Abstract
The acidic interior of neuroendocrine secretory vesicles provides both an energy gradient for amine-proton exchangers (VMATs) to concentrate small transmitter molecules, for example catecholamines, and an optimal pH for the prohormone convertases which cleave hormone precursors. There is evidence that VMAT activity modulates prohormone cleavage, but in the absence of measurements of pH in secretory vesicles in intact cells, it has not been possible to establish whether these effects are attributable to raised intravesicular pH due to proton transport through VMATs.
Clones were generated of the hamster insulinoma cell line HIT-T15 expressing a pH-sensitive form of green fluorescent protein (GFP-F64L/S65T) targeted to secretory vesicles, with and without co-expression of VMAT2. In order to study prohormone cleavage, further clones were generated that expressed preprogastrin with and without co-expression of VMAT2.
Confocal microscopy of GFP fluorescence indicated that the pH in the secretory vesicles was 5.6 in control cells, compared with 6.6 in cells expressing VMAT2; the latter was reduced to 5.8 by the VMAT inhibitor reserpine.
Using a pulse-chase labelling protocol, cleavage of 34-residue gastrin (G34) was found to be inhibited by co-expression with VMAT2, and this was reversed by reserpine. Similar effects on vesicle pH and G34 cleavage were produced by ammonium chloride.
We conclude that VMAT expression confers the linked abilities to store biogenic amines and modulate secretory vesicle pH over a range influencing prohormone cleavage and therefore determining the identity of regulatory peptide secretory products.
The lumen of secretory vesicles in endocrine cells and neurones is generally recognised to be approximately pH 5.5 due to the activity of the vacuolar proton pump (vH+-ATPase) (Mellman et al. 1986; Njus et al. 1986). In many neuroendocrine cells, cleavage of regulatory peptide precursors occurs in acidic compartments of the secretory pathway, i.e. vesicles and trans-Golgi network (TGN), and is mediated by prohormone convertases with acidic pH optima (Rhodes et al. 1987; Davidson et al. 1988; Xu & Shields, 1994; Urbéet al. 1997). The electrochemical gradient across secretory vesicle membranes also provides energy for the transport of small transmitter molecules such as serotonin (5-HT), dopamine, histamine and acetylcholine (Njus et al. 1986; Schuldiner et al. 1995; Liu & Edwards, 1997). In the case of biogenic amines, vesicular uptake is mediated by vesicular monoamine transporter (VMAT) types 1 and 2. These function as proton-amine exchangers with a stoichiometry of two protons to one amine (Liu et al. 1992; Erickson et al. 1992). The two VMATs differ in their ability to transport histamine, and in their sensitivity to certain inhibitors, for example reserpine blocks both, but tetrabenzine is selective for VMAT2 (Peter et al. 1994).
It has been recognised for many years that peptide-secreting neuroendocrine cells also have the capacity to take up biogenic amine precursors, decarboxylate them and store the product in secretory vesicles (Pearse, 1969). In part these properties are attributable to the widespread expression of VMATs in peptide-secreting endocrine cells (Weihe et al. 1994). Interestingly, inhibition of VMAT activity by reserpine increased the cleavage of secretory peptides including chromogranin A (Watkinson & Robinson, 1992; Wolkersdorfer et al. 1996), the opioid peptide precursor proenkephalin (Eiden et al. 1984; Lindberg, 1986; Adams & Boarder, 1987; Wilson, 1991) and the precursor of the gastric hormone, gastrin (Voronina et al. 1997). In the latter case, cleavage of a 34-residue gastrin (G34) yields a 17-residue peptide (G17); since the metabolic clearance rate of G17 is five times greater than G34 (Walsh et al. 1974), cleavage leads to lower and more transient changes in plasma concentrations. The mechanisms by which VMAT activity might influence prohormone cleavage are uncertain. In particular it is not clear whether vH+-ATPase activity in intact cells is able to maintain secretory vesicle pH in the presence of VMAT activity, or whether VMAT activity causes a rise in intravesicular pH which is reflected in decreased prohormone convertase activity. The analysis is, in any case, complicated by the fact that secretory vesicle pH falls as vesicles mature (Urbe et al. 1997), and there has been little attention given to the estimation of pH in vesicles of defined age in intact cells. In order to examine pH in defined populations of secretory vesicles expressing VMAT2 in living cells, we took advantage of a pH-sensitive form of green fluorescent protein (GFP-F64L/S65T) (Kneen et al. 1998) targeted to vesicles in the form of a chimera with preprogastrin (Fig. 1). We selected the hamster insulinoma cell line HIT-T15 for these experiments, since (a) these cells exhibit a polarised phenotype, characterised by the extension of processes the terminals of which are enriched in secretory vesicles which facilitates imaging studies, (b) they do not normally express VMATs, so that the consequences of co-expression of VMAT and progastrin are readily observed experimentally, and (c) with regard to progastrin processing they execute a program of post-translational cleavage closely resembling that in normal G-cells (Bishop et al. 1998). The results presented here show directly, and for the first time, that expression of VMAT2 leads to an increase in secretory vesicle pH, and reserpine-sensitive inhibition of G34 cleavage.
Figure 1. Schematic representation of the organisation of the Gas-GFP chimera.
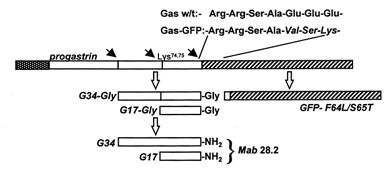
The progastrin cleavage sites (filled arrows) are retained in the chimera, and on proteolysis yield the Gly-gastrins which are then converted to the amidated gastrins for which Mab 28.2 is specific. For the generation of the amidated gastrin epitope, cleavage at Arg94,95 is essential, and this also liberates GFP with two NH2-terminal residues derived from progastrin (Ser-Ala97).
METHODS
DNA constructs and expression vectors
A 1.7 kb fragment containing the entire coding region of rat VMAT2 was generated by PCR from rat gastric corpus cDNA (obtained as described in Dimaline & Struthers, 1996) and ligated into pGEM-Teasy (Promega, Madison, WI, USA) The insert was excised with Apa 1 and Sal 1 and subcloned into the Apa 1 and Xho 1 sites of pcDNA3.1Zeo (InVitrogen, Groningen, The Netherlands), to generate pVMAT2zeo. A preprogastrin-GFP chimera (Gas-GFP) was generated by amplification of the coding region of GFP-F64L/S65T from pEGFP-C1 (Clontech, Basingstoke, UK). Primers were used as previously described (Kneen et al. 1998), except that an engineered Eco RI site in the sense primer was replaced by an Eco 47III site. The GFP encoding sequence was subcloned in-frame into the expression vector pcDNA3 containing rat preprogastrin (Bishop et al. 1998), between the Eco 47III site of rat preprogastrin cDNA and Xba 1 in the multiple cloning site. The construct, pGas-GFP, therefore contained the entire coding region giving rise to the COOH-terminal amidated gastrins together with the associated COOH-terminal cleavage site, upstream of, and in-frame with, GFP (Fig. 1).
Cells and transfections
Hamster insulinoma cells (HIT-T15) permanently transfected with rat preprogastrin cDNA (HIT-T15/23) (Bishop et al. 1998) were maintained in RPMI 1640 medium (Life Technologies, Paisley, UK) supplemented with 11 mm glucose, 10 % v/v fetal bovine serum, penicillin (100 U ml−1), streptomycin (100 μg ml−1) and fungizone (2.5 μg ml−1) (Life Technologies), at 37 °C in 5 % CO2. Permanently transfected cells co-expressing VMAT2 and preprogastrin were generated by electroporation of 5 x 106 HIT-T15/23 cells in 0.8 ml RPMI medium at 200 V and 1070 μF with 10 μg linearised pVMAT2zeo construct or empty vector. Cells were grown in supplemented RPMI medium for 48 h before selection with G418-sulphate (0.4 mg ml−1) (Calbiochem, Beeston, UK) and zeocin (0.75 mg ml−1) (Invitrogen). Colonies were screened for gastrin expression by radioimmunoassay (Varro et al. 1990) and VMAT2 expression by Northern blotting and immunohistochemistry. Permanently transfected cells expressing Gas-GFP and VMAT2 were generated from the parental HIT-T15 cell line using pGas-GFP and pVMAT2zeo, or pGas-GFP and empty vector, using Transfast lipofection reagent (Promega) and selection in G418 and zeocin. Cells were screened for amidated gastrin, GFP and VMAT2 after fixation using GFP fluorescence and detection of gastrin or VMAT2 by immunocytochemistry.
Northern analysis
Total mRNA was extracted from transfected HIT-T15 cells (3 x 106) using the Quickprep total RNA extraction kit (Amersham Pharmacia Biotech, Little Chalfont, UK). Total RNA (10 μg) was denatured, loaded onto a 1.0 % agarose-formaldehyde gel run in 1 x Mops at 65 V for 4 h, and transferred to nylon membranes (Roche Diagnostics Ltd, Lewes, Sussex, UK) that were hybridized as previously described with a [32P]cRNA probe for VMAT2 (Dimaline & Struthers, 1996), and the signal analysed using a PhosphorImager (Molecular Dynamics, Seven Oaks, UK).
Immunocytochemistry
Cells (20 x 103) were plated onto 13 mm poly-l-lysine-coated coverslips and incubated for 24-48 h. In some experiments cells were incubated with the serotonin (5-hydroxytryptamine, 5-HT) precursor 5-hydroxytryptophan (5-HTP, 10 μm), or with the vH+-ATPase inhibitors concanamycin and bafilomycin. Cells were washed in phosphate-buffered saline (PBS), fixed in 2 % w/v paraformaldehyde in PBS at 22 °C for 30 min, rinsed in PBS and permeablised with 0.2 % v/v Triton in PBS containing 0.3 % w/v bovine serum albumin (BSA). Coverslips were incubated (4 °C, 18 h) with primary antibody diluted in 0.1 m PBS, 2 % w/v BSA, 0.25 % w/v NaN3 and (1 h, 22 °C) with the appropriate anti-species IgG conjugated to fluorescein isothiocyanate or Texas Red (1:100; Vector Laboratories Ltd, Peterborough, UK); they were mounted in Vectashield (Vector Laboratories), and viewed under an epifluorescence microscope (Zeiss, Jena, Germany) with fluorescein or Texas Red filter sets. Images were captured using KS300 imaging software (Imaging Associates, Oxford, UK). The rabbit antiserum L450, raised to the synthetic C-terminal decapeptide of VMAT2, was used at 1:300 and rabbit antiserum L425 specific for the COOH-terminus of gastrin/cholecystokinin was used at 1:400 (antisera provided by the late Dr J. H. Walsh, UCLA, USA and Professor G. Banting, University of Bristol, UK); the mouse monoclonal antibody 28.2, specific for COOH-terminally amidated gastrins was used at 1:2000 (gift from J. H. Walsh), TGN-38 antibody was used at 1:100 (gift from G. Banting) and anti-5-HT antibody (Dako) was used at 1:50.
Confocal microscopy
Transfected HIT-T15 cells (0.2 x 106) were plated on 22 mm poly-l-lysine-coated coverslips, incubated at 37°C for 18 h, followed by 31 °C for 48 h. Coverslips were washed in buffer containing 130 mm NaCl, 4.7 mm KCl, 1.13 mm MgCl2.6H2O, 20 mm Hepes, 10 mm glucose and 1 mm CaCl2 (pH 7.4), mounted on a perfusion chamber and visualised under an oil-immersion objective (Nikon Plan-Apo, x 40, NA 1.3). Live-cell imaging was carried out on a Noran Odyssey confocal system (Noran Instruments, Bicester, UK) at 20 °C. Acidic compartments were initially identified by loading with LysoSensor Blue DND-167 (4 μm) or LysoTracker Green DND-26 (50 nm) (Molecular Probes, Leiden, The Netherlands) immediately prior to imaging and excited with the 363 nm line of the UV laser and 400 nm dichroic (LysoSensor Blue) or 488 nm line of the argon ion laser with 510 nm dichroic (LysoTracker Green). Coverslips were then removed and cells processed for immunohistochemistry as described above. Cells expressing GFP were visualised by excitation at 488 nm with a 510 nm dichroic and 525 ± 45 nm band-pass filter to reduce cellular autofluorescence. A live-cell pH-titration curve was constructed by perfusing cells with buffer containing 125 mm KCl, 20 mm NaCl, 0.5 mm CaCl2, 0.5 mm MgCl2.6H2O, 25 mm of either Mes or Hepes, and 2-5 μm concentrations of the ionophores valinomycin and nigericin. The effects of ammonium chloride (5 mm) and reserpine (1 μm) were examined on vesicles of defined age generated by incubating cells at 22 °C for 2 h, and then at 31 °C for 6 h. In each experiment signals were normalised to the maximum fluorescence at pH 8.0, and fitted to an equation derived from normalised calibration data using FigP (Biosoft, Cambridge, UK), and calibrations after perfusion with 125 mm K+ buffered at pH 8.0 and pH 5.0.
Biochemical labelling studies
Cells (3 x 106) were depleted for 3 h in sulphate-free medium, labelled for 15 min with 100 μCi [35S]sulphate (Amersham Pharmacia Biotech), and chased for up to 160 min in RPMI medium (containing ascorbate, 50 mg l−1). In some experiments cells were incubated with ammonium chloride (5 mm), amine precursors (5-HTP, and l-DOPA, 12.5 μm) or VMAT inhibitors (reserpine, 1 μm; tetrabenazine, 1 μm), during the chase. In release experiments, cells were chased for 160 min, and release stimulated with 55 mm K+ for 15 min. Progastrin-derived peptides were concentrated as previously described and immunoprecipitated with antibodies to COOH-terminal amidated gastrins (Varro et al. 1994). Precipitates were solubilised and separated by reverse-phase high-performance liquid chromatography (HPLC) on a 300 Å PLRP-S column (8 μm, 150 mm x 4.6 mm) (Polymer Laboratories, Church Stretton, UK) with a gradient of acetonitrile in 50 mm NH4HCO3 with on-line scintillation counting (Canberra Packard, Pangbourne, UK) (Varro et al. 1994).
RESULTS
Cellular localisation of VMAT2, gastrin and acidic compartments
First, we asked whether cells co-transfected with VMAT2 and gastrin co-localised these proteins to acidic secretory vesicles capable of loading biogenic amines (i.e. that the expressed VMAT was functional). In double-labelling immunocytochemistry of co-transfected cells, the distribution of VMAT2 overlapped with that of amidated gastrin, which is the mature product of preprogastrin processing (Fig. 2). In cells incubated with LysoSensor and LysoTracker dyes, which are sequestered in acidic compartments, the dyes were co-localised with gastrin. To establish the function of VMAT2, cells were incubated with the 5-HT precursor 5-HTP (10 μm), which is converted to 5-HT by the cytosolic enzyme aromatic l-amino acid decarboxylase thereby providing a substrate for VMAT. In VMAT2-expressing cells incubated in 5-HTP for 10-20 min, but not in the parental cell line (not shown), secretory vesicles were labelled with 5-HT, and double-labelling immunocytochemistry co-localised 5-HT with both gastrin and VMAT2 (Fig. 2). The uptake of 5-HT was inhibited by 1 μm reserpine, and by inhibition of vH+-ATPase with 10 nm concanamycin, or 1 μm bafilomycin (not shown). The data therefore indicate that gastrin and VMAT2 are localised to acidic secretory vesicles, and that VMAT2 is functional.
Figure 2. Gastrin, VMAT2 and 5-HT are localised in acidic compartments identified by LysoSensor and LysoTracker dyes in transfected HIT-T15 cells.
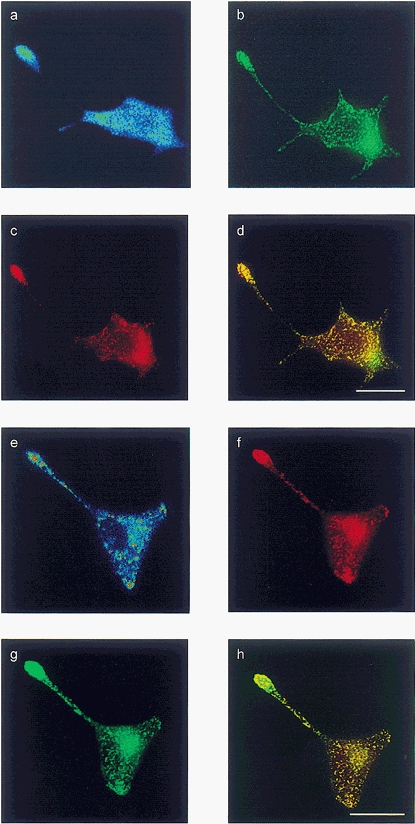
The top four panels show a single cell co-transfected with preprogastrin and VMAT2, loaded with LysoSensor Blue and stained for VMAT2 and 5-HT; the bottom four panels show a single co-transfected cell loaded with LysoTracker, and stained for gastrin and 5-HT. a, LysoSensor Blue; b, VMAT2; c, 5-HT; d, overlay of VMAT2 and 5-HT; e, LysoTracker; f, 5-HT; g, gastrin; h, overlay of gastrin and 5-HT. Scale bars, 20 μm.
Targeting of a preprogastrin-GFP chimera to secretory vesicles
In order to target a pH reporter specifically and directly to secretory vesicles containing gastrin, we constructed a chimera of a pH-sensitive form of GFP (F64L/S65T) inserted in-frame and immediately downstream of a rat preprogastrin sequence retaining all but the C-terminal seven amino acid residues. In fixed cells, amidated gastrin and GFP co-localised to secretory vesicles that were abundant in terminal regions (Fig. 3). In addition, however, there was a perinuclear GFP-rich region in which amidated gastrin was poorly represented. The same region exhibited TGN-38 immunoreactivity and so presumably includes the TGN as well as more proximal compartments of the Golgi complex and endoplasmic reticulum. The amidated gastrin epitope is only liberated after cleavage at Arg94,95 in secretory vesicles (Varro et al. 1994), so that preprogastrin acted to target GFP to secretory vesicles where the chimeric protein was cleaved. In cells expressing both Gas-GFP and VMAT2, there was co-localisation of VMAT2 and GFP (Fig. 3).
Figure 3. Localisation of GFP, amidated gastrin, TGN38 and VMAT2 in HIT-T15 cells.
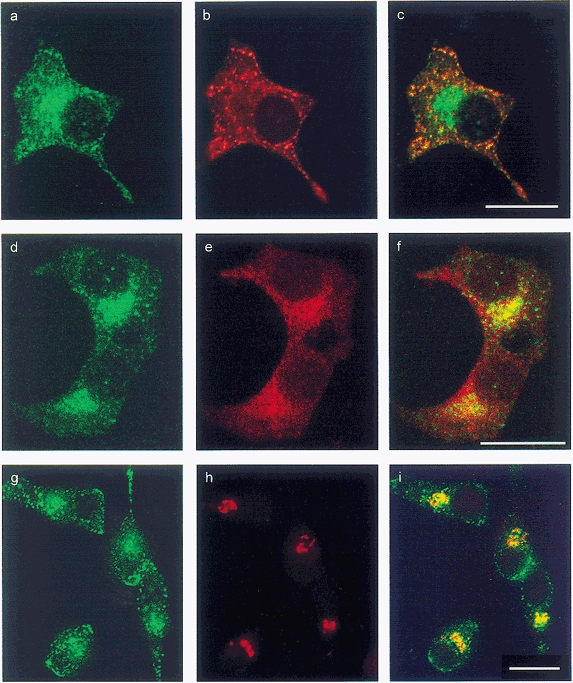
Left (a, d and g), GFP; centre, gastrin (b), VMAT2 (e) and TGN-38 (h); right (c, f and i), overlay of left and centre panels. Note presence of VMAT2 and GFP in terminals and a perinuclear zone that includes the TGN, while gastrin is predominantly localised to terminals. Scale bars, 20 μm.
To establish that GFP in the terminal regions was derived from the perinuclear zone we made use of the fact that exit of progastrin from TGN is blocked at 22 °C (Varro et al. 1994), while there is continuous unstimulated exocytosis from mature vesicles. We found that after 2-4 h at 22 °C there was loss of GFP and gastrin immunoreactivity from the terminal regions, but GFP accumulated in the Golgi complex. When cells were then incubated at 31°C, newly formed GFP-containing vesicles could be observed migrating towards terminal regions, and the vesicle pool in the terminal regions was re-established within 4-6 h (Fig. 4).
Figure 4. Synchronisation of GFP-containing vesicles in terminal regions.
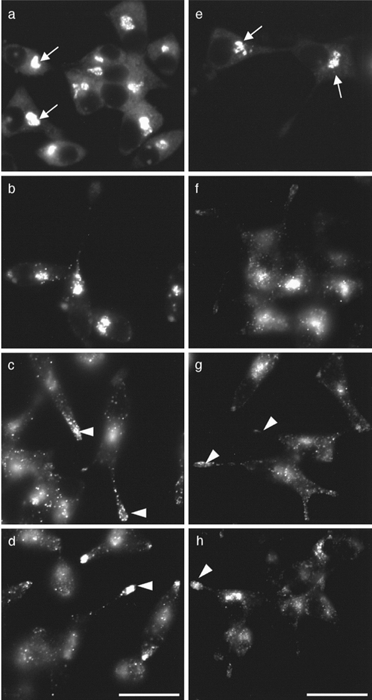
a and e, after 2 h at 22 °C there is loss of vesicles from terminal regions, and GFP is localised to a perinuclear zone (arrows). When cells are then incubated at 31 °C, GFP-positive vesicles appear over the following 2-6 h and progress along processes to populate the expanded terminal region (arrowheads). b and f, 2 h; c and g, 4 h; d and h, 6 h. Sham (a-d) and VMAT2-co-transfected clones (e-h). Scale bars, 20 μm.
pH calibration of GFP
The pH sensitivity of GFP-F64L/S65T in the secretory pathway was determined by permeabilising cells with the ionophores nigericin and valinomycin in media containing 125 mm K+ over the pH range 5-8 (Fig. 5), thereby equilibrating vesicular pH with that of the external buffer. The resulting calibration curve of GFP fluorescence corresponded closely to that derived for GFP-F64L/S65T previously reported; thus the pKa determined in the present study was 6.03, compared with reports of 5.9 to 6.15 (Kneen et al. 1998; Haupts et al. 1998; Llopis et al. 1998) (Fig. 6). The calculated pH in the vesicle-enriched terminal region in two clones of GFP-expressing cells was estimated to be 5.68 ± 0.07 and 5.48 ± 0.11 (mean ±s.e.m., n = 10 and 16, respectively). In the region that includes the Golgi complex, the pH in these clones was 6.19 ± 0.08 and 6.06 ± 0.07 (n = 9 and 11, respectively) (Fig. 7). This region is likely to include the Golgi stack, TGN and some immature vesicles. In cells incubated in 5 mm ammonium chloride, the pH in the terminal regions increased from 5.70 ± 0.17 to 6.55 ± 0.29 (n = 5, P < 0.05, Student's t test), and there was also a significant increase in the Golgi region (6.23 ± 0.09 vs. 6.69 ± 0.09, P < 0.05). These data suggest that GFP-F64L/S65T targeted to secretory vesicles can be used as a pH reporter in living cells.
Figure 5. Confocal microscopy of GFP in cells co-transfected with pGas-GFP and an empty vector (sham co-transfection), or pGas-GFP and pVMAT2zeo.
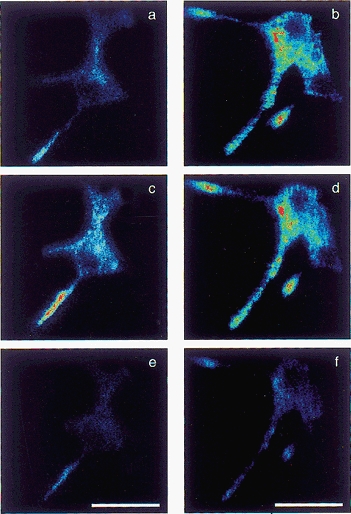
a, a sham co-transfected cell; c and e, the same field after permeabilising cells with buffer at pH 8 and pH 5, respectively. b, d and f, a cell co-transfected with pVMAT2zeo and pGas-GFP, in control conditions (b) and after permeabilisation at pH 8 (d) and pH 5 (f), respectively. Scale bars, 25 μm.
Figure 6. Calibration of vesicular pH using confocal microscopy of GFP.
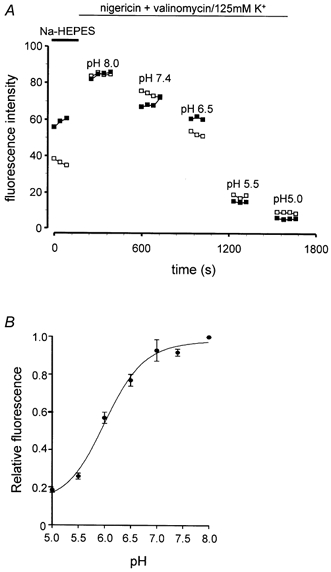
A, representative calibration experiment for Gas-GFP, showing fluorescence in the presence of 4 μm nigericin and 5 μm valinomycin in 125 mm K+ and external pH from 5.0 to 8.0. The fluorescence in a field corresponding to vesicles in a terminal region (□) is shown and in a perinuclear region (▪). B, calibration curve for secretory vesicles; 12-40 experiments at each point; data are means ±s.e.m.
Figure 7. Determination of pH from GFP fluorescence in vesicles and in the Golgi region of sham co-transfected (□) and VMAT2 co-transfected (▪) cells.
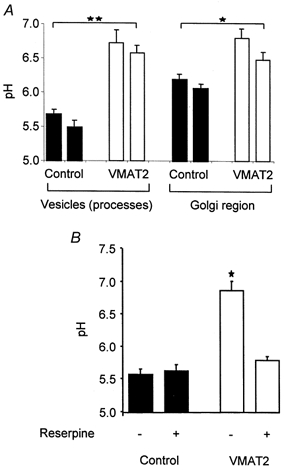
A, in two clones each, the pH in both vesicles and Golgi region was significantly higher in VMAT2-expressing cells. B, reserpine depressed pH in vesicles of VMAT2-expressing cells, but not control cells; mean of at least 3 independent experiments. *P < 0.05,**P < 0.01.
Intravesicular pH after co-transfection of progastrin-GFP and VMAT2
To determine whether VMAT expression was associated with increased intravesicular pH we compared fluorescence in clones expressing Gas-GFP alone, and both Gas-GFP and VMAT2 (Fig. 5). The pH in secretory vesicles in two clones expressing Gas-GFP and VMAT2 was 6.57 ± 0.11 and 6.72 ± 0.20 (n = 21 and 13, respectively), and was significantly higher than in clones expressing Gas-GFP alone (see above, P < 0.01) (Fig. 7). Similarly, in the region that includes the Golgi complex, the pH in two VMAT2-expressing clones (6.48 ± 0.11 and 6.80 ± 0.14) was significantly greater than in control clones (see above, P < 0.05, n = 9-15) (Fig. 7).
Modulation of vesicular pH by VMAT2 activity
We attributed the raised pH in secretory vesicles expressing VMAT2 to the transport activity of the protein. To test this idea, we examined the effect of reserpine on vesicular pH. In these experiments we studied vesicles of defined age produced by incubation at 22 °C followed by a chase at 31 °C for 6 h in the presence or absence of reserpine (see above). Incubation with reserpine significantly lowered intravesicular pH in Gas-GFP- and VMAT2-expressing cells to 5.79 ± 0.06, in keeping with the idea that VMAT2 produced an increase in granular pH (P < 0.05) (Fig. 7). In contrast, reserpine had no effect on vesicular pH in cells expressing Gas-GFP but not VMAT2.
Expression of VMAT2 inhibits cleavage of G34
In order to determine whether VMAT2 expression regulated prohormone cleavage we conducted pulse-chase labelling studies of progastrin cleavage using [35S]sulphate. The appearance and relative abundance of labelled G34 and G17 in transfected HIT-T15 cells were consistent with progressive cleavage of G34 to G17 from 40-160 min after labelling. Moreover, the cleavage of G34 was dependent on vesicle acidification as it was inhibited by 5 mm NH4Cl (control: 73.4 ± 5.3 % G34 cleaved after 80 min chase, compared with 35.5 ± 1.8 % in ammonium chloride; P < 0.01, t test). Co-expression of preprogastrin and VMAT2 was associated with inhibition of G34 cleavage at each time point (Fig. 8). The expression of VMAT2 was not associated with changes in the initial rate of appearance of G34, or of total counts in G34/G17, indicating no effect on cleavage at other sites in progastrin, or COOH-terminal amidation (not shown). The VMAT2 inhibitors reserpine (1 μm) and tetrabenazine (1 μm) reversed the delayed cleavage of G34 in VMAT2-expressing clones (Fig. 8), but not in control clones (expressing preprogastrin but not VMAT2). These data therefore indicate that in HIT-T15 cells, G34 cleavage is inhibited by raising vesicle pH and by co-expression of VMAT2.
Figure 8. Post-translational processing of progastrin in HIT-T15 cells transfected with preprogastrin and VMAT2.
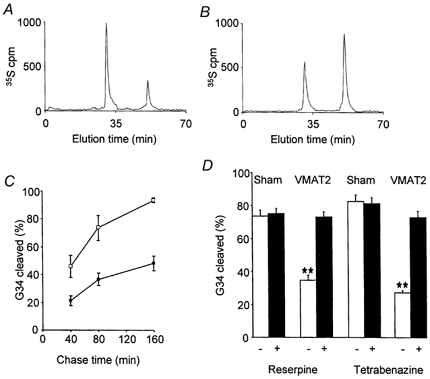
A and B, representative HPLC traces of [35S]sulphate-labelled G17 and G34, after 80 min chase following 15 min pulse labelling in a control clone expressing preprogastrin (A), and in a clone co-expressing VMAT2 (B). C, the time course of cleavage of G34 in VMAT2-expressing cells (•) and in control cells (□) (bars are means ±s.e.m. for 3 independent experiments). D, the inhibition of G34 cleavage in cells expressing VMAT2 by 1 μm reserpine or tetrabenazine (▪); **P < 0.01 (t test) vs. control; in sham co-transfected cells, reserpine and tetrabenazine had no effect. Mean of at least 3 independent experiments; similar results were obtained with 2 separate clones of preprogastrin/VMAT2 expressing cells, and of preprogastrin/sham co-transfected cells.
VMAT2 expression influences the identity of secreted gastrins
Finally, we asked whether VMAT2-dependent inhibition of prohormone cleavage influenced the identity of the secreted peptide (i.e. is functionally significant), by examining the ratio of [35S]G34:[35S]G17 released into the medium following a depolarising stimulus. The basal release of [35S]gastrins over 15 min was virtually undetectable, but [35S]gastrin released by depolarisation of both control and VMAT2-expressing clones, was approximately 40 % of the total labelled pool. The ratio G34:G17 in medium was similar to that in cell extracts, and was significantly greater in the VMAT2-expressing cells (Fig. 9). Thus VMAT2 expression inhibits cleavage in a pool of secretory granules available for evoked secretion, thereby determining the identity of the secreted peptide product.
Figure 9. Depolarisation-evoked secretion of gastrin from control and VMAT2 co-transfected clones.
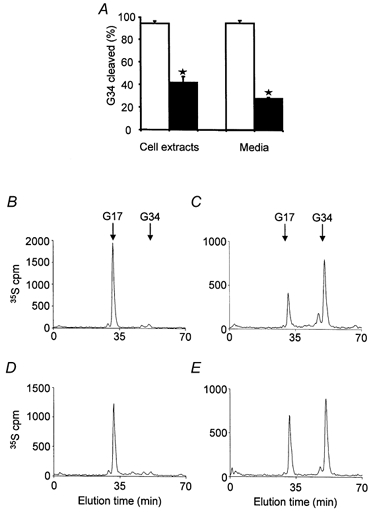
A, cleavage (% total) of [35S]G34 in cell extracts and in media after stimulating release with 50 mm K+ in sham co-transfected cells (□) and VMAT2 co-transfected cells (▪); means of 4-5 independent experiments, *P < 0.01.B and C, representative traces for [35S]peptides in cell extracts of control and VMAT2-expressing cells, respectively. D and E, representative HPLC traces of media after depolarisation for 15 min from control and VMAT2 co-transfected cells, respectively.
DISCUSSION
Communication between cells in the nervous and endocrine systems depends on the evoked exocytosis of transmitter molecules, including regulatory peptides and biogenic amines. At target cells, there are many examples of interaction between these two classes of signalling molecule (Hokfelt, 1991). The results of the present study suggest a novel class of interaction that determines the identity of the regulatory peptide secreted. We have shown that expression of VMAT2, which is responsible for biogenic amine loading in secretory granules, is associated with modulation of a pH-sensitive cleavage step in progastrin processing. Moreover, VMAT activity leads to an increase in secretory vesicle pH as revealed by the fluorescence of a pH-sensitive form of GFP. We propose, therefore, that in neuroendocrine cells the VMATs responsible for loading biogenic amines may also modulate internal pH over a range that influences prohormone cleavage. There is widespread expression of VMATs in neuroendocrine cells (Weihe et al. 1994), so that this mechanism may influence the cleavage of many different regulatory peptide precursors and would account for the observation that the VMAT inhibitors reserpine and tetrabenazine increased cleavage of chromogranin A and proenkephalin as well as gastrin (Wilson et al. 1980; Eiden et al. 1984; Wilson, 1991; Watkinson & Robinson, 1992; Wolkersdorfer et al. 1996; Voronina et al. 1997).
The HIT-T15 cells used in this study exhibit many similarities to human G-cells, and some important differences that are experimentally useful. Wild-type G-cells and HIT-T15 cells express both prohormone convertase type 1/3 and type 2 (PC1/3 and PC2) (Macro et al. 1997; Bishop et al. 1998). Unlike normal G-cells, however, HIT-T15 cells do not express VMATs, which offers the opportunity to experimentally restore the capacity for amine transport; moreover their morphology readily allows detection of secretory vesicles progressing from the Golgi to terminal regions. The present findings extend previous studies in native G-cells by showing that (a) expression of VMATs is sufficient to confer the capacity to inhibit precursor cleavage, (b) that VMAT expression is capable of raising pH in secretory vesicles approximately 1 pH unit, (c) that the VMAT inhibitor reserpine blocks both the inhibition of G34 cleavage and the increase in vesicle pH, and (d) the effects of VMAT on pH and prohormone cleavage are similar to those produced by ammonium chloride.
Studies using immunocytochemical detection of the weak base 3(2,4-dinitroanilino-)-3′-amino-N-methyldipropylamine (DAMP) showed that secretory vesicles in β-cells of the islets of Langerhans were acidic and that proinsulin cleavage was associated with an acidic interior in secretory vesicles (Rhodes et al. 1987). Recent studies of the pH in the secretory pathway have made use of targeted fluorescent probes as well as a pH-sensitive form of GFP (Kim et al. 1996; Demaurex et al. 1998; Han et al. 1999). A variety of secretory vesicle proteins have been used as targeting vectors for the sequestration of GFP in secretory vesicles, including chromogranin B, neuropeptide Y, tissue plasminogen activator and atrial natriuretic peptide (Kaether et al. 1997; Lang et al. 1997; Lochner et al. 1998; Han et al. 1999). The preprogastrin-GFP chimera used here retained the Arg94,95 of progastrin which is normally cleaved rapidly when the precursor leaves the TGN (Varro et al. 1994) and which is not sensitive to raising secretory vesicle pH. The detection of amidated gastrin generated from the chimera therefore provides an indicator of the targeting and liberation of GFP in secretory vesicles. The present estimates of pH in secretory vesicles in HIT cells are consistent with previous reports using other experimental approaches (Hutton, 1982; Mellman et al. 1986; Orci et al. 1986; Johnson, 1988), but the use of GFP as a pH reporter offers the advantage of allowing pH determination in secretory vesicles in intact living cells.
It is clear that vH+-ATPase activity is required for cleavage of prosomatostatin in the TGN (Xu & Shields, 1994), and secretogranin II (Urbéet al. 1997), insulin (Rhodes et al. 1987) and progastrin (Voronina et al. 1997) in secretory vesicles. The pH optima of both PC1/3 and PC2 is between 5.5 and 6.0 (Bailyes et al. 1992, 1995; Zhou & Lindberg, 1993, 1994). However, the relationships between pH and the activity of prohormone convertases are complex. For example, over the range pH 6-7 the catalytic activities of the 88 and 78 kDa forms of PC1/3 are reported to differ (Zhou & Lindberg, 1994). Moreover, the effect of pH on the activity of PC1/3 and PC2 was modified when these proteins were expressed in a membrane-tethered form compared with the soluble forms (Bruzzaniti et al. 1999). Inhibition of cleavage of G34 may reflect a direct modulation of PC activity by raised intravesicular pH. However, G34 cleavage is thought to be mediated by PC2 (Dickinson et al. 1995), the maturation of which is reported to depend on secretory vesicle acidification (Lamango et al. 1999). In addition, there are other pH-dependent events within secretory vesicles that potentially influence prohormone cleavage. For example, the electron-dense core of secretory vesicles in G-cells is dissipated by increased pH (Voronina et al. 1997), and vesicle proteins such as the chromogranins exhibit pH- and calcium-dependent aggregation, and membrane association (Yoo & Lewis, 1992). Moreover transport of other small molecules, e.g. ATP, depends on the proton gradient (Njus et al. 1986). An increase in secretory vesicle pH may therefore be expected to influence several aspects of vesicle function, including the organisation of the secretory vesicle matrix, the capacity for transport of other small molecules and prohormone convertase maturation and activity. Thus while the present findings indicate a mechanism for modulating secretory vesicle pH over a range influencing prohormone cleavage, further work will be required to determine the nature of this control mechanism.
The vH+-ATPase generates a proton electrical gradient (Δψ) and a pH gradient (Mellman et al. 1986). The vesicular monoamine-proton exchangers are thought to use the chemical gradient more than the electrical gradient (Njus et al. 1986; Schuldiner et al. 1995). Possibly, VMAT activity leads to proton extrusion such that the proton pump is unable to maintain internal pH; alternatively re-protonation of accumulated amines transported by VMAT may raise intravesicular pH. The present data suggest that VMAT2 activity influences vesicular pH during the period that secretory vesicles mature and their peptide contents are cleaved. These effects are therefore distinct from those reported to occur in vesicles just prior to exocytosis. Thus in PC12 cells, calcium-dependent vesicle alkalinization was linked to exocytosis and solubilisation of the peptide core of secretory vesicles (Han et al. 1999). However, glutamate transport into vesicles in β-cells has been reported to stimulate exocytosis possibly by a mechanism involving vesicle acidification, so that the relationship between vesicle pH and exocytosis may vary between cell types.
Cleavage of G34 is functionally important because although G34 and G17 have similar affinities for the gastrin-CCKB receptor, they differ 5-fold in metabolic clearance rates (Walsh et al. 1974, 1976). Cleavage of G34 therefore results in lower and more transient increases in plasma gastrin concentration. The importance of maintaining concentrations of gastrin in plasma in the physiological range is emphasised by recent observations in mice in which the gastrin gene has been deleted, where there is little or no gastric acid secretion and there is impaired development of the gastric glands (Koh et al. 1997; Friis-Hansen et al. 1998). Conversely, in transgenic mice that overexpress the gastrin gene, and have modestly elevated plasma gastrin concentrations, there is a long-term progression to gastric cancer (Wang et al. 2000). Moreover, increased plasma gastrin is a risk factor for colon cancer (Thorburn et al. 1998). The identification of a novel mechanism that determines the ratio of G34 to G17 released by endocrine cells therefore has clinical as well as physiological implications.
Acknowledgments
We are grateful to the Wellcome Trust, and Medical Research Council for grant support. We thank Cathy McLean for help with radioimmunoassays, Susan Jones for some help with immunocytochemistry, Oleg Gerasimenko and Alexei Tepikin for useful discussions regarding confocal microscopy. John Walsh and George Banting generously donated antibodies and Len Best kindly provided HIT-T15 cells.
References
- Adams M, Boarder MR. Secretion of [Met]enkephalyl-Arg6-Phe7-related peptides and catecholamines from bovine adrenal chromaffin cells: modification by changes in cyclic AMP and by treatment with reserpine. Journal of Neuroscience. 1987;49:208–215. doi: 10.1111/j.1471-4159.1987.tb03416.x. [DOI] [PubMed] [Google Scholar]
- Bailyes EM, Shennan KIJ, Seal AJ, Smeekens SP, Steiner DF, Hutton JC, Docherty K. A member of the eukaryotic subtilisin family (PC3) has the enzymic properties of the type 1 proinsulin-converting endopeptidase. Biochemical Journal. 1992;285:391–394. doi: 10.1042/bj2850391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailyes EM, Shennan KI, Usac EF, Arden SD, Guest PC, Docherty K, Hutton JC. Differences between the catalytic properties of recombinant human PC2 and endogenous rat PC2. Biochemical Journal. 1995;309:587–594. doi: 10.1042/bj3090587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop L, Dimaline R, Blackmore C, Deavall D, Dockray GJ, Varro A. Modulation of the cleavage of the gastrin precursor by phosphorylation. Gastroenterology. 1998;115:1154–1162. doi: 10.1016/s0016-5085(98)70086-1. [DOI] [PubMed] [Google Scholar]
- Bruzzaniti A, Marx R, Mains RE. Activation and routing of membrane-tethered prohormone convertases 1 and 2. Journal of Biological Chemistry. 1999;274:24703–24713. doi: 10.1074/jbc.274.35.24703. [DOI] [PubMed] [Google Scholar]
- Davidson HW, Rhodes CJ, Hutton JC. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic β cell via two distinct site-specific endopeptidases. Nature. 1988;333:93–96. doi: 10.1038/333093a0. [DOI] [PubMed] [Google Scholar]
- Demaurex N, Furuya W, D'Souza S, Bonifacino JS, Grinstein S. Mechanism of acidification of the trans-Golgi network (TGN): in situ measurements of pH using retrieval of TGN38 and furin from the cell surface. Journal of Biological Chemistry. 1998;273:2044–2051. doi: 10.1074/jbc.273.4.2044. [DOI] [PubMed] [Google Scholar]
- Dickinson CJ, Sawada M, Guo YJ, Finniss S, Yamada T. Specificity of prohormone convertase endoproteolysis of progastrin in AtT-20 cells. Journal of Clinical Investigation. 1995;96:1425–1431. doi: 10.1172/JCI118178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimaline R, Struthers J. Expression and regulation of a vesicular monoamine transporter (VMAT2) in rat stomach: a putative histamine transporter. Journal of Physiology. 1996;490:249–256. doi: 10.1113/jphysiol.1996.sp021140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden LE, Giraud P, Affolter HU, Herbert E, Hotchkiss AJ. Alternative modes of enkephalin biosynthesis regulation by resperpine and cyclic AMP in cultured chromaffin cells. Proceedings of the National Academy of Sciences of the USA. 1984;81:3949–3953. doi: 10.1073/pnas.81.13.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JD, Eiden LE, Hoffman BJ. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proceedings of the National Academy of Sciences of the USA. 1992;89:10993–10997. doi: 10.1073/pnas.89.22.10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis-Hansen L, Sundler F, Li Y, Gillespie PJ, Saunders TL, Greenson JK, Owyang C, Rehfeld JF, Samuelson LC. Impaired gastric acid secretion in gastrin-deficient mice. American Journal of Physiology. 1998;274:G561–568. doi: 10.1152/ajpgi.1998.274.3.G561. [DOI] [PubMed] [Google Scholar]
- Han W, Li D, Stout AK, Takimoto K, Levitan ES. Ca2+-induced deprotonation of peptide hormones inside secretory vesicles in preparation for release. Journal of Neuroscience. 1999;19:900–905. doi: 10.1523/JNEUROSCI.19-03-00900.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupts U, Maiti S, Schwille P, Webb WW. Dynamics of fluorescence fluctuations in green fluorescent protein observed by fluorescence correlation spectroscopy. Proceedings of the National Academy of Sciences of the USA. 1998;95:13573–13578. doi: 10.1073/pnas.95.23.13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T. Neuropeptides in perspective: The last ten years. Neuron. 1991;7:867–879. doi: 10.1016/0896-6273(91)90333-u. [DOI] [PubMed] [Google Scholar]
- Hutton JC. The internal pH and membrane potential of the insulin-secretory granule. Biochemical Journal. 1982;204:171–178. doi: 10.1042/bj2040171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RGJ. Accumulation of biological amines into chromaffin granules: a model for hormone and neurotransmitter transport. Physiological Reviews. 1988;68:232–307. doi: 10.1152/physrev.1988.68.1.232. [DOI] [PubMed] [Google Scholar]
- Kaether C, Salm T, Glombik M, Almers W, Gerdes HH. Targeting of green fluorescent protein to neuroendocrine secretory granules: a new tool for real time studies of regulated protein secretion. European Journal of Cell Biology. 1997;74:133–142. [PubMed] [Google Scholar]
- Kim JH, Lingwood CA, Williams DB, Furuya W, Manolson MF, Grinstein S. Dynamic measurement of the pH of the Golgi complex in living cells using retrograde transport of the verotoxin receptor. Journal of Cell Biology. 1996;134:1387–1399. doi: 10.1083/jcb.134.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneen M, Farinas J, Li Y, Verkman AS. Green fluorescent protein as a noninvasive intracellular pH indicator. Biophysical Journal. 1998;74:1591–1599. doi: 10.1016/S0006-3495(98)77870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TJ, Goldenring JR, Ito S, Mashimo H, Kopin AS, Varro A, Dockray GJ, Wang TC. Gastrin deficiency results in altered gastric differentiation and decreased colonic proliferation in mice. Gastroenterology. 1997;113:1015–1025. doi: 10.1016/s0016-5085(97)70199-9. [DOI] [PubMed] [Google Scholar]
- Lamango NS, Apletalina E, Liu J, Lindberg I. The proteolytic maturation of prohormone convertase 2 (PC2) is a pH- driven process. Archives of Biochemistry and Biophysics. 1999;362:275–282. doi: 10.1006/abbi.1998.1033. [DOI] [PubMed] [Google Scholar]
- Lang T, Wacker I, Steyer J, Kaether C, Wunderlich I, Soldati T, Gerdes HH, Almers W. Ca2+-triggered peptide secretion in single cells imaged with green fluorescent protein and evanescent-wave microscopy. Neuron. 1997;18:857–863. doi: 10.1016/s0896-6273(00)80325-6. published erratum appears in Neuron (1997) 19, following p. 463. [DOI] [PubMed] [Google Scholar]
- Lindberg I. Reserpine-induced alterations in the processing of proenkephalin in cultured chromaffin cells. Journal of Biological Chemistry. 1986;261:16317–16322. [PubMed] [Google Scholar]
- Liu Y, Edwards RH. The role of vesicular transport proteins in synaptic transmission and neural degeneration. Annual Review of Neuroscience. 1997;20:125–156. doi: 10.1146/annurev.neuro.20.1.125. [DOI] [PubMed] [Google Scholar]
- Liu Y, Peter D, Roghani A, Schuldiner S, Prive GG, Eisenberg D, Brecha N, Edwards RH. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992;70:539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- Llopis J, McCaffery JM, Miyawaki A, Farquhar MG, Tsien RY. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proceedings of the National Academy of Sciences of the USA. 1998;95:6803–6808. doi: 10.1073/pnas.95.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner JE, Kingma M, Kuhn S, Meliza CD, Cutler B, Scalettar BA. Real-time imaging of the axonal transport of granules containing a tissue plasminogen activator/green fluorescent protein hybrid. Molecular Biology of the Cell. 1998;9:2463–2476. doi: 10.1091/mbc.9.9.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macro JA, Bate GW, Varro A, Vaillant C, Seidah NG, Dimaline R, Dockray GJ. Regulation by gastric acid of the processing of progastrin-derived peptides in rat antral mucosa. Journal of Physiology. 1997;502:409–419. doi: 10.1111/j.1469-7793.1997.409bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annual Review of Biochemistry. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Njus D, Kelley PM, Hardanek GJ. Bioenergetics of secretory vesicles. Biochimica et Biophysica Acta. 1986;853:237–265. doi: 10.1016/0304-4173(87)90003-6. [DOI] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Amherdt M, Madsen O, Perrelet A, Vassalli J-D, Anderson RGW. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. Journal of Cell Biology. 1986;103:2273–2281. doi: 10.1083/jcb.103.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse AGE. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. Journal of Histochemistry and Cytochemistry. 1969;17:303–313. doi: 10.1177/17.5.303. [DOI] [PubMed] [Google Scholar]
- Peter D, Jimenez J, Liu Y, Kim J, Edwards RH. The chromaffin granule and synaptic vesicle amine transporters differ in substrate recognition and sensitivity to inhibitors. Journal of Biological Chemistry. 1994;269:7231–7237. [PubMed] [Google Scholar]
- Rhodes CJ, Lucas CA, Mutkoski RL, Orci L, Halban P. Stimulation by ATP of proinsulin to insulin conversion in isolated rat pancreatic islet secretory granules. Journal of Biological Chemistry. 1987;262:10712–10717. [PubMed] [Google Scholar]
- Schuldiner S, Shirvan A, Linial M. Vesicular neurotransmitter transporters: from bacteria to humans. Physiological Reviews. 1995;75:369–392. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- Thorburn CM, Friedman GD, Dickinson CJ, Vogelman JH, Orentreich N, Parsonnet J. Gastrin and colorectal cancer: A prospective study. Gastroenterology. 1998;115:275–280. doi: 10.1016/s0016-5085(98)70193-3. [DOI] [PubMed] [Google Scholar]
- Urbé S, Dittie AS, Tooze SA. pH-dependent processing of secretogranin II by the endopeptidase PC2 in isolated immature secretory granules. Biochemical Journal. 1997;321:65–74. doi: 10.1042/bj3210065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varro A, Henry J, Vaillant C, Dockray GJ. Discrimination between temperature- and brefeldin A-sensitive steps in the sulfation, phosphorylation, and cleavage of progastrin and its derivatives. Journal of Biological Chemistry. 1994;269:20764–20770. [PubMed] [Google Scholar]
- Varro A, Nemeth J, Bridson J, Lee CM, Moore S, Dockray GJ. Processing of the gastrin precursor: modulation of phosphorylated, sulfated and amidated products. Journal of Biological Chemistry. 1990;265:21476–21481. [PubMed] [Google Scholar]
- Voronina S, Henry J, Vaillant C, Dockray GJ, Varro A. Amine precursor uptake and decarboxylation: significance for processing of the rat gastrin precursor. Journal of Physiology. 1997;501:363–374. doi: 10.1111/j.1469-7793.1997.363bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JH, Debas HT, Grossman MI. Pure human big gastrin: immunochemical properties, disappearance half time and acid stimulating action in dogs. Journal of Clinical Investigation. 1974;54:477–485. doi: 10.1172/JCI107783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JH, Isenberg JI, Ansfield J, Maxwell V. Clearance and acid-stimulating action of human big and little gastrins in duodenal ulcer subjects. Journal of Clinical Investigation. 1976;57:1125–1131. doi: 10.1172/JCI108379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TC, Dangler CA, Chen D, Goldenring JR, Koh TJ, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ, Fox JG. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- Watkinson A, Robinson I. Reserpine-induced processing of chromogranin A in cultured bovine adrenal chromaffin cells. Journal of Neurochemistry. 1992;58:877–883. doi: 10.1111/j.1471-4159.1992.tb09338.x. [DOI] [PubMed] [Google Scholar]
- Weihe E, Schafer MKH, Erickson JD, Eiden LE. Localization of vesicular monoamine transporter isoforms (VMAT1 and VMAT2) to endocrine cells and neurons in rat. Journal of Molecular Neuroscience. 1994;5:149–164. doi: 10.1007/BF02736730. [DOI] [PubMed] [Google Scholar]
- Wilson SP. Processing of proenkephalin in adrenal chromaffin cells. Journal of Neurochemistry. 1991;57:876–881. doi: 10.1111/j.1471-4159.1991.tb08232.x. [DOI] [PubMed] [Google Scholar]
- Wilson SP, Chang K-J, Viveros OH. Synthesis of enkephalins by adrenal medullary chromaffin cells: reserpine increases incorporation of radiolabeled amino acids. Proceedings of the National Academy of Sciences of the USA. 1980;77:4364–4368. doi: 10.1073/pnas.77.7.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkersdorfer M, Laslop A, Lazure C, Fischer-Colbrie R, Winkler H. Processing of chromogranins in chromaffin cell culture: effects of reserpine and a α-methyl-p-tyrosine. Biochemical Journal. 1996;316:953–958. doi: 10.1042/bj3160953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Shields D. Prosomatostatin processing in permeabilized cells: endoproteolytic cleavage is mediated by a vacuolar ATPase that generates an acidic pH in the trans-Golgi network. Journal of Biological Chemistry. 1994;269:22875–22881. [PubMed] [Google Scholar]
- Yoo SH, Lewis MS. Effects of pH and Ca2+ on monomer-dimer and monomer-tetramer equilibria of chromogranin A. Journal of Biological Chemistry. 1992;267:11236–11241. [PubMed] [Google Scholar]
- Zhou Y, Lindberg I. Purification and characterization of the prohormone convertase PC1 (PC3) Journal of Biological Chemistry. 1993;268:5615–5623. [PubMed] [Google Scholar]
- Zhou Y, Lindberg I. Enzymatic properties of carboxyl-terminally truncated prohormone convertase 1 (PC1/SPC3) and evidence for autocatalytic conversion. Journal of Biological Chemistry. 1994;269:18408–18413. [PubMed] [Google Scholar]


