Abstract
There is a great interest in the behavior of diatomic molecular solids under extremely high-pressure conditions that lead to pressure-induced metallization, molecular dissociation, and formation of atomic phase. The consensus has been that the phase-transition sequence that happened in both solid bromine and iodine is from a molecular phase (phase I), to an incommensurate phase (phase V), and then to an atomic phase (phase II), with increasing pressure. However, a puzzle remains unresolved for both solids: pressure-induced X and Y bands were observed in the Raman spectra in the molecular phase at low pressures, even before the onset of phase V. Here, we suggest a phase for solid iodine in such a low-pressure range (designated as phase I′) in which two different covalent intramolecular bonds coexist, based on first-principles calculations and later corroborated by x-ray diffraction experiments. The pressure dependence of the X and Y bands and other vibrational frequencies measured experimentally can be explained nicely by combining the vibrational modes of phase I and phase I′. These results help improve our understanding on the pressure-induced molecular dissociation and metallization in diatomic solids and may shed some light on the investigation of similar phenomena in solid H2.
Keywords: high pressure, molecular dissociation
Molecular hydrogen was predicted to undergo a transition from a proton-paired insulator to a monatomic metal under sufficiently strong compression by Wigner and Huntington in 1935 (1). Although many experiments have tried to achieve this (2–5), a direct and convincing experimental observation of metallic hydrogen in the solid form has yet to be seen. However, there has been remarkable progress in the study of other diatomic molecular solids at high density, especially for bromine and iodine. This includes the observation of pressure-induced metallic transition, molecular dissociation, and atomic phase (6–14). The consensus has been that the phase-transition sequence that happened in both bromine and iodine is from a molecular phase (phase I), to an incommensurate phase (phase V), and then to an atomic phase (phase II), with increasing pressure (6–8).
However, a puzzle remains unresolved. Two bands, X band and Y band, have been observed experimentally (7, 12) in the Raman spectra for both iodine and bromine at a much lower pressure before the onset of phase V, implying that some structural change may have occurred. These Raman peaks could not be explained by phase I alone. The pressure dependence of X band behaves like Ag(L) mode, and that of Y band like B3g(L) mode of phase I, but they could not be assigned to the vibrational modes of either phase I or phase V. Recent x-ray absorption spectroscopy experiments (15) indicate a possibility that a phase may exist between phase I and phase V in solid bromine, but there is no detailed structural information.
Here, we present results of our study that a phase of solid iodine may exist between phase I and V based on first-principles calculations and later corroborated by x-ray diffraction (XRD) experiments. This phase has two different covalent intramolecular bonds in molecular solid iodine (hereafter designated as phase I′), and it exists before the onset of phase V. This finding provides us with a key step toward the understanding of how the molecular phase changes to the incommensurate phase and delineates a picture for the process of pressure-induced molecular dissociation, which could have a significant impact on the investigation of similar phenomena in solid H2.
Results and Discussion
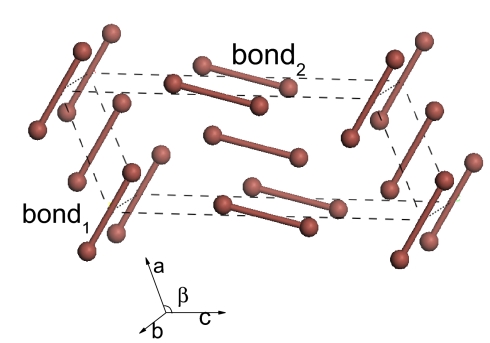
The new phase (I′) exists between ≈12.5 GPa, the pressure at which the X and Y bands start to emerge in the Raman spectra, and ≈23.5 GPa, the pressure at which the molecular phase starts to transform to phase V. This phase I′ has a crystal structure as shown in Fig. 1. It is a C-centered monoclinic Bravais lattice (space group of C2/M) with eight atoms in a unit cell. The calculated lattice parameters at 23 GPa are: a = 3.951 Å, b = 5.768 Å, and c = 9.787 Å with β = 113.719°. The two nonequivalent iodine atoms in the unit cell are at (0.3331, 0.0000, 0.1244) and (0.5849, 0.5, 0.3760). The structure of phase I′ can be understood as a distorted phase I, where C2/M is a subgroup of Cmca.
Fig. 1.
The unit cell of phase I′ at 23 GPa (bond1 = 2.775 Å and bond2 = 2.767 Å).
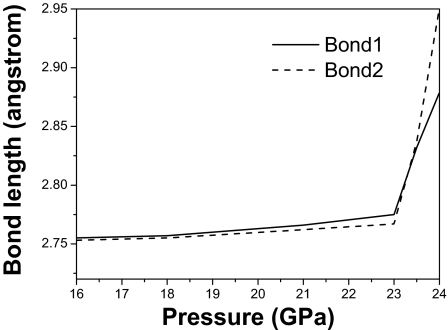
Two covalent intramolecular bonds exist in phase I′. Fig. 2 shows the pressure dependence of the two bond lengths (bond 1 and bond 2). The calculated bond lengths of bond 1 and bond 2 are slightly different at pressures <16 GPa. They both become longer and the difference between their lengths becomes larger as the pressure increases. The bond lengths increase abruptly at ≈23.5 GPa, indicating the first-order phase transition from molecular phase to phase V. Different bonds coexist in phase I′ that do not occur in phase I or phase II. This phase is an important intermediate step for the pressure-induced molecular dissociation and the cause for the emergence of X and Y bands in the Raman measurements.
Fig. 2.
Pressure dependence of two calculated bond lengths in phase I′ with pressure.
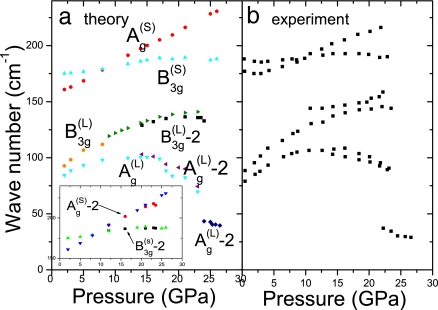
Pressure dependence of our calculated vibrational frequencies of Raman-active modes is shown in Fig. 3. Our results show that both the X and Y bands can be assigned to the vibrational modes of phase I′. From the phonon eigenvectors, we find that the Ag(L)-2 (or X band) mode and the B3g(L)-2 (or Y band) mode of phase I′ are essentially the same as the soft mode [Ag(L) mode] and B3g(L) mode of phase I, respectively. The calculated pressure dependence of vibrational frequencies agrees well with experiments if the effect of this phase is taken into account. The difference of both high-frequency Ag(S) and B3g(S) modes between phase I and phase I′ is not significant here, as shown in Fig. 3a Inset, but it was observed in the experiments (7). The analogies between the modes of both phases can be easily understood because the interatomic distances in the two systems are very similar. At ≈23.5 GPa, solid iodine starts to transform to phase V. The calculated frequency of Ag(L)-2 changes abruptly to ≈40 cm−1 at pressures >23.5 GPa, showing softening behavior. It can be concluded that phase I and phase I′ coexist in the pressure range from ≈12.5 to 23.5 GPa before the onset of phase V.
Fig. 3.
Pressure dependence of vibrational frequencies of Raman-active modes. (a) Our calculated results of phase I and phase I′. (b) The experimental data from ref. 7.
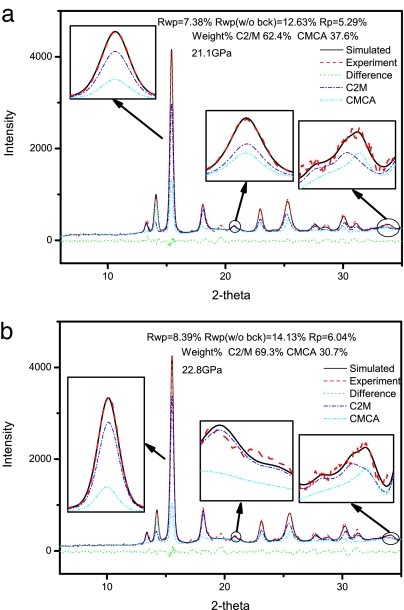
The XRD experiments of iodine under several pressure points were performed. The XRD patterns at 21.1 and 22.8 GPa are shown in Fig. 4 a and b, respectively. The XRD spectra were refined by the quantitative phase analysis Rietveld method. The refined analysis shows that solid iodine at these pressure points is a mixture of phase I with a Cmca space group and phase I′ with a C2/M space group, corroborating our conjecture. Phase I has a single covalent bond whereas phase I′ has two different covalent bonds. Our XRD experimental results also show that the content of phase I′ increases with increasing pressure, suggesting that phase I′ is indeed an intermediate phase coexisting with phase I before the onset of phase V.
Fig. 4.
The XRD patterns of solid iodine at 21.1 GPa (a) and 22.8 GPa (b).
Conclusions
We have used both first-principles calculations and high-pressure x-ray diffraction experiments to show that a new phase (I′) of solid iodine exists in the pressure region ≈12.5–23.5 GPa. This new phase has two different covalent intramolecular bonds, and it coexists with phase I until the onset of phase V at 23.5 GPa. The existence of such new phase nicely explains the emergence of the X and Y bands in the Raman spectra and other vibrational frequencies observed experimentally.
Methods
We used the pseudopotential plane wave method together with Norm-conserving pseudopotentials (16–18) to perform the calculation and the generalized gradient approximation (GGA) (19) to describe the exchange-correlation effect among electrons. The GGA results are in better agreement with the experimental results than those of local density approximation (LDA) for this system, especially under high pressures (20). Phonons at Γ point were calculated by using the finite-displacement method (21). The XRD data were obtained by using a Bruker's SMART-APEX with 4K CCD and 2KW sealed-tube molybdenum target x-ray generator, and a diamond anvil cell (DAC) was used to obtain high pressures.
Acknowledgments.
This work was supported by National Basic Research Program of China Grants 2005CB724400 and 2001CB711201, National Natural Science Foundation of China Grants 10574053 and 10674053, the 2004 National Center for Educational Technology and 2003 Excellent Young Teachers' Program of the Minster of Education of China, and The Cultivation Fund of the Key Scientific and Technical Innovation Project 2004-295.
Footnotes
The authors declare no conflict of interest.
References
- 1.Wigner E, Huntington HB. On the possibility of a metallic modification of hydrogen. J Chem Phys. 1935;3:764–770. [Google Scholar]
- 2.Narayana C, Luo H, Orloff J, Ruoff AL. Solid hydrogen at 342 GPa: No evidence for an alkali metal. Nature. 1998;393:46–49. [Google Scholar]
- 3.Mao HK, Hemley RJ. Optical studies of hydrogen >200-gigapascals—Evidence for metallization by band overlap. Science. 1989;244:1462–1465. [Google Scholar]
- 4.Goncharov AF, Gregoryanz E, Hemley RJ, Mao HK. Spectroscopic studies of the vibrational and electronic properties of solid hydrogen to 285 GPa. Proc Natl Acad Sci USA. 2001;98:14234–14237. doi: 10.1073/pnas.201528198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loubeyre P, Occelli F, LeToullec R. Optical studies of solid hydrogen to 320 GPa and evidence for black hydrogen. Nature. 2002;416:613–617. doi: 10.1038/416613a. [DOI] [PubMed] [Google Scholar]
- 6.Duan DF, et al. Ab initio studies of solid bromine under high pressure. Phys Rev B. 2007;76:104113. [Google Scholar]
- 7.Kume T, Hiraoka T, Ohya Y, Sasaki S, Shimizu H. High-pressure raman study of bromine and iodine: Soft phonon in the incommensurate phase. Phys Rev Lett. 2005;94:065506. doi: 10.1103/PhysRevLett.94.065506. [DOI] [PubMed] [Google Scholar]
- 8.Takemura K, Sato K, Fujihisa H, Onoda M. Modulated structure of solid iodine during its molecular dissociation under high pressure. Nature. 2003;423:971–974. doi: 10.1038/nature01724. [DOI] [PubMed] [Google Scholar]
- 9.Congeduti A, Postorino P, Nardone M, Buontempo U. Raman spectra of a high-pressure iodine single crystal. Phys Rev B. 2001;65:014302. [Google Scholar]
- 10.Yamaguchi K, Miyagi H. Structural properties of molecular solid iodine under pressure: First-principles study of Raman-active Ag modes and hyperfine parameters. Phys Rev B. 1998;57:11141–11148. [Google Scholar]
- 11.Fujihisa H, Fujii Y, Takemura K, Shimomura O. Structural aspects of dense solid halogens under high pressure studied by x-ray diffraction—Molecular dissociation and metallization. J Phys Chem Solids. 1995;56:1439–1444. [Google Scholar]
- 12.Olijnyk H, Li W, Wokaun A. High-pressure studies of solid iodine by Raman spectroscopy. Phys Rev B. 1994;50:712–716. doi: 10.1103/physrevb.50.712. [DOI] [PubMed] [Google Scholar]
- 13.Fujii Y, et al. Evidence for molecular dissociation in bromine near 80 GPa. Phys Rev Lett. 1989;63:536–539. doi: 10.1103/PhysRevLett.63.536. [DOI] [PubMed] [Google Scholar]
- 14.Takemura K, Minomura S, Shimomura O, Fujii Y, Axe JD. Structural aspects of solid iodine associated with metallization and molecular dissociation under high pressure. Phys Rev B. 1982;26:998–1004. [Google Scholar]
- 15.San-Miguel A, et al. New phase transition of solid bromine under high pressure. Phys Rev Lett. 2007;99:015501. doi: 10.1103/PhysRevLett.99.015501. [DOI] [PubMed] [Google Scholar]
- 16.Kohn W, Sham LJ. Self-consistent equations including exchange and correlation effects. Phys Rev. 1965;140:A1133–A1138. [Google Scholar]
- 17.Segall MD, et al. First-principles simulation: Ideas, illustrations and the CASTEP code. J Phys Condens Matter. 2002;14:2717–2744. [Google Scholar]
- 18.Ackland GJ, Warren MC, Clark SJ. Practical methods in ab initio lattice dynamics. J Phys Condens Matter. 1997;9:7861–7872. [Google Scholar]
- 19.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 20.Miao MS, Van Doren VE, Martins JL. Density-functional studies of high-pressure properties and molecular dissociations of halogen molecular crystals. Phys Rev B. 2003;68:094106. [Google Scholar]
- 21.Hsueh HC, et al. Vibrational properties of the layered semiconductor germanium sulfide under hydrostatic pressure: Theory and experiment. Phys Rev B. 1996;53:14806–14817. doi: 10.1103/physrevb.53.14806. [DOI] [PubMed] [Google Scholar]