Abstract
The role of the cGMP pathway in the modulation of the cardiac L-type Ca2+ current (ICa,L) by nitric oxide (NO) was examined in rat ventricular myocytes.
The NO donors DEANO, SIN-1, SNP, SNAP and GSNO had no significant effects on basal ICa,L. However, DEANO (100 μM) inhibited ICa,L after the current had been previously stimulated by either isoprenaline (Iso, 1–10 nM), a β-adrenergic agonist, or isobutylmethyl-xanthine (IBMX, 10–80 μM), a wide spectrum phosphodiesterase (PDE) inhibitor.
The anti-adrenergic effect of DEANO on ICa,L was not mimicked by other NO donors (SIN-1, SNAP and SPNO).
The NO-sensitive guanylyl cyclase inhibitor ODQ (10 μM), antagonized the inhibitory effect of DEANO on ICa,L. Likewise, inhibitors of the cGMP-dependent protein kinase (cG-PK), Rp-8-chloro-phenylthio-cGMP (10 μM) and KT5823 (0.1 and 0.3 μM), also abolished the inhibitory effect of DEANO on Iso (1–;10 nM)-stimulated ICa,L.
Intracellular dialysis with exogenous cAMP (10–100 μM) blunted the inhibitory effect of DEANO (10 and 100 μM) on ICa,L. SNAP and SNP also had no effect on the cAMP-stimulated ICa,L.
Pre-treatment of the myocytes with pertussis toxin (0.5 μg ml−1, 4–6 h at 37 °C) eliminated the inhibitory effect of DEANO (100 μM) on ICa,L, in the presence of either Iso (0.01 and 1 nM) or IBMX (10–80 μM).
These results demonstrate that DEANO produces anti-adrenergic effects in rat ventricular myocytes. This effect of DEANO occurs in a cGMP-dependent manner, and involves activation of cG-PK and regulation of a pertussis toxin-sensitive G protein.
The synthesis of nitric oxide (NO) plays an important role in the endothelium-dependent relaxation of various blood vessels. In the heart, the role of NO synthesis is rather controversial (reviewed in Méry et al. 1997; Feron et al. 1999; Kojda & Kottenberg, 1999). NO was soon recognized as an activator of cGMP synthesis in the whole heart, as well as in purified cardiac myocytes. In turn, cGMP co-ordinates the activity of various proteins, such as the cGMP-stimulated phosphodiesterase (PDE2), the cGMP-inhibited-PDE (PDE3) and the cGMP-dependent protein kinase (cG-PK). Accordingly, several NO donors can modulate myocyte shortening, ionic currents and contractile proteins, in a cGMP-dependent manner. More recently, nitrosothiols (SNAP, SNAC, GSNO) or the sydnonymine SIN-1 were found to elicit cGMP-independent effects in cardiac myocytes, including the modulation of calcium channels (Hu et al. 1997; Xu et al. 1998), the inhibition of the creatine kinase (Gross et al. 1996), and the modulation of mitochondrial respiration (Wolin et al. 1997).
While these results tend to support a physiological role of NO in heart muscle, a number of questions remain unanswered. First, in several studies performed on isolated cardiac myocytes, NO donors had no effect on cell shortening (Stein et al. 1993; McDonell et al. 1995, 1997), L-type Ca2+ current (ICa,L) (Thomas et al. 1997), or the cAMP-activated chloride current (Zakharov et al. 1996). Thus, subtle differences between cardiac preparations can blunt the effects of NO at the single cell level. Second, the exact mechanism by which NO or cGMP produces its effect in a given cardiac preparation is not clear. For instance, the inhibitory effect of NO or cGMP on ICa,L in mammalian cardiac myocytes may take place either via activation of PDE2 (Feron et al. 1999) or activation of cG-PK (Méry et al. 1991; Sumii & Sperelakis, 1995; Whaler & Dollinger, 1995). Third, while the effect of cG-PK on ICa,L appears to take place at the level of the L-type Ca2+ channel (or a closely associated protein Méry et al. 1991; Sumii & Sperelakis, 1995), the possibility exists that cG-PK might also act upstream from Ca2+ channel phosphorylation. Indeed, in smooth muscle cells, cG-PK was shown to directly phosphorylate αi subunits of GTP-binding proteins (G proteins) and/or receptors (Pfeiffer et al. 1995; G.-R. Wang et al. 1998). In addition, cG-PK can increase the spontaneous binding of GTP on α subunits and reduce the stimulatory effects of receptor agonists on their GTPase activity (Pfeiffer et al. 1995; Miyamoto et al. 1997; G.-R. Wang et al. 1998).
In the present study, we examined the effects of different NO donors on basal and stimulated ICa,L in ventricular myocytes isolated from rat hearts. In this preparation, we had found previously that cG-PK mediates the inhibitory effect of exogenous cGMP on ICa,L (Méry et al. 1991). While none of the NO donors tested produced any effect on basal ICa,L, the NONOate DEANO produced a profound inhibition of the current stimulated by isoprenaline. We show that cG-PK mediates this effect through the activation of a pertussis toxin-sensitive G protein.
Part of this work has been presented in an abstract form (Abi-Gerges et al. 1997c).
METHODS
The investigation conforms with our institution guidelines which are defined by the European Community guiding principles in the care and use of animals (86/609/CEE, CE Off J no. L358, 18 December, 1986) and the French decree no. 87/848 (J Off République Française, 20 October, 1987, pp. 12245-12248). Authorizations to perform animal experiments according to this decree were obtained from the French Ministère de l’Agriculture et de la Forêt (no. 04226, 12 April, 1991).
Cell isolation and storage
Male Wistar rats (160-250 g) were anaesthetized by intraperitoneal injection of urethane (2 g kg−1) and heparin (2.5 mg kg−1). After all reflex activity had ceased, the animal was killed by opening the chest and rapidly removing the heart. Myocytes were dispersed using collagenase A (0.255 mg ml−1; Boehringer-Mannheim Biochemica, Mannheim, Germany) as previously described (Scamp et al. 1990; Abi-Gerges et al. 1997a).
Electrophysiology
The whole-cell configuration of the patch-clamp technique was used to record the L-type Ca2+ current (ICa,L) on Ca2+-tolerant cells, as described (Scamps et al. 1990; Abi-Gerges et al. 1997a, 1999). The routine protocol consisted of a depolarizing pulse to 0 mV test potential (400 ms duration) elicited every 8 s from a holding potential of −50 mV. The test potential was set at 0 mV because, at this potential, the ICa,L amplitude (Scamps et al. 1990; Méry et al. 1991; Abi-Gerges et al. 1999; see Fig. 2) and steady-state activation are at their maximal values in rat myocytes (Scamps et al. 1990). Current-voltage relationships and inactivation curves were performed as described (Abi-Gerges et al. 1999). The experiments were carried out at room temperature (22-32°C, mean value 25.6 ± 0.1°C, n = 346), and the temperature did not change by >2°C in any given experiment.
Figure 2. DEANO inhibits the β-adrenergic stimulation of ICa,L in rat ventricular myocytes.
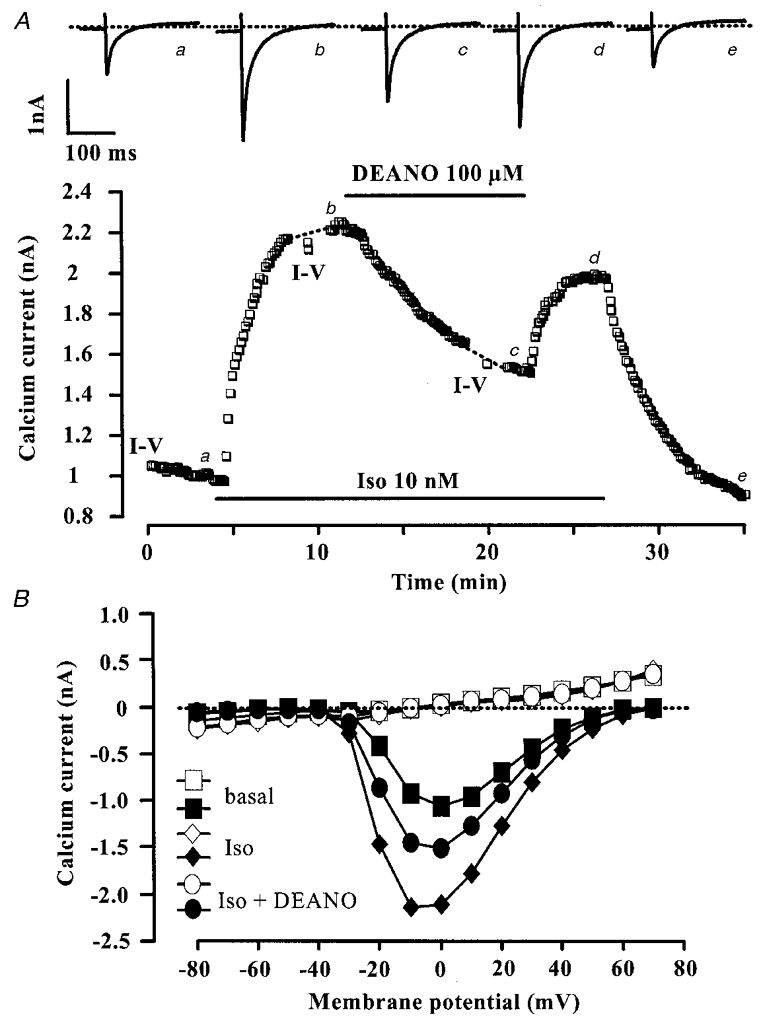
A, a myocyte was first exposed to control intracellular and extracellular solutions. Applications of isoprenaline (Iso, 10 nM) and DEANO (100 μM) are indicated by the horizontal lines. Current traces on top were recorded at the times indicated by the corresponding letters on the main graph. The dotted line indicates the zero-current level. B (same experiment as in A), current-voltage relationships of ICa,L (filled symbols) and of the steady-state current at the end of the pulse (open symbols) obtained under basal conditions, and in the presence of Iso (10 nM) with or without DEANO (100 μM).
During patch-clamp experiments, the maximal amplitude of ICa,L and the current at the end of the 400 ms test potential were measured as described (Abi-Gerges et al. 1997a). Currents were not compensated for capacitive and leak currents. The density of basal ICa,L was 5.25 ± 0.14 pA pF−1 and the density of the current at the end of the 400 ms pulse was 0.37 ± 0.02 pA pF−1 (n = 266). The steady-state value of the end-pulse current was stable over the time course of the experiments (see individual current traces in figures). The effects of the agonists used in this study were not correlated with the amplitude of the end-pulse current (data not shown). The decay of the capacitive transient was fast (< 3 ms), and did not interfere significantly with the activation of the calcium current (mean time to peak 6.2 ± 0.1 ms, n = 266). On-line analysis of the recordings was made possible by programming a PC-compatible 486/50 microcomputer in Assembly language (Borland) to determine, for each membrane depolarization, peak and steady-state current values.
Solutions for patch-clamp recordings
The extracellular solution contained (mM): 107 NaCl, 10 Hepes, 20 CsCl, 4 NaHCO3, 0.8 NaH2PO4, 1.8 MgCl2, 1.8 CaCl2, 5 D-glucose, 5 sodium pyruvate, and 6 × 10−4 tetrodotoxin, pH 7.4 adjusted with CsOH (294 mosmol kg−1). Solutions were superfused onto floating myocytes as described (Abi-Gerges et al. 1997a). The patch pipettes (0.5-1.0 MΩ) were filled with an intracellular solution containing (mM): 119.8 CsCl, 5 EGTA (acid form), 4 MgCl2, 5 sodium phosphocreatine, 3.1 Na2ATP, 0.42 Na2GTP, 0.062 CaCl2 (pCa 8.5) and 10 Hepes, pH 7.3 adjusted with CsOH (292 mosmol kg−1).
Drugs
3-Morpholino-sydnonimine (SIN-1) was a generous gift from Dr J. Winicki (Hoechst-Houdé Laboratories, Paris-La Défense). Spermine-NONOate (SPNO) and S-nitroso-L-glutathione (GSNO) were from Alexis Corp. (La Jolla, CA, USA); S-nitroso-N-acetyl-d,L-penicillamine (SNAP), 2-(N,N-diethylamino)-diazenolate-2-oxide (DEANO), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxaline-1-one (ODQ) from Tocris-Cookson (Bristol, UK) or Alexis Corp.; 8-(4-chlorophenylthio)guanosine-3′-5′-cyclic monophosphorothioate, Rp-isomer (Rp8cG) were from Biolog L.S.I. (Bremen, Germany); 8-(4-chlorophenylthio)-guanosine-3′,5′-cyclic monophosphate (8-p-CPT-cGMP) and KT5823 were from Calbiochem-France Biochem (Meudon, France); pertussis toxin (from Bordetella pertussis) was from Sigma-Aldrich (Saint Quentin Fallavier, France); tetrodotoxin was from Latoxan (Rosans, France). All other drugs were from Sigma-Aldrich. Drugs were prepared and used according to manufacturer’s instructions. Mock DEANO consisted of a DEANO (100 μM)-containing solution left at room temperature for >20 h. Solutions were prepared by dilution to the desired concentration in the physiological solution at the beginning of each experiment.
Statistical analysis
Results are expressed as means ±s.e.m. Differences between mean values were tested for statistical significance by Student’s paired t test, as indicated. In the text, the ‘basal’ condition for ICa,L refers to the absence of extracellular isoprenaline or IBMX, or intracellular cAMP.
RESULTS
Effect of NO donors on basal ICa,L
NO donors can modulate basal ICa,L in some, but not all cardiac myocytes (Méry et al. 1993; Kirstein et al. 1995; Campbell et al. 1996; Y. G. Wang et al. 1998). In the experiment of Fig. 1A, a rat myocyte was first exposed to control intracellular and extracellular solutions. Under this condition, the amplitude of the basal ICa,L declined slowly (∼ -5.4 pA min−1), a phenomenon known as ‘run-down’. Superfusion of the myocyte with DEANO (100 μM) barely modified the amplitude and the kinetics of the basal ICa,L. As summarized in Fig. 1B, the basal ICa,L amplitude was not significantly modified by different NO donors. These included the NONOate DEANO (100 μM), the nitrosothiols GSNO (1 mM) and SNAP (1 mM), the ferrocyanate SNP (500 μM), and the sydnonymine SIN-1 (1 mM). Lower concentrations of these compounds (down to 1 μM) also had no effect on basal ICa,L (data not shown).
Figure 1. NO donors do not regulate basal ICa,L in rat ventricular myocytes.
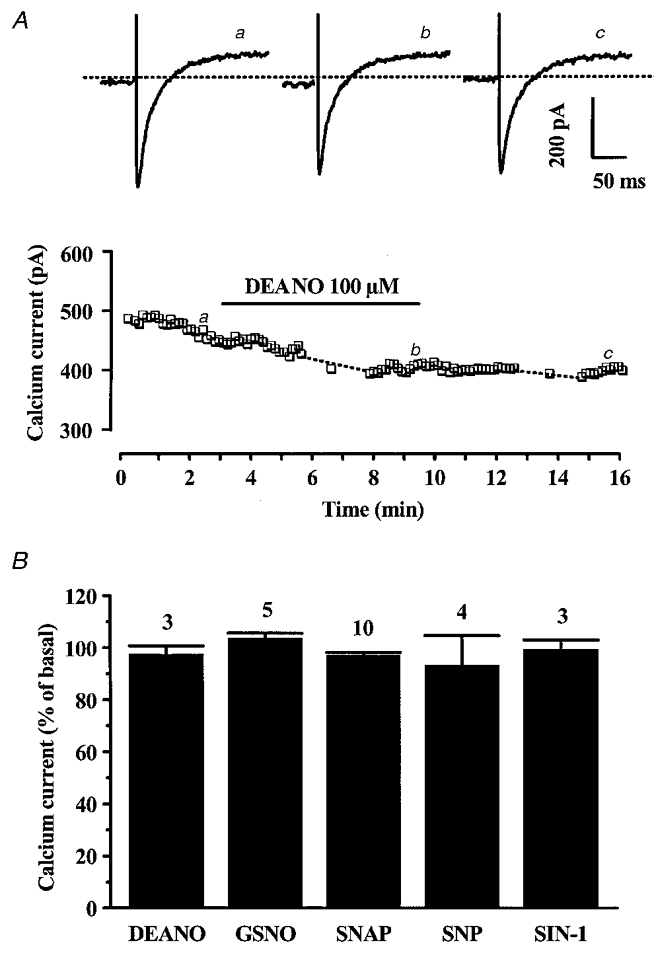
A, a myocyte was first exposed to control intracellular and extracellular control solutions. ICa,L (□) was elicited at 0 mV from a holding potential of −50 mV. Superfusion of the myocyte with 100 μM DEANO is indicated by the horizontal line. Current traces on top were recorded at the times indicated by the corresponding letters on the main graph. The dotted line indicates the zero-current level. B, summary of the effects of DEANO (100 μM), GSNO (1 mM), SNAP (1 mM), SNP (500 μM) and SIN-1 (1 mM) on basal ICa,L amplitude. The amplitude of ICa,L in the presence of NO donors was normalized to the amplitude of basal ICa,L in control conditions (set to 100 %). Bars are the means and lines are the s.e.m. of the number of experiments indicated near the bars.
Since nitrosylation and/or oxidation might account for effects of NO donors on the basal ICa,L in other preparations (Campbell et al. 1996; Hu et al. 1997) we further investigated the sensitivity of basal ICa,L in rat myocytes to the effects of reducing or oxidizing agents. The basal ICa,L was not changed when the myocyte was superfused with 1 mM reduced glutathione (5.9 ± 2.6 % over basal, n = 5), 1 mM dl-dithiothreitol (dl-DTT) (2.3 ± 1.6 % over basal, n = 6), 0.1 mM N-acetyl-penicillamine (-1.6 ± 0.9 % over basal, n = 4). In contrast, extracellular application of a superoxide anion generator LY83583 (10 μM) inhibited the basal ICa,L (-36.9 ± 2.5 % over basal, n = 17, P < 0.001). Therefore, the basal activity of L-type Ca2+ channels in rat myocytes was sensitive to an oxidative treatment but not to NO donors.
Inhibitory effect of DEANO on the β-adrenergic stimulation of ICa,L
NO donors can reduce the β-adrenergic stimulation of ICa,L in frog and guinea-pig ventricular myocytes (Méry et al. 1993; Levi et al. 1994; Whaler & Dollinger, 1995; reviewed in Méry et al. 1997). We first investigated the effects of DEANO in the presence of isoprenaline (Iso), a non-selective β-adrenergic agonist. In the experiment of Fig. 2A, a myocyte was exposed to control solutions and the ICa,L amplitude reached a steady state 11.8 min after the beginning of the recording of ICa,L. The myocyte was then superfused with 10 nM Iso, which induced a ∼2-fold increase in ICa,L. In the continuing presence of Iso, superfusion with 100 μM DEANO elicited a pronounced reduction in the response of ICa,L to Iso. The inhibitory effect of DEANO was reversible. Figure 2B shows the current-voltage relationships of ICa,L (filled symbols) and of the steady-state current measured at the end (400 ms) of the test pulse (open symbols) in the same experiment as in Fig. 2A. The effects of Iso and DEANO on ICa,L were homogeneous across the voltage range and neither drug produced a change in the steady-state current (similar findings were obtained in 10 other experiments). In addition, DEANO did not change the inactivation curve of ICa,L (data not shown). Thus, DEANO produced an anti-adrenergic effect on ICa,L which occurred in a voltage-independent manner.
The inhibitory effect of DEANO (100 μM) was studied at three different Iso concentrations (1, 3 and 10 nM). As summarized in Fig. 3, increasing the Iso concentration progressively reduced the inhibitory effect of DEANO on ICa,L suggesting that the inhibitory effect of DEANO was somewhat competitive with the stimulatory effect of Iso. The involvement of NO in the anti-adrenergic effect of DEANO was investigated using mock DEANO (see Methods). The Iso (1 nM) stimulation of ICa,L was identical in the absence and in the presence of 100 μM mock DEANO (176.8 ± 11.9 % and 188.9 ± 10.1 % of control, respectively, n = 4). In addition, the stimulatory effect of 1 nM Iso on ICa,L was not changed in the presence of 100 μM diethylamine (179.1 ± 16.0 % and 177.0 ± 20.0 % of control, respectively, n = 4), the other metabolite of DEANO. Thus, the inhibitory effect of DEANO on Iso-stimulated ICa,L was mediated by NO and not by one of its metabolites.
Figure 3. Summary of the effects of DEANO on the β-adrenergic stimulation of ICa,L in rat ventricular myocytes.
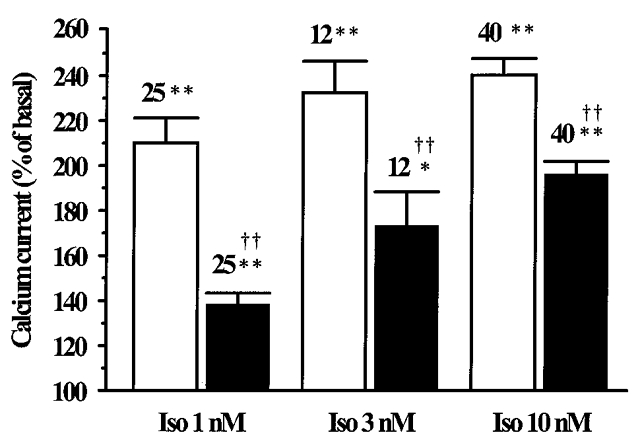
The amplitude of ICa,L in the presence of Iso (1, 3 or 10 nM), without (□) or with DEANO (100 μM, ▪), was normalized to the basal ICa,L amplitude (set to 100 %). Bars are the means and lines are the s.e.m. of the number of experiments indicated near the bars. Significant differences from basal (*) or Iso levels (†) are indicated as: *P < 0.001; **, ‡P < 0.0001.
Effect of other NO donors on the β-adrenergic stimulation of ICa,L
For a comparison with the effect of DEANO, we examined the effects of other NO donors on the Iso-stimulated ICa,L, namely SPNO (a NONOate like DEANO), SIN-1 (a sydnonymine) and SNAP (a nitrosothiol). Figure 4A shows a typical experiment in which SIN-1 (100 μM) had no effect on ICa,L stimulated by 10 nM Iso. As summarized in Fig. 4B, SIN-1 (100 μM) as well as the two other NO donors, SPNO (100 μM) and SNAP (100 μM), produced inconsistent and non-significant effects on ICa,L stimulated with either 1 or 10 nM Iso. In addition, the shape of the current-voltage relationship of the Iso-stimulated ICa,L was not modified in the presence of SIN-1, SNAP, or SPNO (n = 8, 5 and 2, respectively, data not shown). These negative results were not due to the wash-out of some cellular component in the whole-cell configuration of the patch-clamp technique since SNAP also failed to modify the Iso-stimulated ICa,L when tested in the perforated-patch configuration (data not shown, see also Thomas et al. 1997). They were not due either to a difference in temperature, since the mean temperatures of the experiments were similar with DEANO (25.3 ± 0.3°C, n = 25), SPNO (28.0 ± 0.5°C, n = 5), SIN-1 (28.5 ± 0.5°C, n = 3) and SNAP (28.3 ± 0.4°C, n = 4) when tested on top of 1 nM Iso. Thus, among a total of four NO donors tested, DEANO was the only one to consistently produce an inhibitory effect on Iso-stimulated ICa,L in rat ventricular myocytes (see Maragos et al. 1991; Feelisch et al. 1991).
Figure 4. Other NO donors do not inhibit the β-adrenergic stimulation of ICa,L in rat ventricular myocytes.
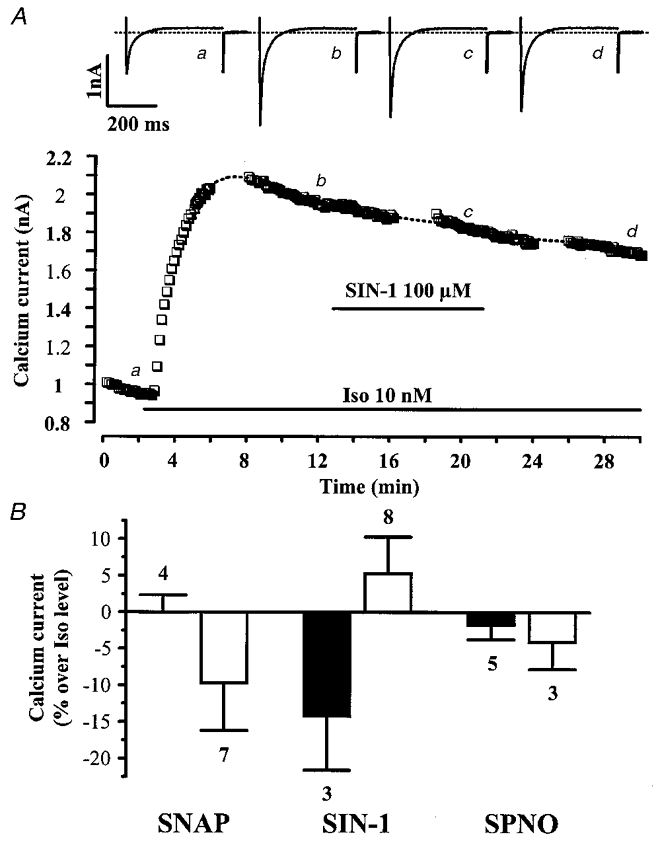
A, a myocyte was first exposed to control intracellular and extracellular solutions. Applications of Iso (10 nM) and SIN-1 (100 μM) are indicated by the horizontal lines. Current traces on top were recorded at the times indicated by the corresponding letters on the main graph. The dotted line indicates the zero-current level. B, summary of the effects of three NO donors (SNAP, SIN-1, SPNO, at 100 μM) on ICa,L in the presence of either 1 nM (▪) or 10 nM Iso (□). The effects of NO donors on ICa,L are presented as percentage variations from the amplitude of the Iso-stimulated ICa,L. Bars are the means and lines are the s.e.m. of the number of experiments indicated near the bars.
Contribution of guanylyl cyclase to the anti-adrenergic effect of DEANO on ICa,L
To evaluate the contribution of cGMP to the modulation of ICa,L by DEANO, we examined the effect of DEANO in the presence of ODQ, an inhibitor of the NO-sensitive guanylyl cyclase (Kojda et al. 1996; Abi-Gerges et al. 1997b). In the typical experiment of Fig. 5A, a myocyte was exposed to control solutions, and then superfused with 3 nM Iso. The stimulatory effect of the β-adrenergic agonist was reduced by about 50 % upon addition of DEANO (100 μM). In the continuing presence of Iso plus DEANO, superfusion with ODQ (10 μM) increased ICa,L, eliminating most of the inhibitory effect of DEANO. The effect of ODQ was slowly reversible upon wash-out of the drug. On average (Fig. 5B), ODQ (10 μM) fully antagonized the inhibitory effects of DEANO on ICa,L, in the presence of 1, 3 or 10 nM Iso. However, ODQ (10 μM) did not modify Iso (10 nM)-stimulated ICa,L in the absence of DEANO (-2.2 ± 2.1 % over Iso level, n = 4). Thus, these results indicate that the inhibitory effect of DEANO on Iso-stimulated ICa,L in rat ventricular myocytes is mediated by activation of the NO-sensitive guanylyl cyclase.
Figure 5. The guanylyl cyclase inhibitor ODQ antagonizes the effect of DEANO on the β-adrenergic stimulation of ICa,L in rat ventricular myocytes.
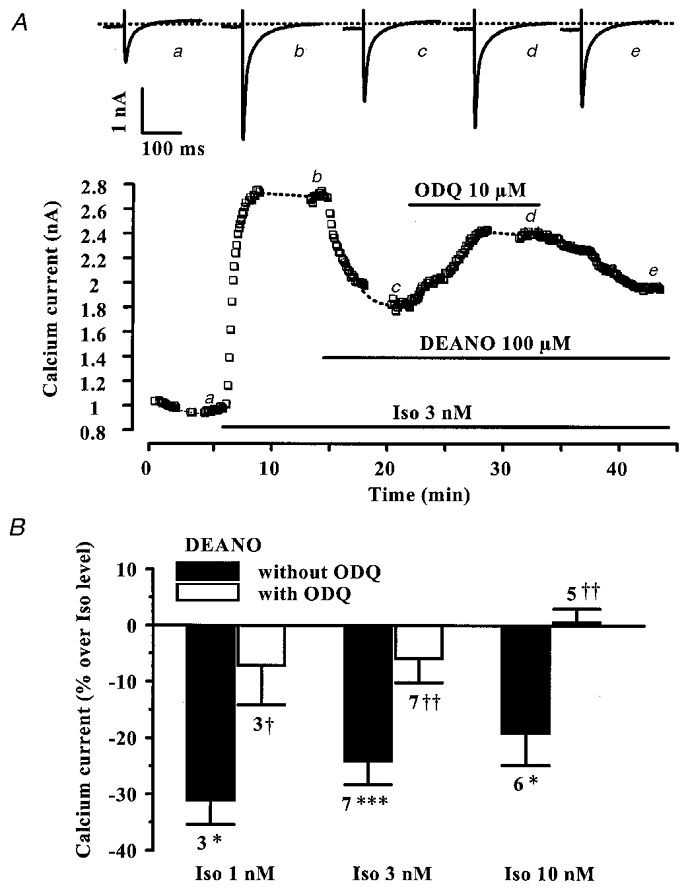
A, a myocyte was first exposed to control intracellular and extracellular solutions. Applications of Iso (3 nM), DEANO (100 μM) and ODQ (10 μM) are indicated by the horizontal lines. Current traces on top were recorded at the times indicated by the corresponding letters on the main graph. The dotted line indicates the zero-current level. B, summary of the effects of DEANO without (▪) or with ODQ (10 μM, □), in the presence of 1, 3 or 10 nM Iso. DEANO was used at 10 μM (in the presence of Iso 10 nM) or 100 μM (in the presence of 1 and 3 nM Iso). The effects of DEANO on ICa,L are presented as percentage variations from the amplitude of the Iso-stimulated ICa,L. Bars are the means and lines are the s.e.m. of the number of experiments indicated near the bars. Significant differences from Iso (*) or Iso + DEANO (†) levels are indicated as: *, †P < 0.05; ‡P < 0.01; ***P < 0.005.
Contribution of cG-PK to the anti-adrenergic effect of DEANO on ICa,L
We have shown previously that an intracellular application of cGMP in rat ventricular myocytes produces an inhibition of cAMP-stimulated ICa,L that involves the activation of cG-PK (Méry et al. 1991). Similarly, the Iso (1 nM) stimulation of ICa,L was strongly reduced in the presence of 8-p-CPT-cGMP (100 μM), a selective activator of cG-PK (from 235.1 ± 8.9 % to 168.2 ± 6.9 % stimulation, n = 4, P < 0.005). Next, the role of the cG-PK in the anti-adrenergic effect of DEANO was examined using (Rp)-8-CPT-cGMP (Rp8cG), a membrane-permeant inactive analogue of cGMP which competes with cGMP for the binding on cG-PK (Butt et al. 1994). In the typical experiment of Fig. 6A, the β-adrenergic stimulation of ICa,L (with 1 nM Iso) was strongly inhibited by DEANO (100 μM). This anti-adrenergic effect of DEANO was fully antagonized by superfusion of the cell with 10 μM Rp8cG, in a reversible manner. Figure 6B summarizes the results of several similar experiments. At 1 and 10 nM Iso, Rp8cG (10 μM) fully reversed the inhibitory effect of DEANO (100 μM) on ICa,L. We also examined the effects of KT5823, another cG-PK inhibitor structurally unrelated to cGMP (Komalavila & Lincoln, 1996). As summarized in Fig. 6B, KT5823 (0.1 or 0.3 μM) also fully reversed the anti-adrenergic effect of DEANO (100 μM). In the absence of DEANO, neither Rp8cG (10 μM) nor KT5823 (0.1-0.3 μM) had any significant effect on Iso-stimulated ICa,L (data not shown). Thus, these results demonstrate that cG-PK mediates the inhibitory effect of DEANO on ICa,L in rat ventricular myocytes.
Figure 6. cG-PK inhibitors antagonize the effect of DEANO on the β-adrenergic stimulation of ICa,L in rat ventricular myocytes.
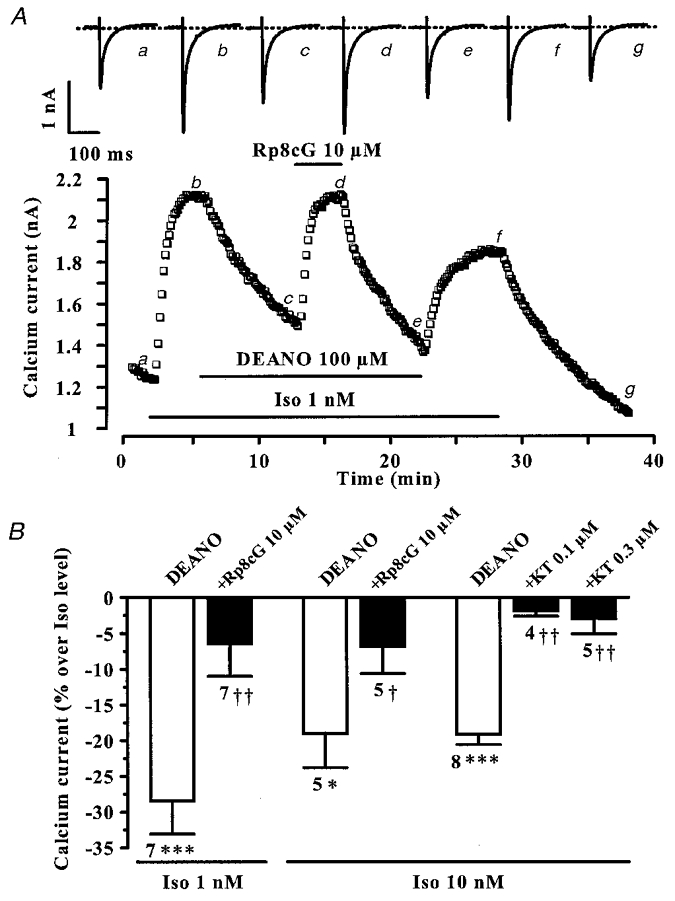
A, a myocyte was first exposed to control intracellular and extracellular solutions. Applications of Iso (1 nM), DEANO (100 μM) and Rp8cG (10 μM) are indicated by the horizontal lines. Current traces on top were recorded at the times indicated by the corresponding letters on the main graph. The dotted line indicates the zero-current level. B, summary of the effects of DEANO (100 μM) without (□) or with cG-PK inhibitors (▪; Rp8cG, 10 μM; KT5823, 0.1 or 0.3 μM), in the presence of 1 or 10 nM Iso. The effects of DEANO on ICa,L are presented as percentage variations from the amplitude of the Iso-stimulated ICa,L. Bars are the means and lines are the s.e.m. of the number of experiments indicated near the bars. Significant differences from Iso (*) or Iso + DEANO (†) levels are indicated as: *, †P < 0.05; ‡P < 0.01; ***P < 0.001.
Effect of DEANO on cAMP-stimulated ICa,L in rat ventricular myocytes
When elicited by intracellular dialysis of cGMP in rat ventricular myocytes, cG-PK-dependent inhibition of ICa,L appeared to take place at the level of the Ca2+ channels (Méry et al. 1991; Sumii & Sperelakis, 1995). In other cell types, cG-PK can modulate the initial steps of signal transduction, at the level of receptors and/or G proteins (Pfeiffer et al. 1995; Miyamoto et al. 1997; G.-R. Wang et al. 1998). To discriminate between these possibilities, we first studied the effect of DEANO in myocytes dialysed with cAMP through the patch pipette, in order to bypass the steps involved in cAMP production. In the experiment of Fig. 7A, the patch pipette was filled with a control intracellular solution supplemented with 30 μM cAMP, and intracellular dialysis of the myocyte started when the membrane patch was ruptured. The diffusion of cAMP into the cytosol induced a large increase in ICa,L (to 44.9 pA pF−1). Surprisingly, superfusion of the myocyte with DEANO (100 μM) did not modify the cAMP-stimulated ICa,L. The effect of another NO donor, SNP, is shown in Fig. 7B. In this experiment, the myocyte was first exposed to control intracellular and extracellular solutions. Intracellular dialysis with cAMP (10 μM) started at the time indicated by the arrow, inducing a 2.5-fold increase in ICa,L (to 26.7 pA pF−1). Superfusion of the myocyte with increasing concentrations of SNP (5, 50 and 500 μM) had no effect on the cAMP-stimulated ICa,L.
Figure 7. DEANO does not inhibit the stimulation of ICa,L induced by exogenous cAMP.
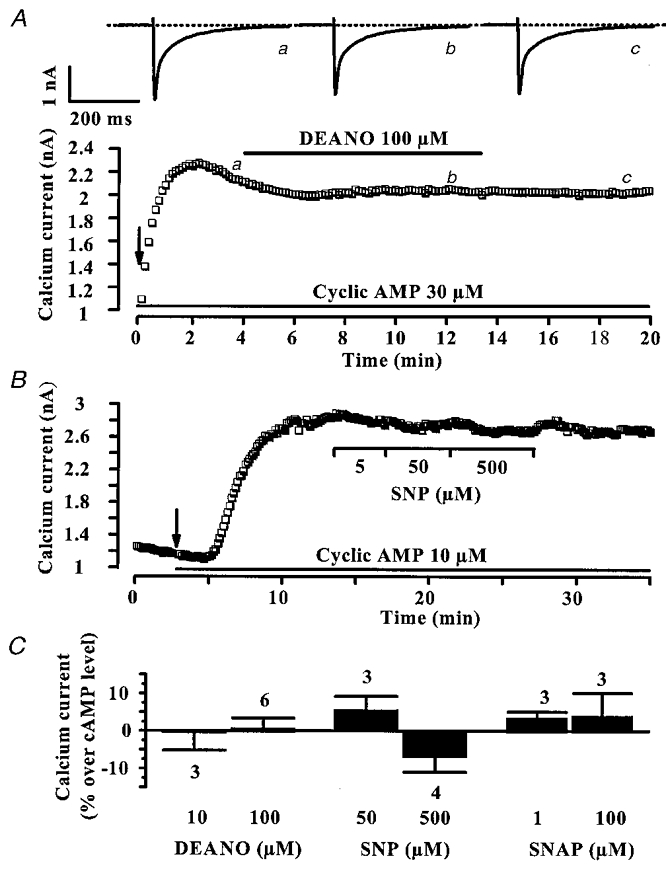
A, the pipette solution contained 30 μM cAMP, and cAMP dialysis started when the patch was ruptured (arrow). B, a myocyte was first dialysed with the control solution, and intracellular dialysis with 30 μM cAMP started at the time indicated by the arrow. Applications of DEANO (100 μM) in A or SNP (5, 50 and 500 μM) in B were performed as indicated by the horizontal lines. In A, current traces on top were recorded at the times indicated by the corresponding letters on the main graph. The dotted line indicates the zero-current level. C, summary of the effects of DEANO, SNP and SNAP on the cAMP (10–100 μM)-stimulated ICa,L. Variations are given as percentage change over the amplitude of the cAMP-stimulated ICa,L. Bars are the means and lines are the s.e.m. of the number of experiments indicated near the bars.
The effects of DEANO, SNP and SNAP on ICa,L in cAMP (10–100 μM)-dialysed myocytes are summarized in Fig. 7C. On average, the density of ICa,L was elevated to 23.02 ± 3.57 pA pF−1 (n = 12). None of the NO donors significantly changed the cAMP-stimulated ICa,L. Thus, since DEANO inhibits the Iso-stimulated ICa,L but not the cAMP-stimulated ICa,L, it must act at a step located upstream from cAMP production in the cAMP signalling cascade leading to ICa,L stimulation. In the following, we tested the hypothesis that DEANO via activation of cG-PK interferes with the receptor-dependent modulation of adenylyl cyclase.
Effect of DEANO on Iso-stimulated ICa,L in pertussis toxin-treated myocytes
In vitro experiments demonstrated that cG-PK could modulate the activity of Gi proteins, which are negatively coupled to adenylyl cyclase in rat cardiac myocytes (Pfeiffer et al. 1995; Miyamoto et al. 1997). Therefore, we studied the effect of DEANO in pertussis toxin (PTX)-treated myocytes, where Gi/Go proteins are irreversibly inactivated. Myocytes were incubated for 4–6 h (before patch-clamp experiments) in the presence of 0.5 μg ml−1 PTX, at 37°C (Hilal-Dandan et al. 1992). In the typical experiment shown in Fig. 8A, Iso (1 nM) was applied to a PTX-treated myocyte, inducing a fast and large rise in ICa,L. However, under these conditions, superfusion of the myocyte with DEANO (100 μM) had no effect on ICa,L. Since the muscarinic receptor agonist acetylcholine (ACh) produces an anti-adrenergic effect on ICa,L which clearly involves activation of PTX-sensitive G proteins (reviewed in Méry et al. 1997; Feron et al. 1999), we tested the efficacy of PTX to inactivate Gi/Go proteins by investigating the effect of a subsequent application of ACh (1 μM) on Iso-stimulated ICa,L. As shown in Fig. 8A, and summarized in Fig. 8B, PTX treatment fully abrogated the anti-adrenergic effect of both ACh (1 μM) and DEANO (100 μM). Note that when normalized to the Iso-stimulated ICa,L amplitude, ACh, but not DEANO, tended to potentiate the effect of the β-adrenergic agonist in PTX-treated myocytes (Fig. 8C).
Figure 8. DEANO does not inhibit the β-adrenergic stimulation of ICa,L in PTX-treated rat ventricular myocytes.
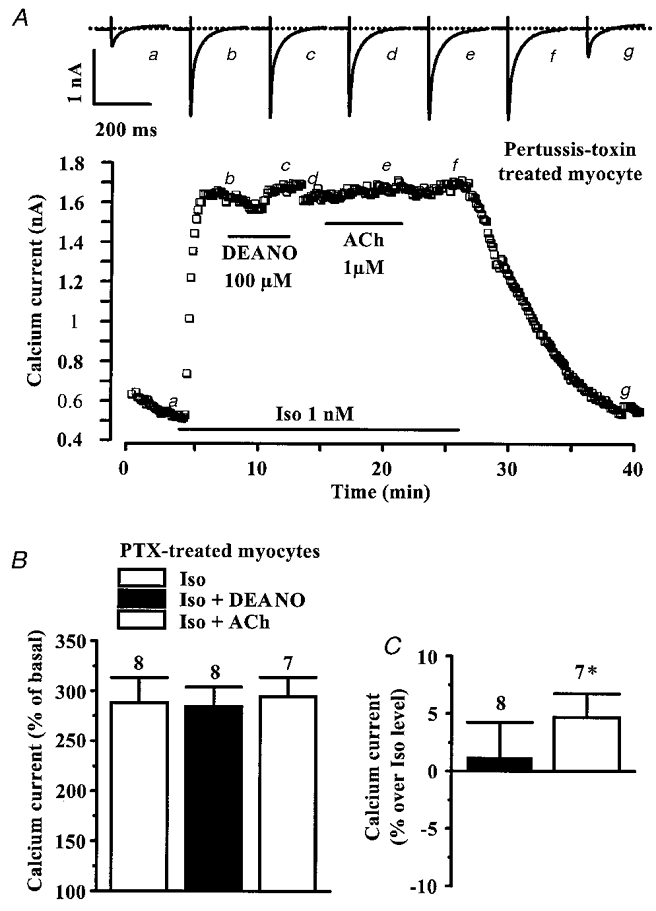
A, a myocyte was incubated with pertussis toxin (PTX, 0.5 μg ml−1, 4 h, 37 °C) prior to the experiment. It was first exposed to control extracellular and intracellular solutions, and applications of Iso (1 nM), DEANO (100 μM) and acetylcholine (ACh, 1 μM) were performed as indicated by the horizontal lines. Current traces on top were recorded at the times indicated by the corresponding letters on the main graph. The dotted line indicates the zero-current level. B and C, summary of the effects of DEANO (100 μM) and ACh (1 μM) on the Iso (0.01 and 1 nM)-stimulated ICa,L in PTX-treated myocytes. The amplitude of ICa,L is presented as percentage increase over basal amplitude (in B) or as percentage variations from the amplitude of the Iso-stimulated ICa,L (in C). Bars are the means and lines are the s.e.m. of the number of experiments indicated near the bars. Significant differences from Iso level are indicated as: *P < 0.05.
We next examined whether the effect of PTX was taking place upstream or downstream from cG-PK activation. For this, we investigated the effect 8-p-CPT-cGMP (100 μM) in PTX-treated myocytes. In four such cells, the stimulation of ICa,L by Iso 1 nM was only slightly attenuated by 8-p-CPT-cGMP (from 192.0 ± 11.0 % to 171.7 ± 11.0 % of control, P = 0.1), demonstrating that PTX treatment blunted the inhibitory effect of a direct activation of the cG-PK on ICa,L. In these cells, the Iso-stimulated ICa,L was also not affected by ACh (1 μM, 198.9 ± 8.0 % of control). Altogether, these data demonstrate that DEANO inhibits ICa,L via a cG-PK modulation of a PTX-sensitive Gi/Go protein.
Effect of DEANO on IBMX-stimulated ICa,L in pertussis toxin-treated myocytes
While PTX treatment of rat ventricular myocytes did not modify basal ICa,L density (5.2 ± 0.4 pA pF−1, n = 23, in PTX vs. 5.3 ± 0.1 pA pF−1, n = 266, in control), it somewhat increased the Iso response (at 0.01 and 1 nM, compare Fig. 8B and Fig. 3). Thus, the anti-adrenergic effect of DEANO might have been blunted in PTX-treated myocytes as a result of a saturating production of cAMP. Therefore, we re-examined the involvement of Gi/Go proteins in the modulation of ICa,L by DEANO under conditions where intracellular cAMP was non-maximally increased. To do this, we used low concentrations of isobutyl-methyl-xanthine (IBMX), a broad spectrum phosphodiesterase inhibitor which, unlike Iso, does not activate cAMP synthesis but increases cAMP levels by reducing its degradation. Figure 9A shows a typical experiment performed in a control (untreated) myocyte. IBMX (40 μM) elicited a 25 % increase in basal ICa,L and this effect was totally abolished by further application of DEANO (100 μM). This effect of DEANO clearly involved NO generation, since the addition of the NO scavenger carboxy PTIO (Akaike et al. 1993) totally antagonized the effect of the NO donor. Indeed, in three myocytes where the stimulation of ICa,L by 40 μM IBMX (154.2 ± 4.9 % of control) had been reduced by 100 μM DEANO (to 118.5 ± 2.9 % of control, P < 0.005), the addition of carboxy PTIO (100 μM) to the DEANO solution restored the stimulation of ICa,L to its level in Iso alone (to 156.8 ± 1.7 % of control).
Figure 9. Effects of DEANO and ACh on IBMX-stimulated ICa,L in rat ventricular myocytes.
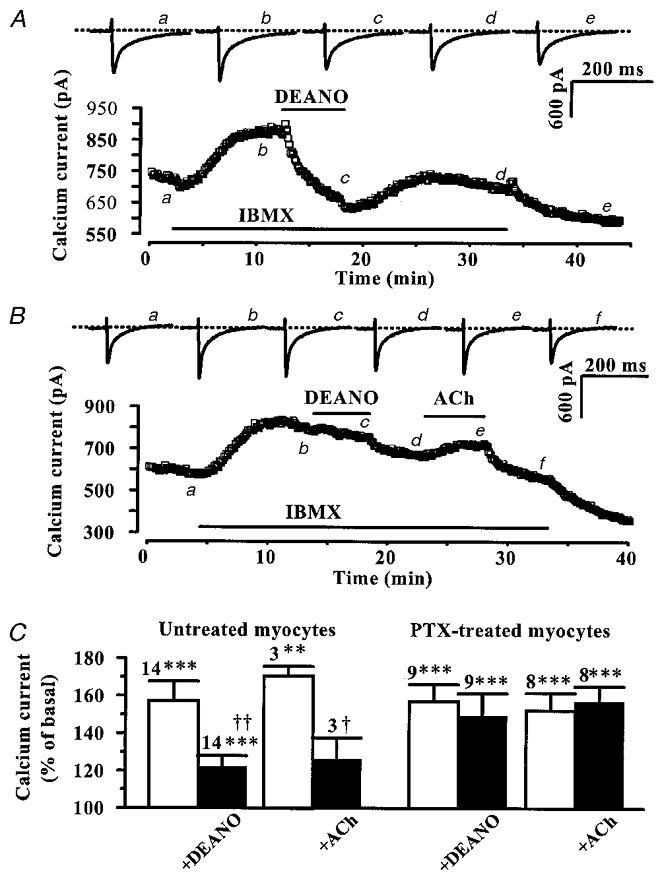
Untreated (A) and PTX-treated (B) myocytes were first exposed to control extracellular and intracellular solutions. Applications of IBMX (40 μM in A, 20 μM in B), DEANO (100 μM, in A and B) and ACh (1 μM, in B) are indicated by the horizontal lines. Current traces on top were recorded at the times indicated by the corresponding letters on the main graphs. The dotted lines indicate the zero-current level. C, summary of the effects of IBMX (10–80 μM) on ICa,L, used either alone (□) or in the presence of DEANO (100 μM) or ACh (1 μM) (▪) in untreated (left) or PTX-treated myocytes (right). The effects of IBMX on ICa,L are presented as percentage variations from the amplitude of basal ICa,L (set to 100 %). Bars are the means and lines are the s.e.m. of the number of experiments indicated near the bars. Significant differences from basal (*) and IBMX (†) levels are indicated as: †P < 0.05; **, ‡P < 0.01; ***P < 0.005.
In contrast to control cells, DEANO failed to inhibit the response of ICa,L to IBMX in PTX-treated myocytes. In the typical experiment of Fig. 9B, neither DEANO (100 μM) nor ACh (1 μM) produced any inhibition of ICa,L stimulated by IBMX (20 μM). The results of several similar experiments are summarized in Fig. 9C. On average, IBMX (10–80 μM) exerted submaximal stimulatory effects on ICa,L. While ACh (1 μM) strongly reduced the IBMX response in control (untreated) myocytes, this inhibition was absent in PTX-treated myocytes indicating that Gi/Go proteins were efficiently blocked by PTX. Similarly, DEANO (100 μM) strongly antagonized the IBMX-stimulated ICa,L in untreated myocytes, and the effect was abolished in PTX-treated myocytes. Thus, the inhibitory effect of DEANO on ICa,L shared some similarity with the effect of ACh, in that both effects required the integrity of PTX-sensitive G proteins. Note that in one out of the nine experiments in PTX-treated myocytes, ACh had no effect on IBMX-stimulated ICa,L while DEANO still elicited an inhibitory effect.
DISCUSSION
In this study, we report that ICa,L is regulated by NO in rat ventricular cardiac myocytes. The NO donor DEANO exerted a pronounced anti-adrenergic effect, which involved the activation of the NO-sensitive guanylyl cyclase and cG-PK. The main locus of action of cG-PK appeared to be a PTX-sensitive G protein.
In agreement with studies in frog and guinea-pig ventricular myocytes (Méry et al. 1993; Levi et al. 1994; Whaler & Dollinger, 1995), we found that a NO donor, DEANO, strongly inhibited the β-adrenergic stimulation of ICa,L in rat ventricular myocytes. The inhibitory effect of DEANO occurred in the micromolar range of concentrations and was not mimicked by mock DEANO or diethylamine. This effect clearly involved NO generation, since the addition of the NO-scavenger carboxy PTIO (Akaike et al. 1993) totally antagonized the effect of the NO donor. In contrast, other NO donors (SIN-1, SNAP and SPNO, a NONOate like DEANO) did not significantly modify the Iso-stimulated ICa,L. However, the rate of NO release by these NO donors is slow compared with that of DEANO (Feelisch et al. 1991; Maragos et al. 1991; Ferrero et al. 1999). As suggested by Kojda et al. (1996), the time course of NO release appears to be critical when comparing the functional effects of different NO donors. Nevertheless, although DEANO is 1000-fold more potent than SIN-1, SNP or SNAP, all these NO donors inhibit the Iso-stimulated ICa,L in frog ventricular myocytes (e.g. see Méry et al. 1993; Abi-Gerges et al. 1997b). A possible explanation for this discrepancy may come from the observation that rat and mouse ventricles exhibit higher myoglobin contents than other species (O’Brien et al. 1992). Since myoglobin is known as a potent NO scavenger, it may hinder the effects of slow NO sources, such as SIN-1, SNAP and SPNO, but not that of a fast NO source like DEANO (Ishibashi et al. 1992; Beckman & Koppenol, 1996).
Another interesting observation was that DEANO behaved as an apparent competitive antagonist of the response of ICa,L to Iso in rat myocytes. We found that the inhibitory effect of DEANO on ICa,L was reduced as the concentration of Iso was increased from 1 to 10 nM. This observation might help to explain the discrepancy between negative and positive results obtained in the literature when comparing the efficacy of a given NO donor in different cardiac preparations (Thomas et al. 1997).
DEANO was shown to enhance cGMP production in isolated rat ventricular myocytes (Kojda et al. 1996). In agreement with this finding, we found that activation of the cGMP pathway accounted for the anti-adrenergic effect of DEANO on ICa,L in the same preparation. Indeed, the effect of DEANO on ICa,L was antagonized by ODQ, a specific guanylyl cyclase inhibitor (Abi-Gerges et al. 1997b; Sandirasegarane & Diamond, 1999). Moreover, the effect of DEANO on ICa,L was mimicked by 8-p-CPT-cGMP, a cG-PK activator, and antagonized by Rp8cG and KT5823, two cG-PK inhibitors (Butt et al. 1994; Komalavila & Lincoln, 1996). Therefore, the endogenous activation of the cG-PK accounted for the anti-adrenergic effect of DEANO on ICa,L.
Earlier studies in rat ventricular myocytes have shown that cG-PK was involved in the inhibitory effects of exogenous cGMP on ICa,L (Méry et al. 1991; Sumii & Sperelakis, 1995). Intracellular dialysis of isolated myocytes with cGMP or with constitutively active cG-PK elicited inhibitory effects on ICa,L when the current was maximally stimulated with 100 μM IBMX or 100 μM intracellular cAMP. In addition, NO donors inhibited IBMX- or cAMP-stimulated ICa,L in guinea-pig myocytes (Levi et al. 1994; Whaler & Dollinger, 1995). Accordingly, L-type Ca2+ channels were viewed as a major target of cG-PK in these cells (Méry et al. 1991; Levi et al. 1994; Whaler & Dollinger, 1995; Sumii & Sperelakis, 1995).
However, we found here that in rat ventricular myocytes the inhibitory effect of DEANO on ICa,L was strongly reduced when the concentration of Iso was increased to 10 nM (Fig. 3) or in the presence of 200 μM IBMX (data not shown). Moreover, DEANO did not inhibit the stimulation of ICa,L induced by intracellular cAMP. Therefore, the major target of cG-PK may differ when activated by endogenous cGMP production (this study) or by intracellular dialysis of exogenous cGMP (Méry et al. 1991). Our present results suggest that cG-PK acts at the level of G proteins when guanylyl cyclase is stimulated by NO since the inhibitory effect of DEANO on ICa,L was antagonized by PTX, which inactivates Gi/Go proteins. Both Gi and Go proteins are involved in the regulation of ICa,L (Méry et al. 1997; Valenzuala et al. 1997; Feron et al. 1999), but only Gi-dependent pathways were shown to be modulated by cG-PK (Pfeiffer et al. 1995). Activation of cG-PK was shown to phosphorylate αi subunits of G proteins (Pfeiffer et al. 1995) and to increase the spontaneous binding of GTP (Miyamoto et al. 1997). Our results strongly suggest that this modulation of αi subunits of Gi proteins accounts for the observed cG-PK-dependent, PTX-sensitive inhibitory effect of DEANO on Iso-stimulated ICa,L in rat myocytes. Yet, we can only speculate on the contribution of this mechanism to the regulation of the Iso-stimulated ICa,L by NO in guinea-pig myocytes (Levi et al. 1994; Whaler & Dollinger, 1995). Interestingly, rat ventricles express higher levels of myoglobin than guinea-pig ventricles (O’Brien et al. 1992), and high myoglobin content blunted cGMP production induced by NO (Ishibashi et al. 1992). Therefore, we propose that only high levels of cGMP can elicit the direct inhibition of L-type calcium channels by cG-PK (as seen during dialysis of cGMP, or in guinea-pig myocytes), while moderate levels of cGMP induce the specific regulation of Gi proteins by cG-PK (as seen in rat myocytes).
In addition to the regulation of cGMP production, NO can regulate directly the activity of several proteins in the heart, including the L-type Ca2+ channels. Nitrosothiols and SIN-1 enhanced ICa,L in a cGMP-independent manner in ferret ventricular myocytes (Campbell et al. 1996), but nitrosothiols inhibited the activity of cloned L-type Ca2+ channels in HEK293 cells (a human embryonic kidney cell line) (Hu et al. 1997). In rat ventricular myocytes, as in cardiac myocytes from other species and tissues (Méry et al. 1993; Levi et al. 1994; Kirstein et al. 1995; Wahler & Dollinger, 1995; Y. G. Wang et al. 1998; Feron et al. 1999), NO donors (including nitrosothiols and SIN-1) did not elicit a cGMP-independent effect on ICa,L. Interestingly, we found that the basal ICa,L in rat ventricular myocytes was not modified by reducing agents but was inhibited by LY83583, a superoxide anion generator. Hu et al. (1997) also reported that the basal activity of the L-type Ca2+ channel was not changed by reducing agents but was inhibited by oxidant treatments. In contrast, ICa,L was inhibited by reducing agents (glutathione, dl-DTT) and enhanced by an oxidant (DTNB) in ferret ventricular myocytes (Campbell et al. 1996). Altogether, these data suggest that the different effects of NO donors on the basal ICa,L might require different redox environments. In addition, it is possible that the ‘direct’ effects of NO donors do not take place on the L-type Ca2+ channel itself, but on other auxiliary proteins and/or on lipids. While the reason for these discrepancies remains to be fully elucidated, our results clearly demonstrate that NO can recruit an endogenous cG-PK in rat ventricular myocytes, leading to a tight control of the β-adrenergic stimulation of L-type Ca2+ channel activity.
Acknowledgments
We thank Patrick Lechêne for skilful technical assistance, Florence Lefebvre for preparation of the cells, Drs Michel Chesnais, Bernd K. Fleischmann and Renzo Levi for helpful discussions. We are grateful to Dr Xinqiang Han for sharing unpublished results. N. Abi-Gerges was supported by the Fondation pour la Recherche Medicale (F.R.M.) and the Association Française contre les Myopathies (A.F.M.).
References
- Abi-Gerges N, Eschenhagen T, Hove-Madsen L, Fischmeister R, Méry P-F. Methylene blue is a muscarinic antagonist in cardiac myocytes. Molecular Pharmacology. 1997a;52:482–490. doi: 10.1124/mol.52.3.482. [DOI] [PubMed] [Google Scholar]
- Abi-Gerges N, Hove-Madsen L, Fischmeister R, Méry P-F. A comparative study of the effects of three guanylyl cyclase inhibitors on the L-type Ca2+ and muscarinic K+ currents in frog cardiac myocytes. British Journal of Pharmacology. 1997b;121:1369–1377. doi: 10.1038/sj.bjp.0701249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Gerges N, Méry P-F, Fischmeister R. The NO-sensitive guanylyl cyclase does not participate in the muscarinic regulation of rat cardiac Ca2+ current. Biophysical Journal. 1997c;72:A34. [Google Scholar]
- Abi-Gerges N, Tavernier B, Mebazaa A, Faivre V, Paqueron X, Payen D, Fischmeister R, Méry P-F. Sequential changes in autonomic regulation of cardiac myocytes after in vivo endotoxin injection in rat. American Journal of Respiratory and Critical Care Medicine. 1999;160:1196–1204. doi: 10.1164/ajrccm.160.4.9808149. [DOI] [PubMed] [Google Scholar]
- Akaike T, Yoshida M, Miyamoto Y, Sato K, Kohno M, Sasamoto K, Miyazaki K, Ueda S, Maeda H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/NO through a radical reaction. Biochemistry. 1993;32:827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide and peroxynitrite: the good, the bad, and the ugly. American Journal of Physiology. 1996;271:C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Butt E, Eigenthaler M, Geneiser H-G. (Rp)-8-pCPT-cGMPS, a novel cGMP-dependent protein kinase inhibitor. European Journal of Pharmacology. 1994;269:265–268. doi: 10.1016/0922-4106(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Campbell DL, Stamler JS, Strauss HC. Redox modulation of L-type calcium channels in ferret ventricular myocytes. Journal of General Physiology. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feelisch M. The biochemical pathways of nitric oxide formation from nitrovasodilators: appropriate choice of exogenous NO donors and aspects of preparation and handling of aqueous NO solutions. Journal of Cardiovascular Pharmacology. 1991;17(suppl. 3):S25–S33. [Google Scholar]
- Feron O, Han X, Kelly RA. Muscarinic cholinergic signaling in cardiac myocytes: dynamic targeting of M2AchR to sarcolemmal caveolae and eNOS activation. Life Sciences. 1999;64:471–477. doi: 10.1016/s0024-3205(98)00590-6. [DOI] [PubMed] [Google Scholar]
- Ferrero R, Rodriguez-Pascual F, Miras-Portugal MT, Torres M. Comparative effects of several nitric oxide donors on intracellular cyclic GMP levels in bovine chromaffin cells: correlation with nitric oxide production. British Journal of Pharmacology. 1999;127:779–787. doi: 10.1038/sj.bjp.0702607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross WL, Bak MI, Ingwall JS, Arstall WA, Smith TW, Balligand J-L, Kelly RA. Nitric oxide inhibits creatine kinase and regulates heart contractile reserve. Proceedings of the National Academy of Sciences of the USA. 1996;93:5604–5609. doi: 10.1073/pnas.93.11.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilal-Dandan R, Urasawa K, Brunton LL. Endothelin inhibits adenylate cyclase and stimulates phosphoinositide hydrolysis in adult cardiac myocytes. Journal of Biological Chemistry. 1992;267:10620–10624. [PubMed] [Google Scholar]
- Hu H, Chiamvimonvat N, Yamagishi T, Marban E. Direct inhibition of expressed cardiac L-type Ca2+ channels by S-nitrosothiol nitric oxide donors. Circulation Research. 1997;81:742–752. doi: 10.1161/01.res.81.5.742. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Hamaguchi M, Kato K, Kawada T, Ohta H, Sasage H, Imai S. Relationship between myoglobin contents and increase in cyclic GMP produced by glyceryl trinitrate and nitric oxide in rabbit aorta, right atrium and papillary muscle. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1993;347:533–561. doi: 10.1007/BF00166750. [DOI] [PubMed] [Google Scholar]
- Kirstein M, Rivet-Bastide M, Hatem S, Bénardeau A, Mercadier J-J, Fischmeister R. Nitric oxide regulates the calcium current in isolated human atrial myocytes. Journal of Clinical Investigation. 1995;95:794–802. doi: 10.1172/JCI117729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojda G, Kottenberg K. Regulation of basal myocardial function by NO. Cardiovascular Research. 1999;41:514–523. doi: 10.1016/s0008-6363(98)00314-9. [DOI] [PubMed] [Google Scholar]
- Kojda G, Kottenberg K, Nix P, Schluter KD, Piper HM, Noack E. Low increase in cGMP induced by organic nitrates and nitrovasodilators improves contractile response of rat ventricular myocytes. Circulation Research. 1996;78:91–101. doi: 10.1161/01.res.78.1.91. [DOI] [PubMed] [Google Scholar]
- Komalavila P, Lincoln TM. Phosphorylation of the inositol 1,4,5-triphosphate receptor. Journal of Biological Chemistry. 1996;271:21933–21938. doi: 10.1074/jbc.271.36.21933. [DOI] [PubMed] [Google Scholar]
- Levi RC, Alloatti G, Penna C, Gallo MP. Guanylate-cyclase-mediated inhibition of cardiac ICa by carbachol and sodium nitroprusside. Pflügers Archiv. 1994;426:419–426. doi: 10.1007/BF00388305. [DOI] [PubMed] [Google Scholar]
- McDonell KL, Diamond J. Cyclic GMP-dependent protein kinase activation in the absence of negative inotropic effects in the rat ventricle. British Journal of Pharmacology. 1997;122:1425–1435. doi: 10.1038/sj.bjp.0701492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell KL, Tibbits GF, Diamond J. cGMP elevation does not mediate muscarinic agonist-induced negative inotropy in rat ventricular cardiomyocytes. American Journal of Physiology. 1995;269:H1905–1912. doi: 10.1152/ajpheart.1995.269.6.H1905. [DOI] [PubMed] [Google Scholar]
- Maragos CM, Morley D, Wink DA, Dunams TM, Saavedra JE, Hoffman A, Bove AA, Isaac L, Hrabie JA, Keefer LK. Complexes of NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. Journal of Medical Chemistry. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- Méry P-F, Abi Gerges N, Vandecasteele G, Jurevicius J, Eschenhagen T, Fischmeister R. Muscarinic regulation of the L-type Ca2+ current in isolated cardiac myocytes. Life Sciences. 1997;60(13/14):1113–1120. doi: 10.1016/s0024-3205(97)00055-6. [DOI] [PubMed] [Google Scholar]
- Méry P-F, Lohmann SM, Walter U, Fischmeister R. Ca2+ current is regulated by cyclic GMP-dependent protein kinase in mammalian cardiac myocytes. Proceedings of the National Academy of Sciences of the USA. 1991;88:1197–1201. doi: 10.1073/pnas.88.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méry P-F, Pavoine C, Belhassen L, Pecker F, Fischmeister R. Nitric oxide regulates cardiac Ca2+ current. Involvement of cGMP-inhibited and cGMP-stimulated phosphodiesterases through guanylyl cyclase activation. Journal of Biological Chemistry. 1993;268:26286–26295. [PubMed] [Google Scholar]
- Miyamoto A, Laufs U, Pardo C, Liao JK. Modulation of bradykinin receptor ligand binding affinity and its coupled G-proteins by nitric oxide. Journal of Biological Chemistry. 1997;272:19601–19608. doi: 10.1074/jbc.272.31.19601. [DOI] [PubMed] [Google Scholar]
- O’Brien PJ, Shen H, McCutcheon LJ, O’Grady M, Byrne PJ, Ferguson HW, Mirsalimi MS, Julian RJ, Sargeant JM, Tremblay RR, Blackwell TE. Rapid, simple and sensitive microassay for skeletal and cardiac muscle myoglobin and hemoglobin: use in various animals indicates functional role of myohemoproteins. Molecular and Cellular Biochemistry. 1992;112:45–52. doi: 10.1007/BF00229642. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Nürnberg B, Kamm S, Uhde M, Schultz G, Ruth P, Hofmann F. Cyclic GMP-dependent protein kinase blocks pertussis toxin-sensitive hormone receptor signaling pathways in chinese hamster ovary cells. Journal of Biological Chemistry. 1995;270:9052–9059. doi: 10.1074/jbc.270.16.9052. [DOI] [PubMed] [Google Scholar]
- Sandirasegarane L, Diamond J. The nitric oxide donors, SNAP and DEA/NO, exert a negative inotropic effect in rat cardiomyocytes which is independent of cyclic GMP elevation. Journal of Molecular and Cellular Cardiology. 1999;31:799–808. doi: 10.1006/jmcc.1998.0919. [DOI] [PubMed] [Google Scholar]
- Scamps F, Mayoux E, Charlemagne D, Vassort G. Calcium current in single cells isolated from normal and hypertrophied rat heart. Circulation Research. 1990;67:199–208. doi: 10.1161/01.res.67.1.199. [DOI] [PubMed] [Google Scholar]
- Stein B, Drögemüller A, Mülsch A, Schmitz W, Scholz H. Ca2+-dependent constitutive nitric oxide synthase is not involved in the cyclic-GMP-increasing effects of carbachol in ventricular myocytes. Journal of Pharmacology and Experimental Therapeutics. 1993;266:919–925. [PubMed] [Google Scholar]
- Sumii K, Sperelakis N. cGMP-dependent protein kinase regulation of the L-type Ca2+ current in rat ventricular myocytes. Circulation Research. 1995;77:803–812. doi: 10.1161/01.res.77.4.803. [DOI] [PubMed] [Google Scholar]
- Thomas GP, Sims SM, Karmazyn M. Differential effects of endothelin-1 on basal and isoprenaline-enhanced Ca2+ currents in guinea-pig ventricular myocytes. The Journal of Physiology. 1997;503:55–65. doi: 10.1111/j.1469-7793.1997.055bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela D, Han X, Mende U, Fankhauser C, Mashimo H, Huang P, Pfeffer J, Neer EJ, Fishman MC. GαO is necessary for muscarinic regulation of Ca2+ channels in mouse heart. Proceedings of the National Academy of Sciences of the USA. 1997;94:1727–1732. doi: 10.1073/pnas.94.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahler GM, Dollinger SJ. Nitric oxide donor SIN-1 inhibits mammalian cardiac calcium current through cGMP-dependent protein kinase. American Journal of Physiology. 1995;268:C45–54. doi: 10.1152/ajpcell.1995.268.1.C45. [DOI] [PubMed] [Google Scholar]
- Wang G-R, Zhu Y, Halushka PV, Lincoln TM, Mendelsohn ME. Mechanism of platelet inhibition by nitric oxide: in vivo phosphorylation of thromboxane receptor by cyclic GMP-dependent protein kinase. Proceedings of the National Academy of Sciences of the USA. 1998;95:4888–4893. doi: 10.1073/pnas.95.9.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YG, Rechenmacher CE, Lipsius SL. Nitric oxide signaling mediates stimulation of L-type Ca2+ current elicited by withdrawal of acetylcholine in cat atrial myocytes. Journal of General Physiology. 1998;111:113–125. doi: 10.1085/jgp.111.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin MS, Hintze TH, Shen W, Mohazzab-H KM, Xie Y-W. Involvement of reactive oxygen and nitrogen species in signaling mechanisms that control tissue respiration in muscle. Biochemical Society Transactions. 1997;25:934–939. doi: 10.1042/bst0250934. [DOI] [PubMed] [Google Scholar]
- Xu L, Eu J, Meissner G, Stamler J. Activation of the cardiac release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- Zakharov SI, Pieramici S, Kumar GK, Prabhakar NR, Harvey RD. Nitric oxide synthase activity in guinea pig ventricular myocytes is not involved in muscarinic inhibition of cAMP-regulated ion channels. Circulation Research. 1996;78:925–935. doi: 10.1161/01.res.78.5.925. [DOI] [PubMed] [Google Scholar]


