Abstract
Monolayer cultures of rat fetal distal lung epithelial (FDLE) cells generated larger spontaneous short circuit currents (ISC) when maintained (48 h) at neonatal alveolar PO2 (100 mmHg) than at fetal PO2 (23 mmHg). When cells were shifted between these atmospheres in order to impose a rise in PO2 equivalent to that seen at birth, no rise in ISC was seen after 6 h but the response was fully established by 24 h.
Studies of basolaterally permeabilised cells revealed a small rise in apical Na+ conductance (GNa) 6 h after PO2 was raised but no further change had occurred by 24 h. A substantial rise was, however, seen after 48 h.
Reporter gene assays showed that no activation of the α-ENaC (epithelial Na+ channel α-subunit) promoter was discernible 24 h after PO2 was raised but increased transcriptional activity was seen at 48 h.
Studies of apically permeabilised cells showed that a small rise in Na+ pump capacity was evident 6 h after PO2 was raised and, in common with the rise in ISC, this effect was fully established by 24 h. The rise in ISC thus develops 6-24 h after PO2 is raised and is due, primarily, to increased Na+ pump capacity.
The increase in GNa thus coincides with activation of the α-ENaC promoter but these effects occur after the rise in ISC is fully established and so cannot underlie this physiological response. The increased transcription may be an adaptation to increased Na+ transport and not its cause.
If breathing is to be established at birth, the liquid secreted into the lung lumen during fetal life (see Olver & Strang, 1974) must first be removed from the potential airspaces. During the final stages of pregnancy, and particularly during labour and birth, the distal lung epithelia thus cease secreting liquid and begin to absorb the fluid already present (Walters & Olver, 1978; Brown et al. 1983). This absorption is driven by the active withdrawal of Na+ from the lung lumen and depends critically upon amiloride-sensitive Na+ channels (Olver et al. 1986; Hummler et al. 1996; Matalon & O’Brodovich, 1999). Although the high levels of fetal adrenaline seen during labour elicit a phenotypic transition from net secretion to net absorption (Brown et al. 1983), the lung retains its Na+-absorbing phenotype throughout adult life despite the rapid fall in circulating adrenaline that occurs post partum. Mechanisms other than acute control via adrenoceptors must, therefore, contribute to the regulation of alveolar Na+ transport during the perinatal period (see review by Matalon & O’Brodovich, 1999). It has recently become clear that raising ambient PO2 from its fetal level evokes increased Na+ absorption in isolated rat fetal distal lung epithelial (FDLE) cells (Pitkänen et al. 1996; Rafii et al. 1998; Ramminger et al. 2000). This observation, together with some earlier data, suggests that the rise in PO2 that occurs in the first hours of independent life (i.e. from 23 to 100 mmHg) might play an important role in the functional maturation of the lung by stabilising the newly acquired Na+-absorbing phenotype (Acarregui et al. 1993; Barker & Gatzy, 1993; Pitkänen et al. 1996; Round et al. 1999; Ramminger et al. 2000). Despite its potential importance, the means by which O2 exerts control over Na+ transport are not well understood. It has, however, been proposed that it involves increased transcription of genes encoding the protein subunits (α, β and γ) which form the epithelial Na+ channel (ENaC, see Canessa et al. 1994) and that this process, in turn, depends upon the activation of nuclear factor κB (NF-κB), a redox-sensitive transcription factor (Pitkänen et al. 1996; Rafii et al. 1998). The aim of the present study was therefore to define the events underlying O2-evoked Na+ transport. Some of the data have been presented to The Physiological Society (Baines et al. 2000b).
METHODS
Solutions
Physiological salt solution was composed of (mm): NaCl, 117; NaHCO3, 25; KCl, 4.7; MgSO4, 1.2; KH2PO4, 1.2; CaCl2, 2.5; d-glucose, 11; pH 7.3-7.4 when bubbled with 5 % CO2. Sodium gluconate solution was prepared by isosmotically replacing all Cl− in this solution with gluconate whereas, in potassium gluconate solution, all Na+ was also replaced by K+. The amount of Ca2+ added to these gluconate-containing solutions was raised to 11.5 mm to maintain Ca2+ activity despite gluconate’s capacity to bind this ion.
Cell culture
Time mated, pregnant Sprague-Dawley rats were anaesthetised (3 % halothane) 72 h before the pregnancy’s full term (22 days). Their fetuses were then delivered by Caesarean section, immediately decapitated and fetal lung tissue removed; the anaesthetised animals were then killed by cervical dislocation/exsanguination without regaining consciousness. These procedures accorded with legislation currently in force in the UK and with the University of Dundee’s animal welfare guidelines. The fetal lung tissue was collected into ice-cold Hank’s balanced salt solution and FDLE cells isolated and cultured on Transwell-col membranes (Costar, High Wycombe, UK) in serum-free medium PC-1 (Biowhittaker, Wokingham, UK). The methods used are described elsewhere (Ramminger et al. 2000). The cells consistently become integrated into coherent epithelial layers under these conditions.
Quantification of ion transport processes
Cultured epithelia were mounted in Ussing chambers and bathed with physiological salt solution (15 ml on each side of the cell layer) so that transepithelial ion transport processes could be quantified electrometrically (see Pitkänen et al. 1996; Ramminger et al. 2000). It is well established that rat FDLE cells generate a spontaneous short circuit current (ISC) under these conditions and that this current is predominantly due to the absorption of Na+ from the apical solution (Barker et al. 1992; Pitkänen et al. 1996; Matalon & O’Brodovich, 1999; Ramminger et al. 1999, 2000). In the present study, changes in ISC were therefore attributed to changes in Na+ transport. In some experiments the cells were first treated with apical amiloride (10 μm) in order to block the Na+ channels in this membrane. Nystatin (50 μm) was then added to the apical bath in order to introduce an exogenous Na+ conductance into this membrane; this consistently evoked a slowly developing (3-5 min) rise in ISC attributable to the extrusion of Na+ across the baslateral membrane (see Lewis et al. 1977). We therefore measured the rapid (≈15 s) fall in ISC evoked by basolateral ouabain (1 mm) in order to estimate the Na+ extrusion capacity of the basolateral Na+ pump. This method is described elsewhere (Ramminger et al. 2000). Experiments were also undertaken using cells that had been permeabilised using basolateral nystatin in order to explore the conductive properties of the apical membrane. In these experiments the cells were first bathed symmetrically with a cytoplasm-like solution which was prepared by mixing the standard physiological salt solution with potassium gluconate solution at a ratio of 8.1:91.9. The ionic composition of the solution produced in this way was thus: Na+, 11.5 mm; K+, 136.4 mm; Cl−, 10.3 mm; gluconate, 122 mm; HCO3−, 25 mm; Mg2+, 1.2 mm; H2PO4−, 1.2 mm; Ca2+, ≈2.5 mm. In these experiments the basolaterally permeabilised epithelia were subsequently exposed to an inwardly directed Na+ gradient that was imposed by withdrawing an aliquot (5 ml) of solution from the apical bath and replacing it with a second solution prepared by mixing the standard physiological salt solution with sodium gluconate solution at a ratio of 8.1:91.9. In this way apical Na+ was raised to 55 mm by selectively replacing K+ whilst the concentrations of all other ions remained constant. In electrometric studies of intact epithelia, positive ISC is defined as the current carried by cations moving from the apical to the basolateral solutions. In studies of nystatin-permeabilised preparations, however, positive currents are defined as those which would be carried by cations leaving the cytoplasm. These are standard electrophysiological conventions.
Luciferase reporter gene assay
The 5′ flanking region of the α-ENaC gene was amplified from 1 μg of genomic DNA, obtained from A549 human adenocarcinoma cells, by the polymerase chain reaction (PCR) using Taq DNA polymerase in a reaction mixture containing 75 mm Tris-HCl (pH 9.0), 200 mm (NH4)2SO4, 0.1 % Tween-20, 15 mm MgCl2, 200 μm dNTPs and 0.5 μm of each primer. The primers (sense: 5′-CACACAGGTACCCAGCACCCAGAGCA-3′, anti-sense: 5′-CACACACTCGAGGGGGTGGCGAGGAAT-3′) were designed by reference to the published human sequence (GenBank accession number U81961) and included restriction enzyme sites (underlined) for Kpn I and Xho I. The PCR reaction was continued for 35 denaturing- annealing- polymerisation cycles (30 s at 94 °C-1 min at 56 °C-1 min at 72 °C) and the resultant products fractionated on 1 % agarose gels. The appropriate DNA product was extracted from the gel and cloned into p GEMT (Promega, Southampton, Herts, UK). Plasmids from several clones were isolated (Wizard Plus SV Miniprep System, Promega, UK) and sequenced to verify their origin. The DNA was then excised from p GEMT using Kpn I and Xho I, and directionally subcloned into a p GL3 Basic Vector (Promega) to produce a luciferase reporter construct (p GL3E2.2) containing the α-ENaC promoter region. Experiments were also undertaken using luciferase reporter construct (p GL3E.control) containing a constitutively active SV40 promoter, and a construct (p GL3E.basal) that did not include any promoter. All plasmids used for transfection experiments were isolated using an Endo Free Plasmid Maxi Kit (Qiagen Ltd, Crawley West Sussex, UK). To transfect these constructs into the FDLE cells, isolated cells on Transwell membranes that had been cultured overnight were bathed with 250 μl of antibiotic-free medium PC1 containing the appropriate reporter construct (1 μg) and 10 μg of lipofectamine. After 5 h, the cells were flooded with ≈2 ml of antibiotic-free PC1 medium and incubated overnight. The following day this medium was replaced and the cells were cultured for a further 24 or 48 h, under the conditions described below, and luciferase formation then quantified using standard techniques. Briefly, the cells were placed on ice, washed twice with ice-cold phosphate-buffered saline, disrupted with 100 μl of reporter lysis buffer and allowed to stand at room temperature for 15 min. The cells were then scraped into 500 μl tubes and vortexed for 2 min. Cellular debris was removed by centrifugation and luciferase activity assayed luminetrically (Luciferase Assay Buffer, Promega, UK) in duplicate, 20 μl aliquots of supernatant. The results of this analysis are presented as counts per second and have been corrected for any differences in protein content, which was determined by the Bradford method. In some experiments cells were co-transfected with the luciferase reporter constructs and 2 μg of p SVβ-gal reporter vector (Promega) which consists of a β-galactosidase gene coupled to the constitutively active SV40 promoter. In these experiments β-galactosidase formation was assessed by using a colorimetric assay system (Promega) to determine the activity of this enzyme in aliquots of cell lysate. These data are presented as absorbance units (a.u.) and have also been corrected for variations in the amount of cellular protein.
Experimental design and data analysis
Experiments were undertaken using a strictly paired protocol in which cells that had originated from the same litters were divided into three groups which were initially cultured (48-120 h) at an ambient PO2 equivalent to that found in either the fetal (23 mmHg) or the neonatal alveolar regions (100 mmHg). These atmospheres were maintained by the regulated introduction of nitrogen into the incubators. In all experiments, one group of cells was transferred from the fetal to the neonatal environment in order to impose a rise in PO2 equivalent to that which occurs during the few hours that follow birth. This manipulation was undertaken 6, 24 or 48 h before the cells were used in experiments. The data derived from cells that had experienced this shift in PO2 were then compared (Student’s paired t test) with the equivalent data derived from control cells, which had been maintained at fetal PO2 throughout the entire culture period. Each such experiment was undertaken in duplicate or in triplicate, and data are presented as means ±s.e.m. Values of n refer to the number of experiments undertaken using cells derived from different litters.
Quantification of NF-κB activity
The effects of increasing PO2 upon the activity of NF-κB was determined using an electrophoretic mobility shift assay which measures the abundance of protein species able to bind specific nucleotide sequences. The method is described elsewhere and so only brief details are presented here (Haddad & Land, 2000; Haddad et al. 2000). The oligonucleotide probe used (5′AGTTGAGGGGACTTTCCCAGGC-3′) contained a consensus-binding site for NF-κB (underlined) and was end-labelled with [γ32P]-ATP. Assays were undertaken using nuclear protein extracted from FDLE cells that had either been maintained at fetal PO2 or transferred to the neonatal alveolar environment up to 96 h before nuclear protein was harvested. Aliquots (1-5 μg) of extracted protein were incubated (30 min, 25 °C) with identical amounts of 32P-labelled probe (22-40 nCi) in 40 μl of DNA binding buffer and proteins then separated from the more mobile oligonucleotides by electrophoresis on 4 % polyacrylamide gels. The amount of oligonucleotide associated with protein was then determined by measuring (Canberra-Packard Instant Imager) the amount of 32P associated with the less mobile band. Data are presented as increases above the binding activity determined for cells maintained at fetal PO2.
RESULTS
Changes in ISC
The data presented in Fig. 1A show that cells maintained continually at neonatal alveolar PO2 generate a larger spontaneous ISC than do cells cultured at fetal PO2 (see also Pitkänen et al. 1996; Ramminger et al. 2000). Moreover, increased ISC was evident in cells that had been exposed to the adult alveolar environment for 24 h (Fig. 1Aic) or 48 h (Fig. 1Aiic) and there was no statistically significant difference between the responses quantified (i.e. as increase in ISC) at these two time points (3.1 ± 0.06 and 4.6 ± 0.8 μA cm−2, respectively. These experiments were undertaken using cells that had been maintained in culture for 96-120 h which is longer that the 48 h period used in our previous study (Ramminger et al. 2000). This difference was introduced so that electrometric experiments (Fig. 1A) and molecular (Fig. 1B) studies could be undertaken using cells maintained under identical conditions. However, despite this difference between the two studies, the present increases in ISC seen 24 and 48 h after PO2 was raised did not differ from the equivalent responses reported in our previous study (Ramminger et al. 2000). The present data thus confirm that a rise in PO2 mimicking that seen at birth evokes increased Na+ absorption in FDLE cells (see also Pitkänen et al. 1996; Ramminger et al. 2000) and show that the time spent in culture has no significant effect upon this response.
Figure 1. O2-evoked changes in ISC and in transcriptional activity of the α-ENaC promoter.
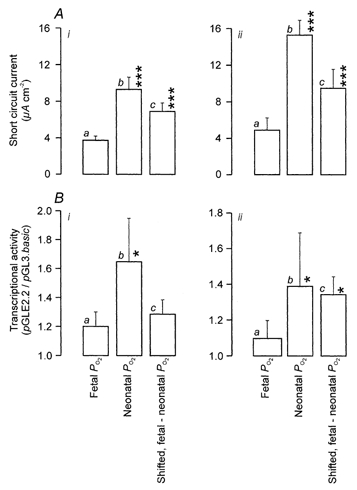
Experiments were undertaken using cells that were maintained continually at either fetal (a) or neonatal alveolar PO2 (b), or were transferred between these environments in order to impose a rise in PO2 equivalent to that seen at birth (c). This shift in PO2 was imposed 24 h (ic) or 48 h (iic) before the cells were used in experiments. The cells were thus maintained in culture for a total of 96 h (i) or 120 h (ii), the prolonged culture period being needed to allow the cells to be transfected with the reporter constructs. A, the total ISC generated by cells maintained under each of the three regimes (n = 5 for each). B, transcriptional activity of the α-ENaC promoter was determined for cells maintained under each regime (n = 5 for each). Luciferase production by cells transfected with p GLE2.2, which contains the α-ENaC promoter region, was determined and is presented as a fraction of the luciferase formation determined in cells derived from the same litters that had been transfected with the promoterless p GLE.basic construct but otherwise treated identically. Asterisks denote values that differed significantly from the appropriate data (ia and iia) derived from cells maintained continually at fetal PO2 (*P < 0.05, ***P < 0.01).
Transcriptional activity of the α-ENaC promoter
Three separate groups of cells (n = 4) were co-transfected with one of the three luciferase reporter constructs, and with pSVβ-gal, and then cultured at neonatal alveolar PO2 for a further 24 h. The activity of each construct was then determined by measuring luciferase and β-galactosidase formation. Cells expressing the pGL3.control construct, which contains the spontaneously active promoter, generated more luciferase activity (327 ± 153 counts s−1, P < 0.05) than did cells transfected with the promoterless p GL3.basic (56 ± 9 counts s−1). Moreover, expression of p GL3E2.2, the construct incorporating the α-ENaC promoter, led to a level of luciferase activity (103 ± 19 counts s−1) lower than that seen in cells expressing p GL3.control (P < 0.05) but greater than that associated with the promoterless p GL3.basic construct. However, despite these variations in luciferase activity, similar levels of β-galactosidase activity were measured in each group of cells (p GL3.control, 0.124 ± 0.004 a.u., p GL3E2.2, 0.123 ± 0.005 a.u., p GL3.basic, 0.120 ± 0.002 a.u.). The differences in luciferase activity cannot, therefore, be attributed to variations in transfection efficiency and so these data show that p GL3.control, which contains the spontaneously active promoter, is ≈6-fold more active than p GL3.basic. This establishes that differences in transcription activity can be discerned using this assay system. The luciferase assays also show that p GL3E2.2 is less active than p GL3.control (P < 0.05) but more active than p GL3.basic (P < 0.05), and so the α-ENaC promoter appears to display a low but significant level of activity (≈1.8-fold above basal) under these conditions. Subsequent experiments using a culture regime identical to that used to study the effects of PO2 upon ISC (Fig. 1A) explored the effects of increasing PO2 upon the transcriptional activity of p GL3E2.2. These experiments (Fig. 1B) indicated that increased activity was always evident in cells maintained at neonatal alveolar PO2. However, raising PO2 for only 24 h had no discernible effect (Fig. 1Bi) although a significant response did become apparent after 48 h (Fig. 2Bii). A rise in PO2 mimicking that seen at birth can thus activate the α-ENaC promoter, but this response, in contrast to the rise in ISC, occurs only after prolonged exposure to the neonatal atmosphere.
Figure 2. Quantification of apical Na+ conductance in a basolaterally permeabilised cell.
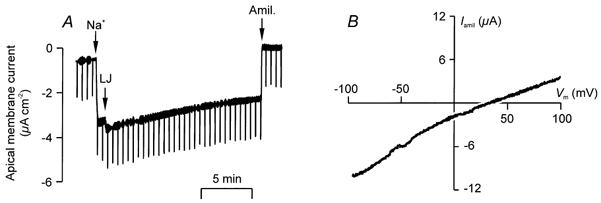
A, cultured FDLE cells were mounted in Ussing chambers and bathed symmetrically with a cytoplasm-like solution in which [Na+], [K+] and [Cl−] were 11.5, 136.4 and 10.3 mm, respectively. At the point indicated by the first arrow (Na+), apical [Na+] was raised to 55 mm by isosmotically replacing K+. The current deflection (LJ) denotes the point at which Vm was adjusted to compensate for the changed liquid junction potential. Amiloride (10 μm) was added to the apical solution as indicated (Amil). Vm was normally held at 0 mV but transepithelial resistance was monitored by recording the currents elicited by 1 mV excursions in Vm. B, apical membrane currents were recorded under the conditions described above and the average response to a series of 4 voltage ramps (from -100 to +100 mV over 20 s) measured under control conditions and 2-3 min after addition of apical amiloride (10 μm). The amiloride-sensitive component of the total current (Iamil) was then calculated and is plotted against Vm.
Quantification of apical Na+ conductance
Figure 2A shows membrane currents recorded from basolaterally permeabilised cells cultured at neonatal alveolar PO2. Vm was held at 0 mV throughout the experiment and, initially, the cells were bathed symmetrically with a cytoplasm-like solution. Under these conditions, the recorded current is close to zero which was anticipated since there is no driving force for the movement of any ion. However, raising apical [Na+] to 55 mm evoked an inwardly directed current that could be essentially abolished by apical amiloride (Fig. 2A). To explore the properties of this current (Iamil) further we undertook experiments to establish its relationship with Vm. These studies (Fig. 2B) showed that Iamil displayed inward rectification and a mean reversal potential (Vrev) of 38.3 ± 7.8 mV (n = 5, range 29.0-42.4 mV, Fig. 2B). The equilibrium potentials for K+ and Na+ (EK and ENa) under these ionic conditions are -10.2 and 41.8 mV, respectively, and so the mean value of Vrev is close to ENa demonstrating that Iamil is due, predominantly, to Na+ influx via a selective pathway. To determine the degree to which the channels underlying Iamil discriminate between Na+ and K+, their sodium permeability (PNa) was assigned a value of unity whilst their potassium permeability (PK) was set to an initial, arbitrary estimate. A computer algorithm then reiteratively adjusted the value of PK in order to provide a solution to the Goldman-Hodgkin-Huxley equation consistent with the observed value of Vrev. This analysis showed that the apical conductance was 14-fold more permeable to Na+ than to K+.
PO2-evoked changes in GNa
Under the conditions shown in Fig. 2A, the magnitude of the amiloride-sensitive Na+ conductance (GNa) can be calculated from the equation GNa=Iamil/(Vm - ENa). Moreover, as Vm and ENa are determined by the experimental conditions, we could use the values of Iamil measured in such experiments to derive estimates of GNa. We therefore used this approach to explore the possibility that the PO2-evoked increase in ISC (Fig. 1A) may reflect changes in GNa. However, these experiments, in contrast to the reporter gene assays, did not require the cells to be maintained in culture for prolonged periods and so were undertaken using cells that were cultured for only 48-72 h. We therefore also characterised the responses to increased PO2 in intact cells cultured under these conditions. These data confirmed once again that cells maintained continually at neonatal alveolar PO2 generate a larger spontaneous short circuit current than cells maintained under fetal conditions (Pitkänen et al. 1996; Ramminger et al. 2000). No statistically significant increase in ISC was seen in cells exposed to increased PO2 for only 6 h (Fig. 3Aic) but clear responses were evident after 24 h (Fig. 3Aiic, ΔISC 2.6 ± 0.6 μA cm−2) and 48 h (Fig. 3Aiiic, ΔISC 4.5 ± 0.8 μA cm−2). The magnitudes of these responses did not differ significantly from those shown in Fig. 1A, providing further evidence that the duration of the culture period has no significant effect upon the response to increased PO2.
Figure 3. Effects of PO2 upon ISC, GNa and the Na+ extrusion capacity of the Na+ pump.
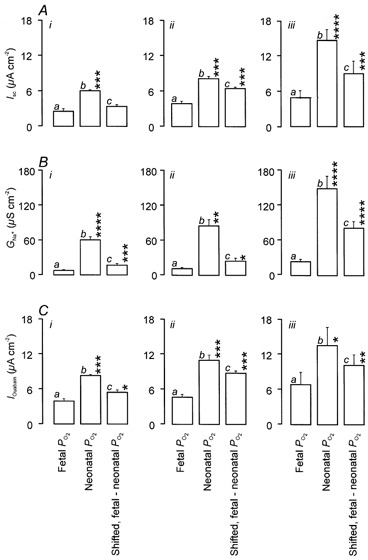
Experiments were undertaken using cultured epithelia maintained continually at either fetal (a) or neonatal alveolar PO2 (b), or exposed to a shift in PO2 equivalent to that seen at birth (c). This manoeuvre was undertaken 6 h (ic), 24 h (iic) or 48 h (iiic) before the cells were used in experiments (n = 5 for each). The cells used had been cultured for a total of 48 h (i and ii) or 72 h (iii). A, the total ISC recorded from cells maintained under the 3 culture regimes. B, estimates of GNa derived from experiments undertaken using basolaterally permeabilised cells. C, the capacity of the basolateral Na+ pump estimated from the ouabain-evoked fall in ISC measured in apically permeabilised cells. Asterisks denote values that differed significantly (*P < 0.05, **P < 0.02, ***P < 0.01, ****P < 0.001) from the appropriate control data derived from cells that had been maintained continually at fetal PO2 (ia, iia and iiia).
Subsequent studies showed that GNa in cells maintained continually under neonatal alveolar conditions was ≈6-fold greater than in cells maintained in the fetal atmosphere (Fig. 3B). Raising PO2 to its neonatal alveolar value for only 6 h caused a small but significant rise in GNa (Fig. 3Bi) but this response (9.3 ± 1.8 μS cm−2) was smaller (≈15 %, P < 0.001) than the increase calculated for the cells that had been maintained continually at neonatal alveolar PO2. A rise in conductance (16.8 ± 2.9 μS cm−2) was also seen 24 h after PO2 was raised (Fig. 3Bii) and this response did not differ significantly from that seen at 6 h but was smaller (≈20 %, P < 0.005) than that recorded in cells maintained continually at neonatal alveolar PO2. A substantial rise in conductance (59.2 ± 12.9 μS cm−2) did, however, become evident once the cells that had been exposed to increased PO2 for 48 h (Fig. 3Biii) and analysis showed that this increase in conductance was larger than that seen in cells that had been exposed to increased PO2 for 6 h (Fig. 3Bi, P < 0.01) or 24 h (Fig. 3Bii, P < 0.01). Increased PO2 thus causes a rise in GNa but, in contrast to the rise in ISC, this response is seen only after PO2 has been raised for 48 h.
Na+ pump capacity
The effects of PO2 upon Na+ pump capacity were investigated by studying cultured epithelia that had been apically permeabilised using nystatin (50 μm) as described elsewhere (Ramminger et al. 2000). The first such experiments confirmed the findings of Ramminger et al. (2000) by showing that larger pump currents were generated by cells maintained at neonatal alveolar PO2 (Fig. 3B). Parallel studies demonstrated that these cells maintain their adenylate energy charge at a value of ≈0.7 at both fetal and neonatal alveolar PO2 (Haddad & Land, 2000; Haddad et al. 2000) and so this effect cannot be simply attributed to reduced availability of ATP. Increased Na+ pump capacity was evident in cells exposed to the neonatal atmosphere for only 6 h but this effect was small (Fig. 3Bi) and a larger (P < 0.01) rise was evident after 24 h (Fig. 3Bii). Indeed, the Na+ pump currents recorded 24 h after PO2 was raised did not differ significantly from those seen after 48 h (Fig. 3Biii).
Activation of NF-κB
Analysis of nuclear protein showed that increased NF-κB activity was evident in cells that had been exposed to the neonatal alveolar atmosphere for only 5 min (Fig. 4). This response reached a clearly defined peak after ≈15 min and, thereafter, there was a slight fall in activity which was followed by a slower and less clearly defined rise to a second peak that was reached after ≈60 h (Fig. 4).
Figure 4. PO2-evoked activation of NF-κB.
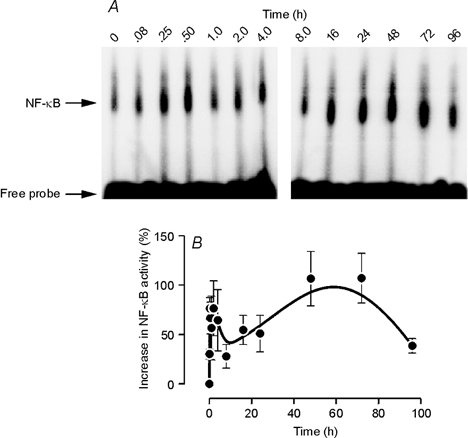
FDLE cells in monolayer culture were first maintained at fetal PO2 and then transferred into the adult alveolar environment in order to impose a shift in PO2 equivalent to that seen at birth. Nuclear protein was extracted 0-96 h after the imposition of this shift in PO2, and the effects of this manoeuvre upon NF-κB activity determined (see Methods). A, representative images which together show the PO2-evoked changes in NF-κB activity over the time course of this experiment. B, pooled data (means ±s.e.m., n = 4) showing the time course of the PO2-evoked changes in NF-κB activity.
DISCUSSION
Previous studies of FDLE cells (Pitkänen et al. 1996) showed that raising PO2 from its fetal level increases the abundance of mRNA encoding α-, β- and γ-ENaC, the proteins which form the channels permitting apical Na+ entry in alveolar epithelia (Canessa et al. 1993, 1994; Hummler et al. 1996). This result raised the possibility that increases in PO2 might stimulate Na+ transport by evoking the expression of genes encoding these proteins and so increasing GNa (Pitkänen et al. 1996). However, not all data are consistent with this hypothesis as the stimulation of Na+ transport precedes a discernible change in mRNA abundance (Pitkänen et al. 1996). Moreover, recent work from this laboratory showed that increases in PO2 also increase the capacity of the Na+ pump and so this basolateral protein may also be involved in the response (Ramminger et al. 2000). The present study therefore explores the effects of increased PO2 upon GNa and Na+ pump capacity, and also upon the transcriptional activity of the promoter region of the α-ENaC gene, the expression of which is vital for lung liquid clearance at birth (Hummler et al. 1996).
Events underlying O2-evoked Na+ transport
Studies of intact cells showed that increasing PO2 for only 6 h had no statistically significant effect upon ISC. However, despite this lack of an overall effect, studies of nystatin-permeabilised cells showed that this relatively brief exposure to increased PO2 had elicited small increases in both Na+ pump capacity and GNa. By 24 h there was an unambiguous rise in ISC that was accompanied by a further rise in Na+ pump capacity but no change in GNa. The PO2-evoked stimulation of Na+ transport thus develops during the 6-24 h period following the imposition of a rise in PO2 and appears to be due to a small rise in GNa (present study) together with a substantial rise in Na+ pump capacity (Ramminger et al. 2000). The latter effect would increase the driving force for apical Na+ entry, both by lowering the internal [Na+] and by hyperpolarising the cell. However, it is important to remember that a fall in intracellular [Na+] may increase GNa by activating apical Na+ channels (see for example, Dinudom et al. 1998). The consequences of such ionic control over GNa would not have been detected in the present study, where the apical membrane’s constitutive Na+ conductance was assessed under constant ionic conditions. Nevertheless, the present study did show that increasing PO2 causes a substantial rise in GNa, and this part of the response coincided with increased activity of the α-ENaC promoter (present study) and with the previously reported increase in ENaC mRNA abundance (Pitkänen et al. 1996). It thus appears that increases in PO2 can lead to increased expression of the genes encoding ENaC subunits but that this does not occur until 24-48 h after PO2 is raised. Increased expression of the α-ENaC gene is thus a relatively late response to increased PO2 and so this event cannot underlie the stimulation of Na+ transport. Furthermore, the products of the ENaC genes are present in the lungs before birth and so it is obvious that the expression of this gene family is controlled by factors other than increased PO2 (Venkatech & Katzberg, 1997; Baines et al. 2000a; Gaillard et al. 2000). Previous studies have shown that delivery is associated with increased capacity of the Na+ pump (Bland & Boyd, 1986; Bland, 1990) and our data suggest that this is an early, physiological response to a rise in PO2 equivalent to that seen at birth (present study; Ramminger et al. 2000). The activation of the α-ENaC promoter which we now report may well be secondary to the resultant changes in the rate of Na+ transport.
Properties of GNa
Studies of basolaterally permeabilised cells showed that Iamil reflects the movement of Na+ through a conductance that could discriminate clearly between Na+ and K+ (see also Jiang et al. 1998) indicating that the spontaneous ISC involves the inward movement of Na+ through selective Na+ channels in the apical membrane. Such channels have been described in Xenopus oocytes co-injected with mRNA encoding the α-, β- and γ-ENaC subunits (Canessa et al. 1994) and are found in many Na+-absorbing tissues (see review by Benos et al. 1997). However, almost all electrophysiological studies of FDLE cells suggest that apical Na+ entry occurs via channels that do not distinguish between Na+ and K+ (Orser et al. 1991; MacGregor et al. 1994; Tohda et al. 1994; Marunaka, 1996, 1999; Matalon & O’Brodovich, 1999). The structural relationship between these non-selective cation channels and the selective Na+ channels is not clear but there is evidence that such channels are formed if α-ENaC is expressed independently of the β- and γ-subunits (Kizer et al. 1997; Jain et al. 1999). Whilst this would reconcile the crucial importance of α-ENaC to lung liquid clearance (Hummler et al. 1996) with the presence of non-selective cation channels, FDLE cells do seem to express all three ENaC subunits (see review by Matalon & O’Brodovich, 1999). Moreover, the spontaneous ISC generated by these cells is reduced by basolateral Ba2+, a K+ channel blocker, suggesting that tonic activity of basolateral K+ channels may maintain a driving force for Na+ entry by hyperpolarising the cell (O’Brodovich & Rafii, 1993). It is difficult to see how this mechanism could operate were a substantial, non-selective cation conductance present in the apical membrane. However, at least one study of cultured FDLE cells has identified small, highly selective Na+ channels essentially identical to those found in the classical Na+-absorbing tissues (Voilley et al. 1994). It is therefore clear that FDLE cells can express true Na+ channels under certain conditions and data from basolaterally permeabilised preparations (present study, Jiang et al. 1998) suggest strongly that it is these channels, rather than non-selective cation channels, that allow apical Na+ entry under basal conditions. It may well be that a polarised cell preparation retains the characteristics of the Na+ channels found in vitro more accurately than isolated cells.
Role of NF-κB
NF-κB is a redox-sensitive transcription factor that becomes active in FDLE cells when PO2 is raised from its fetal level (Rafii et al. 1998; Haddad & Land, 2000; Haddad et al. 2000). Structural analysis of the α-ENaC promoter has revealed an NF-κB binding site raising the possibility that activation of this transcription factor may underlie the PO2-evoked increase in ENaC expression (Rafii et al. 1998; Otulakowski et al. 1999). The present study shows, however, that the physiological response to increased PO2 is relatively slow whilst the activation of NF-κB is essentially instant. There is thus a discrepancy between the time required for activation of NF-κB and for the increased transcription of genes encoding ENaC subunits (present study; Pitkänen et al. 1996). Whilst this does not preclude a role for NF-κB in the response, it is difficult to see how this discrepancy could arise were NF-κB the only signalling factor controlling α-ENaC transcription in O2-stimulated cells. However, the α-ENaC promoter does contain binding sites for other transcription factors (Otulakowski et al. 1999) and it may be that several of these must be activated simultaneously before increased transcription can proceed.
Biological significance of present findings
Studies of fetal lambs showed that the absorption of liquid from the lung begins only during the very last stages of labour and occurs in response to the high levels of fetal adrenaline seen at this time (Brown et al. 1983; Olver et al. 1986). These experiments also indicated that an appreciable volume of liquid remains in the lung at birth, and so liquid absorption must continue into the postnatal period despite falling adrenaline levels (Brown et al. 1983). Furthermore, studies of fetal guinea-pigs (Baines et al. 2000a) showed that animals delivered prematurely by Caesarean section initially had abnormally large amounts of liquid present in the lungs and displayed both respiratory distress and reduced lung liquid absorption. Subsequently, even though the animals had not experienced labour and delivery, α-ENaC mRNA levels rose to levels greater than those seen in normally delivered animals and this expression of α-ENaC was associated with the removal of the excess lung liquid, stimulation of amiloride-sensitive fluid absorption and resolution of the respiratory distress (Baines et al. 2000a). O2-evoked fluid absorption may thus be particularly important to the prematurely delivered infant in which β-adrenoceptor-mediated control of fluid absorption is poorly developed (Brown et al. 1983). The distal lung epithelium’s inherent sensitivity to O2 (present study; Pitkänen et al. 1996; Rafii et al. 1998; Ramminger et al. 2000) also implies that the rise in alveolar PO2 occurring at birth may be an important maturational stimulus that provides a drive for continued Na+ absorption in the early newborn period and allows the lung to maintain a Na+-absorbing phenotype throughout adult life in the absence of sustained adrenergic stimulation.
Acknowledgments
The authors are grateful to the Wellcome Trust and to Tenovus (Scotland) for their financial support which made this study possible; to Drs S. Jovanovi’c, S. K. Inglis and A. Jovanovi’c for their helpful comments on the manuscript, and to H. L. Murphie and H. McHardy for their skilled technical assistance.
References
- Acarregui MJ, Snyder JM, Mendeson CR. Oxygen modulates the differentiation of human fetal lung in vitro and its responsiveness to cAMP. American Journal of Physiology. 1993;264:L465–474. doi: 10.1152/ajplung.1993.264.5.L465. [DOI] [PubMed] [Google Scholar]
- Baines DL, Folkesson HG, Norlin A, Bingle CD, Yuan HT, Olver RE. The influence of mode of delivery, hormonal status and postnatal O2 environment on epithelial sodium channel (ENaC) expression in guinea-pig lung. Journal of Physiology. 2000a;522:147–157. doi: 10.1111/j.1469-7793.2000.t01-2-00147.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines DL, Ramminger SJ, Collett A, Best OG, Olver RE, Wilson SM. PO2-evoked increases in epithelial Na+ channel α-subunit gene transcription Na+ transport in fetal distal lung epithelial cells. Journal of Physiology. 2000b;527.P:24P. [Google Scholar]
- Barker PM, Gatzy JT. Effect of gas composition on liquid secretion by explants of distal lung of fetal rat in submersion culture. American Journal of Physiology. 1993;265:L512–517. doi: 10.1152/ajplung.1993.265.5.L512. [DOI] [PubMed] [Google Scholar]
- Barker PM, Styles AD, Boucher RC, Gatzy JT. Bioelectric properties of cultured epithelial monolayers from distal lung of 18-day fetal rat. American Journal of Physiology. 1992;262:L628–636. doi: 10.1152/ajplung.1992.262.5.L628. [DOI] [PubMed] [Google Scholar]
- Benos DJ, Fuller CM, Shlyonsky VG, Berdiev BK, Ismailov II. Amiloride-sensitive Na+ channels: insights and outlooks. News in Physiological Sciences. 1997;12:55–61. [Google Scholar]
- Bland RD. Lung epithelial ion transport and fluid movement during the perinatal period. American Journal of Physiology. 1990;259:L30–37. doi: 10.1152/ajplung.1990.259.2.L30. [DOI] [PubMed] [Google Scholar]
- Bland RD, Boyd CAR. Cation transport in lung epithelial cells derived from fetal, newborn and adult rabbits. Journal of Applied Physiology. 1986;61:507–515. doi: 10.1152/jappl.1986.61.2.507. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Olver RE, Ramsden CA, Strang LB, Walters DV. Effects of adrenaline and of spontaneous labour on the secretion and absorption of lung liquid in the fetal lamb. Journal of Physiology. 1983;344:137–152. doi: 10.1113/jphysiol.1983.sp014929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa CM, Horisberger JD, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–466. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Dinudom A, Harvey KF, Komwatana P, Young JA, Kumar S, Cook DI. Nedd4 mediates control of an epithelial Na+ channel in salivary duct cells by cytosolic Na+ Proceedings of the National Academy of Sciences of the USA. 1998;95:7169–7173. doi: 10.1073/pnas.95.12.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard D, Hinnrasky J, Coscoy S, Hofman P, Matthay MA, Puchelle E, Barbry P. Early expression of β- and γ-subunits of epithelial sodium channel during human airway development. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2000;278:L177–184. doi: 10.1152/ajplung.2000.278.1.L177. [DOI] [PubMed] [Google Scholar]
- Haddad JJE, Land SC. O2-evoked regulation of HIF-α and NF-κB in perinatal lung epithelium requires glutathione biosynthesis. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2000;278:L492–503. doi: 10.1152/ajplung.2000.278.3.L492. [DOI] [PubMed] [Google Scholar]
- Haddad JJE, Olver RE, Land SC. Antioxidant/pro-oxidant equilibrium regulates HIF-1α and NF-κB redox sensitivity: evidence for inhibition by glutathione oxidation in alveolar epithelial cells. Journal of Biological Chemistry. 2000;275:21130–21139. doi: 10.1074/jbc.M000737200. [DOI] [PubMed] [Google Scholar]
- Hummler E, Baker P, Gatzy J, Berrmann F, Verdumo C, Schmidt A, Boucher R, Rossier RC. Early death due to defective neonatal lung liquid clearance in α-ENaC-deficient mice. Nature Genetics. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- Jain L, Chen X-C, Malik B, Al-Khalili O, Eaton DC. Antisense oligonucleotides against the α-subunit of ENaC decrease lung epithelial cation channel activity. American Journal of Physiology. 1999;276:L1046–1051. doi: 10.1152/ajplung.1999.276.6.L1046. [DOI] [PubMed] [Google Scholar]
- Jiang X, Ingbar DH, O’Grady SM. Adrenergic stimulation of Na+ transport across alveolar epithelial cells involves activation of apical Cl- channels. American Journal of Physiology. 1998;275:C1610–1620. doi: 10.1152/ajpcell.1998.275.6.C1610. [DOI] [PubMed] [Google Scholar]
- Kizer N, Guo XL, Hruska K. Reconstitution of stretch activated cation channels by expressing alpha-subunit of the epithelial sodium channel cloned from osteoblasts. Proceedings of the National Academy of Sciences of the USA. 1997;94:1013–1018. doi: 10.1073/pnas.94.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SA, Eaton DC, Clausen C, Diamond JM. Nystatin as a probe for investigating the electrical properties of a tight epithelium. Journal of General Physiology. 1977;70:427–440. doi: 10.1085/jgp.70.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor GG, Olver RE, Kemp PJ. Amiloride-sensitive Na+ channels in fetal type II pneumocytes are regulated by G proteins. American Journal of Physiology. 1994;267:L1–8. doi: 10.1152/ajplung.1994.267.1.L1. [DOI] [PubMed] [Google Scholar]
- Marunaka Y. Amiloride-blockable Ca2+-activated Na+-permeant channels in the fetal distal lung epithelium. Pflügers Archiv. 1996;431:748–756. doi: 10.1007/BF02253839. [DOI] [PubMed] [Google Scholar]
- Marunaka Y, Niisato N, O’Brodovich H, Eaton DC. Regulation of an amiloride-sensitive Na+-permeable channel by a β2-adrenergic agonist, cytosolic Ca2+ and Cl- in fetal rat alveolar epithelium. Journal of Physiology. 1999;515:669–683. doi: 10.1111/j.1469-7793.1999.669ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon S, O’Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties and physiological significance. Annual Review of Physiology. 1999;61:627–661. doi: 10.1146/annurev.physiol.61.1.627. [DOI] [PubMed] [Google Scholar]
- O’Brodovich H, Rafii B. Effect of K channel blockers on basal and β-agonist stimulated ion transport by fetal distal lung epithelium. Canadian Journal of Physiology and Pharmacology. 1993;71:54–57. doi: 10.1139/y93-008. [DOI] [PubMed] [Google Scholar]
- Olver RE, Ramsden CA, Strang LB, Walters DV. The role of amiloride-blockable sodium transport in adrenaline-induced lung liquid reabsorption in the fetal lamb. Journal of Physiology. 1986;376:321–340. doi: 10.1113/jphysiol.1986.sp016156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olver RE, Strang LB. Ion fluxes across the pulmonary epithelium and the secretion of lung liquid in the fetal lamb. Journal of Physiology. 1974;241:327–357. doi: 10.1113/jphysiol.1974.sp010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orser BA, Bertlik M, Fedorko L, O’Brodovich H. Cation selective channel in fetal alveolar type II epithelium. Biochimica et Biophysica Acta. 1991;1094:19–26. doi: 10.1016/0167-4889(91)90021-o. [DOI] [PubMed] [Google Scholar]
- Otulakowski G, Raffii B, Bremner HR, O’Brodovich H. Structure and hormone responsiveness of the gene encoding the α-subunit of the rat amiloride-sensitive epithelial sodium channel. American Journal of Respiration - Lung Cellular and Molecular Physiology. 1999;20:L1028–1040. doi: 10.1165/ajrcmb.20.5.3382. [DOI] [PubMed] [Google Scholar]
- Pitkänen OM, Tanswell AK, Downey G, O’Brodovich H. Increased PO2 alters the bioelectric properties of the fetal distal lung epithelium. American Journal of Physiology. 1996;270:L1060–1066. doi: 10.1152/ajplung.1996.270.6.L1060. [DOI] [PubMed] [Google Scholar]
- Rafii B, Tanswell AK, Otulakowski G, Pitkänen O, Belcastro-Taylor R, O’Brodovich H. O2-induced ENaC expression is associated with NF-κB activation and blocked by superoxide scavenger. American Journal of Physiology. 1998;275:L764–770. doi: 10.1152/ajplung.1998.275.4.L764. [DOI] [PubMed] [Google Scholar]
- Ramminger SJ, Baines DL, Olver RE, Wilson SM. The effects of PO2 upon transepithelial ion transport in fetal rat distal lung epithelial cells. Journal of Physiology. 2000;524:539–547. doi: 10.1111/j.1469-7793.2000.t01-1-00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramminger SJ, Collett A, Baines DL, Murphie H, McAlroy HL, Olver RE, Inglis SK, Wilson SM. P2Y2 receptor-mediated inhibition of ion transport in distal lung epithelial cells. British Journal of Pharmacology. 1999;128:293–300. doi: 10.1038/sj.bjp.0702767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JEC, Junor RWJ, Gallacher ME, Walters DV. The effects of in vivo pulmonary oxygenation on lung liquid production in near-term fetal sheep. Experimental Physiology. 1999;84:725–738. [PubMed] [Google Scholar]
- Tohda H, Foskett JK, O’Brodovich H, Marunaka Y. Cl- regulation of a Ca2+-activated nonselective cation channel in β-agonist-treated fetal distal lung epithelium. American Journal of Physiology. 1994;266:C104–109. doi: 10.1152/ajpcell.1994.266.1.C104. [DOI] [PubMed] [Google Scholar]
- Venkatech VC, Katzberg HD. Glucocorticoid regulation of epithelial sodium channel genes in human fetal lung. American Journal of Physiology. 1997;273:L227–233. doi: 10.1152/ajplung.1997.273.1.L227. [DOI] [PubMed] [Google Scholar]
- Voilley N, Lingueglia E, Champigny G, Mattei MG, Waldmann R, Lazdunski M, Barbry P. The lung amiloride-sensitive Na+ channel: biophysical properties, pharmacology, ontogenesis, and molecular cloning. Proceedings of the National Academy of Sciences of the USA. 1994;91:247–251. doi: 10.1073/pnas.91.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters DV, Olver RE. The role of catecholamines in lung liquid absorption at birth. Pediatric Research. 1978;12:239–242. doi: 10.1203/00006450-197803000-00017. [DOI] [PubMed] [Google Scholar]


