways to regulate metabolic activity and growth in Drosophila and other species (Vogt et al. 2005), and overexpression of Foxo caused short DAs and thin eggshells (Figure 3H). Lilli is the only member of the Fragile X/Burkitt's lymphoma family of transcription factors found in Drosophila. The lilli gene is required for normal cytoskeleton organization, cell size, and embryo segmentation and interacts genetically with each of the Wnt, AKT, and EGFR pathways (Su et al. 2001; Tang et al. 2001; Wittwer et al. 2001; Gu and Nelson 2003).
Wnt signaling is critical for tubulogenesis and has several direct cytoskeletal targets relevant to moving cells (summarized in Figure 1). Overexpression of the antagonistic Wnt receptor gene fz3 (Chen et al. 2004) caused overly long DAs (Table 1). Wnt signaling through GSK3 is known to directly modify β-catenin, which in turn acts both as a transcription factor and a component of the adherens junction. DA structure was disrupted by overexpression of arc, which encodes a component of the adherens junction, and osa, which is a Wnt pathway trancription factor target. GTPases such as Rho and nucleoside kinases including nm23 (awd) act downstream of Wnt and other pathways to directly regulate cytoskeleton organization and membrane dynamics. In Drosophila nm23 has been shown to regulate tracheal cell motility and FGFR recycling (Dammai et al. 2003). Overexpression of a gene designated veil caused thin eggshells and short DAs. The veil gene is predicted to encode a 5′-nucleotidase and overexpression of veil can affect growth of mutant cells in the wing (Raisin et al. 2003). Taken together the data suggest a possible role for veil in the [GTP] signaling pathway (summarized in Figure 1).
The [Ca2+]/calcineurin pathway regulates integrin gene expression, as well as focal adhesion assembly/disassembly and recycling of integrin proteins to the leading edge of many types of moving cells (summarized in Figure 1). Calreticulin is a Ca2+ binding protein thought to function as a molecular chaperone component of the endoplasmic reticulum (ER) quality control machinery and in exocytosis. In mice, calreticulin has been found to be a critical regulator of calcineurin activity, particularly important in development of the heart (Lynch et al. 2005). Here overexpression of the Drosophila calreticulin gene was found to cause both thin eggshells and short DAs, consistent with a role in ECM patterning. A similar thin eggshell and short DA phenotype was observed for overexpression of a gene designated peg. The peg gene encodes a homolog of a signal recognition particle (SRP) factor involved in protein folding at the ER, and it is tempting to speculate that peg might also act in the calcineurin pathway.
One common aspect of Drosophila DAs, Drosophila tracheoles, human lungs, and human vasculature is they all serve as tubular conduits for oxygen supply to various tissues. It is then perhaps no surprise that cellular oxygen-sensing pathways are found to affect the development of these structures. Mammalian HIF-1α regulates cellular metabolism and transcription in response to oxygen concentration and functions in angiogenesis (Kim et al. 2006; Papandreou et al. 2006). The Drosophila homolog of HIF-1α (Sima) (Nambu et al. 1996; Reiling and Hafen 2004) regulates tracheole branch extension and termination. Here overexpression of HIF-1α (Sima) was found to affect DA length and structure (Table 1). Intriguingly, the Foxo transcripton factor implicated here as a regulator of DA length is also involved in oxygen-stress sensing pathways in various organisms (Lehtinen et al. 2006; Wolff et al. 2006).
In summary, while it is possible that one or more of the genes identified in this study represent a nonspecific background, many of the mutations were found to affect pathways critical for ECM patterning and should be useful reagents for further genetic analyses (summarized in Figure 1). Mutations were identified in genes known to be essential for DA formation such as broad (Deng and Bownes 1997; Tzolovsky et al. 1999; Chen and Schüpbach 2006), as well as new genes. Both overexpression and loss-of-function tubulogenesis phenotypes were identified for the potentially important signaling gene pterodactyl (related to mammalian STRAP),for which no mutant phenotype had previously been available. In addition, phenotypes could be observed for genes that have no detectable loss-of-function phenotype such as fz3. The set of ECM patterning genes identified here overlaps and complements those identified for trachea development. Finally, since many interesting genes were hit only once, it is likely that this screen (3000 insertions) has only scratched the surface of potential ECM regulatory switches.
The P element has allowed numerous types of genomic alterations and engineering, such as germ-line transformation, targeted deletions, local transposition, and enhancer-trapping (Bellen et al. 2004). In the past, the low frequency of mutation has limited the feasibility of large-scale P-element screens. As shown here, the dominant and conditional nature of PdL mutations allows for increased scale through the scoring and recovery of conditional mutations at the level of the individual (F1) mutant fly. Saturation-type P-element screens (50–100,000 events) should be possible in the future for groups interested in identifying Drosophila mutations affecting ECM patterning and other reiterated events in the adult such as gametogenesis, movement, behavior, and learning.
Acknowledgments
We thank Andy Dillin for suggesting the name pterodactyl. This research was supported by grants from the Department of Health and Human Services to J.T. (GM48449 and AG11833).
This article is dedicated to the memory of Harminder Kaur.
References
- Allikian, M. J., D. Deckert-Cruz, M. R. Rose, G. N. Landis and J. Tower, 2002. Doxycycline-induced expression of sense and inverted-repeat constructs modulates phosphogluconate mutase (Pgm) gene expression in adult Drosophila melanogaster. Genome Biol. 3 research0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo, H., E. Negreiros and E. Bier, 2003. Integrins modulate Sog activity in the Drosophila wing. Development 130 3851–3864. [DOI] [PubMed] [Google Scholar]
- Bellen, H. J., R. W. Levis, G. Liao, Y. He, J. W. Carlson et al., 2004. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics 167 761–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg, C. A., 2005. The Drosophila shell game: patterning genes and morphological change. Trends Genet. 21 346–355. [DOI] [PubMed] [Google Scholar]
- Bieschke, E. T., J. C. Wheeler and J. Tower, 1998. Doxycycline-induced transgene expression during Drosophila development and aging. Mol. Gen. Genet. 258 571–579. [DOI] [PubMed] [Google Scholar]
- Cabernard, C., M. Neumann and M. Affolter, 2004. Cellular and molecular mechanisms involved in branching morphogenesis of the Drosophila tracheal system. J. Appl. Physiol. 97 2347–2353. [DOI] [PubMed] [Google Scholar]
- Chen, C. M., W. Strapps, A. Tomlinson and G. Struhl, 2004. Evidence that the cysteine-rich domain of Drosophila Frizzled family receptors is dispensable for transducing Wingless. Proc. Natl. Acad. Sci. USA 101 15961–15966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., and T. Schüpbach, 2006. The role of brinker in eggshell patterning. Mech. Dev. 123 395–406. [DOI] [PubMed] [Google Scholar]
- Crozatier, M., B. Glise and A. Vincent, 2004. Patterns in evolution: veins of the Drosophila wing. Trends Genet. 20 498–505. [DOI] [PubMed] [Google Scholar]
- Dai, P., H. Akimaru and S. Ishii, 2003. A hedgehog-responsive region in the Drosophila wing disc is defined by debra-mediated ubiquitination and lysosomal degradation of Ci. Dev. Cell 4 917–928. [DOI] [PubMed] [Google Scholar]
- Dammai, V., B. Adryan, K. R. Lavenburg and T. Hsu, 2003. Drosophila awd, the homolog of human nm23, regulates FGF receptor levels and functions synergistically with shi/dynamin during tracheal development. Genes Dev. 17 2812–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta, P. K., and H. L. Moses, 2000. STRAP and Smad7 synergize in the inhibition of transforming growth factor beta signaling. Mol. Cell. Biol. 20 3157–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta, P. K., A. Chytil, A. E. Gorska and H. L. Moses, 1998. Identification of STRAP, a novel WD domain protein in transforming growth factor-beta signaling. J. Biol. Chem. 273 34671–34674. [DOI] [PubMed] [Google Scholar]
- De Celis, J. F., 2003. Pattern formation in the Drosophila wing: the development of the veins. BioEssays 25 443–451. [DOI] [PubMed] [Google Scholar]
- Deng, W. M., and M. Bownes, 1997. Two signalling pathways specify localised expression of the Broad-Complex in Drosophila eggshell patterning and morphogenesis. Development 124 4639–4647. [DOI] [PubMed] [Google Scholar]
- Dorman, J. B., K. E. James, S. E. Fraser, D. P. Kiehart and C. A. Berg, 2004. bullwinkle is required for epithelial morphogenesis during Drosophila oogenesis. Dev. Biol. 267 320–341. [DOI] [PubMed] [Google Scholar]
- Fossett, N., S. G. Tevosian, K. Gajewski, Q. Zhang, S. H. Orkin et al., 2001. The Friend of GATA proteins U-shaped, FOG-1, and FOG-2 function as negative regulators of blood, heart, and eye development in Drosophila. Proc. Natl. Acad. Sci. USA 98 7342–7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghabrial, A. S., and M. A. Krasnow, 2006. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature 441 746–749. [DOI] [PubMed] [Google Scholar]
- Glesne, D. A., W. Zhang, S. Mandava, L. Ursos, M. E. Buell et al., 2006. Subtractive transcriptomics: establishing polarity drives in vitro human endothelial morphogenesis. Cancer Res. 66 4030–4040. [DOI] [PubMed] [Google Scholar]
- Gu, Y., and D. L. Nelson, 2003. FMR2 function: insight from a mouse knockout model. Cytogenet. Genome Res. 100 129–139. [DOI] [PubMed] [Google Scholar]
- Han, H. J., W. J. Sigurdson, P. A. Nickerson and M. Taub, 2004. Both mitogen activated protein kinase and the mammalian target of rapamycin modulate the development of functional renal proximal tubules in matrigel. J. Cell Sci. 117 1821–1833. [DOI] [PubMed] [Google Scholar]
- Jung, A. C., B. Denholm, H. Skaer and M. Affolter, 2005. Renal tubule development in Drosophila: a closer look at the cellular level. J. Am. Soc. Nephrol. 16 322–328. [DOI] [PubMed] [Google Scholar]
- Kim, J. W., I. Tchernyshyov, G. L. Semenza and C. V. Dang, 2006. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3 177–185. [DOI] [PubMed] [Google Scholar]
- Kleve, C. D., D. A. Siler, S. K. Syed and E. D. Eldon, 2006. Expression of 18-wheeler in the follicle cell epithelium affects cell migration and egg morphology in Drosophila. Dev. Dyn. 235 1953–1961. [DOI] [PubMed] [Google Scholar]
- Landis, G., D. Bhole, L. Lu and J. Tower, 2001. High-frequency generation of conditional mutations affecting Drosophila melanogaster development and life span. Genetics 158 1167–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis, G. N., D. Bhole and J. Tower, 2003. A search for doxycycline-dependent mutations that increase Drosophila melanogaster life span identifies the VhaSFD, Sugar baby, filamin, fwd and Cctl genes. Genome Biol. 4 R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenburg, K. R., J. Ivey, T. Hsu and R. C. Muise-Helmericks, 2003. Coordinated functions of Akt/PKB and ETS1 in tubule formation. FASEB J. 17 2278–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit, T., and F. Pilot, 2003. Developmental control of cell morphogenesis: a focus on membrane growth. Nat. Cell Biol. 5 103–108. [DOI] [PubMed] [Google Scholar]
- Lehtinen, M. K., Z. Yuan, P. R. Boag, Y. Yang, J. Villen et al., 2006. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 125 987–1001. [DOI] [PubMed] [Google Scholar]
- Levi, B. P., A. S. Ghabrial and M. A. Krasnow, 2006. Drosophila talin and integrin genes are required for maintenance of tracheal terminal branches and luminal organization. Development 133 2383–2393. [DOI] [PubMed] [Google Scholar]
- Lynch, J., L. Guo, P. Gelebart, K. Chilibeck, J. Xu et al., 2005. Calreticulin signals upstream of calcineurin and MEF2C in a critical Ca(2+)-dependent signaling cascade. J. Cell Biol. 170 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis, J., and A. C. Spradling, 1995. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development 121 3797–3807. [DOI] [PubMed] [Google Scholar]
- Meyer, T. N., C. Schwesinger, K. T. Bush, R. O. Stuart, D. W. Rose et al., 2004. Spatiotemporal regulation of morphogenetic molecules during in vitro branching of the isolated ureteric bud: toward a model of branching through budding in the developing kidney. Dev. Biol. 275 44–67. [DOI] [PubMed] [Google Scholar]
- Montell, D. J., 2006. The social lives of migrating cells in Drosophila. Curr. Opin. Genet. Dev. 16 374–383. [DOI] [PubMed] [Google Scholar]
- Nambu, J. R., W. Chen, S. Hu and S. T. Crews, 1996. The Drosophila melanogaster similar bHLH-PAS gene encodes a protein related to human hypoxia-inducible factor 1 alpha and Drosophila single-minded. Gene 172 249–254. [DOI] [PubMed] [Google Scholar]
- Neumann, M., and M. Affolter, 2006. Remodeling epithelial tubes through cell rearrangements: from cells to molecules. EMBO Rep. 7 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell, P., and M. Rosbash, 1984. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 12 5495–5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios, F., J. K. Schweitzer, R. L. Boshans and C. D'Souza-Schorey, 2002. ARF6-GTP recruits Nm23–H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat. Cell Biol. 4 929–936. [DOI] [PubMed] [Google Scholar]
- Papadia, S., G. Tzolovsky, D. Zhao, K. Leaper, D. Clyde et al., 2005. emc has a role in dorsal appendage fate formation in Drosophila oogenesis. Mech. Dev. 122 961–974. [DOI] [PubMed] [Google Scholar]
- Papandreou, I., R. A. Cairns, L. Fontana, A. L. Lim and N. C. Denko, 2006. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 3 187–197. [DOI] [PubMed] [Google Scholar]
- Peña-Rangel, M. T., I. Rodriguez and J. R. Riesgo-Escovar, 2002. A misexpression study examining dorsal thorax formation in Drosophila melanogaster. Genetics 160 1035–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit, V., C. Ribeiro, A. Ebner and M. Affolter, 2002. Regulation of cell migration during tracheal development in Drosophila melanogaster. Int. J. Dev. Biol. 46 125–132. [PubMed] [Google Scholar]
- Pilot, F., and T. Lecuit, 2005. Compartmentalized morphogenesis in epithelia: from cell to tissue shape. Dev. Dyn. 232 685–694. [DOI] [PubMed] [Google Scholar]
- Raisin, S., S. Pantalacci, J. P. Breittmayer and P. Leopold, 2003. A new genetic locus controlling growth and proliferation in Drosophila melanogaster. Genetics 164 1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiling, J. H., and E. Hafen, 2004. The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Genes Dev. 18 2879–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya, T., and H. Clevers, 2005. Wnt signalling in stem cells and cancer. Nature 434 843–850. [DOI] [PubMed] [Google Scholar]
- Robertson, H. M., C. R. Preston, R. W. Phillis, D. M. Johnson-Schlitz, W. K. Benz et al., 1988. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics 118 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorth, P., 1998. Gal4 in the Drosophila female germline. Mech. Dev. 78 113–118. [DOI] [PubMed] [Google Scholar]
- Rorth, P., K. Szabo, A. Bailey, T. Laverty, J. Rehm et al., 1998. Systematic gain-of-function genetics in Drosophila. Development 125 1049–1057. [DOI] [PubMed] [Google Scholar]
- Rosario, M., and W. Birchmeier, 2003. How to make tubes: signaling by the Met receptor tyrosine kinase. Trends Cell Biol. 13 328–335. [DOI] [PubMed] [Google Scholar]
- Samakovlis, C., N. Hacohen, G. Manning, D. C. Sutherland, K. Guillemin et al., 1996. Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development 122 1395–1407. [DOI] [PubMed] [Google Scholar]
- Seong, H. A., H. Jung, H. S. Choi, K. T. Kim and H. Ha, 2005. Regulation of transforming growth factor-beta signaling and PDK1 kinase activity by physical interaction between PDK1 and serine-threonine kinase receptor-associated protein. J. Biol. Chem. 280 42897–42908. [DOI] [PubMed] [Google Scholar]
- Soriano, J. V., N. Liu, Y. Gao, Z. J. Yao, T. Ishibashi et al., 2004. Inhibition of angiogenesis by growth factor receptor bound protein 2-Src homology 2 domain bound antagonists. Mol. Cancer Ther. 3 1289–1299. [PubMed] [Google Scholar]
- Stapleton, M., J. Carlson, P. Brokstein, C. Yu, M. Champe et al., 2002. A Drosophila full-length cDNA resource. Genome Biol. 3 RESEARCH0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, M. A., R. G. Wisotzkey and S. J. Newfeld, 2001. A screen for modifiers of decapentaplegic mutant phenotypes identifies lilliputian, the only member of the Fragile-X/Burkitt's lymphoma family of transcription factors in Drosophila melanogaster. Genetics 157 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, A. H., T. P. Neufeld, G. M. Rubin and H. A. Muller, 2001. Transcriptional regulation of cytoskeletal functions and segmentation by a novel maternal pair-rule gene, lilliputian. Development 128 801–813. [DOI] [PubMed] [Google Scholar]
- Tseng, A. S., and I. K. Hariharan, 2002. An overexpression screen in Drosophila for genes that restrict growth or cell-cycle progression in the developing eye. Genetics 162 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzolovsky, G., W. M. Deng, T. Schlitt and M. Bownes, 1999. The function of the broad-complex during Drosophila melanogaster oogenesis. Genetics 153 1371–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering, M., E. Sancho, C. Verweij, W. de Lau, I. Oving et al., 2002. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111 241–250. [DOI] [PubMed] [Google Scholar]
- Vogt, P. K., H. Jiang and M. Aoki, 2005. Triple layer control: phosphorylation, acetylation and ubiquitination of FOXO proteins. Cell Cycle 4 908–913. [DOI] [PubMed] [Google Scholar]
- Ward, E. J., X. Zhou, L. M. Riddiford, C. A. Berg and H. Ruohola-Baker, 2006. Border of Notch activity establishes a boundary between the two dorsal appendage tube cell types. Dev. Biol. 297 461–470. [DOI] [PubMed] [Google Scholar]
- Wittwer, F., A. van der Straten, K. Keleman, B. J. Dickson and E. Hafen, 2001. Lilliputian: an AF4/FMR2-related protein that controls cell identity and cell growth. Development 128 791–800. [DOI] [PubMed] [Google Scholar]
- Wolff, S., H. Ma, D. Burch, G. A. Maciel, T. Hunter et al., 2006. SMK-1, an essential regulator of DAF-16-mediated longevity. Cell 124 1039–1053. [DOI] [PubMed] [Google Scholar]
- Yamada, T., M. Okabe and Y. Hiromi, 2003. EDL/MAE regulates EGF-mediated induction by antagonizing Ets transcription factor Pointed. Development 130 4085–4096. [DOI] [PubMed] [Google Scholar]